Abstract
Clinical evidence links increased aortic collagen content and stiffness to abdominal aortic aneurysm (AAA) formation. However, the possibility that excess collagen contributes to AAA formation remains untested. We investigated the hypothesis that augmentd collagen promotes AAA formation, and employed apoE-null mice expressing collagenase-resistant mutant collagen (ColR/R/apoE−/−), heterozygote (ColR/+/apoE−/−), or wild-type collagen (Col+/+/apoE−/−) infused with angiotensin II to induce AAA. As expected, the aortas of ColR/R/apoE−/− mice contained more interstitial collagen than those from the other groups. Angiotensin II treatment elicited more AAA formation in ColR/R/apoE−/− mice than ColR/+/apoE−/− or Col+/+/apoE−/− mice. Aortic circumferences correlated positively with collagen content, determined by picrosirius red and Masson trichrome staining. Mechanical testing of aortas of ColR/R/apoE−/− mice showed increased stiffness and susceptibility to mechanical failure compared to those of Col+/+/apoE−/− mice. Optical analysis further indicated altered collagen fiber orientation in the adventitia of ColR/R/apoE−/− mice. These results demonstrate that collagen content regulates aortic biomechanical properties and influences AAA formation.
Keywords: aneurysm, collagen, metalloproteinases, pathology, remodeling
The pathogenesis of abdominal aortic aneurysm (AAA) remains uncertain, and the lack of definitive insight suggests a complex, multi-factorial process.1–4 AAA possesses major alterations in the content of extracellular matrix proteins: collagen and elastin.3, 5–7 Interstitial collagen increases arterial stiffness, a property linked to AAA formation.8, 9 Clinical evidence links increased arterial stiffness or collagen content to aortic aneurysms, while nonaneurysmal portions of aortas in AAA patients have showed increased collagen accumulation.10–20 However, whether collagen accumulation contributes causally to aneurysm formation or merely represents an epiphenomenon remains untested. To unravel the complex pathogenesis of AAA, we hypothesized that examination of both collagen content and its mechanical properties in mice with a genetic abnormality would provide novel insight.
To test directly in vivo whether aortic stiffness due to excess collagen accumulation promotes aneurysm formation, we used collagenase-resistant knock-in (ColR/R) mice.21, 22 This mouse strain bears a mutation in type I collagen, which comprises two-thirds of aortic collagen, at a cleavage site of the α1 (I) chain (Ile776 to Pro776) shared by collagenases of the matrix metalloproteinase (MMP) family (MMP-1/collagenase-1, MMP-8/collagenase-2 and MMP-13/collagenase-3). This mutation thus confers resistance to degradation, resulting in excess arterial collagen accumulation.22 Our study used angiotensin II (AngII) infusion to provoke AAA formation in apolipoprotein E-deficient (apoE−/−) mice as established by Daugherty et al.23 AngII also stimulates collagen synthesis in vitro and induces collagen turnover and remodeling in the aortic wall of apoE−/− mice with features similar to human AAA.24–26
Our results revealed that augmented collagen content induced by collagenase resistance modified the mechanical properties of the abdominal aortas of apoE−/− mice, and accelerated AAA formation. These data connect excess collagen accumulation and aortic stiffness to AAA formation, providing a novel potential mechanism for the pathogenesis of this complex disease process.
Methods
Animal experiments and tissue preparation
All mouse experiments conformed to protocols approved by the Standing Committee on Animals at Harvard Medical School. ColR/R mice, backcrossed seven generations into C57BL/6, were crossed into apoE−/− mice (C57BL/6), yielding littermates of ColR/R/apoE−/−, ColR/+/apoE−/−, and Col+/+/apoE−/− mice.22 Five-month-old male mice received AngII (1.44 mg/kg/day) infusions for four weeks with subcutaneously implanted osmotic mini-pumps (Alzet). Mice were anesthetized and perfused with 4% paraformaldehyde at a constant pressure of 100 mmHg through the heart. Aortas were dissected in the region of the superior mesenteric and right renal arteries and embedded with paraffin.
Histological analyses
Sections (6 μm-thick) were stained with Hematoxylin-Eosin, Masson trichrome, and picrosirius red. Aortic circumferences were measured on the histological sections by tracing the internal elastic lamina at the level of superior mesenteric artery in the aortas of AngII-infused Col+/+/apoE−/−, ColR/+/apoE−/−, and ColR/R/apoE−/− mice (n=8, n=6, and n=8, respectively), using the NIH Image software (Research Services Branch, National Institute of Mental Health). Aortic diameter of each mouse was then obtained based on the circumference, which determined the incidence of AAA (>1.5-fold greater than the aortas of mice untreated with AngII). Picrosirius red staining was viewed under polarized light to detect fibrillar collagen.22, 27–29 Using picrosirius red- or Masson-stained sections, adventitial collagen was measured in the tissue within 0.1 mm of the adventitial-medial border. Immunohistochemistry employed mouse monoclonal antibodies against human α-smooth muscle actin (1A4, Dako) and human cathepsin S (Serotec) with a kit for immunohistochemistry using mouse primary antibodies on mouse tissues (Iso-IHC AEC kit, InnoGenex) and rat monoclonal antibody against mouse macrophages (Mac3, Pharmingen). Quantitative histological analysis on immunostaining and collagen staining used a digital imaging system (ImagePro Plus v5.1, Media Cybernetics).22, 28
RNA extraction and Real-time RT-PCR
Total RNA extraction employed RNA-Bee (Tel-Test) and RNeasy (Qiagen) followed by reverse transcription using SuperScript II reverse transcriptase (Invitrogen). Real time RT-PCR employed PRISM7900 with SYBR green Master Mix (Applied Biosystems). Primer sequences are available upon request.
Mechanical testing of aortas
To examine the effects of collagenase resistance on the mechanical properties of the aortas, strain experiments employed freshly isolated, unfixed pre-aneurysmal abdominal aortas of Col+/+/apoE−/− (n=3) and ColR/R/apoE−/− (n=4) mice after AngII infusion (2 weeks). Parker MX80L electromechanical positioning system (Daedal Division) generated repetitive, controlled strains and ULC-1N load cell (Interface) recorded forces. Model SGA Strain Gauge Transducer Amplifier (Interface) passed through load cell signals. After determining a zero-point for each aorta, i.e., when the aorta began to experience significant changes in restoring force, aorta length at zero-point was defined as the rest length. This measurement served to calculate subsequent strains for this aorta. The aorta was preconditioned with 300 cycles of 20% strain, then stressed for 10 cycles at 70% strain, both at 0.5 Hz frequency, and finally stretched to fracture (strain-to-break) by 5% strain increments up to 250% strain. The aorta’s restoring forces was measured 20 times per cycle (10 times on extension and 10 times on relaxation), except for the strain-to-break experiment, which measured the forces at every 5% step. To determine the ultimate strength of the aortas, the strain-to-break experiment determined the stress and strain at the maximum force. The stresses, calculated by dividing the measured forces by the cross-sectional areas of the aorta, were reported as relative stresses to control stresses (which are normalized to 100%) for each experiment. Stiffness was approximated linearly using the stress at 70% strain, normalized to the control conditions.
Collagen fiber orientation
To probe further the mechanisms that link increased aortic adventitial collagen content to mechanical failure in collagenase-resistant mice, we performed additional qualitative analysis of collagen organization in the non-aneurysm portions (in Col+/+/apoE−/− and ColR/R/apoE−/− mice after 2 weeks of Ang II treatment) and aneurysm portions (in ColR/R/apoE−/− mice after 4 weeks of Ang II) of the aortic tunica adventitia in a subset of animals (n=3 for each group). Specifically, we examined and compared, using linearly polarized light and picrosirius red-stained sections, the collagen fiber extinction patterns seen in the three groups as the tissue sections were rotated on the microscope stage. When a fiber (or part of a fiber) is aligned parallel to the transmission axis of either of the microscope’s two polarizing filters, it appears dark (said to be at extinction); at other orientations, the fiber appears bright.30 Thus, the extinction pattern provides information on collagen fiber organization.
Statistics
Differences between two groups and among three groups were determined using the unpaired Student’s t-test and One-way ANOVA followed by Tukey’s test, respectively. Pearson’s test examined the correlation between collagen content and aortic size. Statistical testing for stretching experiments was determined using the unpaired Student’s t-test. To compare relative changes, we used a single-group comparison t-test to test the hypothesis that the mean relative change differs from zero.
Results
Collagenase resistance accelerated AAA formation in AngII-treated apoE−/− mice
Body weight, plasma total cholesterol and triglyceride levels in all three mouse strains treated with AngII did not differ significantly (Table). Elevated blood pressure may promote AAA formation. Among Col+/+/apoE−/−, ColR/+/apoE−/−, and ColR/R/apoE−/− strains, conscious blood pressure did not differ (Table). AngII increased the diameter of abdominal aortas at the level of the superior mesenteric artery in Col+/+/apoE−/− mice (Figure 1A, left), as demonstrated by Daugherty et al.23 However, ColR/R/apoE−/− mice exhibited greater aneurysm formation than did Col+/+/apoE−/− mice (Figure 1A, right). Histological sections showed that ColR/R/apoE−/− aorta dilated more than those of similarly treated Col+/+/apoE−/− mice (Figure 1B). Notably, the tunica intima rarely developed in the abdominal aortas of these mice. We also observed no sign of rupture or thrombus formation in the aneurysmal aorta. Quantitative analysis demonstrated that the circumference of the aortas in ColR/R/apoE−/− mice exceeded that of ColR/+/apoE−/− or Col+/+/apoE−/− mice (Figure 1C). AAA (>1.5-fold increases in diameter) developed in 50% of AngII-infused Col+/+/apoE−/− mice (Figure 1D). The incidence was greater in ColR/+/apoE−/− (83%) and ColR/R/apoE−/− mice (100%). These results indicate that collagenase resistance accelerated AAA formation in a gene dosage-dependent manner.
Table.
Characteristics of mice
| Col+/+/apoE−/− | ColR/+/apoE−/− | ColR/R/apoE−/− | ||
|---|---|---|---|---|
| n=8 | n=6 | n=8 | ||
| Body Weight (g) | 31.6±0.6 | 32.6±1.1 | 30.9 ±0.9 | n.s. |
| Cholesterol (mg/dL) | 606.3±27.5 | 635.3±63.9 | 620.2±28.5 | n.s. |
| Triglycerides (mg/dL) | 141.5±10.9 | 118.0±25.2 | 120.6±14.5 | n.s. |
| Systolic Blood Pressure* (mmHg) | 130.5±17.0 | 134.6±23.8 | 130.2±9.5 | n.s. |
Values are mean ± SEM.
measured in 4 animals of Col+/+/apoE−/−, 4 animals of ColR/R/apoE−/−, and 3 animals of ColR/+/apoE−/−.
Figure 1. Collagenase resistance accelerated AAA formation.
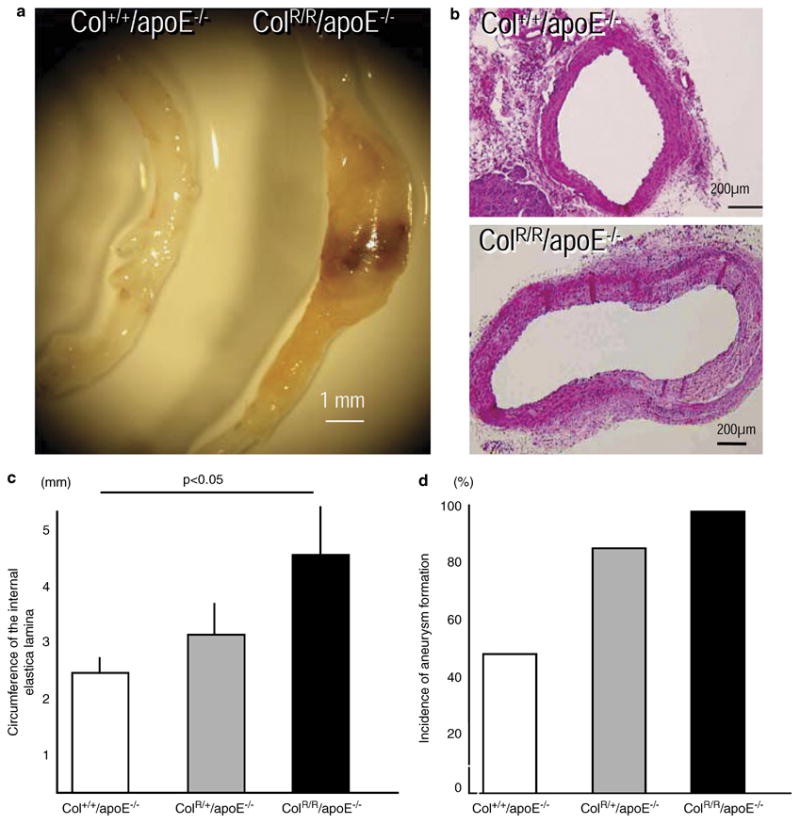
A, Representative gross appearance of abdominal aortas of AngII-treated Col+/+/apoE−/− (left) or ColR/R/apoE−/− (right) mice. B, Representative Hematoxylin-Eosin staining of the abdominal aortas from Col+/+/apoE−/− (left) and ColR/R/apoE−/− (right) mice. The aortic wall protrudes and extends to the right in the ColR/R/apoE−/− mouse. C, Quantitative analysis of circumference of the internal lamina. ColR/R/apoE−/− mice (n=8) had greater abdominal aortas compared with Col+/+/apoE−/− (n=6) or Col+/+/apoE−/− mice (n=8). Bars and error bars represent means and S.E.M, respectively. D, Collagenase resistance also increased the incidence of AAA formation (>1.5-fold increase in diameter).
Collagenase resistance induced excess collagen content in the aortic adventitia
Defining whether collagenase resistance with AngII treatment promotes excess collagen accumulation entailed picrosirius red staining for quantification of the collagen content in the aortas. Quantification of picrosirius red staining viewed with polarization measures the content of fibrillar collagen, which comprises more than 90% of aortic collagen.5 ColR/R/apoE−/− mice had more fibrillar collagen in aortas localized in the adventitia than Col+/+/apoE−/− mice (Figure 2A). Masson trichrome staining, another histological assay for collagen, also demonstrated more prominent collagen accumulation in the adventitia of ColR/R/apoE−/− mice (Figure 2B). Further, quantitative analysis of collagen content determined by picrosirius red and Masson trichrome staining demonstrated more abundant adventitial collagen in the aortas of ColR/R/apoE−/− mice vs. ColR/+/apoE−/− and Col+/+/apoE−/− mice (Figure 2C). Real-time RT-PCR showed slightly decreased levels of type I procollagen α1 mRNA in the aortas of ColR/R/apoE−/− mice compared to those of Col+/+/apoE−/− mice (Figure 2D). These results suggest that impaired collagen degradation by collagenases of the MMP family, but not increased synthesis, accounts for the greater collagen accumulation in the aortic adventitia of ColR/R/apoE−/− mice.
Figure 2. Collagen accumulates in the adventitia.
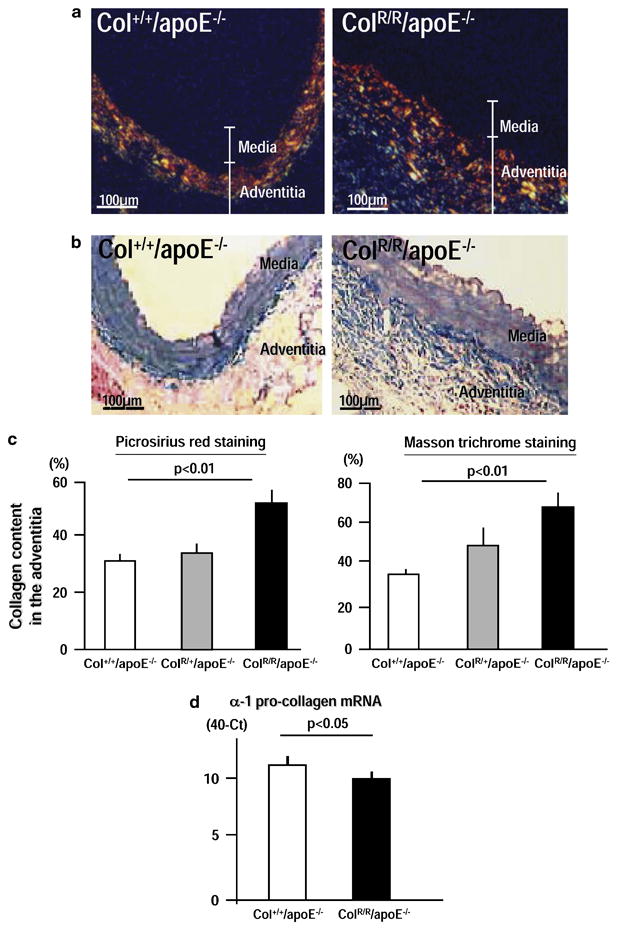
A, Representative cross-sections stained with picrosirius red viewed under polarization. B, Masson trichrome staining on cross-sections adjacent to those shown in A. C, Quantitative analysis of collagen content in the adventitia, determined by picrosirius red (left) and Masson trichrome (right) staining. Collagen increased in the adventitia of ColR/R/apoE−/− (n=8) compared to those of Col+/+/apoE−/− mice (n=8). D, Real-time RT-PCR demonstrated that α-1 collagen expression did not increase in the aortas of ColR/R/apoE−/− compared to those of ColR/R/apoE−/− mice. The data were obtained from three independent pooled total RNA samples of the aorta (n=2). Bars and error bars represent means and S.E.M, respectively.
Collagen content in the adventitia correlated with size of the aorta
Since ColR/R/apoE−/− mice had both increased collagen accumulation and accelerated formation of AAA, we tested the hypothesis that aortic collagen content correlates with abdominal aortic dimension. Regression analysis demonstrated that adventitial collagen content correlated positively with aorta circumferences in AngII-treated apoE−/− mice, as determined by both picrosirius red and Masson trichrome staining (Figure 3).
Figure 3. Collagen content in the adventitia correlates with the size of aortas.
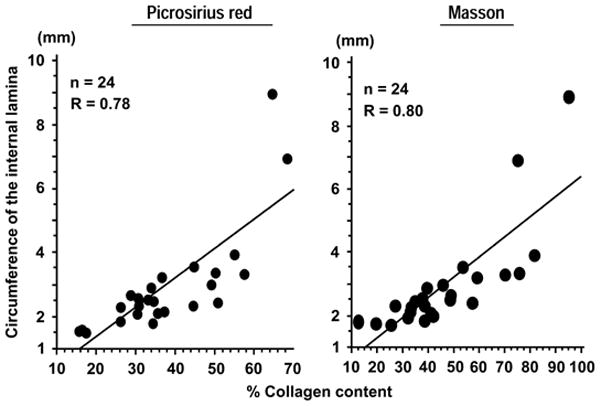
Left, correlation between circumference of the internal lamina and percentage of picrosirius-red positive areas in the adventitia. Right, correlation between circumference of the internal lamina and the percentage of Masson-trichrome positive areas in the adventitia. The data were obtained from all apoE−/− mice studied (n=24).
Collagenase resistance promoted disruption of medial elastic laminae, but did not induce expression of elastases or collagenases
Aneurysms characteristically have disrupted medial elastic laminae. In this study, the aortas of ColR/R/apoE−/− mice exhibited more prominent, but focal, medial disruption compared to the aortas of Col−/−/apoE−/− mice (Figure 4A). However, medial disruption of ColR/R/apoE−/− mice aortas did not colocalize with either macrophage accumulation, a major source of various elastolytic enzymes, or immunoreactive cathepsin S, a key elastase in vascular remodeling (Figure 4B).
Figure 4. Elastin disruption in aortas of ColR/R/apoE−/− mice following AngII infusion.
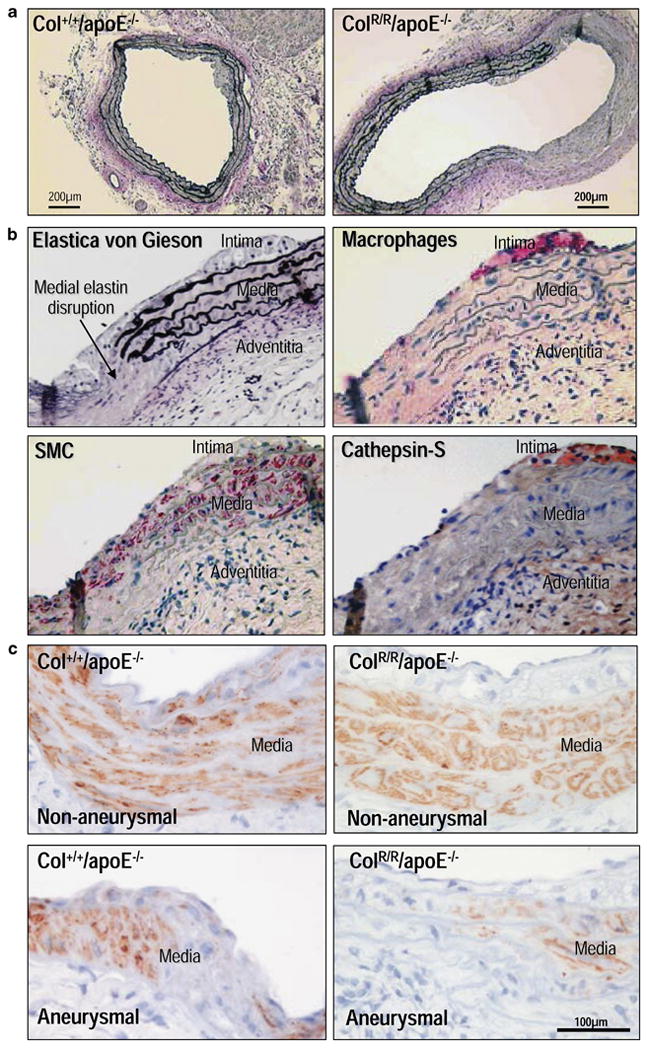
A, Elastica von Gieson staining demonstrates disrupted medial elastin in aortas of Col+/+/apoE−/− and ColR/R/apoE−/− mice. Note that ColR/R/apoE−/− showed marked loss of medial elastin (right side). B, Immunohistochemistry for macrophages (Mac-3), smooth muscle cells (α-actin), and cathepsin S in aortic wall of ColR/R/ApoE−/− mouse. The same section was stained with Elastica von Gieson. Disrupted medial elastin (arrow) did not colocalize with macrophages or immunoreactive cathepsin S. C, In non-aneurysmal regions, the majority of medial smooth muscle cells stained positively for α-actin in both Col+/+ and ColR/R mice. In contrast, in aneurysmal segments of both groups, the disrupted medial smooth muscle cell layer did not contain detectable levels of α-actin. These results were reproducible in all animals with no substantial differences between Col+/+ and ColR/R mice (n=8 each).
To address the potential role of the changes in smooth muscle cells, we performed immunohistochemistry for smooth muscle α-actin in the aortas of mice treated with angiotensin II. In all non-aneurysmal regions, the majority of medial smooth muscle cells stained positively for α-actin in both groups (Figure 4C). In contrast, in aneurysmal segments of Col+/+ and ColR/R mice, the disrupted medial smooth muscle cell layer did not contain detectable levels of α-actin. These results were reproducible in all animals with no substantial differences between wild-type and collagenase-resistant mutant mice.
Germline manipulation of a gene in mice could cause “compensatory” changes in other genes that share similar functions with the targeted gene. Such responses could hinder data interpretation and require careful consideration. Indeed, our previous study of acute myocardial infarction in MMP-9-deficient mice yielded unexpected results likely due to increases in other MMPs.31 Therefore, this study examined expression of several elastases including MMP-9, MMP-12 (MMP family elastases), and cathepsin S (a major non-MMP elastase) to evaluate whether compensatory increases could contribute to elastolysis. Quantitative real-time RT-PCR demonstrated that GAPDH-adjusted levels of mRNAs encoding MMP-9, MMP-12, and cathepsin S in the AngII-treated aortas did not differ between ColR/R/apoE−/− and Col+/+/apoE−/− mice (Figure 5A). Additionally, we evaluated the expression of these elastases in macrophages, the major source of matrix-degrading proteinases in aortas. Collagenase resistance did not affect elastase gene expression in peritoneal macrophages from ColR/R/apoE−/− mice compared to those from Col+/+/apoE−/− mice (Figure 5B). We further evaluated expression of interstitial collagenases (MMP-8 and MMP-13). In particular, MMP-13, the major interstitial collagenase in mice (a species that lacks MMP-1), has elastase activity32 and converts pro-MMP-9 into its active form.33 AngII-treated aortas and peritoneal macrophages of ColR/R/apoE−/− and Col+/+/apoE−/− mice expressed similar levels of MMP-8 and MMP13 mRNAs (Figures 5A, 5B).
Figure 5. Collagenase resistance did not cause compensatory changes in elastolytic and collagenolytic enzymes.
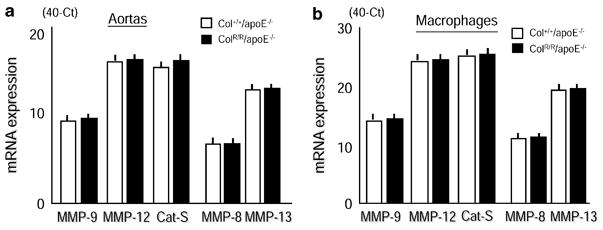
Real-time PCR demonstrated similar mRNA levels of MMP-elastases (MMP-9 and MMP-12), MMP-collagenase (MMP-8 and MMP-13), and cathepsin S (Cat-S) in aortas treated with AngII for 2 weeks from Col+/+/ApoE−/− or ColR/R/apoE−/− mice (B) and in thioglycollate-treated peritoneal macrophages (C) from Col+/+/apoE−/− or ColR/R/apoE−/− mice. The data were obtained from three independent pooled total RNA samples of the aorta (n=2) or three different preparations of peritoneal macrophages. Bars and error bars represent mean cycle numbers (ΔCT: 40 – threshold cycle numbers) and S.E.M.
Collagenase resistance increased aortic stiffness and promoted susceptibility to mechanical failure
To test further the hypothesis that aortic stiffness due to excess collagen accumulation impairs resistance of the aorta to mechanical stresses, we examined the biomechanical properties of pre-aneurysmal aortas obtained from Col+/+/apoE−/− and ColR/R/apoE−/− mice after two weeks of AngII infusion. The 70%-strain cycles assessed durability, while the strain-to-break experiments aimed to gauge the ultimate strength of the aortas (Figure 6A). As expected, the aortas of ColR/R/apoE−/− mice exhibited increased initial stiffness at the first 70% strain (after preconditioning) compared to those of Col+/+/apoE−/− mice (Figure 6B). However, stiffness decreased more rapidly in the aortas of ColR/R/apoE−/− mice after 10 cycles of the 70% strain than those of Col+/+/apoE−/− mice, suggesting higher susceptibility to fatigue-type failure in collagenase-resistant aortas (Figure 6C).
Figure 6. Mechanical testing of the aorta.
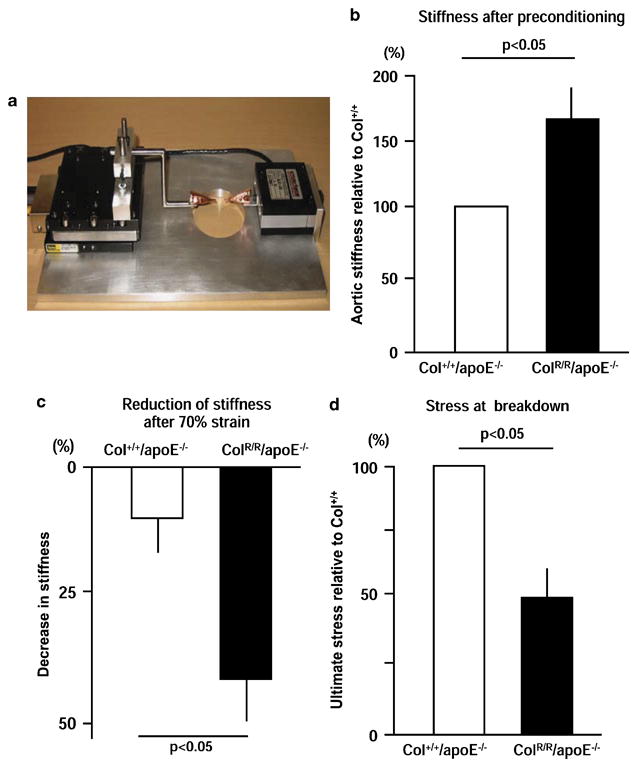
A, The strain-device used to measure the mechanical response of aortas to cyclic strain and strain failure. The load cell is on the right and the electromechanical positioning system is on the left. The aorta is clamped in the center above the hole. B, Aortic stiffness of ColR/R/apoE−/− mice is 65% higher, on average, compared to Col+/+/apoE−/− mice. C, After 10 cycles of 70% strain, ColR/R/apoE−/− aortas showed a significant reduction in stiffness compared to Col+/+/apoE−/− aortas (ColR/R/apoE−/−; 32% decrease, ColR/R/apoE−/−; 8% decrease in stiffness before 70% strain test). D, Aortas of ColR/R/apoE−/− mice failed under stresses at 48% of the failure, compared to Col+/+/apoE−/− aortas. These assays employed a subset of Col+/+/apoE−/− (n=3) and ColR/R/apoE−/− mice (n=4).
Examining whether the aortas of ColR/R/apoE−/− mice have impaired resistance to stretch required the strain-to-break test. Based on the stresses at failure, we found significantly lower ultimate stresses on the aortas of ColR/R/apoE−/− mice vs. Col+/+/apoE−/− mice (Figure 6D). Thus, the collagen-enriched aortas of the collagenase-resistant animals are more fragile and more easily broken by deformation than wild-type aortas.
Collagenase resistance altered collagen orientation in the aortic adventitia
To address the potential mechanism for the paradoxical association of increased collagen accumulation in the aortas of ColR/R mice and susceptibility to rapid fatigue and ultimate disruption, we subsequently examined whether collagen orientation changed in collagenase-resistant mice. Figure 7 illustrates both schematic and actual representative extinction patterns seen with different collagen fiber architecture. The extinction pattern observed in the aneurysm wall corresponded to that expected for straight fibers (Figure 7A and B, top panels), while the banded extinction pattern frequently observed in the adventitia of ColR/R aortas indicated two-dimensional fiber waviness (Figure 7A and B, middle panels). In contrast, the narrow band of extinction frequently observed to sweep across fibers in the adventitia of the wild type samples echoed fibers whose waviness possesses a three-dimensional helical component (Figure 7A and B, bottom panels). These results reveal major differences in collagen fiber architecture in the adventitia. In contrast, we observed no structural changes or differences between wild-type and collagenase-resistant mutant mice in the medial collagen fibers of the non-aneurysmal segments of the aorta (data not shown).
Figure 7. Collagen extinction patterns viewed with linearly polarized light provide an indication of fiber organization.
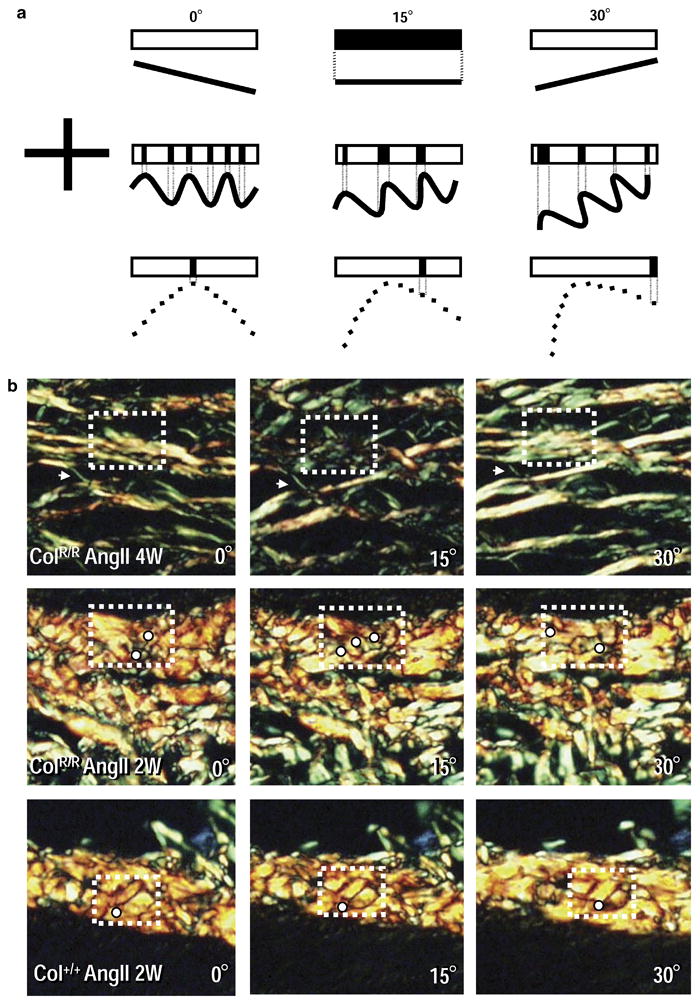
A, Schematic extinction patterns for three fiber configurations at three orientations (the cross indicates the alignment of the polarizers’ transmission axes). When collagen is aligned parallel to either transmission axis, it appears dark. At all other orientations, it appears bright. The upper row of each schematic shows the extinction pattern that reflects fiber orientation illustrated in the lower row (dotted line connects fiber segments aligned parallel to a transmission axis to corresponding extinction pattern). Top: No portion of the straight fiber in the first column (0°) is parallel to a transmission axis and so appears bright. When rotated by 15° (second column), the entire fiber is parallel to the horizontal transmission axis and appears dark. When rotated an additional 15° (third column), the fiber again appears bright. Middle: Portions of the wavy fiber are parallel to a transmission axis and hence a banded extinction pattern results. A 15° rotation brings different portions parallel to the transmission axes, producing a different banded pattern. Additional rotation changes the pattern again. Bottom: A fiber with three-dimensional waviness, i.e., a helical structure (dashed line). In the first column, only a small portion of this fiber is aligned parallel to a transmission axis and a single narrow extinction band results; the rest of the fiber appears bright. Additional 15° rotations bring different regions to extinction – a narrow extinction band sweeps across the fiber. B, Actual aortic tissue extinction patterns. Top: Aneurysm collagen from a collagenase-resistant mouse (4-weeks AngII). An arrow marks the fiber of interest and an arrowhead a fiber that appears bright in each panel. The entire marked fiber appears dark in the middle panel, but bright in the others – consistent with a straight fiber. Middle: Pre-aneurysmal ColR/R tissue (2-weeks AngII). Different fiber portions appear dark at each orientation (white dots placed immediately below the dark area) – consistent with two-dimensional waviness. Bottom: Wild-type (2-weeks AngII). With rotation, a narrow extinction band sweeps across the marked fiber – consistent with three-dimensional waviness. The white dot lies approximately at the end of the band of extinction in each panel. These data represent three different mice per group that produced similar results.
Discussion
Multiple biomechanical and biological factors, including smoking, hypertension, shear or circumferential stress, aging, and atherosclerosis, may contribute to the development of AAA.5, 6, 19 Such risk factors can influence the metabolism of the aortic extracellular matrix and promote ongoing structural matrix remodeling, a characteristic of AAA. Collagen and elastin comprise the two major extracellular matrix constituents of the aorta. Regulation of both elastin and collagen content in the abdominal aorta appears dynamic.5, 6, 19 Clinical evidence indicates an important role for aortic stiffness and/or excess collagen in aneurysm formation.10–16 Therefore, the present study tested the hypothesis that excess collagen accumulation due to impaired collagen catabolism during aortic remodeling promotes AAA formation.
Daugherty et al. found that AngII treatment in apoE−/− mice produces AAA.23 AngII also increases arterial collagen turnover: production and degradation.24–26 Our study used AngII-treated apoE−/− mice bearing a mutation that renders collagen type I collagenase-resistant to induce greater aortic collagen accumulation than those expressing wild-type collagen and to test whether excess collagen accelerates AAA formation. Interstitial collagens, especially type I collagen, consist of a triple helix of polypeptide chains that resist degradation by most proteases except the interstitial collagenases.21, 34 Unperturbed collagenase-resistant mice show no overt vascular phenotype.21 Indeed, without AngII treatment, ColR/R/apoE−/− mice do not develop aneurysm (data not shown). We found more prominent AAA formation and collagen accumulation in AngII-treated ColR/R/apoE−/− mice.
Collagen provides mechanical integrity to the arterial wall and regulates various biological functions of vascular cells.8, 35 Adventitial fibrillar collagen generally confers tensile mechanical strength on the aorta.5 Collagen also contributes to aortic stiffness.8 Compared to the aortas of Col+/+/apoE−/− mice, increased collagen in the aortas of ColR/R/apoE−/− led to increased stiffness, as demonstrated by our mechanical testing (Figure 6B). However, this increased stiffness did not provide durability to the aorta (Figures 6C and 6D). Rather, compared to Col+/+/apoE−/− mice, the aortas of ColR/R/apoE−/− mice experienced more rapid loss of stiffness as well as mechanical failure at lower stresses, indicating that excess collagen accumulation promoted susceptibility to mechanical failure.
The apparent paradoxical association of increased aortic collagen accumulation in ColR/R mice and susceptibility to rapid mechanical fatigue and eventual tissue disruption requires further consideration, even though our findings concur with clinical evidence linking aortic stiffness and increased collagen with aneurysm formation.10–15 Collagen’s mechanical properties depend not only on its quantity and biochemical properties, but also on fiber architecture. We indeed found substantial architectural differences between groups in the subset of mice studied in this regard. Normal arterial adventitial collagen organization displays a three-dimensional helical waviness36; the extinction patterns observed in our wild-type samples reflect such an organization (Figure 7). In our study, aortic adventitial collagen assumed a more two-dimensional, but still wavy, structure in ColR/R mice (probably to accommodate the collagen increase). Additional remodeling resulted in fiber straightening in the aneurysm wall. Assuming the absence of concurrent biochemical changes, such structural alterations would associate with increased tissue stiffness. In tissues subject to pulsatile or multi-axial deformation, the compliance provided by three-dimensional fiber waviness probably provides mechanical advantage. For example, skin possesses a three-dimensional structure and exhibits extinction patterns37 similar to those of the Col+/+ adventitia. In contrast, collagen fibers in tendons, prone to rupture due to mechanical stresses, have a predominantly two-dimensional structure and extinction patterns30 similar to those seen in the adventitia and aneurysm of ColR/R tissue. We propose that these architectural changes in the adventitia agree with our mechanical data and explain why collagen accumulation induced by collagenase resistance paradoxically increases susceptibility to mechanical failure. We observed no structural differences between groups in the collagen fibers or smooth muscle cells in the media of the non-aneurysmal aortic segments. Nonetheless, these observations were not made in regions directly involved in aneurysm formation and therefore whether medial changes occur during the initial development of the aneurysm remains to be determined.
Accumulating evidence suggests that MMPs participate in various aspects of cardiovascular remodeling including aneurysm formation.2, 3, 22, 27–29, 38–44 Previous studies including our own showed that MMP-elastases and collagenases increase in the wall of human AAA.2, 38–40 Degradation of the extracellular matrix in the aortic wall may cause aneurysmal changes, and animal studies suggest that MMPs participate in aneurysm formation.2, 3, 38–44 Understanding further mechanisms of aneurysm formation involving MMPs should provide important insights into therapeutic strategies for this disease. The present study did not attempt to determine the role of MMPs in the pathogenesis of aneurysms, but tested the specific hypothesis that excess collagen accelerates AAA formation, using mice that resist cleavage by MMP-family collagenases. While testing our hypothesis, however, we were also aware that if MMP-collagenases play a role in AAA formation, introducing a loss-of-function phenotype due to collagenase resistance could decrease the incidence of AAA, contrary to our originally anticipated results. The data showed that collagenase resistance yielded foci of substantial aortic dilatation in AngII-treated apoE−/− mice, supported further by mechanical testing data. In addition, collagenase resistance did not cause compensatory increases of mRNA that encode major elastases. Collectively, these results suggest that augmented formation of AAA in this particular study depends primarily on accumulation of uncleavable fibrillar collagen, but not directly on changes in matrix-degrading enzymes.
Our study demonstrates that collagenase resistance promotes excessive collagen accumulation in aortic adventitia and modifies aortic mechanical properties, in turn accelerating aneurysm formation. These results in genetically-altered mice provide mechanistic insights into the pathogenesis of AAA formation, specifically linking collagen content and organization to the aorta’s mechanical properties. Furthermore, these associations implied by our results combined with the novel finding of altered collagen architecture provide a basis for future investigation of the structural and mechanical aspects of AAA formation.
Acknowledgments
This work was in part supported by grants from the NHLBI (HL-56985 to Drs. Libby and Aikawa, HL-66086 to Dr. Aikawa, HL-80472 to Dr. Libby, HL-67249 to Dr. Sukhova), from the Donald W. Reynolds Foundation (Dr. Libby), and an unrestricted research grant from Kowa Company (Dr. Aikawa). Dr. Deguchi received a fellowship from the Reynolds Foundation. We acknowledge Dr. Stephen M. Krane for creating ColR/R mice and helpful suggestions, Mr. Francisco U. Cruz for his assistance with stretching experiments, and Ms. Joan Perry for her editorial assistance.
Contributor Information
Jun-o Deguchi, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Hayden Huang, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Peter Libby, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Elena Aikawa, Center for Molecular Imaging Research, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA.
Peter Whittaker, Department of Emergency Medicine, Wayne State University, Detroit, MI.
Jeremy Sylvan, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Richard T. Lee, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA
Masanori Aikawa, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
References
- 1.Ernst CB. Abdominal aortic aneurysm. N Engl J Med. 1993;328:1167–1172. doi: 10.1056/NEJM199304223281607. [DOI] [PubMed] [Google Scholar]
- 2.Thompson RW. Aneurysm treatments expand. Nat Med. 2005;11:1279–1281. doi: 10.1038/nm1205-1279. [DOI] [PubMed] [Google Scholar]
- 3.Allaire E, Forough R, Clowes M, Starcher B, Clowes AW. Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model. J Clin Invest. 1998;102:1413–1420. doi: 10.1172/JCI2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Touat Z, Ollivier V, Dai J, Huisse MG, Bezeaud A, Sebbag U, Palombi T, Rossignol P, Meilhac O, Guillin MC, Michel JB. Renewal of mural thrombus releases plasma markers and is involved in aortic abdominal aneurysm evolution. Am J Pathol. 2006;168:1022–1030. doi: 10.2353/ajpath.2006.050868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilson MD, Gregory AK, Hingorani AP. Aneurysmal Disease of the Abdominal Aorta. New York: Futura Publishing Company; 1997. [Google Scholar]
- 6.Thompson RW, Geraghty PJ, Lee JK. Abdominal aortic aneurysms: basic mechanisms and clinical implications. Curr Probl Surg. 2002;39:110–230. doi: 10.1067/msg.2002.121421. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu K, Libby P, Mitchell RN. Local cytokine environments drive aneurysm formation in allografted aortas. Trends Cardiovasc Med. 2005;15:142–148. doi: 10.1016/j.tcm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Lee RT, Kamm RD. Vascular mechanics for the cardiologist. J Am Coll Cardiol. 1994;23:1289–1295. doi: 10.1016/0735-1097(94)90369-7. [DOI] [PubMed] [Google Scholar]
- 9.Sumner DS. Essential Hemodynamic Principles. In: Rutherford RB, editor. Vascular Surgery. 5. Philadelphia: W.B. Saunders company; 2000. pp. 73–120. [Google Scholar]
- 10.Baxter BT, Davis VA, Minion DJ, Wang YP, Lynch TG, McManus BM. Abdominal aortic aneurysms are associated with altered matrix proteins of the nonaneurysmal aortic segments. J Vasc Surg. 1994;19:797–802. doi: 10.1016/s0741-5214(94)70004-4. discussion 803. [DOI] [PubMed] [Google Scholar]
- 11.Sonesson B, Hansen F, Lanne T. Abdominal aortic aneurysm: a general defect in the vasculature with focal manifestations in the abdominal aorta? J Vasc Surg. 1997;26:247–254. doi: 10.1016/s0741-5214(97)70185-x. [DOI] [PubMed] [Google Scholar]
- 12.Simons PC, Algra A, Bots ML, Banga JD, Grobbee DE, van der Graaf Y. Common carotid intima-media thickness in patients with peripheral arterial disease or abdominal aortic aneurysm: the SMART study. Second Manifestations of ARTerial disease. Atherosclerosis. 1999;146:243–248. doi: 10.1016/s0021-9150(99)00142-2. [DOI] [PubMed] [Google Scholar]
- 13.Dijk JM, van der Graaf Y, Grobbee DE, Banga JD, Bots ML. Increased arterial stiffness is independently related to cerebrovascular disease and aneurysms of the abdominal aorta: the Second Manifestations of Arterial Disease (SMART) Study. Stroke. 2004;35:1642–1646. doi: 10.1161/01.STR.0000130513.77186.26. [DOI] [PubMed] [Google Scholar]
- 14.Cheng KS, Tiwari A, Morris R, Hamilton G, Seifalian AM. The influence of peripheral vascular disease on the carotid and femoral wall mechanics in subjects with abdominal aortic aneurysm. J Vasc Surg. 2003;37:403–409. doi: 10.1067/mva.2003.52. [DOI] [PubMed] [Google Scholar]
- 15.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 16.He CM, Roach MR. The composition and mechanical properties of abdominal aortic aneurysms. J Vasc Surg. 1994;20:6–13. doi: 10.1016/0741-5214(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 17.Menashi S, Campa JS, Greenhalgh RM, Powell JT. Collagen in abdominal aortic aneurysm: typing, content, and degradation. J Vasc Surg. 1987;6:578–582. doi: 10.1067/mva.1987.avs0060578. [DOI] [PubMed] [Google Scholar]
- 18.Minion DJ, Davis VA, Nejezchleb PA, Wang Y, McManus BM, Baxter BT. Elastin is increased in abdominal aortic aneurysms. J Surg Res. 1994;57:443–446. doi: 10.1006/jsre.1994.1168. [DOI] [PubMed] [Google Scholar]
- 19.Glickman BS, Rehm JP, Baxter BT. Arterial Aneurysm: Etiologic Consideration. In: Rutherford RB, editor. Vascular Surgery. 5. Philadelphia: W. B. Sanders Company; 2000. pp. 373–386. [Google Scholar]
- 20.Rizzo RJ, McCarthy WJ, Dixit SN, Lilly MP, Shively VP, Flinn WR, Yao JS. Collagen types and matrix protein content in human abdominal aortic aneurysms. J Vasc Surg. 1989;10:365–373. doi: 10.1067/mva.1989.13151. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Wu H, Byrne M, Jeffrey J, Krane SM, Jaenisch R. A targeted mutation at the known collagenase cleavage site in mouse type I collagen impairs tissue remodeling. J Cell Biol. 1995;130:227–237. doi: 10.1083/jcb.130.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukumoto Y, Deguchi J, Libby P, Rabkin-Aikawa E, Sakata Y, Chin MT, Hill CC, Lawler PR, Varo N, Schoen FJ, Krane SM, Aikawa M. Genetically determined resistance to collagenase action augments interstitial collagen accumulation in atherosclerotic plaques. Circulation. 2004;110:1953–1959. doi: 10.1161/01.CIR.0000143174.41810.10. [DOI] [PubMed] [Google Scholar]
- 23.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 24.Ford CM, Li S, Pickering JG. Angiotensin II stimulates collagen synthesis in human vascular smooth muscle cells. Involvement of the AT(1) receptor, transforming growth factor-beta, and tyrosine phosphorylation. Arterioscler Thromb Vasc Biol. 1999;19:1843–1851. doi: 10.1161/01.atv.19.8.1843. [DOI] [PubMed] [Google Scholar]
- 25.Tham DM, Martin-McNulty B, Wang YX, Da Cunha V, Wilson DW, Athanassious CN, Powers AF, Sullivan ME, Rutledge JC. Angiotensin II injures the arterial wall causing increased aortic stiffening in apolipoprotein E-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1442–1449. doi: 10.1152/ajpregu.00295.2002. [DOI] [PubMed] [Google Scholar]
- 26.Satta J, Juvonen T, Haukipuro K, Juvonen M, Kairaluoma MI. Increased turnover of collagen in abdominal aortic aneurysms, demonstrated by measuring the concentration of the aminoterminal propeptide of type III procollagen in peripheral and aortal blood samples. J Vasc Surg. 1995;22:155–160. doi: 10.1016/s0741-5214(95)70110-9. [DOI] [PubMed] [Google Scholar]
- 27.Deguchi J, Aikawa E, Libby P, Vachon JR, Inada M, Krane SM, Whittaker P, Aikawa M. Matrix metalloproteinase-13/collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques. Circulation. 2005;112:2708–2715. doi: 10.1161/CIRCULATIONAHA.105.562041. [DOI] [PubMed] [Google Scholar]
- 28.Aikawa M, Rabkin E, Okada Y, Voglic SJ, Clinton SK, Brinckerhoff CE, Sukhova GK, Libby P. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: a potential mechanism of lesion stabilization. Circulation. 1998;97:2433–2444. doi: 10.1161/01.cir.97.24.2433. [DOI] [PubMed] [Google Scholar]
- 29.Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 30.Whittaker P, Canham PB. Demonstration of quantitative fabric analysis of tendon collagen using two-dimensional polarized light microscopy. Matrix. 1991;11:56–62. doi: 10.1016/s0934-8832(11)80227-1. [DOI] [PubMed] [Google Scholar]
- 31.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- 33.Knauper V, Smith B, Lopez-Otin C, Murphy G. Activation of progelatinase B (proMMP-9) by active collagenase-3 (MMP-13) Eur J Biochem. 1997;248:369–373. doi: 10.1111/j.1432-1033.1997.00369.x. [DOI] [PubMed] [Google Scholar]
- 34.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 35.Pickering JG. Regulation of vascular cell behavior by collagen: form is function. Circ Res. 2001;88:458–459. doi: 10.1161/01.res.88.5.458. [DOI] [PubMed] [Google Scholar]
- 36.Finlay HM, Whittaker P, Canham PB. Collagen organization in the branching region of human brain arteries. Stroke. 1998;29:1595–1601. doi: 10.1161/01.str.29.8.1595. [DOI] [PubMed] [Google Scholar]
- 37.Beck LS, DeGuzman L, Lee WP, Xu Y, Siegel MW, Amento EP. One systemic administration of transforming growth factor-beta 1 reverses age- or glucocorticoid-impaired wound healing. J Clin Invest. 1993;92:2841–2849. doi: 10.1172/JCI116904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995;96:318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest. 1998;102:1900–1910. doi: 10.1172/JCI2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knox JB, Sukhova GK, Whittemore AD, Libby P. Evidence for altered balance between matrix metalloproteinases and their inhibitors in human aortic diseases. Circulation. 1997;95:205–212. doi: 10.1161/01.cir.95.1.205. [DOI] [PubMed] [Google Scholar]
- 41.Allaire E, Hasenstab D, Kenagy RD, Starcher B, Clowes MM, Clowes AW. Prevention of aneurysm development and rupture by local overexpression of plasminogen activator inhibitor-1. Circulation. 1998;98:249–255. doi: 10.1161/01.cir.98.3.249. [DOI] [PubMed] [Google Scholar]
- 42.Lemaitre V, Soloway PD, D’Armiento J. Increased medial degradation with pseudo-aneurysm formation in apolipoprotein E-knockout mice deficient in tissue inhibitor of metalloproteinases-1. Circulation. 2003;107:333–338. doi: 10.1161/01.cir.0000044915.37074.5c. [DOI] [PubMed] [Google Scholar]
- 43.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]


