Abstract
Sickle cell disease is a chronic illness that impacts patients physically and emotionally and can do so at an early age. An ecological model of palliative care that involves improved communication among the health care team, patients, and their families can be beneficial. Open and honest communication regarding advance care planning, disease management, relief of pain and other symptoms, and bereavement and grief are all important for the patient, family, and health care team. Given the multiple acute and chronic complications of sickle cell disease, an approach to care that is holistic and comprehensive may help to improve a patient’s biological function and the perceived health, functional status, and quality of life of the patient and family.
Keywords: Sickle cell, pain, symptoms, advance care planning, grief, bereavement, palliative care
Introduction
People living with sickle cell disease (SCD) face many types of morbidity and early mortality. SCD is an inherited hemoglobin disorder characterized by chronic hemolytic anemia, increased susceptibility to infections, end organ damage, and intermittent episodes of vascular occlusion that result in acute and chronic pain.1 In some African countries, nearly all babies born with SCD die in early childhood. In the United States (U.S.), newborn screening, prophylactic penicillin treatment in childhood and other aggressive treatments for pain and disease complications have increased the average life expectancy for the 100,000 people with SCD to age 42 for men and 48 for women.2 Recent advances in the treatment of SCD, such as hydroxyurea, prolong and improve the quality of life for many people, with some living into their eighties. Unfortunately, people with SCD live with many threats to the quality of life for them and their families. Palliative care offers the hope of reducing these threats. The purpose of this article is to profile the many opportunities for implementation of palliative care concepts across the life span, towards the goal of further improving the quality of life for infants, children, adolescents, adults, and older adults who inherited SCD, and for their families. Specifically, we discuss an ecological model of palliative care that is patient-centered and family-focused, as a way to improve awareness and communication about SCD, understand the challenges of SCD, manage pain and other symptoms over a lifetime, and cope with loss, grief and bereavement.
Ecological Model of Care for People with SCD
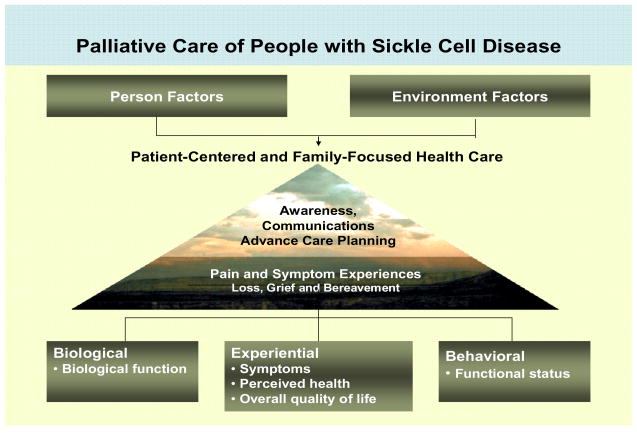
Patient-centered and family-focused care for people with SCD and their families. A health ecological model is useful for planning comprehensive care of people with SCD because it can be a means to improve quality of life with a special focus on patient-centered and family-focused care (Figure 1). Within a health ecological model, it is important to consider personal and environmental factors as they influence palliative care needs. Focus should include awareness of the disease, advance care planning, symptom experiences throughout the life span, and bereavement. Based on the human response model,3, 4 a health ecological model allows incorporation of the essential components of palliative care for individuals and families across the life span.5, 6, 7, 8, 9, 10 A health ecological model is also consistent with the National Quality Forum’s priorities for high-quality palliative care.10 It is important for the comprehensive care of people living with SCD that clinicians recognize the palliative care needs and life-threatening nature of this disease.
Figure 1.
Ecological Framework for Palliative Care of People with SCD. (Modified with permission: University of Illinois College of Nursing, Center for End-of-Life Transition Research, © 2007).
As shown in Figure 1, palliative care for the person with SCD requires that clinicians have biological expertise to better manage symptoms and to maintain or improve functional status. Similarly, clinicians with behavioral and community expertise are needed to deal with (a) personal (individual) factors, such as awareness of the genetic transmission of the disease and effective communication; and (b) external (environmental) factors, such as cultural norms that contribute to behavior, advance care planning or adherence to prescribed analgesic therapies. Attention to salient personal and environmental factors is important for implementing interventions at the personal or community level, thereby maximizing patient-centered and family-focused palliative care of people with SCD.
Model components. Personal factors and environmental factors are keys to understanding the constellation of factors that contribute to palliative care. Personal factors are either fixed characteristics or modifiable dimensions,7 such as age and ethnicity (fixed characteristics) and physical symptoms, and a sense of purpose and meaning (modifiable dimensions). Environmental factors include home, community and health care system factors that exist outside the person to influence health care outcomes.7 Palliative care is directed toward assessing and changing personal (individual) vulnerability or resilience factors that are modifiable, and it is directed toward manipulating or helping individuals to manipulate environmental factors that constitute risks or resources, with the goal of improving the quality of life. Care system interventions exist on several levels: the family, the larger social group (communities) and health care institutions, including health care providers, to facilitate awareness about the challenges of SCD and the life-threatening nature of the disease.
As shown in Figure 1, awareness of SCD transmission and disease trajectory and effective communication is central to advance care planning to allow the individual or the parents of a child to plan in advance of a critical event the type of care preferred. Ideally, advance care planning occurs before the patient and family are facing death. It also recognizes that individuals’ previous experiences with end-of-life experiences can influence subsequent advance care planning. Advance care planning indicates the type of care desired when one approaches death, be it aggressive medical treatment (e.g., mechanical ventilation, tube feeding, and cardiopulmonary resuscitation in persons with SCD) or be it solely aggressive palliative care to promote symptom control and maintain function. Providing comfort to the dying individual is important for managing the pain and symptom experience and to reducing complications during the bereavement process. Bereavement refers to having experienced the loss of someone significant through death.11 Although bereavement is viewed as a normal life event, and most bereaved people transition through the grieving process without any outside intervention, various personal and/or environmental factors can make certain individuals vulnerable to negative physical and mental health outcomes.12 For example, the death of a child, even one with SCD, is often seen as an unnatural event that can place a parent at risk for a negative health outcome, such as major depression. Offering support groups for bereaved parents, siblings, and friends or schoolmates or for raising awareness of and coping with SCD is a palliative care-oriented intervention, which is typically offered by communities (e.g., faith communities, organizations) or Comprehensive SCD Centers.
Within the health ecological framework and in the context of palliative care, nurses and other health care providers focus on related components, including biological function, symptoms, perceived health, quality of life, and functional status. These components represent realms that can be measured physiologically, experientially (personally reported) or behaviorally (observed). Awareness about the transmission of SCD and communication issues that occur across the life span is vital to understanding the importance of assessing these components and providing palliative care as appropriate to the life stage.
Awareness and Communication Issues
Inherited genetic disease. SCD results from the inheritance of a gene for abnormal hemoglobin (sickle hemoglobin or hemoglobin S [Hb S]), which, upon deoxygenation, forms an intracellular polymer that transforms the red blood cell to a dense and rigid “sickle” cell. The sickle cells have difficulty flowing through small blood vessels and can block effective blood flow, which results in tissue damage and death. Several closely related genetic conditions can each lead to a form of SCD, with more than 400 hemoglobin variants in the U.S. In each case, the patient inherits a gene for hemoglobin S from one parent, and a second abnormal hemoglobin gene from the other parent. The commonest (and generally the most severe) form of SCD is homozygous hemoglobin S (Hb SS). Other common variants include sickle hemoglobin C disease (Hb SC) and sickle beta thalassemia (Hb Sβ thal). SCD is most common in people descendant from Africa, the Mediterranean area, Middle East, and India.
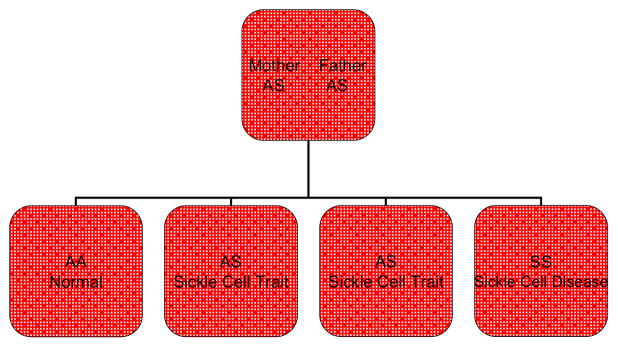
Because SCD is a recessive genetic disease, both parents must be carriers (sometimes referred to as having the “trait”) of an abnormal hemoglobin (at least one being Hb S) and/or have the disease (Hb SS) to produce offspring with SCD. In the U.S., SCD primarily affects African Americans (AA). The incidence is 1 in 500 births, and 10% (1 in 10) of AAs are carriers of the trait. If both parents are carriers of an abnormal hemoglobin gene, then with each pregnancy there is a 25% chance that the offspring will be born with SCD. There is also a 25% chance that the offspring will be born with normal hemoglobin, and a 50% chance that the offspring will be born with the trait that was carried by the parent(s).13 This inheritance pattern is illustrated in Figure 2. Gallo and colleagues, however, found that many parents are unaware of their potential to transmit SCD to their children.14
Figure 2.
Inheritance Pattern of Parents with Heterozygous Hemoglobin Variant S. A normal hemoglobin gene; S abnormal hemoglobin gene
Awareness of SCD transmission. The Comprehensive Sickle Cell Center at the University of Illinois at Chicago (UIC), in partnership with board members of the Have A Heart For Sickle Cell Anemia Foundation, a not-for-profit community sickle cell advocate program, conducted an informal survey about sickle cell awareness. The overwhelming majority of people surveyed were not aware of the possibility of screening for SCD nor of whether they were carriers of sickle cell trait (SCT) or of other hemoglobin variants.15 Widespread community education and screening events are crucially needed to increase awareness in people at risk for SCT. Additionally, discussions with individuals with SCD and SCT should address a number of topics, for example: (a) disease burdens and consequences for both the affected individual as well as the caregiver; (b) the genetic transmission of SCD; and (c) the reproductive risks of having an affected child, as well as reproductive options that will allow for informed reproductive decisions and then the practice of appropriate reproductive health behaviors.16 This lack of awareness of the genetic transmission of the disease and lack of awareness of one’s own trait carrier status are both major areas requiring improved communication within families, communities, and the public health and health care systems.
There remains a lack of awareness of SCD transmission and care needs not only among the general population, but also (disturbingly) among health care providers and those who are affected by this devastating disease. Griffin17 reported to the SCD Association of America-Dallas Chapter’s newborn screening program that challenges for proper SCD care included “a pervasive lack of awareness of sickle cell risk among the target population; a lack of effective parental notification and referral mechanisms to ensure family access to required testing, education, counseling and support services; inadequate parental health knowledge and health supervision to improve outcomes for children affected by SCD; and inadequate medical homes limiting children’s access to care that is accessible, family centered, culturally competent, compassionate, comprehensive and continuous.”17, page 1 Clearly, awareness of the key facts, decisions, and practices related to SCD transmission is an important area for a public health approach to palliative care that has been advocated by the International Workgroup on Death, Dying and Bereavement.18
Care communication issues. When parents give birth to a child with SCD, a number of communication issues become critically important for a palliative care approach to SCD. SCD is characterized by chronic hemolytic anemia, increased susceptibility to infections, end organ damage, and intermittent episodes of vascular occlusion that result in acute and chronic pain.1 All of these complications compromise the quality of life for the person with SCD, as well as for the family. Helping each patient to attain optimal quality of life is the goal of palliative care,19 and this occurs through open and effective communication.20 Effective communication among the health care professional team, patient and family is essential to positive patient outcomes, well-being, and satisfaction. Conversely, all too often, poor communication occurs between patients and health care professionals, which results in suspicion and mistrust.21 Patients perceive themselves as labeled as drug seekers (though occasionally they are), and they feel that the medical community is merely tolerant of them. These patients believe they are confronted by an unsympathetic system and hence respond to health care professionals in a hostile fashion. The end result is what we commonly see today, an adversarial relationship between the two groups. Utilization of good communication skills by health care professionals is vital to good health practice and healthy patient-provider relationships.21
Advance care planning. Ethical decision-making is another core value of integrated palliative care that should be integrated into the care of patients with SCD. For example, not only must SC patients make decisions on reproductive choices, but also they should make decisions regarding advance care planning. Advance care planning includes informed consent, surrogate decision-making, advance directives and “do not resuscitate” orders (DNR). Much progress has been made in the management of SCD, especially among the pediatric population; however, these patients’ life expectancy is significantly lower than that of the general population.2 Integrated palliative care facilitates advance care planning discussions across the life span.
Individuals with decision-making capacity have a legal and ethical right to participate in health care decision-making. Ethical guidelines require five elements in order for informed consent to occur: (1) person must have capacity to make decisions; (2) all relevant facts must be disclosed; (3) person must understand what has been disclosed; (4) the decision must be voluntary; and (5) the person must give consent.22 For the person lacking capacity for decision-making, there are basic standards for surrogate decision-making that ensure that the person’s previously stated wishes are honored. Two examples are substituted judgment standard and best interest standard. Substituted judgment standard is doing what the patient would have chosen if he or she were able to choose. Written evidence such as advance directives; verbal evidence, such as conversations with family, friends, or professionals regarding their wishes; and relational evidence, personal knowledge of an individual, are acceptable forms of evidence for the substituted judgment standard. The best interest standard applies if there is no available information of what a person would have wanted: then surrogates are obligated to do what is in the patient’s best interest.22
Advance directives are a way that individuals can make their wishes for end-of-life treatment known in advance of losing their decision-making capacity. Advance directives usually take the form of written evidence of a person’s wishes. There are two basic types of advance directives: one that stipulates one’s treatment preferences (e.g., living wills); and the other that stipulates who will be the surrogate decision makers (e.g., a durable power of attorney for health care). Typically, advance directives are completed more frequently by white, middle- to upper-class individuals than by individuals of ethnic and minority populations. Generally, some groups in the U.S. fear that they may be denied treatment at the end of life and who therefore are unlikely to complete an advance directive. A DNR order serves as written evidence that informed consent discussion has occurred between the health care professional and the patient or his/her legal surrogate.22
Similar to adults, children with SCD must have advance care planning as well. Decision-making needs to occur, and parents are considered to be the best surrogate decision makers for a minor child. Because children do not have a history of prior choices, parents are held to the best interest standard. The model for informed parental consent includes parental informed permission combined with the assent of the child. The purpose of an assent is to gain the child’s cooperation and agreement as much as possible. Also, assent considers the developmental capabilities of the child and assesses the child’s knowledge and response to tests and treatments.22 Advance care planning is needed early in the life of individuals with SCD and throughout their lives as they encounter the many challenges of SCD. Living one’s entire life with the realities and burdens of SCD provides experience that leaves each SCD patient able to contribute realistically to discussions about clinical decisions.
Challenges of SCD
Ever since SCD was first described in the Western literature a century ago,23 understanding of the disease mechanisms and sequelae has been important to guide care of people with the disease. There was a time when SCD was considered primarily a pediatric condition because of extremely high childhood morbidity and mortality. In the 1960s, only 50% of SCD patients survived to age 20;24 however, in the first decade of the twenty-first century, the probability of survival to adulthood has improved to nearly 95%,25 with a parallel reduction in life-threatening complications. These improvements can be attributed to a number of medical advances, including the introduction of penicillin prophylaxis for prevention of pneumococcal infections,26, 27 conjugated Haemophilus,28, 29 and pneumococcal30 vaccines in early childhood; universal newborn screening for hemoglobinopathies;31, 32 and prevention of primary strokes.33, 34
Currently, newborn screening for hemoglobinopathies is practiced in all 50 states. Hence, in contrast to previous years, the parents of virtually all SCD patients in this country become aware of their child’s condition shortly after birth. Upon being informed for the first time of the diagnosis of SCD in their newborn child, parents face a multitude of issues. Although most have heard of SCD, unless they or another family member also have the disease, parents are likely to have little understanding of the condition or its potential impact on the health and life expectancy of their children. Many new parents are surprised to learn that they were at risk of having a child with SCD, and were unaware that they and/or their partner were carriers of a genetic abnormality. In addition to feeling guilt for having passed on a genetic illness to their child, they may feel helpless or uncertain for the future.
From early in life and throughout their lives, people with SCD have great need for palliative care. Although a child with SCD is asymptomatic at birth (due to high levels of fetal hemoglobin), by three to six months of age the child is at risk for early complications of the disease. Frequently, dactylitis or “hand-and-foot syndrome” is the first clinical manifestation in an infant or toddler with SCD. Dactylitis is characterized by swelling of the dorsum of the hands or feet, or swelling of the fingers or toes. Progressive loss of splenic function begins in infancy and leads to a lifelong risk for potentially deadly bacterial infections, such as septicemia, pneumonia, meningitis, and osteomyelitis. Because of infection risk, young children with SCD are frequently hospitalized when they develop a fever. Infants or young children may develop potentially fatal splenic sequestration crises, due to trapping of the blood by the dysfunctional spleen, or aplastic crises associated with common viral infections (e.g., parvovirus). In these situations, red blood cell transfusions may be life-saving. Parents may feel ambivalent or fearful of the risks of transfusions, or may have religious objections, lending further emotional trauma to such situations. Families find themselves needing to adapt their lifestyles to accommodate frequent visits to the doctor or hospital, daily medications, blood tests and other medical procedures.
In addition to infections, the risk for certain complications of SCD begins early in life and continues throughout life. Examples35 include painful vaso-occlusive crises and acute chest syndrome, which is an acute illness characterized by fever and respiratory symptoms in an SCD patient and accompanied by a new pulmonary infiltrate on a chest X-ray.36 This syndrome of pulmonary infarction may arise from a variety of causes, including bacterial or viral pneumonitis, pulmonary infarction, fat embolus from marrow necrosis during vaso-occlusive crisis, or acute respiratory compromise. Although more common in young children, the condition is much more likely to be fatal in adults.37
School-aged children with SCD face additional challenges. Painful crises or hospitalizations may lead to frequent school absences, with difficulty in maintaining school performance and passing grades. Risk for debilitating strokes is high throughout the school years, with a peak incidence for cerebral infarction at about 7 years of age.38 The vascular pathology leading to infarctive strokes in SCD children is incompletely understood, but frequently involves the large cerebral arteries. In this case, inflammatory endothelial damage results from adherent sickle cells or the products of hemolysis in conjunction with high blood flow rates due to anemia and vasoconstriction from nitric oxide depletion. Intimal hyperplasia ensues, the caliber of the affected vessels narrows, and a thrombus ultimately occludes the vessel.39 A child having a stroke or found through screening to be at high risk for a stroke may start a chronic red blood cell transfusion program. This involves transfusions approximately monthly; this is about 90% effective in reducing stroke risk.33 Because discontinuing a program of regular transfusions is associated with return of high stroke risk,40 chronic transfusion therapy may be maintained for many years. This is not without risks. Chronic transfusion therapy leads to excessive iron accumulation in body organs (transfusional hemosiderosis), which results in liver, cardiac or endocrine abnormalities, and potentially contributes to premature mortality.41 In addition to overt strokes, a different CNS lesion known as “silent infarction” may be found in children with SCD.42 These children may experience attention deficit, hyperactivity or various learning disabilities. Silent infarcts, which can be diagnosed based upon characteristic brain lesions seen on MRI,43, 44 may progress to overt stroke.45 Neuropsychiatric testing and development of an individualized education plan at school may help a child perform to his or her fullest potential.
Children and teens with SCD face additional physical and social challenges. The renal damage caused by SCD leads to loss of urinary-concentrating ability during childhood.46 As a result, young people with SCD have a high incidence of primary enuresis, which may be embarrassing. Body image issues are common in adolescents and teens with SCD; these frequently include short stature and delayed puberty,47 as well as chronic jaundice. Participation in sports and physical activities is frequently limited. Not only do chronic anemia and propensity to dehydration limit the exercise capacity of SCD patients, but also painful crises may be triggered by vigorous exercise or environmental extremes, including summer heat, swimming in cold water, or travel to high altitudes. Despite these limitations, physical activity may be beneficial in SCD patients48 and should be judiciously encouraged. Denial, or a desire to be like other teens, may induce a patient with SCD to engage in risky behaviors,49 or to forgo his or her medications or medical treatments, sometimes with serious consequences.
As teens grow into adulthood, the transition to being an adult living with a chronic medical condition is difficult.50 The young adult must leave the familiar setting of the pediatric physicians and nurses who have cared for the patient for his or her entire life, and establish a relationship with an unfamiliar medical care team. In addition, the responsibility for managing the patient’s medical condition, taking medicines, making appointments, etc., is transferred from the parent to the patient. Medicaid or parental private insurance coverage terminates at the age of majority, and the patient must secure new medical coverage through an employer, Medicare or SSI (Supplemental Security Income) disability. If the pediatric sickle cell center has a transition program, that program may be a valuable resource in guiding the patient through this process.51
Certain medical complications of SCD become increasingly frequent in the teenage to young adult years. Because of sickle vasculopathy and poor wound healing, cutaneous leg ulcers may arise from apparently trivial injury, such as insect bites or minor abrasions.52 Ulcers may become large and debilitating, sometimes lasting for years. Avascular necrosis of bones occurs, most frequently in the femoral head, leading to chronic hip disease.53, 54 The patient ultimately may need a hip replacement in order to relieve pain and restore function. Avascular necrosis also may occur in the humeral head, leading to shoulder dysfunction. Collapse of infarcted vertebral bodies may lead to chronic back pain. Vasculopathy also leads to sickle retinopathy,55 which includes retinal hemorrhages, neovascularization and retinal detachment, and may lead to blindness. This complication is particularly frequent in patients with hemoglobin SC disease.56 Frequent eye exams are essential to identifying and treating retinal vascular lesions early.57 Cholelithiasis is a frequent complication of SCD. If asymptomatic, no intervention is required; however, cholelithiasis may progress to symptomatic cholecystitis requiring cholecystectomy.58
Priapism, a painful, prolonged involuntary erection, is a potentially serious condition in teens and young men with SCD. It may occur repeatedly; recurrent priapism leads to lifelong impotence in many cases.59 Priapism frequently begins at night, and prompt interventions that patients may try at home include voiding, ejaculation, warm baths and analgesics.60 However, the occurrence of a painful erection lasting more than two hours should be considered an emergency, and the patient should seek medical attention immediately, in order to reduce the risk for impotence. There is no universal approach to the medical management of priapism, but intervention may include oral vasoactive agents, transfusion, penile aspiration and injection with epinephrine or phenylephrine, and surgical shunting.59
Although many women with SCD can safely bear children, pregnancy is considered high-risk61 due to a high frequency of sickling complications, as well as spontaneous miscarriage, preeclampsia, premature delivery and low birth weight infants. Teens and young adults should be counseled to use effective methods of birth control to avoid unintended pregnancies. When they become pregnant, women should initiate prenatal care promptly from an obstetrician experienced in the management of SCD. Teens and young adults with SCD should receive genetic counseling, in order to understand the risk of having children with SCD. Their partners should also be tested for a hemoglobinopathy; this testing should be by means of hemoglobin electrophoresis or HPLC (high-performance liquid chromatography), and not merely a sickle prep, as the latter will miss other hemoglobinopathies such as hemoglobin C or beta thalassemia.
Due to chronic pain, physical disabilities, frequent acute pain episodes or other complications resulting in hospitalization, SCD patients may be fired for excessive job absences, and may have difficulty in securing employment. Employed patients should be encouraged to file federal Family and Medical Leave Act papers with their employer, to protect their jobs whenever possible. Similarly, patients may experience difficulties with marital and other personal relationships.
Despite medical advances, SCD in adults is a debilitating disorder leading ultimately to premature mortality. The Cooperative Study of SCD (CSSCD), a national study of the natural history of SCD, found in 1994 that patients with persistently high levels of fetal hemoglobin (Hb F) had prolonged survival compared to those with low Hb F. The protective effect of Hb F has been observed for many years62 and forms the basis for the treatment of patients with hydroxyurea,63 a chemotherapy agent capable of inducing Hb F production in some SCD patients. Long-term follow-up of patients who participated in a definitive trial of hydroxyurea therapy64 has provided some preliminary evidence that use of this drug may prolong survival. Patients often succumb to chronic organ failure, dying from an illness that under other circumstances would not be fatal. Common causes of death include pulmonary hypertension, sudden death due to unknown causes, renal failure, ACS or acute multi-organ failure, often in the context of hospitalization for painful crisis, sepsis, cardiac disease, thromboembolism, stroke, or iron overload.65 Frequently, more than one of these conditions contributes to the death.
Despite the multitude of challenges facing persons living with SCD and their loved ones, many adults are able to lead successful careers and fulfilling family lives. Such patients’ optimism has been eloquently expressed by the late Linda Collins, a beloved and respected Chicago sickle cell activist, UIC patient and the founder of the Have a Heart for Sickle Cell Foundation, whose motto was, “SCD is not who you are; it is what you have.” With the focus on the person with SCD, effective management of the pain and other symptoms of the disease is important for patient-centered and family-focused care and central to high quality of life, as advocated by a palliative care approach to SCD management.
SCD Pain and Symptom Management
Pain and SCD are so intertwined that ancient African tribal words for SCD are onomatopoeic for pain. The first patient reported with SCD in the Western literature was hospitalized for 62 days for “muscular rheumatism.”66 Whereas SCD has been associated with many groundbreaking discoveries, our understanding of pain syndromes associated with it, the pathophysiology of the pain, and its treatment have not advanced nearly to the same degree. Pain syndromes in those with SCD do not generally start until after the first few months of life, when the amount of fetal hemoglobin in their red blood cells decreases, and sickle hemoglobin increases, an observation first reported by Watson in 1948.67 The importance of elevated fetal hemoglobin in adults to decrease disease severity was first reported by Perrine, in 1972.68 Both of these findings are the underpinning for the development of therapies that increase fetal hemoglobin production to ameliorate pain and other the symptoms of the disease. The consequences of unrelieved pain in those with SCD are substantial.69
Many different pain syndromes are associated with the sickle hemoglobinopathies; however, the most common is the acute vaso-occlusive crisis, which represents over 90% of admissions to the hospital for SCD.35 Acute vaso-occlusive crisis is a complicated physiologic event, involving endothelial red blood cell interaction, leukocyte adhesion, hemoglobin polymerization, and coagulation activation, to mention a few.70 It has been described as having four phases. The first phase is prodromal, with symptoms of numbness, aches, and paresthesias that develop in areas subsequently affected by pain. This phase can last up to two days. The second phase, the initial infarct, shows increasing pain that usually peaks by the second or third day. This phase is followed by the post-infarction phase, with persistent and severe pain associated with signs and symptoms of inflammation, followed by the resolution phase.35 The pain may involve any part of the body, and its severity and time from development to resolution can vary greatly. Someone can be well, and a few minutes later in severe pain, needing admission to the hospital, whereas for others their pain can be easily controlled at home. Other causes of acute pain include the acute chest syndrome, hepatic crisis, cholecystitis, priapism, hand-and-foot syndrome, and splenic sequestration syndrome. Chronic pain can be associated with avascular necrosis of joints, skin ulcers, osteomyelitis, and chronic pain syndromes, including neuropathic pain.35
The CSSCD’s prospective study of the natural history of pain in people with SCD is the largest prospective study of the natural history of pain in subjects with SCD.71 The study followed 3,578 children and adults and documented 12,290 pain episodes in 18,356 patient years. A pain episode was defined as pain in the extremities, back, abdomen, chest, or head that lasted at least 2 hours, required a visit to the hospital, and could only be explained by SCD. Subjects with hemoglobin SS and those with hemoglobin sickle beta thalassemia0 had higher mean pain frequency (0.8 and 1.0 episodes/year) compared to subjects with hemoglobin SC disease or hemoglobin sickle beta thalassemia+ (0.4 episodes/year). These investigators also showed the wide variability in the frequency of these painful episodes within each phenotype. As defined for the study, 39% of subjects had no pain episodes/year, while 1% of subjects had 6 or more/year.
The CSSCD researchers also found significant differences in the frequency in pain episodes between age groups, with children 4 years of age or less having 0.4 episodes/year and those 25 to 29 years of age having 1.21 episodes/year. 71 A lower fetal hemoglobin and higher hemoglobin were independent risk factors for these pain events. Although there is little doubt as to the importance of the CSSCD pain study, it may not represent fully the pain experience of those with SCD. In order to be considered a painful event, the subject’s pain needed to be severe enough to be seen by a health care provider, and if they had more than one event in a week, it was considered as one event. Therefore, the number of painful episodes may be under-represented.
The pain experiences of those with SCD are much more frequent and complex than the CSSCD study suggested. Nurse scientists have contributed substantially to understanding the sensory, emotional, cognitive, temporal characteristics of acute pain in children with SCD by using the Adolescent Pediatric Pain Tool.72–74 Dampier et al. reported on 37 child and adolescent subjects with SCD who completed pain diaries at home, for a total of 18,377 pain diary days.75 Only two subjects did not report pain during the study, whereas the remaining 35 reported 518 pain episodes. Smith et al.76 prospectively studied 232 adults with SCD for up to six months, using a daily self-reported pain diary. They found that subjects reported pain on more than 50% of the days, and 30% experienced pain on more than 95% of the study days. Importantly, only 22% of days that the subjects reported acute pain resulted in an admission.
Our group at UIC recently reported on the pain of 145 adults who used PAINReportIt,®77 an electronic version of the McGill Pain Questionnaire.78 We found that the SCD pain at a usual clinic visit was more severe than pain severity reported for cancer pain or childbirth pain.79 Furthermore, our findings79 suggested that the pain experienced by adults with SCD may include a component that is neuropathic (pain arising as a direct consequence of a lesion or disease affecting the somatosensory system),80 in addition to the nociceptive pain (pain from injury to somatic or visceral tissue) commonly associated with a vaso-occlusive event.
Pain in people with SCD also has a significant impact on quality of life. Schwartz et al. studied 40 adolescent subjects with SCD and found that they miss 12% of the school year, and 35% missed at least one month of school.81 These investigators also reported that parent-reported pain frequency and attendance at clinic appointments were significantly associated with the school absenteeism.81
It is not clear why some people have more severe disease. Ballas, however, first suggested that there were two distinct phenotypes of sickle cell:82 those with frequent painful episodes and fewer leg ulcers, and those with fewer painful episodes, but more leg ulcers. Serjeant and others were also able to develop a twofold model with either predominantly painful crises or leg ulcer phenotypes in sickle cell subjects from Jamaica.83 They also found that the painful crisis group had higher frequencies of dactylitis, meningitis, septicemia, acute chest syndrome, and stroke.83 More recently, others have found a hyper-hemolysis phenotype to be more associated with elevated blood pressure, priapism, leg ulcer, and pulmonary hypertension, whereas osteonecrosis and pain occurred less often in this group.84 Some pain syndromes can be addressed with specific procedures, such as arthroplasty for severe avascular necrosis, and surgery for cholecystitis, but the management of the acute vaso-occlusive event and chronic pain syndromes can be much more difficult.
Opioids are the mainstay for management of severe vaso-occlusive events; however, health care providers are often reluctant to provide adequate therapy. The reasons for inadequate medication use are complex.85 A disturbing survey found that 53% of emergency department physicians and 23% of hematologists believed that more than 20% of those with SCD are addicts.86 Using non-pain-related symptoms, however, Elander et al. showed that only 2% met DSM IV (Diagnostic and Statistical Manual of Mental Disorders, fourth edition) criteria for dependence,87 whereas others found an incidence of 0.2% to 2.0%.88 Studies are needed in people with SCD to determine the effect of drugs commonly used for neuropathic pain, such as gabapentin, on the pain that they describe with neuropathic descriptors. A palliative care approach that includes attention to the complete pain experience of SCD may help to improve outcomes and quality of life for this vulnerable population.
When patients cannot control their pain at home and need to go to the emergency department for care, Tanabe et al. found that the median time to administration of an initial analgesic was 90 minutes (25th to 75th interquartile range, 54–159 minutes).89 These investigators also found that 92% received the recommended dose, based on American Pain Association Guidelines, but only 55% received the drug by the recommended route (intravenously or subcutaneously).89
A major advance in the care of patients with SCD has been the acute care facility, or day hospital. In 1984, Grady Hospital in Atlanta opened a center, 24 hours, seven days a week, for adult patients with SCD. In addition to providing outpatient care, this facility substituted for the emergency department for most of the adult patients with SCD who were having painful events that they could not control at home (obstetric cases were triaged to obstetrics, trauma cases to trauma). The admission rate for those who now use this facility is only 20%, which reduced their admission rate from 2.5 to 0.5 active subjects/year, and improved patient satisfaction (personal communication with Alan Platt, PA). In 2000, Benjamin et al. reported on the Day Hospital of the Bronx Comprehensive Sickle Cell Center at the Montefiore Medical Center, Bronx, NY.90 During their first five years of operation, they, too, were able to discharge more than 80% of the patients to home, compared to the emergency department’s 94% hospital admission rate.90 Similar findings have been reported in the UK,91 in Jamaica,92 and for pediatric patients.93 Unfortunately, there are few such facilities in the U.S.
Though it has been almost 100 years since SCD was first reported in the Western literature,66 we are woefully behind in our understanding of the physiology and complicated nature of pain in SCD, as well as its impact on the quality of life for patients. Overall, the delivery of health care and adequate pain management for those with SCD is lacking. We now understand that pain is almost a daily experience in those with SCD, yet patients often have problems in getting appropriate care. Most find themselves in an adversarial relationship with many health care providers, who do not understand the severity of their pain. This breakdown in the patient-client relationship undermines self-reliance and self-knowledge and reduces the capacity for self-management.94 The palliative care philosophy of a partnership between patients and their health care providers, starting at the earliest of ages, may be the most effective health care model for those with SCD.95 A palliative care focus is needed not only to foster adequate pain management but also to facilitate coping with the losses associated with SCD, as well as the grief and bereavement related to death in this vulnerable population.
Coping with Loss, Grief and Bereavement
Individuals and families living with SCD face many kinds of loss, not only the possibility of death at a young age but also the multiple losses along the way as the person experiences the challenges of the disease. For example, the husband whose wife has SCD and has had multiple strokes will be faced with the loss of a partner who shared the household responsibilities, and he may need to take on the task of paying the bills, doing the gardening and grocery shopping, and more. Because he must assume the responsibilities that his wife once did for the both of them, his grieving process starts long before her actual death. At the same time, as his wife’s illness and disability worsens over time, she too grieves the loss of roles, activities and experiences in which she is no longer able to participate. For example, her work as a bank vice president was a key part of her identity, but her health challenges associated with SCD force her to miss work, a big loss for her. Similarly, she grieves for not being able to continue her active support of her teenage daughter’s involvement in competitive gymnastics. As patients and families focus on the challenges of the illness, they experience multiple losses.
The spectrum of losses includes those that often come to mind, as well as those rarely considered (Table 1). Grief is a normal response to loss. People of all ages have grief responses, but the responses differ by age, gender, and other factors (Table 2). Some grief responses persist and interfere with the individual’s health and functioning to the extent that the person is considered to have complicated grief. Uncomplicated grief is managed by the person and his/her communities (e.g., work, faith, neighbors) without need for professional help. In contrast, people with complicated grief often require counseling support from health professionals.
Table 1.
The Spectrum of Losses in People Living with SCD. Modified with permission D. J. Wilkie, © 2001, D. J. Wilkie and TNEEL Investigators.22
Loss of a Significant Loved or Valued Person through Death
|
Loss of Significant People through Other Means
|
|
Loss of a Part of Self Physical loss
|
Psychological Loss
|
Social Loss
|
|
Loss of External Material Objects Loss of all kinds of possessions: both those with economic, practical or convenience value and those with sentimental, emotional value can elicit grieving responses. |
|
Developmental Losses All growing involves relinquishing--i.e., losing certain ways of functioning in order to gain new skills, patterns and modes of functioning. These losses occur across the entire life span, from birth and infancy through older age. |
Table 2.
Differences in Grief Responses by Age, Gender and Person. Modified with permission D. J. Wilkie, © 2001, D. J. Wilkie and TNEEL Investigators.22
| Age | Responses |
|---|---|
| Babies | May withdraw and stop eating, or be listless and fussing. |
| Toddlerhood | Toddlers’ sense of what they want guides how they see the situation around them. They believe that people can read their minds and that wishing for something will make it happen. Frequently, they will blame themselves for what does happen, including the death of a family member. Toddlers may not distinguish between going away and death. They may show their distress in nonverbal ways as agitated behaviors, body language or in dreams. Sometimes in play situation. Toddler may cry one moment and run outside and play in the next moment. |
| Preschool-Kindergarten Age | Children between the ages of 3–6 years begin to recognize their own behavior even though they may not be able to fully control their feelings and actions. By age 4, children may have a limited understanding of the word “death.” They may think the dead may come back to life. There is an interaction with the social environment. For example, if the mother died when the child was 2 years old and the father remarries, the child at the age of 4 feels cared for by this second mother but still may want to visit the grave of his mother. |
| Elementary School Age | Children are on a continuum of development, and they learn quickly not only about relationships but also how to read and develop more resources for themselves. These children are able to separate their point of view from that of others. Elementary school age children realize that there is a connection between events, that their father being sick led to his death. They have concerns as to who will take their father’s place. These children understand death as the cessation of functions. Death processes and funerals are very much on their minds. Older children know that death is universal. There can be emotional disturbances such as poor school performance, appetite changes, shortened attention span, depression, guilt and fear. They may complain of stomachache and other somatic symptoms. They frequently will not bring up their concerns. |
| Adolescence | This period of time is one of rapid cognitive, emotional and physical development. They act more independently and are able to think abstractly, to also recognize their own feelings and point of view as well as others’ feelings and points of view. Maintaining relationships and receiving approval from their peers are vitally important to them. When death occurs in the family, they can talk about their feelings. We see the differences between boys and girls at this time. Boys begin to contain their feelings and are less likely to talk about them, while girls do. Questions about death are natural and an integral part of an attempt to reach a new understanding of life. Initial response to death may be shock, confusion, depression, anger, fear, blaming, lethargy and guilt, which decrease over time. Acting out behavior such as driving fast or taking risks may be seen. |
| Gender | |
| Feminine (conventional) | Sharing intense feelings with others, expressing feelings through crying, responding favorably to interventions that are traditional, affect-intensive strategies, e.g., open sharing of feelings and group support. |
| Masculine | Remaining silent, engaging in solitary mourning, “secret grief,” taking physical or legal action, becoming immersed in activity, exhibiting addictive behavior, seeking companionship (in line of support), using humor or other ways of expressing feelings (but managing anger and aggression) |
| Other Factors | |
| Personal Factors | Past experiences with loss and completion of grief work, when in the life cycle the loss occurred, relationship with deceased, how loss occurred (sudden, expected, unexpected, preventable), health history (physical health, mental health, suicide attempts, life crises) |
Many health professionals commonly express feelings of inadequacy at helping patients with SCD and their families cope with loss, grief and bereavement. Two simple strategies can be implemented to help patients, families, and health professionals cope with the loss, grief, and bereavement that is common in the face of SCD. One strategy is to recognize the importance of rituals to assist in coping with loss and grief. Rituals are embedded within our cultures and offer comfort, especially at the time of a death. Some health care professionals intentionally attend funerals of their patients as a means of offering support to the bereaved but also to help them cope with their sense of loss. It is also helpful for health professionals in clinics or inpatient units to participate in an annual remembrance of patients who died during the year with a recognition event.
Another strategy that many health professionals might find helpful is to write condolence letters or notes to their patients who have experienced a loss (e.g., stroke) or to a bereaved family after a death. Often, though, health professionals lack confidence in their ability to say the right thing, and they fear that their words will cause more distress to a patient with a loss or the family after a death. Table 3 shows a basic structure for a condolence letter or note with example language suggested by grief and bereavement experts.96 Writing the letter or note is therapeutic for the health care professional, and receiving the letter or note is comforting to the family. Using a palliative care approach to care of people living with SCD and their families, health care professionals can incorporate condolence letters and notes into their routines. Additionally, health care professionals should come to understand the benefits that a condolence letter or note has for themselves and the comfort it can bring to the bereaved.
Table 3.
Zunin’s and Zunin’s96 Elements of a Letter of Condolence or Condolence Note. Modified with permission D. J. Wilkie, © 2001, D. J. Wilkie and TNEEL Investigators.22
| Element | Example Language |
|---|---|
| 1. Salutation. | Dear John, |
| 2. Acknowledge the loss. | I was saddened when Troy called this evening to tell me the news of Bobbie’s death. Even though I had been expecting it, the final word was still difficult. |
| 3. Express your sympathy. | Words are so inadequate, but with this letter comes my deep sympathy on the loss of your beloved wife. I, too, cared deeply for her. |
| 4. Note special qualities of the deceased. | Bobbie was a vibrant, talented, caring, and funny woman. Everyone cherished her, but for me, she was even more. She was a rare and treasured friend. |
| 5. Recount a memory about the deceased. | As I write, I recall the precious memories of the day when Bobbie and I were driving to the lake for what we thought would be a lazy afternoon of fishing. Instead, our car broke down and the two of us had quite a time trying to fix it. We were covered in grease and laughing like little girls, but we fixed it! And we made it to the lake just in time to catch the biggest fish either of ever caught. |
| 6. Note special qualities of the bereaved. | I know you will miss her so very much, but I also know that you will find strength remembering the beautiful years you shared. Bobbie loved you so and you were always a source of strength and courage to her. I recall her once saying that your love of life and enduring optimism gave her much peace. I hope that these same qualities will help support and guide you during this difficult time. |
| 7. Offer assistance. | Please know that you have my sympathy and my friendship. I would appreciate if you would turn to me for any help I might give. I’ll call soon to see if there’s anything I can do. |
| 8. Close with a thoughtful word or phrase. | My thoughts are with you, Or I send you my deepest sympathy, Or My prayers are with you, |
Summary
Patients with SCD often have painful episodes and other health challenges, but how they manage those issues can determine the quality of their lives, their successes and productivity. An ecological model of care that is patient-centered and family-focused may better equip a patient and his/her family and health care providers for success across the patient’s life span. Personal factors, including improved communication and education, can benefit patients. Additionally, environmental factors like support groups and advance care planning can help patients navigate the minefields of having a chronic, oftentimes debilitating disease. This palliative care approach can facilitate patient involvement and advocacy in their own care and ultimately help to improve the patient’s quality of life and functional status.
Patients with SCD are at increased risk for morbidity and mortality. The life span of patients has improved over the years, which can be attributed to multiple factors; however, living with SCD still has numerous challenges. Patients must deal with having a chronic disease that often requires frequent hospital and outpatient visits. Patients with SCD live on average into their mid-forties and can have multiple physical and emotional struggles along the way, including leg ulcers, chronic hip pain, relationship challenges, marital discord, and difficulty maintaining insurance coverage. A person-centered and family-focused approach to care can help patients manage the multifaceted challenges of living with SCD.
Communication is important between the health care team and families with SCD. Oftentimes, parents with SCT may not be aware of the potential to transmit SCD to their offspring. Adolescents and young adults, therefore, must be aware of the potential of producing a child with SCD, who will have a decreased life span compared to his/her peers. Advance care planning, including informed consent, surrogate decision-making and the development of advance directives, can aid in improving the patient’s quality of life, which is the ultimate goal of a palliative care approach.
Patients with SCD and pain crises make up the vast majority of admissions to the hospital. Although the exact genetic mutation that causes SCD has been known for years, there has been a lack of quality pain research. Researchers are just beginning to understand the broad impact of frequent admissions to the hospital for pain, including routinely missing school and work. With the advent of recent research and establishment of day hospitals for managing patients’ pain, admission rates can be significantly decreased.
Grief responses may differ for patients, depending on age and gender. Some patients “lose” their ability to provide for their families and fulfill their family roles. When grief responses interfere with an individual’s ability to function, counseling from health care professionals may help. Additionally, health care professionals may be better equipped to manage their patients’ pain by improved communication and connection with their families.
Taken together, the needs of the person with SCD and the family are amenable to intervention and support that is consistent with a simultaneous care plan that involves management of the SCD as well as palliative care. Such an approach could dramatically improve the experience of living with SCD.
Acknowledgments
This publication was made possible by Grant Numbers 1R01 HL078536 and U54 HL090513 from the National Institutes of Health, National Heart Lung and Blood Institute, and P30 NR010680 from the National Institute of Nursing Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart Lung and Blood Institute or the National Institute of Nursing Research. The final peer-reviewed manuscript is subject to the National Institutes of Health Public Access Policy. The authors thank Kevin Grandfield for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Diana J. Wilkie, Email: diwilkie@uic.edu, Professor and Harriet H. Werley Endowed Chair for Nursing Research, Director, Center for End-of-Life Transition Research, Department of Biobehavioral Health Science (MC 802), Member, Comprehensive Sickle Cell Center, University of Illinois at Chicago, 845 S. Damen Ave., Room 660, Chicago, IL 60612-7350, Voicemail: 312.413.5469, Fax: 312.996.1819.
Bonnye Johnson, Email: bonnye@uic.edu, Community Health Education and Outreach Coordinator, College of Medicine and Comprehensive Sickle Cell Center, 820 S. Wood St., Suite 172 (MC 712), Chicago, IL 60612-7350, Voicemail: 312.996.5267, Fax: 312.996.5984.
A. Kyle Mack, Email: a-mack@northwestern.edu, Assistant Professor of Pediatrics, Northwestern University-Feinberg School of Medicine, Division of Pediatric Hematology/Oncology/Stem Cell Transplantation, Children’s Memorial Hospital, Chicago, IL 60614, Phone: 773-880-4562, Fax: 773-880-4209.
Richard Labotka, Email: richardl@uic.edu, Professor, College of Medicine and Comprehensive Sickle Cell Center, 820 S. Wood St., Suite 172 (MC 712), Chicago, IL 60612-7350, 312-996-6143.
Robert E. Molokie, Email: remoloki@uic.edu, Assistant Professor, College of Medicine, College of Pharmacy, and Comprehensive Sickle Cell Center, 820 S. Wood St., Suite 172 (MC 712), Chicago, IL 60612-7350, Jesse Brown VA Medical Center, Chicago, IL, 312-569-6855.
References
- 1.Center TSCI. Sickle cell information-Clinician summary. [Accessed September 13, 2009.];The Georgia Comprehensive Sickle Cell Center at Grady Health System. Available at: http://www.scinfo.org/prod05.htm.
- 2.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994 Jun 9;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 3.Heitkemper MM, Shaver JF. Nursing research opportunities in enteral nutrition. Nurs Clin North Am. 1989 Jun;24(2):415–426. [PubMed] [Google Scholar]
- 4.Mitchell PH, Gallucci B, Fought SG. Perspectives on human response to health and illness. Nurs Outlook. 1991 Jul–Aug;39(4):154–157. [PubMed] [Google Scholar]
- 5.Ruland CM, Moore SM. Theory construction based on standards of care: A proposed theory of the peaceful end of life. Nursing Outlook. 1998;46:169–175. doi: 10.1016/s0029-6554(98)90069-0. [DOI] [PubMed] [Google Scholar]
- 6.Widger KA, Wilkins K. What are the key components of quality perinatal and pediatric end-of-life care? A literature review. Journal of Palliative Care. 2004;20(2):105–112. [PubMed] [Google Scholar]
- 7.Emanuel EJ, Emanuel LL. The promise of a good death. The Lancet. 1998;351:sII21–29. doi: 10.1016/s0140-6736(98)90329-4. [DOI] [PubMed] [Google Scholar]
- 8.Field MJ, Cassel CK, editors. Approaching death: improving care at the end of life. Washington, D.C: National Academy Press; 1997. [PubMed] [Google Scholar]
- 9.Field MJ, Behrman RE, editors. When Children Die: Improving Palliative and End-of-Life Care for Children and their Families. Washington, D.C: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 10.Forum NQ. A National Framework and Preferred Practices for Palliative and Hospice Care Quality. Washington, D.C: National Quality Forum; 2006. [Google Scholar]
- 11.Stroebe MS, Hansson RO, Stroebe W, Schut H. Introduction: concepts and issues in comtemporary research on bereavement. In: Stroebe MS, Hansson RO, Stroebe W, Schut H, editors. Handbook of Bereavement Research. Washington, D.C: American Psychological Association; 2001. [Google Scholar]
- 12.Schut H, Stroebe MS. Interventions to Enhance Adaptation to Bereavement. Journal of Palliative Medicine. 2005;8(Supplement 1):S-140–S147. doi: 10.1089/jpm.2005.8.s-140. [DOI] [PubMed] [Google Scholar]
- 13.Eckman JR, Platt AF. Problem Oriented Management of Sickle Syndromes. 1997 [Google Scholar]
- 14.Gallo AM, Knafl KA, Angst DB. Information management in families who have a child with a genetic condition. J Pediatr Nurs. 2009 Jun;24(3):194–204. doi: 10.1016/j.pedn.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson B, Bilder J, Molokie R. Unpublished raw data. Chicago: University of Illinois at Chicago Medical Center; 2009. Community-University partnership for sickle cell screening. [Google Scholar]
- 16.Gallo AM, Wilkie DJ, Suarez ML, et al. Focus Group Findings about Reproductive Health Decisions in People with Sickle Cell Disease or Trait. In review. [Google Scholar]
- 17.Griffin MF. Improving sickle cell disease newborn notification and follow-up services in North Texas. http://www.sicklecelldisease.net/index.php?option=com_content&task=view&id=83&Itemid=54.
- 18.Becker C, Clark E, DeSpelder LA, et al. A Call to Action:An IWG Charter for a Public Health Approach to Dying, Death, and Loss. doi: 10.2190/OM.69.4.d. In review. [DOI] [PubMed] [Google Scholar]
- 19.Beider S. An Ethical Argument for Integrated Palliative Care. Evid Based Complement Alternat Med. 2005 Jun;2(2):227–231. doi: 10.1093/ecam/neh089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anghelescu DL, Oakes L, Hinds PS. Palliative care and pediatrics. Anesthesiol Clin. 2006 Mar;24(1):145–161. ix. doi: 10.1016/j.atc.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Thomas VJ, Cohn T. Communication skills and cultural awareness courses for healthcare professionals who care for patients with sickle cell disease. J Adv Nurs. 2006 Feb;53(4):480–488. doi: 10.1111/j.1365-2648.2006.03741.x. [DOI] [PubMed] [Google Scholar]
- 22.Wilkie DJ, Brown MA, Corless I, et al. Toolkit for nursing excellence at end of life transition for nurse educators (TNEEL-NE) 1.0. Chicago: University of Illinois Chicago College of Nursing; 2001. [Google Scholar]
- 23.Herrick JB. Peculiar elongated and sickle-shaped red blood corpuscles in a case of severe anemia. Arch Intern Med. 1910;6:517–521. [PMC free article] [PubMed] [Google Scholar]
- 24.Scott RB. Health care priority and sickle cell anemia. Jama. 1970 Oct 26;214(4):731–734. [PubMed] [Google Scholar]
- 25.Quinn CT, Rogers ZR, Buchanan GR. Survival of children with sickle cell disease. Blood. 2004 Jun 1;103(11):4023–4027. doi: 10.1182/blood-2003-11-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falletta JM, Woods GM, Verter JI, et al. Discontinuing penicillin prophylaxis in children with sickle cell anemia. Prophylactic Penicillin Study II. J Pediatr. 1995 Nov;127(5):685–690. doi: 10.1016/s0022-3476(95)70154-0. [DOI] [PubMed] [Google Scholar]
- 27.Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986 Jun 19;314(25):1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 28.Marcinak JF, Frank AL, Labotka RL, et al. Immunogenicity of Haemophilus influenzae type b polysaccharide-diphtheria toxoid conjugate vaccine in 3- to 17-month-old infants with sickle cell diseases. J Pediatr. 1991 Jan;118(1):69–71. doi: 10.1016/s0022-3476(05)81847-5. [DOI] [PubMed] [Google Scholar]
- 29.Schoendorf KC, Adams WG, Kiely JL, Wenger JD. National trends in Haemophilus influenzae meningitis mortality and hospitalization among children, 1980 through 1991. Pediatrics. 1994 Apr;93(4):663–668. [PubMed] [Google Scholar]
- 30.Halasa NB, Shankar SM, Talbot TR, et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007 Jun 1;44(11):1428–1433. doi: 10.1086/516781. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham G. Mortality among children with sickle cell disease identified by newborn screening during 1990–1994--California, Illinois and New York. MMWR Morb Mortal Wkly Rep. 1998;47(9):169–172. [PubMed] [Google Scholar]
- 32.Yanni E, Grosse SD, Yang Q, Olney RS. Trends in pediatric sickle cell disease-related mortality in the United States, 1983–2002. J Pediatr. 2009 Apr;154(4):541–545. doi: 10.1016/j.jpeds.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 33.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998 Jul 2;339(1):5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 34.Fullerton HJ, Adams RJ, Zhao S, Johnston SC. Declining stroke rates in Californian children with sickle cell disease. Blood. 2004 Jul 15;104(2):336–339. doi: 10.1182/blood-2004-02-0636. [DOI] [PubMed] [Google Scholar]
- 35.Ballas SK. Pain management of sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):785–802. v. doi: 10.1016/j.hoc.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000 Jun 22;342(25):1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 37.NIH Publication No. 02-2117. 2002. The Management of Sickle Cell Disease. [Google Scholar]
- 38.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998 Jan 1;91(1):288–294. [PubMed] [Google Scholar]
- 39.Switzer JA, Hess DC, Nichols FT, Adams RJ. Pathophysiology and treatment of stroke in sickle-cell disease: present and future. Lancet Neurol. 2006 Jun;5(6):501–512. doi: 10.1016/S1474-4422(06)70469-0. [DOI] [PubMed] [Google Scholar]
- 40.Adams RJ, Brambilla D. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005 Dec 29;353(26):2769–2778. doi: 10.1056/NEJMoa050460. [DOI] [PubMed] [Google Scholar]
- 41.Kushner JP, Porter JP, Olivieri NF. Secondary iron overload. Hematology Am Soc Hematol Educ Program. 2001:47–61. doi: 10.1182/asheducation-2001.1.47. [DOI] [PubMed] [Google Scholar]
- 42.Hindmarsh PC, Brozovic M, Brook CG, Davies SC. Incidence of overt and covert neurological damage in children with sickle cell disease. Postgrad Med J. 1987 Sep;63(743):751–753. doi: 10.1136/pgmj.63.743.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moser FG, Miller ST, Bello JA, et al. The spectrum of brain MR abnormalities in sickle-cell disease: a report from the Cooperative Study of Sickle Cell Disease. AJNR Am J Neuroradiol. 1996 May;17(5):965–972. [PMC free article] [PubMed] [Google Scholar]
- 44.Steen RG, Emudianughe T, Hankins GM, et al. Brain imaging findings in pediatric patients with sickle cell disease. Radiology. 2003 Jul;228(1):216–225. doi: 10.1148/radiol.2281020943. [DOI] [PubMed] [Google Scholar]
- 45.Miller ST, Macklin EA, Pegelow CH, et al. Silent infarction as a risk factor for overt stroke in children with sickle cell anemia: a report from the Cooperative Study of Sickle Cell Disease. J Pediatr. 2001;139(3):385–390. doi: 10.1067/mpd.2001.117580. [DOI] [PubMed] [Google Scholar]
- 46.Allon M. Renal abnormalities in sickle cell disease. Arch Intern Med. 1990 Mar;150(3):501–504. [PubMed] [Google Scholar]
- 47.Platt OS, Rosenstock W, Espeland MA. Influence of sickle hemoglobinopathies on growth and development. N Engl J Med. 1984 Jul 5;311(1):7–12. doi: 10.1056/NEJM198407053110102. [DOI] [PubMed] [Google Scholar]
- 48.Al-Rimawi H, Jallad S. Sport participation in adolescents with sickle cell disease. Pediatr Endocrinol Rev. 2008 Oct;6 (Suppl 1):214–216. [PubMed] [Google Scholar]
- 49.Britto MT, Garrett JM, Dugliss MA, et al. Risky behavior in teens with cystic fibrosis or sickle cell disease: a multicenter study. Pediatrics. 1998 Feb;101(2):250–256. doi: 10.1542/peds.101.2.250. [DOI] [PubMed] [Google Scholar]
- 50.Kinney TR, Ware RE. The adolescent with sickle cell anemia. Hematol Oncol Clin North Am. 1996 Dec;10(6):1255–1264. doi: 10.1016/s0889-8588(05)70398-1. [DOI] [PubMed] [Google Scholar]
- 51.Bryant R, Walsh T. Transition of the chronically ill youth with hemoglobinopathy to adult health care: an integrative review of the literature. J Pediatr Health Care. 2009 Jan–Feb;23(1):37–48. doi: 10.1016/j.pedhc.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Eckman JR. Leg ulcers in sickle cell disease. Hematol Oncol Clin North Am. 1996;10(6):1333–1344. doi: 10.1016/s0889-8588(05)70404-4. [DOI] [PubMed] [Google Scholar]
- 53.Aguilar C, Vichinsky E, Neumayr L. Bone and joint disease in sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):929–941. viii. doi: 10.1016/j.hoc.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Smith JA. Bone disorders in sickle cell disease. Hematol Oncol Clin North Am. 1996 Dec;10(6):1345–1356. doi: 10.1016/s0889-8588(05)70405-6. [DOI] [PubMed] [Google Scholar]
- 55.Charache S. Eye disease in sickling disorders. Hematol Oncol Clin North Am. 1996 Dec;10(6):1357–1362. doi: 10.1016/s0889-8588(05)70406-8. [DOI] [PubMed] [Google Scholar]
- 56.Downes SM, Hambleton IR, Chuang EL, Lois N, Serjeant GR, Bird AC. Incidence and natural history of proliferative sickle cell retinopathy: observations from a cohort study. Ophthalmology. 2005 Nov;112(11):1869–1875. doi: 10.1016/j.ophtha.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 57.Emerson GG, Lutty GA. Effects of sickle cell disease on the eye: clinical features and treatment. Hematol Oncol Clin North Am. 2005 Oct;19(5):957–973. ix. doi: 10.1016/j.hoc.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 58.Williams CI, Shaffer EA. Gallstone disease: current therapeutic practice. Curr Treat Options Gastroenterol. 2008 Mar;11(2):71–77. doi: 10.1007/s11938-008-0018-6. [DOI] [PubMed] [Google Scholar]
- 59.Rogers ZR. Priapism in sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):917–928. viii. doi: 10.1016/j.hoc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Fowler JE, Jr, Koshy M, Strub M, Chinn SK. Priapism associated with the sickle cell hemoglobinopathies: prevalence, natural history and sequelae. J Urol. 1991 Jan;145(1):65–68. doi: 10.1016/s0022-5347(17)38248-4. [DOI] [PubMed] [Google Scholar]
- 61.Hassell K. Pregnancy and sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):903–916. vii–viii. doi: 10.1016/j.hoc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Sunshine HR, Hofrichter J, Eaton WA. Requirement for therapeutic inhibition of sickle haemoglobin gelation. Nature. 1978 Sep 21;275(5677):238–240. doi: 10.1038/275238a0. [DOI] [PubMed] [Google Scholar]
- 63.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995 May 18;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 64.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. Jama. 2003 Apr 2;289(13):1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 65.Darbari DS, Kple-Faget P, Kwagyan J, Rana S, Gordeuk VR, Castro O. Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006 Nov;81(11):858–863. doi: 10.1002/ajh.20685. [DOI] [PubMed] [Google Scholar]
- 66.Herrick JB. Peculiar elongated and sickle-shaped red blood corpuscles in a case of severe anemia. 1910. Yale J Biol Med. 2001 May–Jun;74(3):179–184. [PMC free article] [PubMed] [Google Scholar]
- 67.Watson J. The significance of the paucity of sickle cells in newborn Negro infants. Am J Med Sci. 1948 Apr;215(4):419–423. doi: 10.1097/00000441-194804000-00008. [DOI] [PubMed] [Google Scholar]
- 68.Brown MJ, Weatherall DJ, Clegg JB, Perrine RP. Benign sickle-cell anaemia. Br J Haematol. 1972 May;22(5):635. [PubMed] [Google Scholar]
- 69.Benjamin L. Pain management in sickle cell disease: palliative care begins at birth? Hematology Am Soc Hematol Educ Program. 2008:466–474. doi: 10.1182/asheducation-2008.1.466. [DOI] [PubMed] [Google Scholar]
- 70.Morris CR. Mechanisms of vasculopathy in sickle cell disease and thalassemia. Hematology Am Soc Hematol Educ Program. 2008:177–185. doi: 10.1182/asheducation-2008.1.177. [DOI] [PubMed] [Google Scholar]
- 71.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991 Jul 4;325(1):11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 72.Franck LS, Treadwell M, Jacob E, Vichinsky E. Assessment of sickle cell pain in children and young adults using the adolescent pediatric pain tool. J Pain Symptom Manage. 2002 Feb;23(2):114–120. doi: 10.1016/s0885-3924(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 73.Jacob E, Beyer JE, Miaskowski C, Savedra M, Treadwell M, Styles L. Are there phases to the vaso-occlusive painful episode in sickle cell disease? J Pain Symptom Manage. 2005 Apr;29(4):392–400. doi: 10.1016/j.jpainsymman.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 74.Jacob E, Hesselgrave J, Sambuco G, Hockenberry M. Variations in pain, sleep, and activity during hospitalization in children with cancer. J Pediatr Oncol Nurs. 2007 Jul–Aug;24(4):208–219. doi: 10.1177/1043454207299875. [DOI] [PubMed] [Google Scholar]
- 75.Dampier C, Ely B, Brodecki D, O’Neal P. Characteristics of pain managed at home in children and adolescents with sickle cell disease by using diary self-reports. J Pain. 2002 Dec;3(6):461–470. doi: 10.1054/jpai.2002.128064. [DOI] [PubMed] [Google Scholar]
- 76.Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008 Jan 15;148(2):94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- 77.Wilkie DJ, Judge MK, Berry DL, Dell J, Zong S, Gilespie R. Usability of a computerized PAINReportIt in the general public with pain and people with cancer pain. J Pain Symptom Manage. 2003 Mar;25(3):213–224. doi: 10.1016/s0885-3924(02)00638-3. [DOI] [PubMed] [Google Scholar]
- 78.Melzack R. The McGill pain questionnaire: Major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 79.Wilkie DJ, Molokie R, Boyd-Seal D, et al. Patient-Reported Outcomes: Nociceptive and Neuropathic Pain and Pain Barriers in Adult Outpatients with Sickle Cell Disease. JNMA. doi: 10.1016/s0027-9684(15)30471-5. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008 Apr 29;70(18):1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 81.Schwartz LA, Radcliffe J, Barakat LP. Associates of school absenteeism in adolescents with sickle cell disease. Pediatr Blood Cancer. 2009 Jan;52(1):92–96. doi: 10.1002/pbc.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ballas SK. Sickle cell anemia with few painful crises is characterized by decreased red cell deformability and increased number of dense cells. Am J Hematol. 1991 Feb;36(2):122–130. doi: 10.1002/ajh.2830360211. [DOI] [PubMed] [Google Scholar]
- 83.Alexander N, Higgs D, Dover G, Serjeant GR. Are there clinical phenotypes of homozygous sickle cell disease? Br J Haematol. 2004 Aug;126(4):606–611. doi: 10.1111/j.1365-2141.2004.05025.x. [DOI] [PubMed] [Google Scholar]
- 84.Taylor JGt, Nolan VG, Mendelsohn L, Kato GJ, Gladwin MT, Steinberg MH. Chronic hyper-hemolysis in sickle cell anemia: association of vascular complications and mortality with less frequent vasoocclusive pain. PLoS One. 2008;3(5):e2095. doi: 10.1371/journal.pone.0002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pack-Mabien A, Labbe E, Herbert D, Haynes J., Jr Nurses’ attitudes and practices in sickle cell pain management. Appl Nurs Res. 2001 Nov;14(4):187–192. doi: 10.1053/apnr.2001.26783. [DOI] [PubMed] [Google Scholar]
- 86.Shapiro BS, Benjamin LJ, Payne R, Heidrich G. Sickle cell-related pain: perceptions of medical practitioners. J Pain Symptom Manage. 1997 Sep;14(3):168–174. doi: 10.1016/S0885-3924(97)00019-5. [DOI] [PubMed] [Google Scholar]
- 87.Elander J, Lusher J, Bevan D, Telfer P. Pain management and symptoms of substance dependence among patients with sickle cell disease. Soc Sci Med. 2003 Nov;57(9):1683–1696. doi: 10.1016/s0277-9536(02)00553-1. [DOI] [PubMed] [Google Scholar]
- 88.Martin JJ, Moore GP. Pearls, pitfalls, and updates for pain management. Emerg Med Clin North Am. 1997 May;15(2):399–415. doi: 10.1016/s0733-8627(05)70307-2. [DOI] [PubMed] [Google Scholar]
- 89.Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007 May;14(5):419–425. doi: 10.1197/j.aem.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 90.Benjamin LJ, Swinson GI, Nagel RL. Sickle cell anemia day hospital: an approach for the management of uncomplicated painful crises. Blood. 2000 Feb 15;95(4):1130–1136. [PubMed] [Google Scholar]
- 91.Wright J, Bareford D, Wright C, et al. Day case management of sickle pain: 3 years experience in a UK sickle cell unit. Br J Haematol. 2004 Sep;126(6):878–880. doi: 10.1111/j.1365-2141.2004.05123.x. [DOI] [PubMed] [Google Scholar]
- 92.Ware MA, Hambleton I, Ochaya I, Serjeant GR. Day-care management of sickle cell painful crisis in Jamaica: a model applicable elsewhere? Br J Haematol. 1999 Jan;104(1):93–96. doi: 10.1046/j.1365-2141.1999.01160.x. [DOI] [PubMed] [Google Scholar]
- 93.Raphael JL, Kamdar A, Beavers MB, Mahoney DH, Mueller BU. Treatment of uncomplicated vaso-occlusive crises in children with sickle cell disease in a day hospital. Pediatr Blood Cancer. 2008 Jul;51(1):82–85. doi: 10.1002/pbc.21483. [DOI] [PubMed] [Google Scholar]
- 94.Wright K, Adeosum O. Barriers to effective pain management in sickle cell disease. Br J Nurs. 2009 Feb 12–25;18(3):158–161. doi: 10.12968/bjon.2009.18.3.39043. [DOI] [PubMed] [Google Scholar]
- 95.McClain BC, Kain ZN. Pediatric palliative care: a novel approach to children with sickle cell disease. Pediatrics. 2007 Mar;119(3):612–614. doi: 10.1542/peds.2006-3580. [DOI] [PubMed] [Google Scholar]
- 96.Zunin LM, Zunin HS. The Art of Condolence. New York: Harper Collins; 1991. [Google Scholar]