Stem cell transplantation is a promising new treatment for ischemic cardiomyopathy, offering the unique opportunity for true cardiac repair and regeneration. Several different types of stem and progenitor cells are being explored for this purpose (1). Among the various cell types, mesenchymal stem cells (MSCs, alternatively named multipotent stromal cells) are potentially attractive, as they are easily isolated and expanded, and they exhibit low immunogenicity, rendering allogeneic applications plausible (2). Despite their potential benefits, results in animal models and in humans have been variable, and little is known regarding their mechanism of action. Are all MSCs created equal? Can they really regenerate heart tissue directly, and, if so, is direct cardiomyogenesis required for a therapeutic effect?
MSCs, first described by Friedenstein in 1961 (3), are self-renewing precursors of non-hematopoietic stromal tissues characterized by: a) adherence to plastic in culture; b) surface expression of CD105, CD90 and CD73; c) lack of expression of hematopoietic markers; and d) the capacity to differentiate into fibroblasts, osteoblasts, adipocytes and chondroblasts under specific in vitro conditions (4). MSCs were originally isolated from the bone marrow (BM), but similar populations have been reported in several other tissues including the heart (5). In vivo, MSCs typically reside in perivascular niches (6) where they create/function as a stromal network, supporting other cell types (e.g. hematopoietic cells in BM) (7) and contributing to the creation and maintenance of connective tissues. Although traditional isolation of MSCs by plastic adherence results in notoriously heterogeneous preparations with respect to cell size, morphology, proliferative capacity and potential for differentiation (8), it is commonly accepted that these cells comprise a multipotent adult stem cell population, but their capacity to differentiate into excitable tissues is not well-established (2).
The ability of MSCs to undergo true cardiomyogenic transdifferentiation, in particular, remains highly controversial (9, 10). In vitro, MSCs can express cardiac-specific proteins but do not display the typical electrical properties of true cardiomyocytes (10). In vivo, there is immunohistologic evidence for low cardiac engraftment and transdifferentiation after MSC transplantation (11), although such findings are not universal (12) even within the same laboratory (13). In order to boost aptitude for cardiomyogenic differentiation, different strategies of MSC ex vivo manipulation have been employed with various degrees of success, including exposure of cells to the DNA demethylating agent 5-azacytidine, pretreatment with growth factors, hypoxic preconditioning and genetic engineering (2). However, there is still lack of convincing evidence that MSCs can differentiate into functional cardiomyocytes.
In this issue of Journal of the American College of Cardiology, landmark work by Behfar and colleagues (14) investigates the feasibility of deriving cardiomyocytes from human MSCs (hMSCs) through mimicry of natural/embryonic cardiogenic signaling. Bone-marrow derived hMSCs were isolated from patients undergoing coronary artery bypass surgery. In their naive state, hMSCs exhibited poor capacity for cardiomyogenic differentiation in vitro as well as limited potential for myocardial repair in vivo. However, ex vivo priming of cells with a cardiogenic cocktail of growth factors (consisting of TGFβ1, BMP-4, Activin-A, retinoic acid, IGF-1, FGF-2, α-thrombin and IL-6) upregulated cytosolic expression and promoted nuclear translocation of cardiac transcription factors, successfully converting weakling hMSCs into ones capable of strong cardiopoiesis. Importantly, this “boot camp” strategy resulted in a dramatic improvement of functional and structural endpoints following intramyocardial injection into nude mice with ischemic cardiomyopathy. The authors provide the first convincing evidence that MSCs, at least in vitro, can in fact become functional cardiomyocytes, exhibiting sarcomerogenesis, mitochondrial maturation and electromechanical coupling. Using a cocktail-based approach (previously employed by the same group to stimulate cardiopoiesis of embryonic stem cells) (15) while avoiding co-culture of MSCs with other cell types, the capacity of bone-marrow derived hMSCs to undergo cardiomyogenic transdifferentiation was convincingly established and distinguished from fusion phenomena.
Behfar et al (14) further noted that naive MSCs demonstrated vast inter-patient heterogeneity in terms of their aptitude for in vitro cardiomyogenic transdifferentiation and potential for myocardial repair. Indeed, only 2 of 11 individuals yielded hMSCs with robust expression of cardiac transcription factors and the ability to boost ejection fraction in injured mouse hearts, but none of the clinical characteristics was predictive of the reparative cytotype. In contrast to some previous reports (11, 12), but confirming others (16), treatment with naive hMSCs did not improve cardiac function relative to saline-treated controls.
Since MSCs can be isolated from a variety of different tissues, including the heart (5, 17), tissue of origin might be of particular importance. It seems plausible that cells may already be primed towards differentiation along lineages specific to tissues in which they reside. Consistent with this idea, the molecular profile, differentiation potential and function can vary widely among MSC preparations depending on their origin (18, 19). If tissue source proves to be important, heart-derived MSCs merit particular investigation, as they may be more predisposed to cardiomyogenesis than are BM MSCs. They may also possess a specialized ability to support cardiac progenitor cells (CPCs) in the heart, just as BM MSCs physiologically support hematopoietic cells in the marrow.
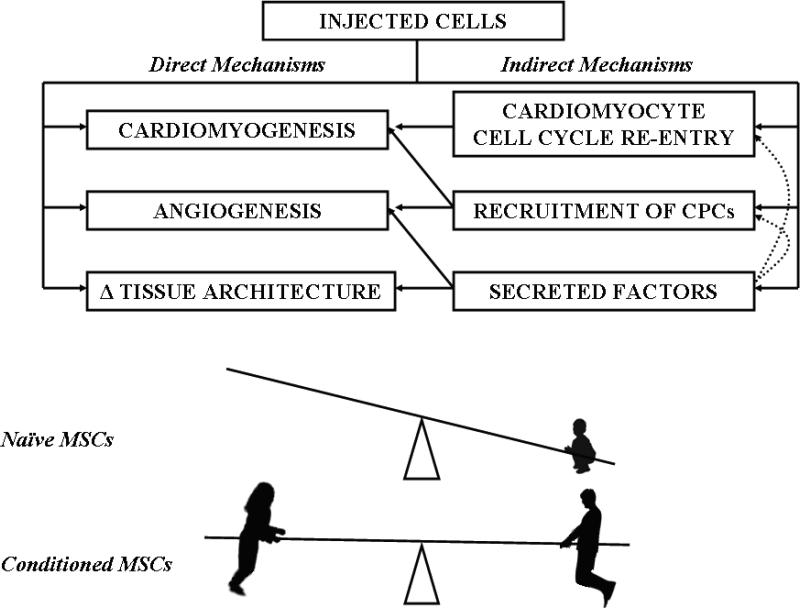
A final notable aspect of the present study (14) is that a MSC-derived cardiopoietic cell phenotype (regardless of whether it was observed spontaneously in rare individuals or as a result of guided cardiopoiesis) was associated with a dramatic increase in reparative efficacy, compared to non-cardiopoietic MSCs. This increase was attributed to more robust direct (cardiomyogenesis, angiogenesis) and indirect (cardiomyocyte cell cycle re-entry, endogenous stem cell recruitment) contributions to infarct repair by conditioned cells compared to naïve MSCs (Fig.1). To date, the majority of in vivo studies have demonstrated at least modest functional improvement following MSC transplantation, despite undetectable to low levels of long-term engraftment and differentiation (12, 20). This implies that MSCs exert their beneficial effects mainly through indirect paracrine actions rather than by contributing directly to tissue regeneration (2, 21). Importantly, the fact that significant long-term engraftment is not required for functional benefit (20), together with the purportedly low immunogenicity (22) of MSCs, support the notion that allogeneic transplantation without immunosuppression may be feasible. On the other hand, the positive correlation between MSC engraftment and functional recovery in post-ischemic cardiomyopathy suggests that some of the benefit may be due to long-term engraftment and tri-lineage differentiation of MSCs (11), even if the absolute survival of transplanted cells is low.
Figure 1. Schematic depiction of the balance of direct (cardiomyogenesis, angiogenesis, and favorable changes in tissue architecture) versus indirect (cell cycle re-entry, recruitment of CPCs and secreted factors) mechanisms underlying the salutary effects of MSCs.
Indirect mechanisms predominate in naïve MSCs, but the benefit is small: a child tips the seesaw. Boot-camp-conditioned MSCs exhibit balanced, robust mechanisms, akin to the interplay of two physically-fit adults.
A particularly important function of cardiac MSCs may be to enhance the survival and/or potency of co-transplanted CPCs in cardiomyoplasty. We have been investigating cardiosphere-derived cells (CDCs) grown from percutaneous endomyocardial biopsies for human therapeutic applications. CDCs are a natural mixture of heart-derived cell sub-populations, including true CPCs (c-kit+/CD90-) as well as cardiac MSCs (c-kit-/CD90+) (23, 24). One logical approach to cardiomyoplasty is the selective purification, expansion and injection of CPCs (25, 26). We find, however, that CDCs outperform purified CPCs. In experiments with intramyocardial injection of human CDCs in a mouse MI model, the spontaneously-emerging unselected mixture of CPCs and cardiac MSCs resulted in a higher ejection fraction at three weeks than either purified c-kit+ or CD90+ cells from the same source (27). These findings hint that cardiac MSCs help CPCs to engraft and/or function, presumably via synergistic paracrine actions as well as direct myocardial regeneration (28).
Sources of funding
Stem cell work in our laboratory is funded by the NHLBI and by the California Institute for Regenerative Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 2.Nesselmann C, Ma N, Bieback K, et al. Mesenchymal stem cells and cardiac repair. J Cell Mol Med. 2008;12:1795–810. doi: 10.1111/j.1582-4934.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedenstein AJ. Osteogenetic activity of transplanted transitional epithelium. Acta Anat (Basel) 1961;45:31–59. doi: 10.1159/000141739. [DOI] [PubMed] [Google Scholar]
- 4.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–17. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 5.Deisher TA. Cardiac-derived stem cells. IDrugs. 2000;3:649–53. [PubMed] [Google Scholar]
- 6.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–99. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 7.Balduino A, Hurtado SP, Frazão P, et al. Bone marrow subendosteal microenvironment harbours functionally distinct haemosupportive stromal cell populations. Cell Tissue Res. 2005;319:255–66. doi: 10.1007/s00441-004-1006-3. [DOI] [PubMed] [Google Scholar]
- 8.Ho AD, Wagner W, Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy. 2008;10:320–30. doi: 10.1080/14653240802217011. [DOI] [PubMed] [Google Scholar]
- 9.Pijnappels DA, Schalij MJ, Ramkisoensing AA, et al. Forced alignment of mesenchymal stem cells undergoing cardiomyogenic differentiation affects functional integration with cardiomyocyte cultures. Circ Res. 2008;103:167–76. doi: 10.1161/CIRCRESAHA.108.176131. [DOI] [PubMed] [Google Scholar]
- 10.Rose RA, Jiang H, Wang X, et al. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26:2884–92. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
- 11.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–7. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davani S, Marandin A, Mersin N, et al. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003;108:II253–8. doi: 10.1161/01.cir.0000089186.09692.fa. [DOI] [PubMed] [Google Scholar]
- 13.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S21–6. doi: 10.1038/ncpcardio0770. Feb. [DOI] [PubMed] [Google Scholar]
- 14.Behfar A, Yamada S, Crespo-Diaz RJ, et al. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol. 2010 doi: 10.1016/j.jacc.2010.03.066. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behfar A, Perez-Terzic C, Faustino RS, et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J Exp Med. 2007;204:405–20. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Bogt KE, Schrepfer S, Yu J, et al. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. 2009;87:642–52. doi: 10.1097/TP.0b013e31819609d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tateishi K, Ashihara E, Honsho S, et al. Human cardiac stem cells exhibit mesenchymal features and are maintained through Akt/GSK-3beta signaling. Biochem Biophys Res Commun. 2007;352:635–41. doi: 10.1016/j.bbrc.2006.11.096. [DOI] [PubMed] [Google Scholar]
- 18.Wagner W, Roderburg C, Wein F, et al. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells. 2007;10:2638–47. doi: 10.1634/stemcells.2007-0280. [DOI] [PubMed] [Google Scholar]
- 19.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 20.Iso Y, Spees JL, Serrano C, et al. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354:700–6. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs). Clin Pharmacol Ther. 2007;82:241–3. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 23.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 24.Davis DR, Zhang Y, Smith RR, et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4(9):e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 26.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith RR, Chimenti I, Marbán E. Unselected human cardiosphere-derived cells are functionally superior to c-Kit- or CD90-purified cardiosphere-derived cells. Circulation. 2008;118:S420. (Abstr) [Google Scholar]
- 28.Chimenti I, Smith RR, Li TS, et al. Relative Roles of Direct Regeneration Versus Paracrine Effects of Human Cardiosphere-Derived Cells Transplanted Into Infarcted Mice. Circ Res. 2010 Jan 28; doi: 10.1161/CIRCRESAHA.109.210682. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]