Abstract
Purpose of Review
To discuss the role of rhinoviruses (HRVs) in early childhood wheezing illnesses and how HRVs contribute to the development of childhood asthma.
Recent Findings
Advanced molecular diagnostics have identified HRVs as pathogens frequently causing wheezing illnesses in infants and young children. Wheezing during HRV infection in early life identifies children at particularly high-risk of asthma development. Plausible mechanisms by which HRV could cause airway damage, promote airway remodeling, and lead to asthma development have recently been identified.
Summary
HRV is a significant source of morbidity in infants and young children. This review identifies mechanisms by which HRV lower respiratory tract infection, particularly in a susceptible host, could promote the development of childhood asthma. Further studies are needed to elucidate the mechanisms underlying the link between HRV wheezing in early childhood and subsequent asthma development, with the critical goal of identifying novel therapeutic and prevention strategies for both early childhood wheezing and asthma.
Keywords: asthma, childhood, rhinovirus, wheezing
Introduction
Wheezing illnesses associated with viral respiratory infections commonly herald the development of asthma in early childhood. Whether these infections are causal in asthma development is controversial. Through the years, the role of respiratory syncytial virus (RSV) bronchiolitis during early childhood in asthma development has been extensively scrutinized. There is evidence to show that children who wheeze with RSV infections during the first few years of life, particularly those who are hospitalized, are at increased risk of childhood asthma.(1;2) With the development of molecular viral diagnostics, much has been learned over the past decade with respect to the role of previously undiscovered or underappreciated viruses as early childhood respiratory pathogens.(3) Perhaps most significantly, human rhinoviruses (HRVs) have been recognized as an extremely common cause of wheezing illnesses in early childhood and beyond.(4–6) In addition, wheezing during HRV infections has been strongly linked to subsequent asthma development.(7) This review will focus on the mechanisms underlying host-susceptibility to HRV infections and the role HRVs play in early childhood wheezing and asthma development.
Epidemiology of Wheezing in Early Childhood
The most common cause of wheezing during the first several years of life is infection with respiratory viruses, most commonly RSV, HRVs, and multiple virus infections. All children are infected with respiratory viruses during early childhood and nearly one-half wheeze during at least one of these infections.(8) A number of risk factors for wheezing with respiratory viruses during early childhood have been identified by birth-cohort studies. Maternal smoking, male gender, older siblings and daycare attendance during infancy increase the risk for early childhood wheezing illnesses, while breastfeeding in the first months of life is likely protective against wheezing respiratory illnesses.(8;9)
While RSV is the predominant lower respiratory pathogen during the winter season in the Northern hemisphere, HRVs cause lower respiratory illnesses throughout the year and are the leading cause of wheezing illnesses during spring and fall.(4) Recently, wheezing illnesses associated with HRV infection, both inpatient and outpatient, have been linked to more dramatic increases in persistent wheezing and asthma risk than wheezing illnesses associated with RSV.(4;7;10)
Human Rhinovirus, an Upper and Lower Respiratory Pathogen
HRVs are the most frequent cause of the common cold and have traditionally been considered a pathogen isolated to the upper airway, in part because the ideal temperature for HRV replication is 33–35°C. However, HRVs can and do invade and replicate in the lower airway following inoculation of the upper airway.(11;12) Historically, HRVs have been divided into 2 groups (A & B) with 101 known serotypes. Tremendous efforts to better understand factors determining pathogenicity of HRVs are ongoing. Recently, full genome sequencing of the known HRV serotypes, revealed a number of areas of the genome that could be important for virulence determination.(13) Another important observation from this sequencing effort was that HRVs can recombine to create new strains.(13) Many HRVs do not grow well in traditional cell culture, and this is particularly true for a novel group of HRVs, categorized as Group C HRVs. Molecular diagnostics and characterization of HRVs has shown clearly that the number of HRV serotypes have previously been dramatically underestimated.(14) In the past several years, there has been an explosion of reports identifying novel Group C HRVs and implicating them in both upper and lower respiratory tract illnesses.(14–18) In fact, Group C HRVs seem to be detected very frequently in children hospitalized for respiratory illnesses and asthma exacerbations.(15–17) Much remains to be learned about this newly discovered group of HRVs.
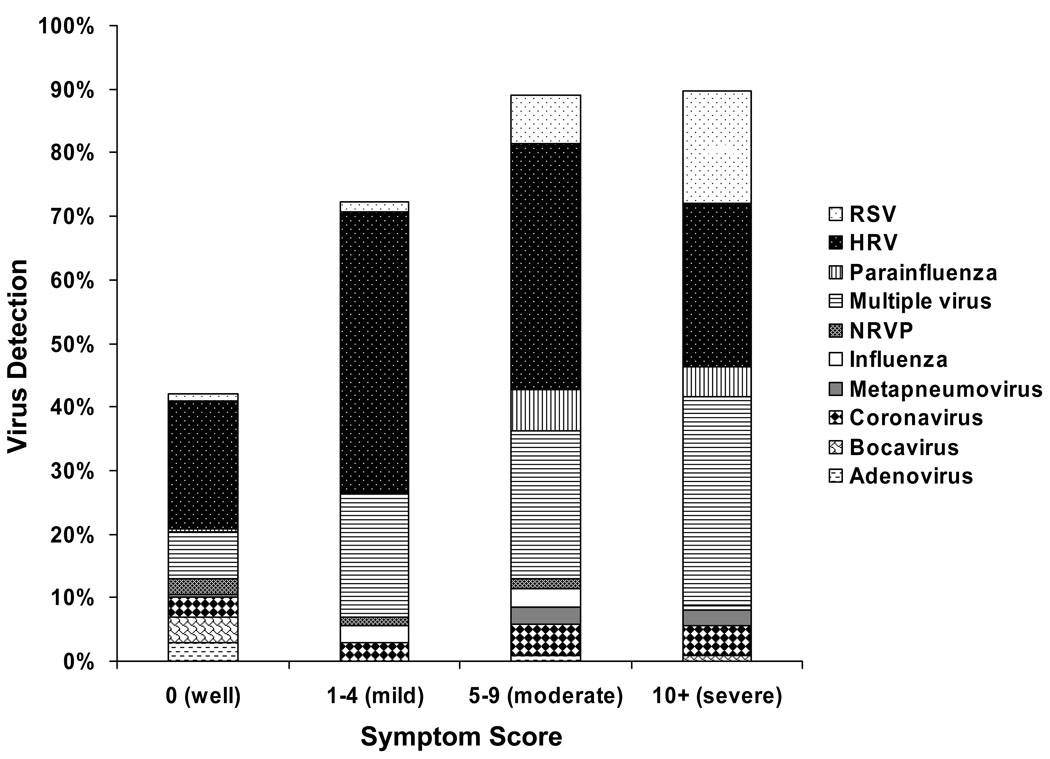
It is also important to note that HRVs (and other respiratory viruses) can lead to a broad spectrum of illness severity (Figure 1). Some infants and children infected with HRV may be asymptomatic, while others may have an illness severe enough to lead to hospitalization. This suggests that there may be host factors that play an important role in the severity of illnesses caused by HRVs. In addition, particular strains of HRV may be particularly pathogenic. A hybrid of these two concepts is that particular strains of HRV may cause more severe illnesses in susceptible hosts. Sorting out the relative contribution of host and virus-related factors toward asthma development are critical ongoing areas of investigation and could lead to new therapeutic and prevention strategies for wheezing illnesses and asthma.
Figure 1. Viral etiology of respiratory illnesses during infancy.
This figure shows the rates of detection of respiratory viruses by illness severity during the first year of life in the Childhood Origins of ASThma (COAST) study and is updated from previously published data(4) to include virus identification by respiratory multicode assay(3). HRV infections are associated with a broad range of symptom severity from asymptomatic to severe respiratory infection. HRV, human rhinovirus; RSV, respiratory syncytial virus; NRVP, non-rhinovirus picornavirus.
The Timing and Etiology of Wheezing Illnesses Impacts Subsequent Asthma Risk
RSV wheezing illnesses during the first few years of life, particularly those leading to hospitalization, have consistently been associated with an increased risk (2 to 3-fold) of subsequent asthma. With the increased ability to detect HRVs, data has emerged which indicates that wheezing with HRV is associated with a more pronounced risk of childhood asthma. Kotaniemi and colleagues have shown that children who are hospitalized with HRV wheezing illnesses during the first 2 years of life are at increased risk (4-fold) of childhood asthma compared to children who were hospitalized with wheezing caused by other viruses.(10)
Data from two birth cohorts have recently identified outpatient HRV-induced wheezing episodes during early childhood as an important risk factor for asthma as well. The Childhood Origins or ASThma (COAST) study is a high-risk birth cohort examining the role of respiratory viruses and immune dysregulation in the development of asthma and other allergic diseases.(19) COAST identified HRV wheezing illnesses during the 1st year of life as significant predictors of wheezing in the 3rd year of life(4) and asthma at age 6 years.(7) In the COAST study, children with HRV wheezing illnesses and aeroallergen sensitization during infancy had the greatest risk of asthma at school age. In a very similar high-risk cohort in Australia, Kusel and colleagues reported that HRV wheezing illnesses during the 1st year of life were associated with increased asthma risk at age 5 years, although this finding was restricted to children who developed aeroallergen sensitization by age 2 years.(20) Thus, two birth cohorts, on separate continents, have identified HRV wheezing illnesses during infancy and aeroallergen sensitization as risk factors for childhood asthma development.
The COAST study has continued to evaluate the timing and specific viral etiology of wheezing illnesses as the subjects have progressed through early childhood. It appears that the age at which HRV wheezing illnesses occur has significant prognostic value with regard to subsequent risk of asthma. Children who wheezed with HRV during year 1 had a ~3-fold risk of having asthma at age 6 years. HRV wheezing in year 2 was associated with a more pronounced increase in asthma risk (OR ~7), while wheezing with HRV infection during year 3 of life was associated with a dramatic (OR ~32) increase in asthma at school age. Indeed, nearly 90% of children who wheezed with HRV during the 3rd year of life had asthma when they reached school age.(7)
Inflammation & Airway Changes in Early Childhood Wheezing
Due to the limited ability to perform controlled invasive airway sampling for research purposes in infants and young children, there is limited data on the inflammatory and structural changes present in the airways of preschool wheezing children. Krawiec and colleagues, utilizing bronchoscopy and bronchoalveolar lavage (BAL), demonstrated that children with persistent wheezing (median age 15 months) had increased cellularity and markers of inflammation compared to healthy controls, but did not have the characteristic eosinophil predominance seen in older children and adults with asthma.(21) Similarly, Saglani and colleagues using bronchoscopy and biopsy in children with persistent wheezing (median age 12 months) showed no eosinophilic inflammation or reticular basement membrane (RBM) thickening, another hallmark of asthma seen in older children and adults.(22) In contrast, children with recurrent wheezing (median age 29 months) had both eosinophilic inflammation and RBM thickening when compared to controls, suggesting that changes typical of asthma in later childhood evolve in the airway between 1 and 3 years of age in recurrent wheezing children. Whether the viruses (frequently HRVs) causing recurrent wheezing illnesses in these children trigger or contribute to these inflammatory changes and RBM thickening in early childhood is currently unknown.
Natural History: Intermittent Virus-Induced Wheezing Becomes Persistent Disease
For years clinicians have noted the progression from generally intermittent wheezing illnesses to persistent asthma during early childhood. Several attempts have been made to alter this “natural history” of asthma development. The Preventing Early Asthma in Kids (PEAK) study(23) and the Prevention of Asthma in Childhood (PAC) study(24) utilized inhaled corticosteroids (ICS) in high-risk children to attempt to prevent asthma development. Both protocols were unsuccessful in altering the natural course of asthma, but PEAK clearly demonstrated symptomatic benefit for use of daily low-dose ICS in high-risk children with recurrent wheezing. In addition, PEAK clearly demonstrated the increased burden of symptoms (i.e. the development of persistent disease) that develops during early childhood.(23) Thus, during the same time frame (year 1 to year 3) that children with recurrent wheezing evolve from having nonspecific airway inflammation(21;22) to having airway changes typical of childhood asthma (eosinophilic predominance and RBM thickening),(25) they begin to develop more persistent symptoms,(23) and the increased risk of having asthma at school-age associated with HRV wheezing illnesses climbs from OR ~3 to OR ~32.(7) All of this clearly suggests that children who wheeze with HRV during the 3rd of life are not just at risk for asthma, but that they have asthma and any interventions directed at prevention need to be implemented before this point. Identifying novel therapeutic targets is critical and a better understanding of the host and virus-specific factors underlying the observed relationships between increased severity of HRV illnesses in early childhood and asthma development is required.
Host and Environmental Factors Associated with Wheezing Illnesses & Asthma
A number of host factors have been associated with increased risk of early childhood virus-induced wheezing illnesses. Some of these same factors have been associated with impaired responses to HRV infection in older individuals with asthma. Impaired peripheral blood interferon-γ responses to both mitogens and viruses at birth have been associated with recurrent wheezing during infancy.(26) Similarly, reduced peripheral blood interferon-γ responses to mitogen at 9 months of age have been linked to an increased risk of wheezing in toddlers and school age children.(27) Interestingly, another important risk factor for asthma development, aeroallergen sensitization in early childhood,(7;8;20;28) is associated with reduced interferon-γ responses as well.
A series of studies utilizing epithelial cells from patients with allergic asthma, suggest that other types of interferon (β and λ) responses are impaired in these individuals as well. In these studies, HRV infection of asthmatic airway epithelial cells led to decreased interferon production compared to controls, leading to increased viral replication and more severe asthma symptoms during infection.(29;30) Thus, deficiencies in interferon responses in the peripheral blood as well as the airway have been associated with both recurrent wheezing and asthma, perhaps through a common mechanism of impaired innate immune response to virus infection.
Barrier function is also critical to the innate immune response, and impaired epithelial barrier function may also increase susceptibility to infection by respiratory viruses. Jakiela and colleagues recently reported that HRV replication is enhanced in epithelial cell culture in an experimental model of airway damage.(31) It is thus plausible to suggest that the variety of host factors (filaggrin mutation(32)) and environmental factors (smoke exposure, pollution, virus infections, and allergens) that lead to disruption of barrier function could lead to enhanced viral replication and increase the severity of viral respiratory illnesses (including HRV) during early childhood, thus leading to airway damage, and asthma development. These same factors may also contribute to asthma exacerbation.
HRV Promotes Inflammation & Airway Remodeling
HRV infections promote the expression of factors that have been associated with airway damage and remodeling. Recent studies have identified a number of potential pathways that could causally link recurrent HRV infection of the lower airway with airway remodeling and asthma development. Leigh and colleagues reported that HRV infection of airway epithelial cells in vitro was associated with increased production of mediators involved in airway remodeling, including amphiregulin, activin A, and VEGF.(33) In this same study, the authors reported increased VEGF in nasal lavage fluid of volunteers with natural HRV infections as well. Recently, Zhu and colleagues demonstrated that HRV infection of epithelial cells also causes TLR-3 dependent mucin production and up-regulation of epidermal growth factor receptor, a prominent component of epithelial repair.(34) Finally, microarray technology was utilized in an attempt to elucidate novel pathways important in response to HRV infection. Two separate studies to assess gene expression in epithelial cells during HRV infection identified up-regulation of many genes in the interferon pathway as well as genes involved in airway remodeling.(35;36) In sum these findings demonstrate that virus infections, and HRV in particular, can activate a number of pro-inflammatory and airway remodeling pathways that one could hypothesize may have deleterious effects on the rapidly growing airways of young children.
HRV and Response to Therapy
In general preschool children with wheezing do not respond as well to typical therapies and utilize a disproportionate number of health care resources when compared to older children and adults with asthma.(37) Conventional therapies do not alter the natural history of the disease.(23;24) Although a recent post hoc analysis suggests that young children with wheezing illnesses associated with HRV may respond differently to prednisolone therapy than children with wheezing associated with other viruses.(38)
Recently, an uncontrolled trial examining the effect of palivizumab (a monoclonal RSV Ab) on the development of recurrent wheezing in young children showed promising results,(39) and a similar strategy aimed at HRV is desirable. To date, vaccines and other prevention strategies for HRV have proven elusive. However, new molecular technologies and the potential for identification of particularly virulent HRV strains could allow for vaccine development in addition to new treatment strategies. Clinical studies attempting to identify particularly virulent groups or strains of HRV are ongoing and critical to the development of novel therapeutic and prevention strategies.
Conclusion
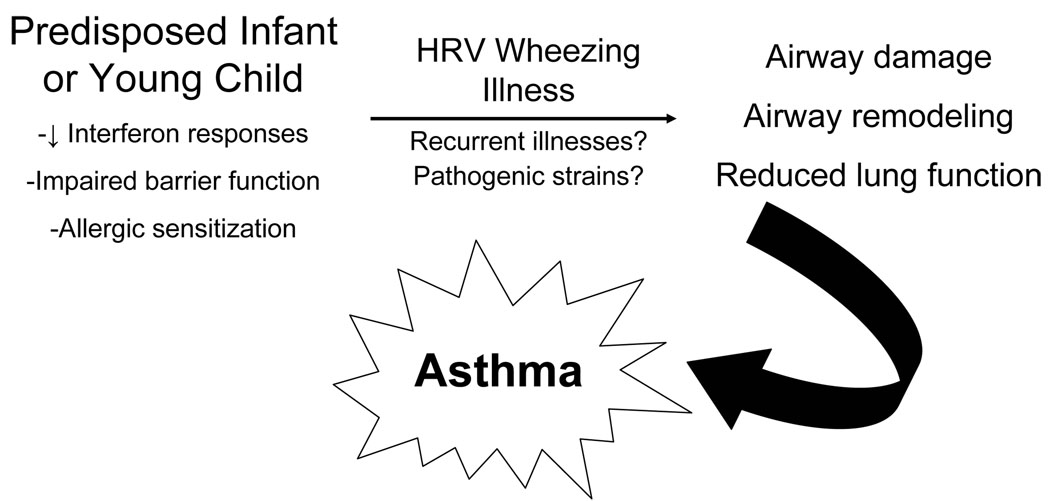
Advanced viral diagnostics have identified the importance of HRVs as both upper and lower respiratory pathogens. Wheezing illnesses caused by HRV in early childhood clearly identify children at increased risk of childhood asthma. Recent studies have identified plausible pathways by which HRV infection could be causal, particularly in a susceptible host, leading to airway damage, remodeling, and asthma (Figure 2). Whether particular strains of HRV are more pathogenic is an open and important question. Much has been learned in recent years regarding the role of HRVs in early childhood wheezing and asthma pathogenesis. However, novel therapeutic targets are needed to more effectively treat virus-induced wheezing, with the ultimate goal of interrupting the progression from intermittent virus-induced wheezing to childhood asthma.
Figure 2. HRV wheezing illnesses & asthma development.
This figure depicts how HRV may be causally involved in asthma pathogenesis in susceptible children.
Acknowledgements
I would like to thank Robert F. Lemanske Jr., MD and James E. Gern, MD for their mentorship and advice.
Supported by NIH grants T32 AI007635 & P01 HL70831 and by a AAAAI/GSK Career Development Award
Reference List
- 1.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 2.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005 Jan 15;171(2):137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 3.Lee WM, Grindle K, Pappas T, Marshall DJ, Moser MJ, Beaty EL, et al. High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol. 2007 Aug;45(8):2626–2634. doi: 10.1128/JCM.02501-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemanske RF, Jr., Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005 Sep;116(3):571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 5.Kistler A, Avila PC, Rouskin S, Wang D, Ward T, Yagi S, et al. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis. 2007 Sep 15;196(6):817–825. doi: 10.1086/520816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller EK, Lu X, Erdman DD, Poehling KA, Zhu Y, Griffin MR, et al. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007 Mar 15;195(6):773–781. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008 Oct 1;178(7):667–672. doi: 10.1164/rccm.200802-309OC. **High-risk birth cohort study identified HRV wheezing in the first three years of life as the most significant predictor of childhood asthma
- 8.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson children's respiratory study: 1980 to present. J Allergy Clin Immunol. 2003 Apr;111(4):661–675. doi: 10.1067/mai.2003.162. [DOI] [PubMed] [Google Scholar]
- 9.Koopman LP, Smit HA, Heijnen ML, Wijga A, van Strien RT, Kerkhof M, et al. Respiratory infections in infants: interaction of parental allergy, child care, and siblings-the piama study. Pediatrics. 2001 Oct;108(4):943–948. doi: 10.1542/peds.108.4.943. [DOI] [PubMed] [Google Scholar]
- 10.Kotaniemi-Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy--the first sign of childhood asthma? J Allergy Clin Immunol. 2003 Jan;111(1):66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gern JE, Galagan DM, Jarjour NN, Dick EC, Busse WW. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med. 1997 Mar;155(3):1159–1161. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 12.Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, Fraenkel DJ, et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000 Jun;181(6):1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 13. Palmenberg AC, Spiro D, Kuzmickas R, Wang S, Djikeng A, Rathe JA, et al. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science. 2009 Apr 3;324(5923):55–59. doi: 10.1126/science.1165557. **Sequenced all known serotypes of HRV, identifying potential regions of the genome involved in pathogenicity and demonstrating that HRVs can recombine.
- 14.Lee WM, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2(10):e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller EK, Edwards KM, Weinberg GA, Iwane MK, Griffin MR, Hall CB, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009 Jan;123(1):98–104. doi: 10.1016/j.jaci.2008.10.007. **Population-based study that identified Group C HRVs as a common cause of hospitalization in young children, particularly those with asthma.
- 16. Miller EK, Khuri-Bulos N, Williams JV, Shehabi AA, Faouri S, Al JI, et al. Human rhinovirus C associated with wheezing in hospitalised children in the Middle East. J Clin Virol. 2009 Sep;46(1):85–89. doi: 10.1016/j.jcv.2009.06.007. *Identified Group C HRVs as a prominent cause of hospitalization of young children in Jordan.
- 17. Tan BH, Loo LH, Lim EA, Kheng Seah SL, Lin RT, Tee NW, et al. Human rhinovirus group C in hospitalized children, Singapore. Emerg Infect Dis. 2009 Aug;15(8):1318–1320. doi: 10.3201/eid1508.090321. *Retrospective study identifying Group C HRVs as a cause of hospitalization in children in Singapore.
- 18. McErlean P, Shackelton LA, Andrews E, Webster DR, Lambert SB, Nissen MD, et al. Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C) PLoS One. 2008;3(4):e1847. doi: 10.1371/journal.pone.0001847. *Performed genomic analyses to characterize new Group C HRVs.
- 19.Lemanske RF., Jr. The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13 Suppl 15:38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 20.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007 May;119(5):1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krawiec ME, Westcott JY, Chu HW, Balzar S, Trudeau JB, Schwartz LB, et al. Persistent wheezing in very young children is associated with lower respiratory inflammation. Am J Respir Crit Care Med. 2001 May;163(6):1338–1343. doi: 10.1164/ajrccm.163.6.2005116. [DOI] [PubMed] [Google Scholar]
- 22.Saglani S, Malmstrom K, Pelkonen AS, Malmberg LP, Lindahl H, Kajosaari M, et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005 Apr 1;171(7):722–727. doi: 10.1164/rccm.200410-1404OC. [DOI] [PubMed] [Google Scholar]
- 23.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006 May 11;354(19):1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 24.Bisgaard H, Hermansen MN, Loland L, Halkjaer LB, Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med. 2006 May 11;354(19):1998–2005. doi: 10.1056/NEJMoa054692. [DOI] [PubMed] [Google Scholar]
- 25.Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007 Nov 1;176(9):858–864. doi: 10.1164/rccm.200702-212OC. [DOI] [PubMed] [Google Scholar]
- 26.Gern JE, Brooks GD, Meyer P, Chang A, Shen K, Evans MD, et al. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006 Jan;117(1):72–78. doi: 10.1016/j.jaci.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007 Oct;120(4):835–841. doi: 10.1016/j.jaci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 28.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006 Aug 26;368(9537):763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 29.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006 Aug 13;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 30.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005 Mar 21;201(6):937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jakiela B, Brockman-Schneider R, Amineva S, Lee WM, Gern JE. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008 May;38(5):517–523. doi: 10.1165/rcmb.2007-0050OC. *In vitro model of epithelial injury demonstrated enhanced HRV replication in damaged bronchial epithelium.
- 32. Basu K, Palmer CN, Lipworth BJ, Irwin McLean WH, Terron-Kwiatkowski A, Zhao Y, et al. Filaggrin null mutations are associated with increased asthma exacerbations in children and young adults. Allergy. 2008 Sep;63(9):1211–1217. doi: 10.1111/j.1398-9995.2008.01660.x. *Mutation in gene associated with barrier function was identified as a risk factor for asthma exacerbation.
- 33. Leigh R, Oyelusi W, Wiehler S, Koetzler R, Zaheer RS, Newton R, et al. Human rhinovirus infection enhances airway epithelial cell production of growth factors involved in airway remodeling. J Allergy Clin Immunol. 2008 May;121(5):1238–1245. doi: 10.1016/j.jaci.2008.01.067. *Identified pathways by which HRV infection may promote airway remodeling.
- 34. Zhu L, Lee PK, Lee WM, Zhao Y, Yu D, Chen Y. Rhinovirus-induced major airway mucin production involves a novel TLR3-EGFR-dependent pathway. Am J Respir Cell Mol Biol. 2009 May;40(5):610–619. doi: 10.1165/rcmb.2008-0223OC. *Identified a mechanism potentially involved in HRV-induced airway inflammation and remodeling.
- 35. Proud D, Turner RB, Winther B, Wiehler S, Tiesman JP, Reichling TD, et al. Gene expression profiles during in vivo human rhinovirus infection: insights into the host response. Am J Respir Crit Care Med. 2008 Nov 1;178(9):962–968. doi: 10.1164/rccm.200805-670OC. **Utilized microarray technology to identify novel pathways involved in the acute response to HRV infection.
- 36. Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Jarjour NN, Gern JE. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2009 Aug 26; doi: 10.1038/mi.2009.109. **Microarray and RT-PCR utilized to identify genes differentially expressed in asthmatics vs. controls prior to and during HRV infection.
- 37.Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002 Aug;110(2 Pt 1):315–322. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- 38.Lehtinen P, Ruohola A, Vanto T, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after a first wheezing episode associated with rhinovirus infection or eczema. J Allergy Clin Immunol. 2007 Mar;119(3):570–575. doi: 10.1016/j.jaci.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007 Jul;151(1):34–42. 42. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]