Abstract
Background
The General Cardiovascular Risk Profile (GCRP) is a multivariable model that predicts global cardiovascular disease risk. Our goal was to assess the ability of the GCRP to identify individuals with advanced coronary artery calcification (CAC), and determine whether identification is improved with family history.
Methods and Results
Using data from the Multi-Ethnic Study of Atherosclerosis, three sex-specific models were developed with ordinal logistic regressions to relate risk factors to CAC scores. Model 1 included covariates in the GCRP. Then family history was added, defined as having at least one first-degree relative with premature coronary heart disease (CHD) (Model 2), or as a weak, moderate or strong family history based on number of relatives with CHD, age at onset, and presence of stroke or diabetes in the family (Model 3). For each model, we estimated mathematical CAC risk functions, derived CAC score sheets, evaluated the ability to discriminate persons having positive CAC scores, and assessed reclassification of individuals with low, intermediate, or high probability of CAC >300. Model 1 worked well to identify women and men with positive CAC scores; c-statistics were 0.752 and 0.718 and X2 values were 821.2 (p<0.0001) and 730.6 (p<0.0001), respectively. Addition of family history improved discrimination and fit of Model 1. However, reclassification of participants with advanced CAC was significantly improved with Model 3 only.
Conclusions
The GCRP identifies advanced CAC, an emerging indication for aggressive risk factor modification. Incorporation of family history, especially comprehensive familial risk stratification, provides incremental prognostic value.
Keywords: coronary artery calcium, family history, risk factors
Coronary artery calcification (CAC) provides prognostic information of proven value regarding the risk of cardiovascular events.1-8 As a result, advanced CAC is increasingly becoming a rationale for aggressive risk factor modification.2-4,9
Significant associations between a positive CAC score and family history of coronary heart disease (CHD)10,11 and family history of diabetes11 have been found. The magnitude of association between CAC and family history of CHD increased as the number of relatives with CHD increased and as the age of onset decreased.11 These results suggest that familial risk assessment using rules that consider the number of relatives affected with CHD, their age at onset, and the presence of related conditions, such as diabetes, might improve the predictive value of family history as a risk factor for CAC compared to a dichotomous assessment of family history based on the presence or absence of premature CHD in first-degree relatives. This type of familial risk stratification has been shown to improve risk assessment for CHD.12,13 However, associations alone are not sufficient to influence prevention strategies for cardiovascular disease (CVD); rather, multivariable assessment has been advocated to estimate absolute CVD risk and to guide treatment of risk factors.14,15
The General Cardiovascular Risk Profile (GCRP) predicts global CVD risk for men and women, including risk for coronary heart disease (CHD), stroke, claudication, and heart failure.16 The ability of the GCRP to predict the individual cardiovascular disease components that reflect atherosclerosis in different vascular territories has been explained by the sharing of a common set of risk factors for these disorders.16 Thus, it seems likely that this model could be useful in identifying individuals with advanced CAC.
The risk factors used in the sex-specific GCRP models include age, total and HDL cholesterol, systolic blood pressure (SBP), antihypertensive medication use, current smoking and diabetes; family history is not included. Recent studies have shown that parental history of a myocardial infarction before age 60 improves global risk prediction for cardiovascular events (myocardial infarction, stroke, coronary revascularization or cardiovascular death).17,.18 Thus, the addition of family history might add to the prognostic ability of the GCRP.
The purpose of this investigation was to assess the ability of the GCRP to identify adults with advanced CAC, and the improvement of the model’s ability to identify individuals with advanced CAC when family history is included according to a traditional definition (i.e., having at least one first-degree relative with premature CHD) or a comprehensive assessment that applies rules that stratify familial risk into weak, moderate and strong categories. We used data from the Multi-Ethnic Study of Atherosclerosis (MESA), a large, epidemiologic study investigating the prevalence, correlates, and progression of subclinical cardiovascular disease that has collected comprehensive family history data.
METHODS
STUDY DESIGN AND SAMPLE
This is an ancillary study to MESA, a multi-ethnic cohort study that enrolled 6,814 participants without clinically apparent atherosclerotic vascular disease, ages 45 to 84 years, from 6 sites across the United States. The purpose of MESA is to study the relationship between risk factors and subclinical atherosclerosis measured periodically using cardiac computed tomography.19
Participants were excluded (n=744) if information about covariates included in the regression models was missing. The majority with missing data (n=618) did not provide family history information. This included participants who dropped out after exam 1 (n=388), gave history of an exclusionary clinical event occurring prior to exam 1 (n=5), missed exam 2 (n=193) or were present for exam 2 but did not complete the family history survey (n=32). Those excluded were slightly older and more likely to be African American, have lower education and income, and more risk factors, including smoking, low HDL and higher SBP. However, these differences should not introduce substantial bias to our results since it is unlikely that missing exam 2 would be associated with family history.
Institutional review board approval for collection of the data used in this ancillary MESA study was obtained from each site, and the RAND Human Subjects Protection Committee approved the data management and analyses of this study.
DEMOGRAPHICS AND PERSONAL RISK FACTORS
Demographic information, medical history and laboratory test results were obtained in exam 1. Current smoking was defined as having smoked a cigarette in the last 30 days. Resting blood pressure was measured three times in the seated position, and the average of the 2nd and 3rd readings was recorded. Hypertension was defined as a SBP ≥140 mmHg, diastolic blood pressure ≥ 90 mmHg, or use of medication prescribed for hypertension. Participants were classified as diabetic if they had a previous diagnosis of diabetes, fasting glucose ≥126 mg/dL or if they used a hypoglycemic medication. BMI was calculated as weight (kg)/height2 (m2).
FAMILY HISTORY
Detailed information on family histories of CHD, stroke, and diabetes was ascertained in exam 2, 18 to 24 months after exam 1. Participants were asked if their mother, father, siblings, or children have had CHD defined as a heart attack or cardiac procedures (coronary bypass surgery, balloon angioplasty, intracoronary stenting); stroke, cerebral hemorrhage or brain attack; or diabetes or high blood sugar. Response options were “yes”, “no”, and “don’t know”. If a participant reported a disease in a relative, the age at diagnosis was also ascertained. “Don’t know” responses were counted as “no” responses.
To evaluate family history as a risk factor, we defined it in two ways. We used a traditional definition of having at least one first-degree relative with premature CHD (occurring before age 55 years in male relatives and before age 65 years in female relatives). We also used rules for familial risk stratification that considered the number, sex, lineage and age at onset of relatives with CHD, stroke and diabetes (Figure 1).13
Figure 1.
Familial risk stratification rules considered the presence of CHD, stroke and diabetes in first-degree relatives. Information regarding second-degree relatives was not collected, though this information may have contributed further to the risk stratification.13 Early-onset CHD was defined as occurring before age 65 years in women and before age 55 years in men. Early-onset stroke was defined as occurring before age 50 years in both women and men. CHD and stroke occurring before age 25 years were not considered, as we suspected these were most likely cases of congenital or hereditary forms of CVD. CHD or stroke after age 85 years was also not considered. Diabetes before age 20 years was excluded, as we considered this more consistent with type 1 diabetes. Type 2 diabetes was of greater interest since it is closely linked to atherosclerosis through insulin resistance. From the same lineage refers to maternal (mother and siblings or children), paternal (father and siblings or children) or nuclear (siblings or children) lineage.
CORONARY ARTERY CALCIUM SCORE
The mean Agatston, phantom-adjusted CAC score measured in exam 1 was the outcome used in this study. Each participant was scanned twice and the mean Agatston score was used. Agreement regarding presence of CAC was high (kappa statistic 0.90 to 0.93 between and within readers), and the intraclass correlation coefficient for the Agatston score between readers was 0.99.20
STATISTICAL ANALYSES
SAS software (version 9.1) was used for all analyses. Basic descriptive statistics described the distribution, mean value, and range of the demographic factors, personal risk factors, family histories, and CAC scores. Chi-square and t-tests assessed differences in the outcomes between groups.
MULTIVARIABLE MODELS AND ESTIMATION OF CAC RISK FUNCTIONS
We selected the GCRP as the global cardiovascular risk assessment model to be used as the baseline model for our study.16 Sex-specific ordinal logistic regressions were used to relate GCRP risk factors to baseline CAC scores (0, 1-100, 101-300, >300) (Model 1). Family history was added to our baseline model; Model 2 included a dichotomous family history variable and Model 3 stratified familial risk. For each model, we estimated mathematical CAC score risk functions (regression coefficients with p-values).
Covariates in the models were similar to those used in the GCRP.16 However, we differed slightly from the GCRP in how we specified use of antihypertensive medication, yet we achieved similar results in our point scales. We used a continuous variable for SBP with a binary variable for use of antihypertensive medication. The GCRP stratified the continuous SBP variable according to antihypertensive medication use. Similar to the GCRP, all the continuous variables were naturally logarithmically transformed to improve discrimination and calibration and to minimize the influence of extreme observations.
DERIVATION OF RISK SCORES FOR CAC
We derived score sheets for having a positive CAC for men and women to describe the relative contribution of family history compared to the other covariates in the GCRP. The regression coefficients from our multivariable models were used to estimate the probability of having a positive CAC score at different threshold values (1-100, 101-300, and >300). We used the method of Sullivan21 to translate these risk functions into sex-specific score sheets (translation tables are provided in the Appendix). To avoid half points, we calibrated the score so that the five years from age 47 to 52 increased the risk score by 3 points. Then each point was associated with a constant B = 0.197 in the regression index. The points for the other continuous risk factors were obtained by multiplying the difference of the log of the midpoint of their interval and the log of the midpoint of the omitted interval by their regression coefficient and dividing by B. We then rounded the result to the nearest integer. For example, for the points for a male with total cholesterol between 200-239 mg/dl, we took [log 220- log 140] × 1.0408/0.197 = 2.33, which rounds to 2.
ASSESSMENT OF MODEL PERFORMANCE
To characterize our baseline model compared to the GCRP, we assessed the correlation between the points generated with the GCRP and Model 1 using the Pearson correlation coefficient. We assessed how well Model 1 identified individuals with different CAC scores relative to Models 2 and 3 by assessing the c-statistic for each model, and by testing for goodness of fit by comparing likelihood ratios. The c-statistic is a variant of Somer’s D index that measures the rank correlation of the ordinal outcome variables, a measure of the discriminative power of the logistic equation. Because Models 2 and 3 are not nested, a X2 test could not be conducted for their equivalence. Thus, we used the Akaike Information Criterion (AIC) to informally compare these models. The AIC is used to describe the tradeoff between bias and variance in model construction. Several competing models may be ranked according to their AIC, with the one having the lowest AIC being the best.
ASSESSMENT OF RECLASSIFICATION
To aid in interpretation and to address clinical issues of risk stratification and reclassification, we generated low (<5%), intermediate (5-25%) and high (>25%) probabilities for having CAC >300 according to each model. We chose this threshold value since it is associated in the MESA cohort with nearly a 10-fold increase in risk for a coronary event in the near future (median follow-up of 3.9 years),8 and individuals with CAC >300 are nearly always targeted for aggressive risk factor modification.9 The categories of probabilities were selected because the distribution of probabilities for CAC >300 ranged from <1% to almost 50% in females and from <2% to almost 60% in males.
The net reclassification improvement (NRI) was calculated as a measure to estimate any overall improvement in reclassification with Models 2 and 3 instead of Model 1, and with Model 3 instead of Model 2.22 This approach separately analyzed the reclassification of persons with CAC >300 and those with CAC ≤300. The NRI estimates the net proportion of participants that correctly move to higher or lower risk strata, and the statistical significance of the overall improvement is assessed with an asymptotic test.
RESULTS
The characteristics of participants at the baseline examinations are shown in Table 1. Having at least one first-degree relative with premature CHD was reported by 18% of the participants, whereas 46% had either a moderate or strong familial risk. Men were less likely to report a family history.
Table 1.
Characteristics of the MESA participants at baseline
| Characteristics | Women (n=3,184) |
Men (n=2,886) |
All participants (n=6,070) |
|---|---|---|---|
| Age: mean(SD), range(years) | 62(10.2) | 62(10.2) | 62(10.2) |
| Ethnicity/race(%) | |||
| African American | 28.2 | 25.7 | 27.0 |
| Asian American | 11.4 | 12.1 | 11.7 |
| Caucasian | 38.9 | 40.5 | 39.6 |
| Hispanic | 21.6 | 21.8 | 21.7 |
| Number of relatives: mean(SD), range | 8.0(3.7),1-26 | 7.9(3.6),1-35 | 7.9(3.6),1-35 |
| Family history of premature CHD(%) | 20.2 | 15.0 | 17.7 |
| Moderate familial CHD risk(%) | 21.6 | 21.4 | 21.5 |
| Strong familial CHD risk(%) | 27.0 | 20.7 | 24.0 |
| Current smoking status(%) | 11.0 | 13.9 | 12.4 |
| Uses antihypertensive medication(%) | 37.3* | 35.9† | 36.6 |
| Hypertension(%) | 45.3 | 42.7 | 44.1 |
| Uses lipid-lowering medication(%) | 16.0 | 16.3 | 16.1 |
| Total cholesterol: mean(SD), range(mg/dl) | 199.2(34.2),89- 365 |
187.3(33.7),65- 408 |
193.6(34.5),65- 408 |
| LDL cholesterol ≥100 mg/dl(%) | 70.4 | 71.7 | 71.1 |
| HDL cholesterol: mean(SD), range(mg/dl) | 56.7(15.2),21- 142 |
45.2(11.5),15- 108 |
51.2(14.8),15- 142 |
| Triglycerides: mean(SD), range(mg/dl) | 123.1(63.2),21- 400 |
128.5(67.9),23- 399 |
125.7(65.5),21- 400 |
| Diabetes, treated | 8.7 | 10.0 | 9.3 |
| Diabetes, untreated | 3.3 | 4.8 | 4.0 |
| 10-year risk of cardiovascular disease using the General Cardiovascular Risk Profile | |||
| <6% | 59.3 | 24.2 | 42.6 |
| 6-20% | 35.9 | 54.6 | 44.8 |
| >20% | 4.8 | 21.2 | 12.6 |
| CAC score: mean(SD), median, range | 71(225),0- 3,571 |
218(523),0- 6,316 |
141(403)0- 6,316 |
| CAC score=0(%) | 61.4 | 39.3 | 50.9 |
| CAC score >0 and ≤100(%) | 23.6 | 28.7 | 26.0 |
| CAC score >100 and ≤300(%) | 8.1 | 14.0 | 10.9 |
| CAC score >300(%) | 6.9 | 18.0 | 12.1 |
MESA, Multi-Ethnic Study of Atherosclerosis; SD, standard deviation; LDL, low density lipoprotein; HDL, high density lipoprotein; BMI, body mass index; CAC, coronary artery calcium
90 women reported they did not have hypertension but took an antihypertensive medication
130 men reported they did not have hypertension but took an antihypertensive medication
The multivariable-adjusted regression coefficients for the outcome of CAC were significant for both definitions of family history and all of the GCRP covariates, except smoking in males (Appendix). The associations between family history and CAC were all highly significant (p <0.0001). For males, the odds ratios were 1.51 given a first-degree relative with premature CHD, and 1.47 and 1.80 given a moderate and strong familial risk, respectively. The corresponding odds ratios for females were 1.70, 1.57 and 1.84.
The variables in Model 1 worked well in identifying both women and men with varying levels of CAC scores, with c-statistics of 0.752 and 0.718, and X2 values of 821.2 (p<0.0001) and 730.6 (p<0.0001), respectively. With Model 2, the c-statistics were 0.756 and 0.721 for women and men, respectively, and the corresponding values for Model 3 were 0.759 and 0.725. In comparing likelihood ratios, for men, the X2 difference between Models 1 and 2 was 17.0 (df, 1), p=0.00004, and for women was 32.1 (df, 1), p<0.00001. The X2 difference between Models 1 and 3 for men was 48.8 (df, 2), p<0.00001, and for women was 51.7 (df, 2), p<0.00001. For Models 2 and 3, the AIC for men was 6,832.2 and 6,802.4, respectively, and for women, they were 5,713.2 and 5,695, respectively. Thus, we can infer Model 3 performed better.
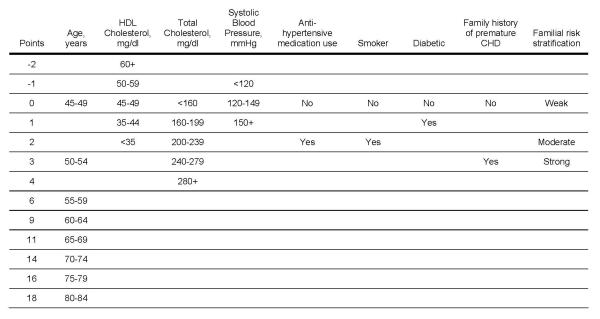
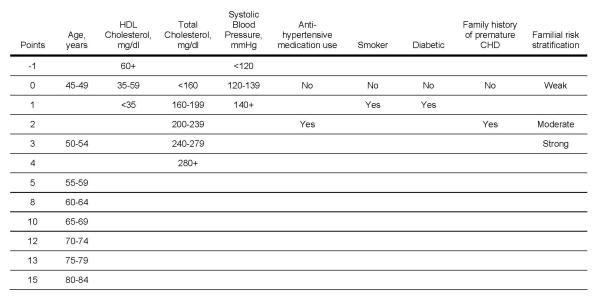
Score sheets that estimate the multivariable risk for a positive CAC score for women and men are shown in Figures 2 and 3, respectively. The correlation between the points assigned using the GCRP and Model 1 was high with values of 0.88 (p<0.0001) in men and 0.84 (p<0.0001) in women, suggesting that the covariates in the GCRP are relevant CAC risk factors.
Figure 2.
Risk scores for coronary artery calcium for women. Current smoking defined as having smoked a cigarette in the last 30 days. Diabetic defined as having a previous diagnosis of diabetes, fasting glucose ≥126 mg/dL or if use of a hypoglycemic medication. Family history of premature coronary heart disease (CHD) was defined as having at least one first-degree male relative with CHD diagnosed before age 55 years and at least one first-degree female relative with CHD diagnosed before age 65 years. Rules for familial risk stratification considered the number, sex, lineage and age at onset of relatives with CHD, stroke and diabetes (as described in Figure 1). HDL = high-density lipoprotein
Figure 3.
Risk scores for coronary artery calcium for men. Current smoking defined as having smoked a cigarette in the last 30 days. Diabetic defined as having a previous diagnosis of diabetes, fasting glucose ≥126 mg/dL or if use of a hypoglycemic medication. Family history of premature coronary heart disease (CHD) was defined as having at least one first-degree male relative with CHD diagnosed before age 55 years and at least one first-degree female relative with CHD diagnosed before age 65 years. Rules for familial risk stratification considered the number, sex, lineage and age at onset of relatives with CHD, stroke and diabetes (as described in Figure 1). HDL = high-density lipoprotein
A total of 220 (6.9%) women were reclassified into higher or lower risk groups with Model 2, and 333 (10.5%) with Model 3 (Table 2). Of those reclassified, the reclassification was correct for 121 (55%) with Model 2 and 192 (57.7%) with Model 3. For women with CAC >300, using Model 2 instead of 1, a total of 12 (3+9) were reclassified correctly into higher risk groups and 10 (3+7) were reclassified incorrectly into lower risk groups. The net estimate for the percentage classified correctly is the difference between these two estimates divided by the total number with CAC >300 [(12-10)/218=0.9%]. Similar calculations for women with CAC ≤300 revealed a total of 109 (91+18) correctly reclassified downward and 89 (70+19) incorrectly reclassified upward. The net estimate for those with CAC ≤300 is [(109-89)/2,966=0.7%]. The NRI was estimated by taking the sum of these percentages, which was 1.6% (p=0.452). The NRI associated with the addition of family history using Model 3 instead of 1 was 6.8% (p=0.015).
Table 2.
Reclassification for CAC score >300 for women comparing Model 1 (based on GCRP variables) to Model 2 (GCRP variables plus dichotomous family history) and Model 3 (GCRP variables plus familial risk stratification)
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| Low probability (<5%) |
Intermediate probability (5-25%) |
High probability (>25%) |
Low probability (<5%) |
Intermediate probability (5-25%) |
High probability (>25%) |
Total Number |
|
| Number (percent) | |||||||
| Participants with CAC >300 | |||||||
| Low probability (<5%) | 18(85.7) | 3(14.3) | 0 | 14(66.7) | 7(33.3) | 0 | 21 |
| Intermediate probability (5-25%) | 3(1.8) | 159(93.0) | 9(5.2) | 4(2.3) | 150(87.7) | 17(9.9) | 171 |
| High probability (>25%) | 0 | 7(26.9) | 19(73.1) | 0 | 8(30.8) | 18(69.2) | 26 |
| Total number | 21 | 169 | 28 | 18 | 165 | 35 | 218 |
| Participants with CAC <300 | |||||||
| Low probability (<5%) | 1,595(96.4) | 70(4.2) | 0 | 1,568(94.7) | 97(5.9) | 0 | 1,655 |
| Intermediate probability (5-25%) | 91(7.3) | 1,143(91.2) | 19(1.5) | 1,41(11.3) | 1,080(86.2) | 32(2.6) | 1,253 |
| High probability (>25%) | 0 | 18(37.5) | 30(62.5) | 0 | 27(56.3) | 21(43.7) | 48 |
| Total | 1,686 | 1,231 | 49 | 1,709 | 1,204 | 53 | 2,966 |
GCRP, general cardiovascular risk profile; CAC, coronary artery calcium
A total of 201 (7%) men were reclassified into higher or lower risk groups with Model 2, and 396 (13.7%) with Model 3 (Table 3). Of the men reclassified, the reclassification was correct for 108 (53.7%) with Model 2 and 214 (54%) with Model 3. For men, the NRI estimates using Models 2 and 3 instead of Model 1 were 0.3% (p=0.803) and 4.2% (p=0.034), respectively.
Table 3.
Reclassification for CAC score >300 for men comparing Model 1 (based on GCRP variables) to Model 2 (GCRP variables plus dichotomous family history) and Model 3 (GCRP variables plus familial risk stratification)
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| Low probability <(5%) |
Intermediate probability (5-25%) |
High probability (>25%) |
Low probability (<5%) |
Intermediate probability (5-25%) |
High probability (>25%) |
Total Number |
|
| Number (percent) | |||||||
| Participants with CAC >300 | |||||||
| Low probability (<5%) | 9 (90.0) | 1(10.0) | 0 | 7(70.0) | 3(30.0) | 0 | 10 |
| Intermediate probability (5-25%) | 2(1.0) | 191(90.5) | 18(8.5) | 2(1.0) | 157(74.4) | 52(24.6) | 211 |
| High probability (>25%) | 0 | 19(6.4) | 279(93.6) | 0 | 34(11.4) | 264(88.6) | 298 |
| Total number | 11 | 211 | 297 | 9 | 194 | 316 | 519 |
| Participants with CAC ≤300 | |||||||
| Low probability (<5%) | 440(93.0) | 33(7.0) | 0 | 417(88.2) | 56(11.8) | 0 | 473 |
| Intermediate probability (5-25%) | 46(3.3) | 1,292(93.8) | 39(2.8) | 78(5.7) | 1,209(87.8) | 90(6.5) | 1,377 |
| High probability (>25%) | 0 | 43(8.3) | 474(91.7) | 0 | 81(15.7) | 436(84.3) | 517 |
| Total | 486 | 1,368 | 513 | 495 | 1,346 | 526 | 2,367 |
GCRP, general cardiovascular risk profile; CAC, coronary artery calcium
We also compared reclassification from Model 2 to 3 for women and men for CAC >300 (Tables 4 and 5). There were 247 (7.8%) women reclassified into lower or higher risk groups. Of the women reclassified, 138 (55.9%) were reclassified correctly and the NRI was 5.2% (p=0.023). For men, 277 (9.6%) were reclassified into lower or higher risk groups, and of these men, 147 (53.1%) were reclassified correctly with an NRI of 3.9% (p=0.022).
Table 4.
Reclassification for CAC score >300 for women comparing Model 2 (GCRP variables plus dichotomous family history) and Model 3 (GCRP variables plus familial risk stratification)
| Model 2 | Model 3 | |||
|---|---|---|---|---|
| Number (percent) | ||||
| Low probability (<5%) |
Intermediate probability (5-25%) |
High probability (>25%) |
Total Number |
|
| Participants with CAC >300 | ||||
| Low probability (<5%) | 16(76.2) | 5(23.8) | 0 | 21 |
| Intermediate probability (5-25%) | 2(1.2) | 155(91.7) | 12(7.1) | 169 |
| High probability (>25%) | 0 | 5(17.9) | 23(82.1) | 28 |
| Total number | 18 | 165 | 35 | 218 |
| Participants with CAC ≤300 | ||||
| Low probability (<5%) | 1,606(95.3) | 80(4.7) | 0 | 1,686 |
| Intermediate probability (5-25%) | 103(8.4) | 1,106(89.8) | 22(1.8) | 1,231 |
| High probability (>25%) | 0 | 18(36.7) | 31(63.3) | 49 |
| Total | 1,709 | 1,204 | 53 | 2,966 |
GCRP, general cardiovascular risk profile; CAC, coronary artery calcium
Table 5.
Reclassification for CAC score >300 for men comparing Model 2 (GCRP variables plus dichotomous family history) and Model 3 (GCRP variables plus familial risk stratification)
| Model 2 | Model 3 | |||
|---|---|---|---|---|
| Number (percent) | ||||
| Low probability (<5%) |
Intermediate probability (5-25%) |
High probability (>25%) |
Total Number |
|
| Participants with CAC >300 | ||||
| Low probability (<5%) | 7(63.6) | 4(36.4) | 0 | 11 |
| Intermediate probability (5-25%) | 2(0.9) | 169(80.1) | 40(19.0) | 211 |
| High probability (>25%) | 0 | 21(7.1) | 276(92.9) | 297 |
| Total number | 9 | 194 | 316 | 519 |
| Participants with CAC ≤300 | ||||
| Low probability (<5%) | 444(91.4) | 42(8.6) | 0 | 486 |
| Intermediate probability (5-25%) | 51(3.7) | 1,252(91.5) | 65(4.8) | 1,368 |
| High probability (>25%) | 0 | 52(10.1) | 461(89.9) | 513 |
| Total | 495 | 1,346 | 526 | 2,367 |
GCRP, general cardiovascular risk profile; CAC, coronary artery calcium
DISCUSSION
The GCRP (Model 1) works well for both women and men for the outcome of CAC, and the addition of family history improves the ability of the GCRP to identify individuals with positive CAC scores. However, significant improvement in reclassification of those with CAC >300 was only achieved when comprehensive familial risk assessment was used in the model.
GCRP AND THE OUTCOME OF CAC
Across the three models in our study, we observed highly statistically significant associations between all risk factors evaluated and CAC except for current smoking in males. The lack of significant association may be attributed to including former smokers in the reference group since both current and former smoking has been associated with CAC in men and women.23
The direction and impact of each variable in the GCRP was the same for CAC in MESA as for cardiovascular events in the Framingham cohort.16 However, age was relatively more important, and blood pressure, smoking and diabetes were relatively less important for CAC than for clinical events. These observed differences may be due to differences in these outcomes.
Finding that the GCRP can identify participants with positive CAC scores is important because advanced subclinical atherosclerosis is increasingly becoming the rationale for aggressive risk factor modification.2-4 Accumulation of CAC occurs almost exclusively in atherosclerotic arteries, particularly in advanced lesions,1,24-28 and several studies have shown that CAC provides prognostic information of proven value regarding the risk of cardiovascular events.1-8 Thus, the addition of measures of subclinical atherosclerosis may be reasonable as a means to improve CHD risk stratification due to the limitations of the current clinical guidelines for primary prevention.14,15,29
FAMILY HISTORY AND CAC MODEL PERFORMANCE
Compared to the simple definition of family history (i.e., having at least one first-degree relative with premature CHD), comprehensive familial risk stratification classified 2.5 times as many participants as having an increased familial risk, or almost half of the cohort. Familial risk stratification also provided quantitatively important risk information for CAC. The only CAC risk factors associated with a greater number of points than strong familial risk were total cholesterol >280 mg/dl and age greater than 55 years. Finally, the addition of family history as a risk factor to the GCRP according to either family history definition (Model 2 or 3) added discriminatory value to the baseline model for identifying participants with positive CAC scores. However, reclassification of individuals with advanced CAC was significantly improved with Model 3 only.
Family history reflects the complex interactions of genetic and non-genetic risk factors (e.g., exposures, behaviors, cultural factors) shared by affected family members.30 As genetic markers for CVD risk are discovered, to understand how best to apply this information for the purpose of risk assessment, it will be important to consider the incremental value of these markers in the context of familial risk. For example, the 9p21 locus is a significant risk factor for CVD; however, when added to a risk prediction model including traditional risk factors, C-reactive protein and family history of premature CHD, it had no effect on model discrimination or reclassification.31 This finding supports omission of 9p21 genotyping in population screening for cardiovascular risk. However, the true incremental predictive value may be found when such testing is restricted to individuals with strong familial risk. Aggregation of genetic risk factors are expected within this subgroup, and genotyping for risk alleles, such as 9p21, may help to further classify risk in this subgroup. Thus, as we enter the “genomic revolution”, family history assessment will likely remain relevant.30
LIMITATIONS
Because our goal was to assess the added value of including family history in an existing global risk assessment model, we worked with data from MESA, as it collected comprehensive family history regarding CHD, stroke and diabetes in first-degree relatives. Family history was ascertained 18 to 24 months after obtaining information on other variables and the baseline CAC score. Thus, there is a potential for bias due to the time elapsed and the possibility that participants were prompted to learn more about their family history after receiving their baseline CAC score. However, we believe this bias is minimal because it is unlikely that the family history changed substantially between Exams 1 and 2, and it is unknown whether participants would be more likely to inquire about family history of CHD, stroke or diabetes given a positive CAC score.
The cross-sectional design of our study prohibits establishment of any temporal associations concerning family history as a risk factor. Moreover, the baseline data do not permit development of a predictive model, which would require prospective data on clinical outcomes. Because our family history models were not nested models, we were only able to informally compare these models using the AIC. We were, however, able to compare reclassification of participants with advanced CAC using NRI estimates.
A major strength of this study is the large, ethnically diverse and well phenotyped MESA cohort. However, these MESA participants are relatively healthy, as individuals with pre-existing CVD were excluded. Thus, those with the strongest risk factors for advanced CAC could have been excluded, which could underestimate the significance of certain familial risk factors and diminish the strength of associations we have observed.
Because our aim was to determine the incremental value of including family history in the sex-specific GCRP models to assess CAC—rather than to create a new model for CAC risk assessment—we did not include other variables. Our analyses showed significant associations between ethnicity/race and CAC; however, there were no significant interactions, suggesting any improvement of model discrimination with the addition of family history would not be limited to specific ethnic/racial groups. Future studies should investigate the added value of ethnicity/race to a global risk assessment model for CAC.
Another limitation of our study is lack of validation of self-reports of family history. Numerous studies have estimated the accuracy of these reports for CHD, stroke, and diabetes in first-degree relatives. In general, sensitivity ranges from 50% to 90% and most specificity values are greater than 90%.32-37 Older individuals are more likely to give inaccurate family history.34,37 However, a personal history of CHD or CHD risk factors generally does not affect the accuracy of the family history report, nor does ethnicity/race34 or sex.34,35,37 Women in our study were much more likely to report a family history consistent with a strong familial risk. Given the sensitivity and specificity of self-reports described above, it seems more likely that men are under-reporting family history rather than women overreporting.
Despite the lower sensitivity of self-reports of family history, positive disease associations are consistently observed and use of family history can help to stratify disease risk in the population. However, given the lower sensitivity values and potential differences in reporting associated with age or other demographic variables, when assessing individual risk, validation of self-reports of family history is desirable, and in the clinical setting validation may be necessary if the familial risk substantially affects management decisions.
CONCLUSIONS
Our findings provide further evidence that the GCRP is a general model for assessing global CVD risk. Incorporation of family history, especially comprehensive familial risk assessment based on the number of relatives with CHD, age at onset, and presence of stroke or diabetes in the family, improves the prediction of advanced CAC.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions.
FUNDING: This work was supported by National Heart, Lung, and Blood Institute grant 1 R21 HL081175-01A1. MESA was supported by contracts NO1-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute.
Footnotes
DISCLOSURES: None of the authors have any conflicts of interest to disclose.
References
- 1.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 2.Kondos GT, Hoff JA, Sevrukov A, Daviglus ML, Garside DB, Devries SS, Chomka EV, Liu K. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5,635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 3.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 4.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, c-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158–165. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean 3-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46:807–814. doi: 10.1016/j.jacc.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 6.Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, Witteman JC. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–577. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 7.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 9.Naghavi M, Falk E, Hecht HS, Jamieson MJ, Kaul S, Berman D, Fayad Z, Budoff MJ, Rumberger J, Naqvi TZ, Shaw LJ, Faergeman O, Cohn J, Bahr R, Koenig W, Demirovic J, Arking D, Herrera VLM, Badimon J, Goldstein J, Rudy Y, Airaksinen J, Schwartz RS, Riley WA, Mendes RA, Douglas P, Shah PK, for the SHAPE Task Force From vulnerable plaque to vulnerable patient – part III: executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol. 2006;98(Suppl):2H–15H. doi: 10.1016/j.amjcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Nasir K, Budoff MJ, Wong ND, Scheuner M, Herrington D, Arnett DK, Szklo M, Greenland P, Blumenthal RS. Family history of premature coronary heart disease and coronary artery calcification. Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;116:619–626. doi: 10.1161/CIRCULATIONAHA.107.688739. [DOI] [PubMed] [Google Scholar]
- 11.Scheuner MT, Messan Setodji C, Pankow JS, Blumenthal RS, Keeler E. Relation of familial patterns of coronary heart disase, stroke, and diabetes to subclinical atherosclerosis: The Multi-Ethnic Study of Atherosclerosis. Genet Med. 2008;10:879–887. doi: 10.1097/GIM.0b013e31818e639b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silberberg JS, Wlodarczyk J, Fryer J, Robertson R, Hensley MJ. Risk associated with various definitions of family history of coronary heart disease. The Newcastle Family History Study II. Am J Epidemiol. 1998a;147:1133–1139. doi: 10.1093/oxfordjournals.aje.a009411. [DOI] [PubMed] [Google Scholar]
- 13.Scheuner MT, Whitworth WC, McGruder H, Yoon PW, Khoury MJ. Familial risk assessment for early-onset coronary heart disease. Genet Med. 2006;8:525–531. doi: 10.1097/01.gim.0000232480.00293.00. [DOI] [PubMed] [Google Scholar]
- 14.Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 15.De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, Ebrahim S, Faergeman O, Graham I, Mancia G, Cats Manger V, Orth-Gomér K, Perk J, Pyorala K, Rodicio JL, Sans S, Sansoy V, Sechtem U, Silber S, Thomsene T, Wood D, for the Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice European guidelines on cardiovascular disease prevention in clinical practice: Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of eight societies and by invited experts) Eur Heart J. 2003;24:1601–1610. doi: 10.1016/s0195-668x(03)00347-6. [DOI] [PubMed] [Google Scholar]
- 16.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women. The Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: The Reynolds Risk Score for Men. Circulation. 2008;118:2243–2251. doi: 10.1161/CIRCULATIONAHA.108.814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 20.Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt-Gray M, Bild DE. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility: MESA study. Radiology. 2005;236:477–484. doi: 10.1148/radiol.2362040513. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan LM, Massaro JM, D’Agostino RB., Sr Presentation of multivariate data for clinical use: the Framingham study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 22.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 23.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the multi-ethnic study of atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 24.Stary HC. Composition and classification of human atherosclerotic lesions. Virchows Arch A Pathol Anat Histopathol. 1992;421:277–290. doi: 10.1007/BF01660974. [DOI] [PubMed] [Google Scholar]
- 25.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 26.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histopathological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 27.Bielak LF, Rumberger JA, Sheedy PF, Schwartz RS, Peyser PA. Probabilistic model for prediction of angiographically defined obstructive coronary artery disease using electron beam computed tomography calcium score strata. Circulation. 2000;102:380–385. doi: 10.1161/01.cir.102.4.380. [DOI] [PubMed] [Google Scholar]
- 28.Nallamothu BK, Saint S, Rubenfire M, Fendrick AM. Electron beam computed tomography in the diagnosis of obstructive coronary artery disease. J Am Coll Cardiol. 2001;37:689–690. doi: 10.1016/s0735-1097(00)01152-9. [DOI] [PubMed] [Google Scholar]
- 29.Smith SC, Jr, Greenland P, Grundy SM. AHA Conference Proceedings. Prevention conference V: Beyond secondary prevention: identifying the high-risk patient for primary prevention: executive summary. Circulation. 2000;101:111–116. doi: 10.1161/01.cir.101.1.111. [DOI] [PubMed] [Google Scholar]
- 30.Guttmacher AE, Collins FS, Carmona RH. The family history – more important than ever. N Engl J Med. 2004;351:2333–2336. doi: 10.1056/NEJMsb042979. [DOI] [PubMed] [Google Scholar]
- 31.Paynter NP, Chasman DI, Buring JE, Shiffman D, Cook NR, Ridker PM. Cardiovascular disease risk prediction with and without knowledge of genetic variation at chromosome 9p21.3. Ann Intern Med. 2009;150:65–72. doi: 10.7326/0003-4819-150-2-200901200-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silberberg JS, Wlodarczyk J, Fryer J, Ray CD, Hensley MJ. Correction for biases in a population-based study of family history and coronary heart disease. The Newcastle Family History Study I. Am J Epidemiol. 1998;147:1123–1132. doi: 10.1093/oxfordjournals.aje.a009410. [DOI] [PubMed] [Google Scholar]
- 33.Friedlander Y, Arbogast P, Schwartz SM, Marcovina SM, Austin MA, Rosendaal FR, Reiner AP, Psaty BM, Siscovick DS. Family history as a risk factor for early onset myocardial infarction in young women. Atherosclerosis. 2001;156:201–207. doi: 10.1016/s0021-9150(00)00635-3. [DOI] [PubMed] [Google Scholar]
- 34.Bensen JT, Liese AD, Rushing JT, Province M, Folsom AR, Rich SS, Higgins M. Genet Epidemiol. 1999;17:141–150. doi: 10.1002/(SICI)1098-2272(1999)17:2<141::AID-GEPI4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 35.Kee F, Tiret L, Robo JY, Nicaud V, McCrum E, Evans A, Cambien F. Reliability of reported family history of myocardial infarction. Br Med J. 1993;307:1528–1530. doi: 10.1136/bmj.307.6918.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hastrup JL, Hotchkiss AP, Johnson CA. Accuracy of knowledge of family history of cardiovascular disorders. Health Psychol. 1985;4:291–306. doi: 10.1037//0278-6133.4.4.291. [DOI] [PubMed] [Google Scholar]
- 37.Murabito JM, Nam BH, D’Agostino RB, Sr, Lloyd-Jones DM, O’Donnell CJ, Wilson PW. Accuracy of offspring reports of parental cardiovascular disease history: the Framingham Offspring Study. Ann Intern Med. 2004;140:434–440. doi: 10.7326/0003-4819-140-6-200403160-00010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.