Abstract
Pancreatic beta cell apoptosis is important in the pathogenesis and potential treatment of Type 1 diabetes. We investigated whether Humanin, a recently described survival factor for neurons, could improve the survival of beta cells and delay or treat diabetes in the NOD model. Humanin reduced apoptosis induced by serum starvation in NIT-1 cells and decreased apoptosis induced by cytokine treatment. Humanin induced Stat3 and ERK phosphorylation over a 24 hour time course. Specific inhibition of Stat3 resulted in nullifying the protective effect of Humanin. Humanin normalized glucose tolerance in diabetic NOD mice treated for 6-weeks and their pancreata revealed decreased lymphocyte infiltration and severity. In addition, Humanin delayed/prevented the onset of diabetes in NOD mice treated for 20 weeks. In summary, Humanin treatment decreases cytokine-induced apoptosis in beta cells in vitro and improved glucose tolerance and onset of diabetes in NOD mice in vivo. This indicates that Humanin may be useful for islet protection and survival in a spectrum of diabetes-related therapeutics.
Keywords: Humanin, beta cell, survival, apoptosis, NOD mouse
Introduction
Type 1 diabetes (T1DM) is characterized by the progressive destruction of pancreatic beta cells following lymphocytic infiltration of the islet, resulting in insulin deficiency. IL-1, TNF-alpha and IFN-gamma are released by T cells and macrophages during this autoimmune response and are important mediators of beta cell destruction [1]. Compelling evidence suggests that apoptosis is the principle mode of beta cell death during the development of T1DM [2]. In addition, beta cell loss by apoptosis also occurs after islet graft [3, 4].
Humanin is a recently discovered, 24 amino acid, potent cell survival peptide originally thought to be encoded from a region within the mitochondrial 16S ribosomal RNA. A recent report suggests the existence of 13 nuclear loci predicted to maintain the open reading frames of 15 distinct full-length Humanin-like peptides [5], thus inviting the question of the true origin(s) of Humanin peptide. It was identified to be a survival factor for dying neurons by screening a cDNA library from brain [6] and antagonizes Bax [7] and Insulin-like Growth Factor Binding Protein-3 (IGFBP-3) [8]. Additional recent work indicates that Humanin is a wide spectrum survival factor [9]. Secondary to the pro-survival effect of Humanin observed in neuronal cells, we hypothesized that Humanin could be a survival factor for neuroendocrine beta cells. Our data demonstrate that Humanin is a survival factor for neuroendocrine beta cells and delays the onset of diabetes in NOD mice.
Methods
Cell lines and reagents
The NOD/Lt mouse-derived pancreatic beta cell line NIT-1 [10] was purchased from ATCC (CRL-2055; Rockville, MD) and maintained in F12K medium supplemented with 10% fetal calf serum (Life Technologies, Carlsbad, California), 100 units of penicillin/ml, and 100 units of streptomycin/ml in a humidified environment with 5% CO2. Antibodies against phospho-STAT3 (Y705), total STAT3, phospho ERK-1/2 (T202/Y204) and total ERK-1/2 were purchased from Cell Signaling Technology (Danvers, MA). Stat3 inhibitor VII was obtained from Calbiochem (San Diego, CA). NIT-1 cells are characterized by glucose-responsive insulin secretion and ultrastructural features of differentiated mouse beta cells. Briefly, cells (2 × 105 per well) were seeded in 24-well plates and precultured for 48 hours. Cells were changed to serum free media for overnight, and exposed to IL-1β, IFN-γ, TNF-α (Sigma). For Stat3 inhibitor experiments, cells were serum starved for 24 hours before treatment with HNG with or without Stat3 inhibitor VII as indicated. Humanin (HN) peptide was synthesized by Genemed Synthesis Biotechnologies (South San Francisco, CA).
Mice
NOD female mice, were purchased from Taconic (Germantown, NY). The mice were housed and fed under specific pathogen-free conditions and were cared for according to an approved protocol from the UCLA ARC. Diabetes onset was diagnosed by the presence of nonfasting blood glucose greater than or equal to 300 mg/dL on 2 consecutive days using a blood glucose meter and test strip (One Touch Ultra; Lifescan, Milpitas, California).
Humanin treatments
9 week old female NOD mice underwent a 6 week injection of Humanin (0.7 mg/kg/d IP qd) or saline (n=12/group) after which they underwent intraperitoneal glucose tolerance test and pancreata were harvested and analyzed. For the diabetes prevention study, 5 week old female NOD mice were injected with 2 mg/kg Humanin or equivalent volume saline IP per day (n=10/group) until the development of diabetes as described above.
Intraperitoneal glucose tolerance test
The intraperitoneal glucose tolerance test (IPGTT) was performed after Humanin treatment (at 15 weeks of age; n=6 in each group). Glucose (1.5 g/kg) was administered intraperitoneally to conscious animals after an overnight fast. Blood samples were collected from the tail vein and the blood glucose level was measured with a glucometer (One Touch Ultra; Lifescan, Milpitas, California).
Immunostaining
Pancreata were resected from 15-week-old female NOD mice after 6 weeks of IP Humanin or saline (n=6, in each group) and fixed in 10% phosphate-buffered neutral formalin. Paraffin-embedded sections were stained with haematoxylin and eosin to observe mononuclear cell infiltration in and around the islet. The grade of insulitis was scored on haematoxylin and eosin-stained sections. For semiquantitative evaluation of infiltration, sections containing 6 or more islets were selected and at least 50 islets per pancreas were evaluated. The degree of cellular infiltration was scored from 0 to 5 as follows: score G2 = intraislet infiltration < 50% of the islet, without islet derangement; score G3= extensive infiltration, > 50% of the islet, cell destruction and prominent cytoarchitectural derangement. The evaluation was carried out by two observers.
Caspase assays
The caspase assay was done using Apo-ONE™ homogenous caspase −3/−7 assay (Promega) and performed according to manufacturer’s instructions.
Apoptosis ELISA Assay
Photometric cell death detection enzyme-linked immunosorbent assay (Roche Molecular Biochemicals, Indianapolis, IN) was performed to quantitate the apoptotic index by detecting the histone-associated DNA fragments (mono- and oligonucleosomes) generated by the apoptotic cells. The reaction products in each 96-well plate were read using a Bio-Rad microplate reader (model 3550-UV). Averages of the values ± SD from double absorbance measurements of the samples were plotted.
Immunoblotting
Cell lysates containing 20 µg protein were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to PVDF membrane (BioRad). Membranes were blocked in 0.2% I-block™ (Applied Biosystems) in PBS containing 0.1% Tween-20 (PBST) for 3 h at room temperature and then probed with the appropriate primary and secondary antibodies. Antibody-antigen complexes were visualized by Chemilucent ECL detection system (Millipore, Billerica, MA) and autoradiography.
Statistical analysis
Mean, SD, and statistical significance (p value) were calculated using Microsoft Excel or GraphPad statistical application. Because the same number of test and control values were compared, a paired two-tailed t test was used unless specified. Log-rank analysis was performed to compare diabetes incidence (hyperglycemia) of the test group with that of the control group. Fisher’s exact test was used for comparing the total number of infiltrated islets in the test groups vs. the control group. A p value of ≤0.05 was considered significant.
Results
Dose-dependent protection of beta cells from serum starvation-induced apoptosis by Humanin
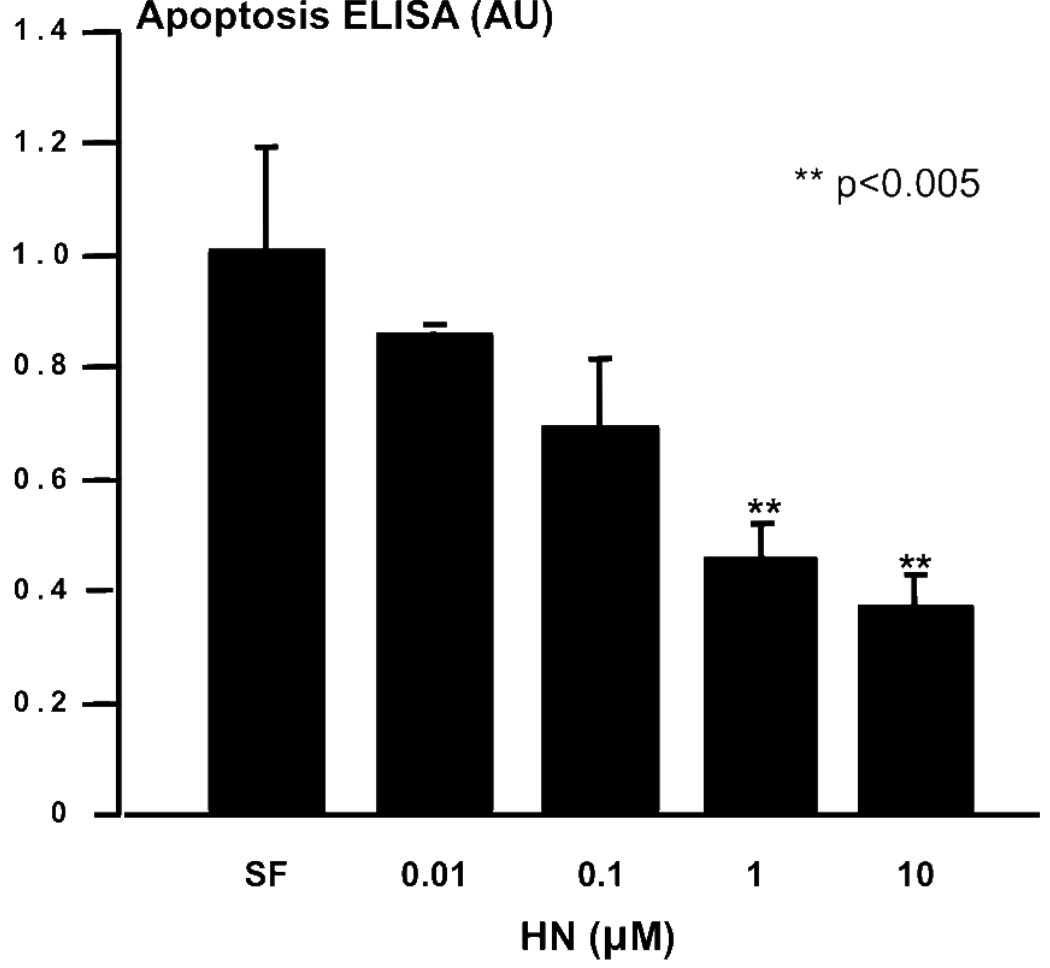
Secondary to the pro-survival effect of Humanin observed in neuronal cells [8] and neuroendocrine PC12 cells [11], we hypothesized that Humanin could be a survival factor for neuroendocrine beta cells. Mouse NIT-1 insulinoma cells were serum starved for 24 hours as control, and compared to cultures co-incubated with increasing doses of Humanin ranging from 1 to 10000 nM. Apoptosis was quantitated by a specific histone-associated DNA ELISA. Humanin potently protected beta cells from serum-starvation induced apoptosis in a dose dependent manner (Fig. 1), decreasing apoptosis by 50% in a similar manner to that for serum-starved PC12 cells [11].
Figure 1.
Humanin protects beta cells from serum starvation-induced apoptosis. NIT-1 cells were serum starved as control (serum free; SF) and compared to cultures co-incubated with increasing doses of Humanin. Apoptosis was quantitated by specific histone-associated DNA ELISA. **p<0.05 compared to SF.
Humanin reduces cytokine-induced apoptosis in beta cells
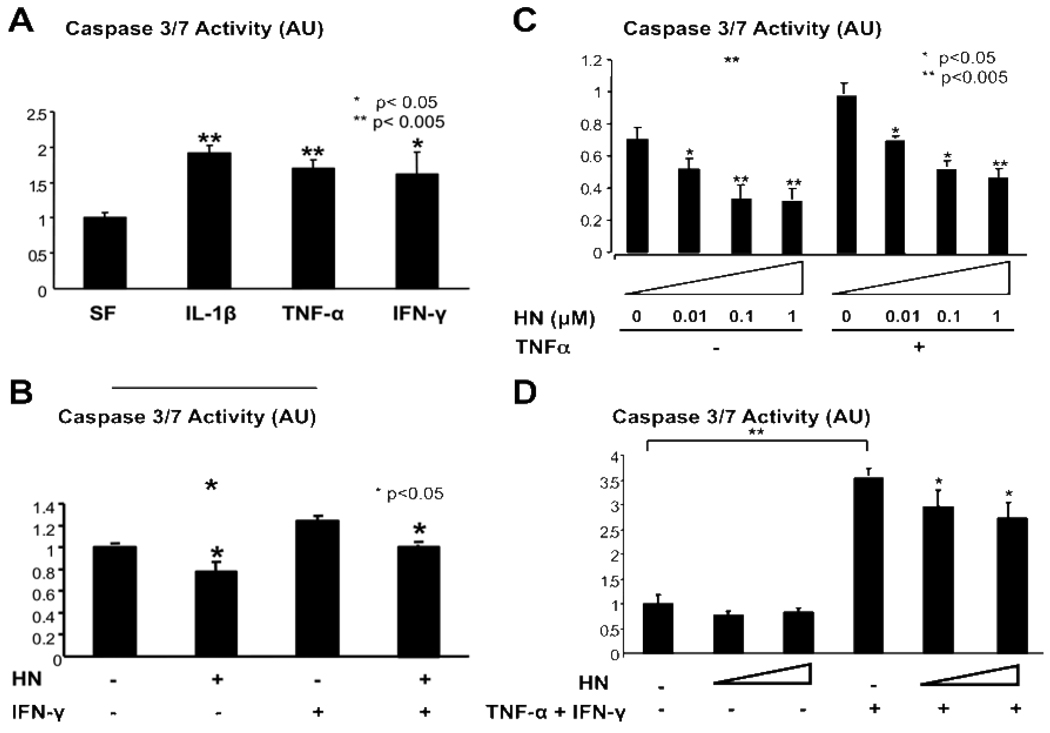
Inflammatory cell infiltration into islets in Type 1 diabetes is a T cell mediated process. In addition, cytokines including interleukin (IL)-1β, tumor necrosis factor (TNF)α, and interferon (IFN)γ, released by T cells and activated macrophages, are present and have a role in beta cell destruction. Other groups have described apoptosis and caspase activation in response to these cytokines alone or in combination [12, 13]. NIT-1 cells were exposed to TNFα (5 ng/ml), IL-1β (1 ng/ml), and IFNγ (5 ng/ml) for 48 hours and apoptosis was measured by caspase activation. All cytokines increased caspase-3/7 activation by 70–93% over untreated control cells (Fig. 2A). Pretreatment of cells for 2 hours with 1000 nM Humanin before IFNγ treatment (5 ng/ml) for 48 hours significantly reduced baseline caspase activation in addition to IFNγ-induced caspase activation (Fig. 2B). Pretreatment with a similar dose of Humanin for 2 hours additionally significantly reduced baseline apoptosis and TNFα-induced caspase activation after 48 hour incubation (Fig. 2C). Combination treatment with TNFα and IFNγ demonstrated robust caspase activation (Fig 2D), and pretreatment with Humanin was able to significantly reduce caspase activation in these cells, albeit to a much reduced degree. Pretreatment with Humanin was ineffective in reducing caspase activation by IL-1β as a sole agent (data not shown).
Figure 2.
Reduction of Cytokine-induced apoptosis by Humanin. (A) NIT-1 cells were exposed to TNFα (5 ng/ml), IL-1β (1 ng/ml), and IFNγ (5 ng/ml) for 48 hours and apoptosis was measured by caspase activation. (B) Pretreatment of cells for 2 hours with 1 µM Humanin before IFNγ treatment (5 ng/ml) for 48 hours (C) Pretreatment with a similar dose of Humanin for 2 hours before TNFα (5 ng/ml) treatment. (D) Humanin Pretreatment (1 and 10 µM) before combination treatment with TNFα (5 ng/ml) and IFNγ (5 ng/ml) for 48 hours.*<0.05, **<0.005 when compared to serum-free (SF).
Humanin activates ERK and STAT3
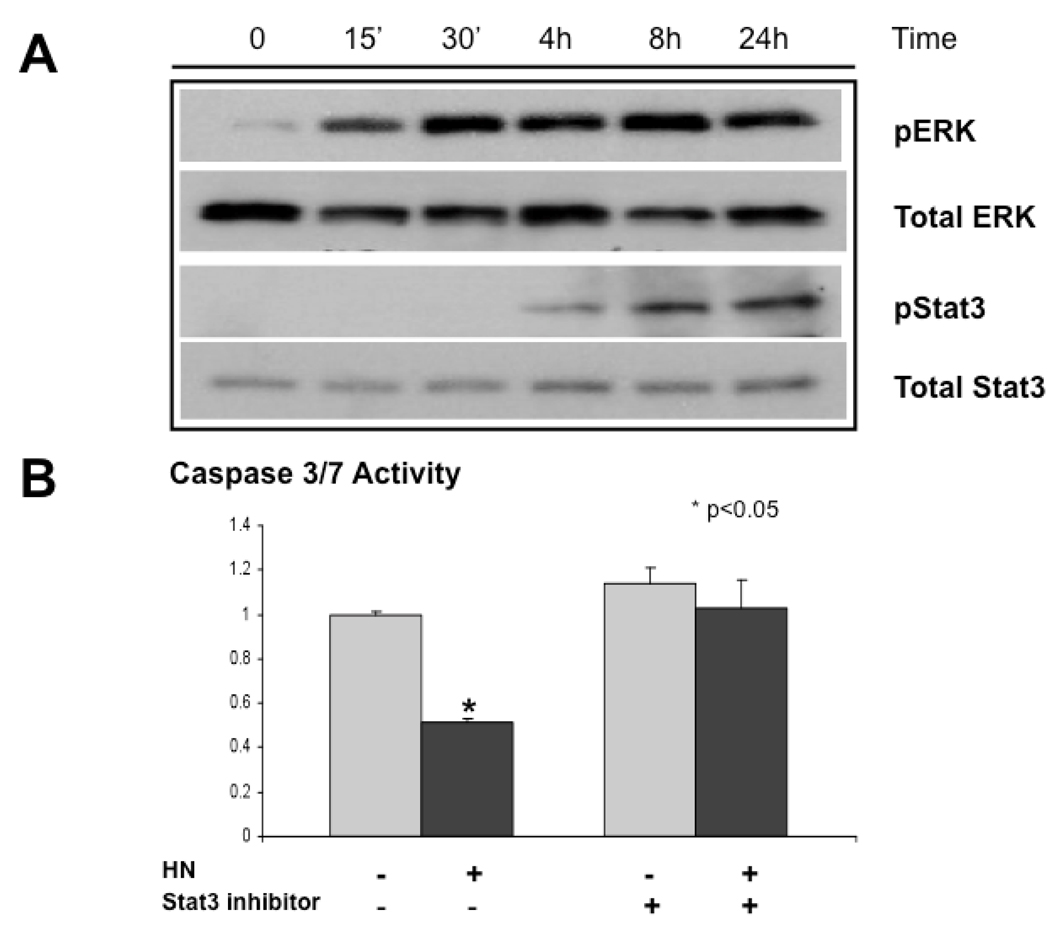
Previous studies implicate that neuroprotection by Humanin in F11 cells is mediated by the STAT3 transcription factor [14]. An ultra-potent Humanin analogue, HNG, has been shown to be neuroprotective in vivo at least in part by inhibiting ERK activation [15]. To investigate the mechanism of Humanin–induced beta cell survival, we treated NIT-1 cells with 100 nM Humanin in SF media. Cell lysates were harvested and phosphorylated extracellular signal-regulated kinase (ERK)1/2 and STAT3 were assessed by immunoblotting. Phosphorylation of ERK (activation) was an early event at 15 minutes after adding Humanin in NIT-1 cells and remained elevated throughout the 24-hour time course. In addition, phosphorylation of STAT3 (activation) was a later event at 4 hours after adding Humanin in NIT-1 cells (Fig. 3A). As ERK activation has been described to decline dramatically in the 24 hours post human, porcine, and canine islet isolation [3]; this initial description of ERK activation by Humanin in beta cells is consistent with a potential role in beta cell survival.
Figure 3.
Activation of ERK and STAT3 by Humanin. (A) NIT-1 cells were treated with 100 nM Humanin in SF media. Cell lysates were harvested and phosphorylated extracellular signal-regulated kinase (ERK)1/2 and STAT3 were assessed by immunoblotting. (B) NIT-1 cells were serum starved for 24 hours and treated with and without HN (1µM) and a specific Stat3 inhibitor (1µM) for an additional 24 hours.
We then asked whether a specific inhibitor of STAT3 activation and transcription of STAT3 target genes that exhibits little effect against pathways involved in Akt/mTOR, Erk1/2, or STAT5 activation [16] would have an effect on protection by Humanin from serum-starvation induced caspase activation. As previously described in Figure 1, treatment with 1 µM Humanin decreased serum-starvation induced apoptosis by about 50%. Co-treatment with a specific STAT3 inhibitor for abolished the protective effect of Humanin (Fig. 3B), indicating activation of STAT3 may be important in apoptosis prevention by Humanin.
Treatment with Humanin in vivo improves glucose tolerance in the NOD mouse
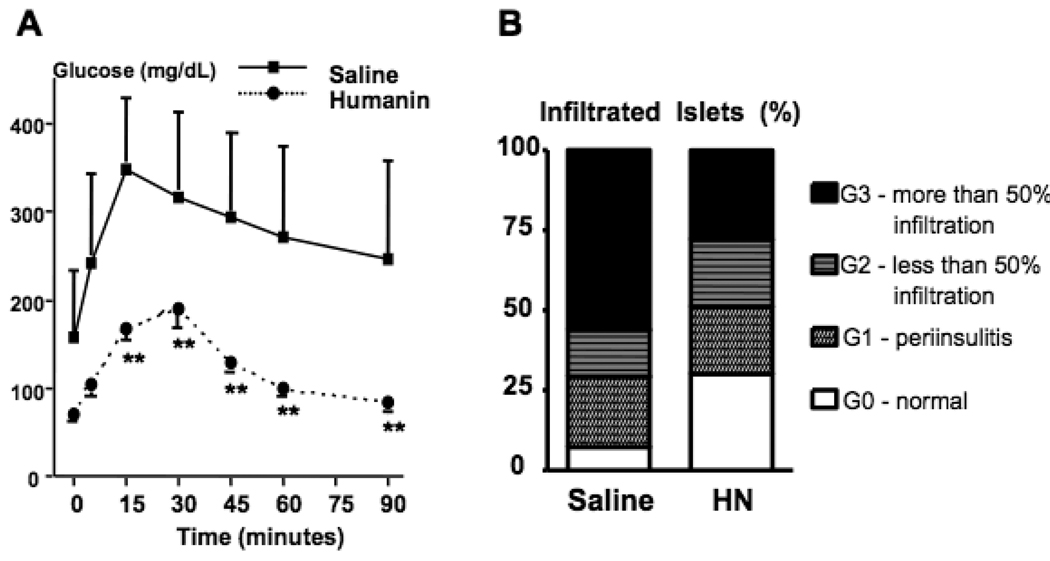
As proof of principle, we examined whether a 6 week course of daily IP injected Humanin (0.7 mg/kg/d) to euglycemic 9-week-old NOD mice could improve glucose tolerance as compared to saline-injected control mice (n=12/group). Daily IP injections were tolerated well. There was no difference in food intake or weight in Humanin-treated vs. control mice (data not shown). At the conclusion of the treatment, mice were fasted overnight and divided into groups that were subjected to IP glucose tolerance testing (n=6/group). Mice that were treated with Humanin showed a non-diabetic response to glucose challenge, while control mice showed the expected diabetic profile (Fig. 4A). Recipients of Humanin injections showed a significantly higher percentage of intact islets (G0 = 27% treated vs. 5% control) with less severe infiltration (G3 = 27% treated vs. 60%) compared with saline-treated control mice (Fig. 4B), suggesting that Humanin reduces islet inflammation during the development of Type 1 diabetes in NOD mice.
Figure 4.
Improvement in Glucose Tolerance in Humanin treated NOD mice. (A) Glucose Tolerance testing post a 6 week course of daily IP injected Humanin (0.7 mg/kg/d) to euglycemic 9-week-old NOD mice (n=12/group) (B) Insulitis scoring of pancreata from Humanin treated NOD mice
Humanin treatment delays diabetes in NOD mice
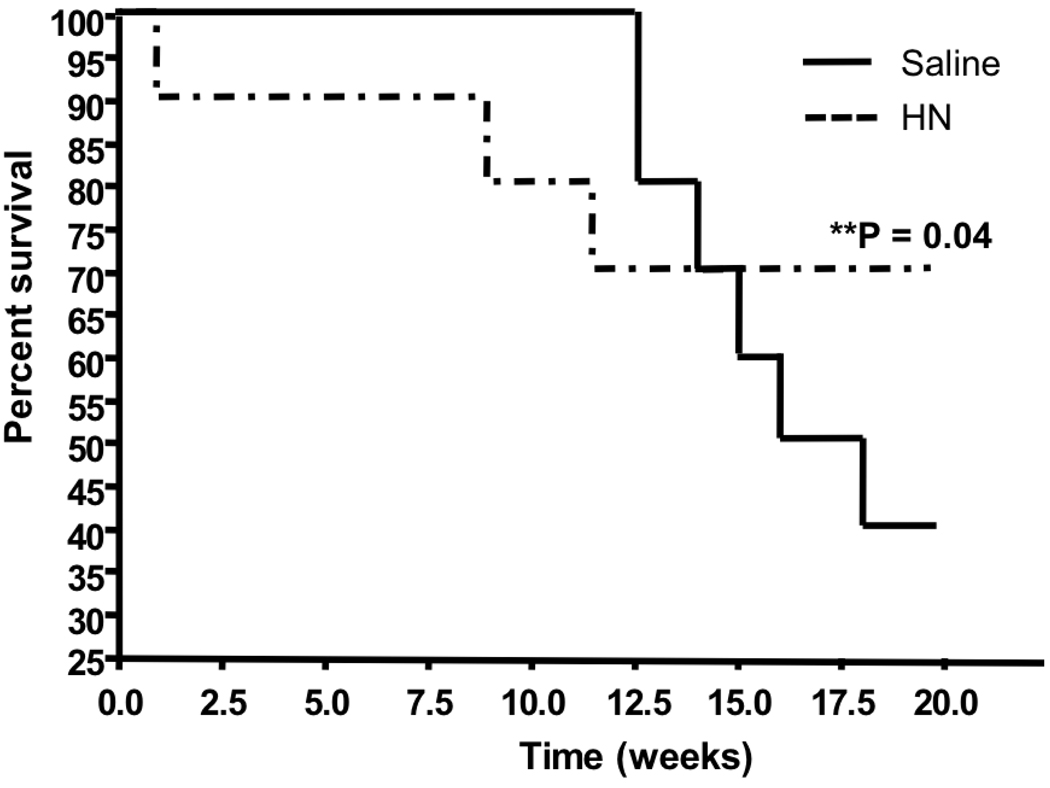
To determine whether Humanin administration delays diabetes in NOD mice, female NOD mice (5 weeks old) were injected with 2 mg/kg Humanin or equivalent volume saline IP per day (n=10/group) until the development of diabetes (glucose >300 mg/dL on 2 consecutive days). There was a marked delay in the group treated with Humanin. After 20 weeks of therapy, only 40% of the control group was normoglycemic, while 70% of the Humanin treatment group was diabetes-free (Fig. 6, p<0.05 by log rank). Humanin treatment reduced diabetes incidence by 50% as only 30% of the treatment cohort became diabetic.
Discussion
The sustenance of viable, robust neuroendocrine beta cells remains a fundamental question that has implications for the prevention of T1DM; the treatment of T1DM post-onset; islet transplantation strategies; and ultimately cure. Humanin is a 24 amino acid peptide that was cloned as a neuroprotective protein that antagonizes apoptosis-related neurotoxicity caused by Alzheimer's disease-relevant insults and is being proposed as a potential therapy for Alzheimer’s [9]. Humanin is thought to bind cell surface receptors (FPRL-1) [17, 18]; to activate kinase signaling and Stat-3 [14]; and has been shown to physically bind and antagonize the Bcl-2 family members, Bax, Bim, and Bid [7, 19, 20] as well as pro-apoptotic IGFBP-3 [8]. Stable transfection of Humanin into K562 human leukemia cells did not reveal survival differences suggesting that this may be a cell type specific phenomena [21].
A pertussis toxin-sensitive G protein-coupled receptor, human formylpeptide receptor-like-1 (FPRL-1) has been reported to be activated by Humanin [17]. To our knowledge, this receptor has not been identified on beta cells or islets. It was thought not to be the sole receptor of Humanin binding as a specific siRNA to the mouse homologue failed to disrupt the protective effect of Humanin in rodent F11 cells [14]. Importantly, FPRL-1 is known to be an immuno-regulatory receptor and it is possible that some of the observed effects of Humanin in the 20 week prevention trial were not simply a result of increased beta cell survival, but possibly due to modulation of the auto-immune process.
Indeed, a recent manuscript reports binding of Humanin to a novel neurocytokine receptor complex of WSX-1, CNTFR, and gp130 [22]. This is particularly intriguing as two sub-modules, among others, of the gp130 transmembrane receptor exist, with one activating Stat3 [23], and the other activating the Ras-ERK pathway [24].
Neither FPRL-1 or the newly described neurocytokine receptor complex has been described in beta cells of yet, but they could well be involved in the mechanism of action described here as a Humanin / gp130 interaction is especially appealing given our observations of Stat3 and ERK activation. In our experiments however, only Stat3 activation is necessary for the protective effect of Humanin against serum starvation.
Stat3 is recognized as an important survival signaling protein in beta cells, mediating the pro-survival effects of various growth factors and cytokines, including hepatocyte growth factor [25, 26], betacellulin [27], Leukaemia Inhibiting Factor (LIF) [28], and heparin-binding EGF-like growth factor [29]. In addition, rat acinar exocrine cells have been “reprogrammed” into beta-cells by a procedure that utilizes epidermal growth factor and LIF, agonists of the JAK2/STAT3 signalling pathway [28, 30]. Enhanced STAT3 signaling protected beta-cells from destruction induced by a genotoxic stress (streptozotocin) [31], and is a mechanism by which islet cell autoantigen 512 (ICA512)/IA-2, an intrinsic tyrosine phosphatase-like protein of the secretory granules, activates a pathway for beta-cell proliferation [32]. In addition, transduction of exogenous constitutively activated Stat3 into dispersed islets induces proliferation of rat pancreatic beta-cells [33]. Clearly, Humanin activates an important beta cell survival pathway. As we have described rapid ERK activation by Humanin in beta cells, ERK activation and phosphorylation have been described to play a key role in glucose-mediated pancreatic beta-cell survival [34]. In contrast, other data suggest that glucose- and IL-1β-induced beta-cell secretory dysfunction and apoptosis are Ca+2 influx and ERK dependent in rat islets [35]. Our observed results of ERK activation are contrary to previous reports [15, 21], and the role of ERK in humanin induced cytoprotection remains unclear.
Recent studies have demonstrated that isolated human islets express the pro-apoptotic protein Bax at higher level than the anti-apoptotic protein Bcl-2, and suggest that the balance between pro-survival and pro-apoptotic molecules to be one of the main mechanisms underlying islet cell death by apoptosis [36]. Additionally, a recent publication describes the Bcl-2 family member, Bid as being essential for death receptor- and inflammatory cytokine-induced apoptosis in beta cells [37]. This is particularly intriguing given that Humanin has been described to physically bind and inactivate both Bax and Bid [7, 20]. We have not observed internalization of Humanin (data not shown) and the possibility of an intracrine mechanism of Humanin has not been explored in this system.
We have previously described the induction of pro-apoptotic IGFBP-3 by IL-1β in RIN cells. In addition, anti-sense IGFBP-3 oligonucleotides inhibit cytokine-and serum withdrawal-induced apoptosis in RIN and HIT cultures. Both exogenous IGFBP-3 and endogenous IGFBP-3 induced apoptosis in the insulin-secreting cell lines studied. IGFBP-3 aggregated in the nuclei of both RIN and HIT nuclei upon exposure to cytokines, supporting a predominantly intracellular action, consistent with data from other systems. Observations that anti-sense IGFBP-3 oligonucleotides inhibit cytokine-induced apoptosis, coupled with confocal data demonstrating cytokine-induced nuclear aggregation, are also consistent with an intracellular (paracrine or autocrine) mechanism for IGFBP-3-induced apoptosis in insulin-secreting cells [38]. Antagonism of IGFBP-3 by Humanin may be an additional mechanism of cell survival. However, IGFBP-3 induction was observed in cell lines and has yet to be reported in primary beta cells.
Re-innervation is another important issue in islet transplantation, since transplanted islets are denervated. Studies of the endocrine response to stress in islet autografted dogs revealed differences consistent with loss of neural regulation [39]. It is an attractive hypothesis that Humanin treatment may promote re-innervation in transplanted islet grafts.
In summary, pancreatic beta-cell function and survival depend on multiple intrinsic and environmental factors. It has been well documented that Humanin potentiates neuronal survival, and we have begun to study the role of Humanin on beta cell survival. We have demonstrated the utility of Humanin in potentiation of beta cell survival by the inhibition of apoptosis. Humanin is a potential treatment for evolving T1DM in the peri-new onset period and delays the onset of diabetes in a genetically prone host. We therefore propose further investigation of the molecular mechanisms by which Humanin promotes beta cell survival as a potential therapeutic in the treatment / prevention of type 1 diabetes.
Figure 5.
Prevention of Diabetes by Humanin. Female NOD mice (5 weeks old) were injected with 2 mg/kg Humanin or equivalent volume saline IP per day (n=10/group) until the development of diabetes (glucose >300 mg/dL on 2 consecutive days).
Acknowledgements
This work was supported in part by an award from the Juvenile Diabetes Research Foundation (Innovative award 5-2006-933) and NIH grants 2P30 DK063491, 2T32 HD007512, and 5T32 GM075776.
Abbreviations
- Stat
Signal Transducers and Activators of Transcription
- ERK
Extracellular Signal-Regulated Kinase
- NOD
Non-obese Diabetic
- GTT
Glucose Tolerance Test
- ITT
Insulin Tolerance Test
- IL
Interleukin
- TNF
Tumor Necrosis Factor
- IFN
Interferon
- ELISA
Enzyme Linked Immunosorbent Assay
- ECL
Enhanced Chemiluminescence
- IP
Intraperitoneal
- IGFBP
Insulin-like Growth Factor Binding Protein
Footnotes
Duality of Interest
The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.Eizirik DL, Mandrup-Poulsen T. A choice of death--the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44:2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien BA, Harmon BV, Cameron DP, Allan DJ. Apoptosis is the mode of beta-cell death responsible for the development of IDDM in the nonobese diabetic (NOD) mouse. Diabetes. 1997;46:750–757. doi: 10.2337/diab.46.5.750. [DOI] [PubMed] [Google Scholar]
- 3.Paraskevas S, Aikin R, Maysinger D, et al. Activation and expression of ERK, JNK, and p38 MAP-kinases in isolated islets of Langerhans: implications for cultured islet survival. FEBS Lett. 1999;455:203–208. doi: 10.1016/s0014-5793(99)00882-0. [DOI] [PubMed] [Google Scholar]
- 4.Tobiasch E, Gunther L, Bach FH. Heme oxygenase-1 protects pancreatic beta cells from apoptosis caused by various stimuli. J Investig Med. 2001;49:566–571. doi: 10.2310/6650.2001.33721. [DOI] [PubMed] [Google Scholar]
- 5.Bodzioch M, Lapicka-Bodzioch K, Zapala B, et al. Evidence for potential functionality of nuclearly-encoded humanin isoforms. Genomics. 2009 doi: 10.1016/j.ygeno.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto Y, Niikura T, Tajima H, et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc Natl Acad Sci U S A. 2001;98:6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo B, Zhai D, Cabezas E, et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 8.Ikonen M, Liu B, Hashimoto Y, et al. Interaction between the Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci U S A. 2003;100:13042–13047. doi: 10.1073/pnas.2135111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arakawa T, Kita Y, Niikura T. A rescue factor for Alzheimer's diseases: discovery, activity, structure, and mechanism. Curr Med Chem. 2008;15:2086–2098. doi: 10.2174/092986708785747616. [DOI] [PubMed] [Google Scholar]
- 10.Hamaguchi K, Gaskins HR, Leiter EH. NIT-1, a pancreatic beta-cell line established from a transgenic NOD/Lt mouse. Diabetes. 1991;40:842–849. doi: 10.2337/diab.40.7.842. [DOI] [PubMed] [Google Scholar]
- 11.Kariya S, Takahashi N, Ooba N, et al. Humanin inhibits cell death of serum-deprived PC12h cells. Neuroreport. 2002;13:903–907. doi: 10.1097/00001756-200205070-00034. [DOI] [PubMed] [Google Scholar]
- 12.Stephens LA, Thomas HE, Ming L, et al. Tumor necrosis factor-alpha-activated cell death pathways in NIT-1 insulinoma cells and primary pancreatic beta cells. Endocrinology. 1999;140:3219–3227. doi: 10.1210/endo.140.7.6873. [DOI] [PubMed] [Google Scholar]
- 13.Zumsteg U, Frigerio S, Hollander GA. Nitric oxide production and Fas surface expression mediate two independent pathways of cytokine-induced murine beta-cell damage. Diabetes. 2000;49:39–47. doi: 10.2337/diabetes.49.1.39. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto Y, Suzuki H, Aiso S, et al. Involvement of tyrosine kinases and STAT3 in Humanin-mediated neuroprotection. Life Sci. 2005;77:3092–3104. doi: 10.1016/j.lfs.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Chua CC, Gao J, et al. Humanin is a novel neuroprotective agent against stroke. Stroke. 2006;37:2613–2619. doi: 10.1161/01.STR.0000242772.94277.1f. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Cole DC, Chang CP, et al. Inhibition of the signal transducer and activator of transcription-3 (STAT3) signaling pathway by 4-oxo-1-phenyl-1,4-dihydroquinoline-3-carboxylic acid esters. J Med Chem. 2008;51:4115–4121. doi: 10.1021/jm701271y. [DOI] [PubMed] [Google Scholar]
- 17.Ying G, Iribarren P, Zhou Y, et al. Humanin, a newly identified neuroprotective factor, uses the G protein-coupled formylpeptide receptor-like-1 as a functional receptor. J Immunol. 2004;172:7078–7085. doi: 10.4049/jimmunol.172.11.7078. [DOI] [PubMed] [Google Scholar]
- 18.Harada M, Habata Y, Hosoya M, et al. N-Formylated humanin activates both formyl peptide receptor-like 1 and 2. Biochem Biophys Res Commun. 2004;324:255–261. doi: 10.1016/j.bbrc.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 19.Luciano F, Zhai D, Zhu X, et al. Cytoprotective peptide humanin binds and inhibits proapoptotic Bcl-2/Bax family protein BimEL. J Biol Chem. 2005;280:15825–15835. doi: 10.1074/jbc.M413062200. [DOI] [PubMed] [Google Scholar]
- 20.Zhai D, Luciano F, Zhu X, et al. Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak. J Biol Chem. 2005;280:15815–15824. doi: 10.1074/jbc.M411902200. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Li H, Yuan H, et al. Humanin delays apoptosis in K562 cells by downregulation of P38 MAP kinase. Apoptosis. 2005;10:963–971. doi: 10.1007/s10495-005-1191-x. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto Y, Kurita M, Aiso S, et al. Humanin Inhibits Neuronal Cell Death by Interacting with a Cytokine Receptor Complex or Complexes Involving CNTF Receptor {alpha}/WSX-1/gp130. Mol Biol Cell. 2009 doi: 10.1091/mbc.E09-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinrich PC, Behrmann I, Muller-Newen G, et al. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Ocana A, Vasavada RC, Cebrian A, et al. Transgenic overexpression of hepatocyte growth factor in the beta-cell markedly improves islet function and islet transplant outcomes in mice. Diabetes. 2001;50:2752–2762. doi: 10.2337/diabetes.50.12.2752. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Ocana A, Takane KK, Syed MA, et al. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem. 2000;275:1226–1232. doi: 10.1074/jbc.275.2.1226. [DOI] [PubMed] [Google Scholar]
- 27.Watada H, Kajimoto Y, Miyagawa J, et al. PDX-1 induces insulin and glucokinase gene expressions in alphaTC1 clone 6 cells in the presence of betacellulin. Diabetes. 1996;45:1826–1831. doi: 10.2337/diab.45.12.1826. [DOI] [PubMed] [Google Scholar]
- 28.De Breuck S, Baeyens L, Bouwens L. Expression and function of leukaemia inhibitory factor and its receptor in normal and regenerating rat pancreas. Diabetologia. 2006;49:108–116. doi: 10.1007/s00125-005-0079-1. [DOI] [PubMed] [Google Scholar]
- 29.Kaneto H, Miyagawa J, Kajimoto Y, et al. Expression of heparin-binding epidermal growth factor-like growth factor during pancreas development. A potential role of PDX-1 in transcriptional activation. J Biol Chem. 1997;272:29137–29143. doi: 10.1074/jbc.272.46.29137. [DOI] [PubMed] [Google Scholar]
- 30.Baeyens L, Bouwens L. Can beta-cells be derived from exocrine pancreas? Diabetes Obes Metab. 2008;10 Suppl 4:170–178. doi: 10.1111/j.1463-1326.2008.00949.x. [DOI] [PubMed] [Google Scholar]
- 31.Mori H, Shichita T, Yu Q, et al. Suppression of SOCS3 expression in the pancreatic beta-cell leads to resistance to type 1 diabetes. Biochem Biophys Res Commun. 2007;359:952–958. doi: 10.1016/j.bbrc.2007.05.198. [DOI] [PubMed] [Google Scholar]
- 32.Mziaut H, Kersting S, Knoch KP, et al. ICA512 signaling enhances pancreatic beta-cell proliferation by regulating cyclins D through STATs. Proc Natl Acad Sci U S A. 2008;105:674–679. doi: 10.1073/pnas.0710931105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsukiyama S, Matsushita M, Matsumoto S, et al. Transduction of exogenous constitutively activated Stat3 into dispersed islets induces proliferation of rat pancreatic beta-cells. Tissue Eng. 2006;12:131–140. doi: 10.1089/ten.2006.12.131. [DOI] [PubMed] [Google Scholar]
- 34.Costes S, Broca C, Bertrand G, et al. ERK1/2 control phosphorylation and protein level of cAMP-responsive element-binding protein: a key role in glucose-mediated pancreatic beta-cell survival. Diabetes. 2006;55:2220–2230. doi: 10.2337/db05-1618. [DOI] [PubMed] [Google Scholar]
- 35.Fei H, Zhao B, Zhao S, Wang Q. Requirements of calcium fluxes and ERK kinase activation for glucose- and interleukin-1beta-induced beta-cell apoptosis. Mol Cell Biochem. 2008;315:75–84. doi: 10.1007/s11010-008-9791-8. [DOI] [PubMed] [Google Scholar]
- 36.Thomas D, Yang H, Boffa DJ, et al. Proapoptotic Bax is hyperexpressed in isolated human islets compared with antiapoptotic Bcl-2. Transplantation. 2002;74:1489–1496. doi: 10.1097/00007890-200212150-00003. [DOI] [PubMed] [Google Scholar]
- 37.McKenzie MD, Carrington EM, Kaufmann T, et al. Proapoptotic BH3-only protein Bid is essential for death receptor-induced apoptosis of pancreatic beta-cells. Diabetes. 2008;57:1284–1292. doi: 10.2337/db07-1692. [DOI] [PubMed] [Google Scholar]
- 38.Shim ML, Levitt Katz LE, Davis J, et al. Insulin-like growth factor binding protein-3 is a novel mediator of apoptosis in insulin-secreting cells. Growth Horm IGF Res. 2004;14:216–225. doi: 10.1016/j.ghir.2003.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Portis AJ, Warnock GL, Finegood DT, et al. Glucoregulatory response to moderate exercise in long-term islet cell autografted dogs. Can J Physiol Pharmacol. 1990;68:1308–1312. doi: 10.1139/y90-196. [DOI] [PubMed] [Google Scholar]