Abstract
Background
Epithelial-mesenchymal transition is a critical early event in the invasion and metastasis of many cancers, including colorectal cancer. Chronic inflammation is an inducer of several cancers and inflammatory cytokines have been implicated in tumor invasion.
Materials and Methods
Human colon cancer cell lines HCT116 and SW480 were transfected with PTEN siRNA or non-targeting control. Invasiveness was measured using a modified Boyden chamber assay and migration was assessed using a scratch assay.
Results
PTEN knockdown increases the invasion and migration of CRC cells and the addition of medium containing TNF-α further enhanced the migration and invasion. PTEN knockdown resulted in nuclear β-catenin accumulation and increased expression of downstream proteins c-Myc and cyclin D1.
Conclusions
Our study supports clinical studies identifying an association of PTEN loss with late stage cancer. Cellular factors secreted from the surrounding tumor milieu likely act in concert with genetic changes in the tumor cells and contribute to enhanced tumor invasion.
Keywords: EMT, PTEN, TNF-α, migration, invasion
Epithelial-mesenchymal transition (EMT) is a process that occurs normally during critical phases of embryonic development in mammals, including embryonic neural crest migration and multi-organ development (1). The migration and subsequent reorganization of embryonic organelles in EMT has led to a comparison with the process of tumor metastasis. Progression from premalignant disease to a malignant phenotype is accomplished by a number of acquired genetic alterations and reversible changes attributable to the tumor-host microenvironment which are exceedingly important in tumor invasion and metastasis (2, 3). During EMT the expression of E-cadherin, referred to as the “caretaker” of the epithelial phenotype, is decreased resulting in loss of cell-cell adhesion and increased migration (4). The nuclear accumulation of β-catenin, a key component of the Wnt signaling pathway, is also noted at the invasive front, a high density area of inflammatory cells that surrounds the primary cancer mass (5).
Tumor necrosis factor-α (TNF-α), an inflammatory cytokine involved in the acute phase reaction, is implicated in a variety of human diseases, including cancers (6). TNF-α is mainly produced by macrophages, but it is also produced by a variety of other cell types including mast cells, lymphoid cells, and fibroblasts (7). The increased density of inflammatory cells, including macrophages that are located at the invasive front of primary tumors, has led to the supposition that TNF-α plays a key role in tumor-host crosstalk. Recent evidence supports the notion that the innate immune system plays a key role in tumor invasion by the release of cytokines mainly from macrophages that are found in high density at the invasive front (8). The effect of TNF-α is well established; however, its involvement as a mediator at the invasive front of primary tumors has yet to be determined.
Activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway is important for normal cellular metabolism and growth and progression of certain cancers (9). PI3K/Akt pathway mutations are found in several human malignancies including breast, ovarian, pancreatic, and colorectal cancer (CRC) (10, 11). Evidence that the PI3K pathway plays a key role in CRC is supported by studies showing that the absence of PTEN (Phosphatase and tensin homolog deleted on chromosome ten), the natural PI3K inhibitor, is associated with increased intestinal mucosal tumors (12-14). Recent studies suggest that loss of PTEN expression is more prevalent in CRCs than originally thought, particularly in later stage cancers. Rychahou et al. (15) from our laboratory demonstrated loss of PTEN expression in approximately 83% of metastatic CRCs. In agreement with these findings, PTEN was noted to be inactivated (expression was decreased or lost in 66-70% of CRCs) and was identified more frequently with microsatellite instability (16, 17). Additionally, TNF-α results in the down-regulation of PTEN (18). Together, the silencing of PTEN and TNF-α stimulation may provide an environment for the migration, invasion, and the subsequent metastasis of CRCs.
The tumor microenvironment is increasingly recognized as a source of the soluble factors and inflammatory cytokines important in the progression of various cancers (19, 20). The purpose of our current study was to evaluate the importance of decreased PTEN expression, coupled with the response of TNF-α, on CRC cell phenotype and invasion. Here, we show that the knockdown of PTEN, in concert with TNF-α, results in cellular changes consistent with EMT and increased invasiveness of CRC cells. We also show that PTEN knockdown results in nuclear β-catenin accumulation and increased expression of downstream proteins c-Myc and cyclin D1 which correlates with the increased expression of β-catenin found at the invasive front of CRC. Together, these findings indicate that PTEN silencing, in concert with factors in the tumor microenvironment, results in an increased invasive phenotype that aids in the invasion and subsequent metastasis of CRC.
Materials and Methods
Antibodies
Antibodies against E-cadherin, and α-catenin, were purchased from BD Transduction Laboratories (San Jose, CA, USA). Antibodies against PTEN, phosphorylated-Akt, Akt, phosphorylated-β-catenin, and β-catenin were purchased from Cell Signaling (Danvers, MA, USA). Antibodies for c-Myc, cyclin D1, phosphorylated-ERK, and ERK were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody for β-Actin was from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Human colon cancer cell lines, HCT116 and SW480, rat intestinal epithelial cell lines (RIE-1 and IEC-6), the murine macrophage cell line RAW, and the human intestinal epithelial cell line (HIE), were obtained from American Type Culture Collection (Manassas, VA, USA). HCT116 cells were maintained in McCoy's 5A medium supplemented with 10% fetal bovine serum (FBS). RAW cells were maintained in DMEM medium supplemented with 10% FBS. RIE-1 cells were maintained in DMEM-H supplemented with 5% FBS. IEC-6 cells were maintained in DMEM with 5% FBS, and HIE cells were maintained in Opti-MEM medium supplemented with 4% FBS. Tissue culture media and reagents were purchased from Life Technologies, Inc. (Grand Island, NY, USA). SW480 cells were maintained in Liebovitz L-15:DMEM (1:1 ratio) with 10% FBS.
To generate the mSW480 PTEN shRNA cell line, SW480 PTEN shRNA green fluorescent protein-tagged cells were injected into the spleen of athymic nude mice. After 4 wks a liver metastasis was isolated, dissected into small fragments, and transferred to a flask. Hank's balanced salt solution was added to the flask and incubated for 3 h. Cells were then passed through a nylon mesh filter, spun down, and plated. Cells were maintained in SW480 media supplemented with ampicillin and streptomycin for 2 wks and then selected with puromycin media with for 2 wks. mSW480 PTEN shRNA cells maintained their green fluorescent protein-tag, and puromycin selection ensured that all other cells were eliminated.
Macrophage conditioned media
For isolation and culture of human macrophages, buffy coats were collected from the blood of healthy donors at The University of Texas Medical Branch Blood Bank. Primary blood mononuclear cells were isolated by density-gradient centrifugation through Ficoll/Hypaque (Amersham Bioscience, Piscataway, NJ, USA), suspended (8 × 106 cell/ml) in RPMI 1640 medium with 15% heat inactivated human serum (Sigma, St. Louis, MO, USA), and seeded into flasks. After incubation for 2 h at 37°C, adherent cells were detached with trypsin and resuspended at 1 × 106 cells/ml in medium supplemented with 40 ng/ml macrophage colony-stimulating factor (Pepro Tech Inc, Rocky Hill, NJ, USA). Cells were allowed to differentiate for seven days in macrophage colony-stimulating factor. On day seven, cell media was replaced with fresh medium without macrophage colony-stimulating factor and cultured for additional 24 h. Human macrophages were finally exposed to lipopolysaccharide (100 ng/ml, Sigma) for another 24 h. The culture medium was collected, centrifuged, stored in aliquots at -80°C, and defined as macrophage conditioned media (MCM).
Cell transfections
For migration and invasion experiments, HCT116 and SW480 cells were transfected with PTEN siRNA (Dharmacon, Lafayette, CO, USA). HCT116 (2.5 × 106), SW480 (4 × 106), RIE-1 (8 × 106), IEC-6 (8 × 106), and HIE (8 × 106) cells underwent electroporation in pre-sterilized 4mm gap electroporation cuvettes (Molecular BioProducts, San Diego, CA, USA). A PTEN non-inducible vector system (Dharmacon) was used to create SW480 and HCT116 polyclonal stable shRNA populations with puromycin antibiotic selection. Stable clones were established for at least 2 wks before they were sorted twice by FACS Aria (Becton Dickinson; Franklin Lakes, NJ, USA). Effective knockdown was confirmed by Western blot and real time RT-PCR as we have previously described (18).
Migration assay
A monolayer scratch assay was used to compare the migratory ability of HCT116, SW480, and metastatic SW480 PTEN shRNA cell lines. PTEN and control NTC siRNA-transfected cell lines or shRNA cell lines were cultured to confluency, scratched and photographed using phase contrast microscopy at 0, 24, and 48 h. In some experiments MCM at different concentrations was added at time 0 h. The minimum distance in millimeters between the wound edges of the scratch area was analyzed using Adobe Photoshop 7.0. All experiments were performed in triplicate.
Invasion assay
A modified Boyden chamber invasion assay with Matrigel-coated Transwell chambers was performed with HCT116 and SW480 cell lines. Cell lines transfected with PTEN siRNA were compared to NTC to determine invasiveness. MCM was used as the chemoattractant and cell line complete media was used as the control. After 72 h the cells were fixed with 3% glutaraldehyde and stained with 4',6-diamidino-2-phenylindole (DAPI) fluorescent staining. Dapi-stained cells were counted in 4 different fields with an inverted fluorescent microscope. For co-culture experiments using SW480 PTEN shRNA with RAW macrophages, TNF-α antibody (R&D, Minneapolis, MN, USA) and/or isotype control IgG was added and renewed every 24 h. All experiments were performed in triplicate.
Soft agar assay
A soft agar assay was used to assess anchorage-independent growth of HCT116 and SW480 PTEN shRNA stable transfectants compared with control cells. Cells were grown in 0.35% Seaplaque agarose gel (Lonza, Switzerland) using 24 well plates. The cells were plated at a concentration of 1.6 × 104 cells/ ml and plated onto the gel. After 2 wks the cells were stained with crystal violet, counted, and photographed. All experiments were performed in triplicate.
Western blot
Western blotting was performed as previously described (21). Briefly, total protein (60 μg) was resolved on a 10% Nu-PAGE Bis-Tris gel and transferred to PVDF membranes. Filters were incubated overnight at 4°C in blotting solution, followed by 1 h incubation with primary antibodies. Filters were washed and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h. After three additional washes, the immune complexes were visualized by enhanced chemiluminescense detection.
Enzyme-linked immunosorbent assay (ELISA)
A monoclonal antibody specific for human TNF-α coated on a 96-well plate was obtained from Bio Scientific (Austin, TX, USA) and the assay was performed according to the manufacturer's instructions. Briefly, standards and samples were added to the wells, the wells were washed, and biotinylated monoclonal anti-human TNF-α antibody was added. After a second wash, avidin-horseradish peroxidase was added, producing an antibody-antigen-antibody conjugate. The wells were again washed and a substrate solution was added, which produces a blue color in direct proportion to the amount of human TNF-α present in the initial sample. The stop solution changes the color from blue to yellow and the wells were read at 450nm. All experiments were performed in triplicate.
Immunostaining
Experiments were performed as described previously (9). For immunofluorescent staining, cells were grown on chamber slides, fixed with 4% paraformaldehyde, and incubated with primary antibodies. Secondary antibodies were FITC-conjugated goat anti-mouse from Invitrogen (Carlsbad, CA, USA).
Statistical analysis
For invasion assay, cell counts were analyzed using analysis of variance for a three-factor experiment. The factors were siRNA (NTC, PTEN siRNA), media (control, MCM) and experiment (conducted at 3 different times). For wound assay, distance were measured at 6 different locations and averaged for each scratch at each time point. The averaged data was analyzed using analysis of variance for a two-factor experiment with repeated measures on time. The two factors were siRNA (NTC, PTEN siRNA) and time (0, 24, 48 h). Main effects and interactions were assessed at the 0.05 level of significance. Multiple comparisons were conducted using a t statistic with the standard error computed from a linear combination of appropriate mean squares in the analysis of variance, with an approximated degrees of freedom using Satterthwaite method, and with Bonferroni adjustment for the number of comparisons. Statistical computations were carried out using PROC GLM and PROC MIXED in SAS®.
Results
PTEN knockdown increases CRC cell migration
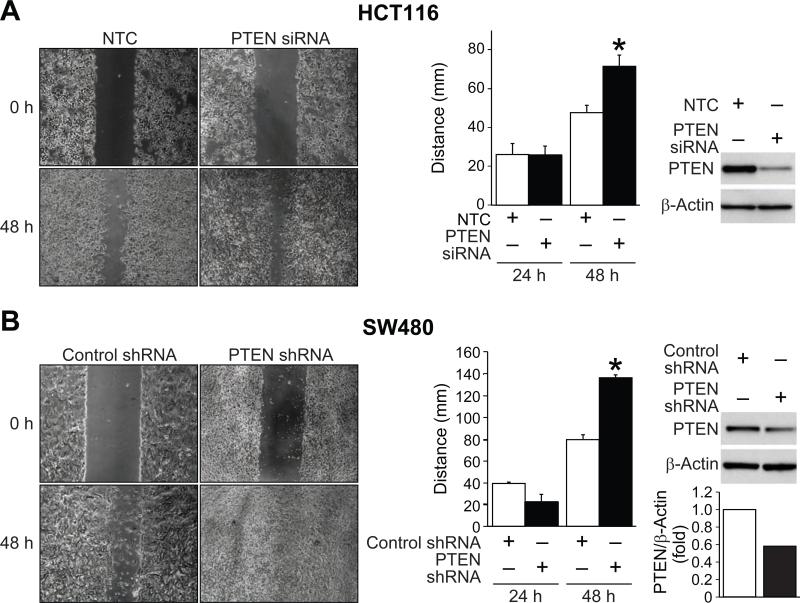
Migration and invasion are both key components of EMT (22). Since the HCT116 CRC cell line is a metastatic cell line that invades through the basement membrane (23), we tested the hypothesis that the knockdown of PTEN would increase migration and invasion. HCT116 cells were transfected with NTC siRNA or PTEN-targeted siRNA and scratch assays were performed. There was near complete closure of the scratch in HCT116 PTEN siRNA cells when compared to control (Figure 1A; left). These results were quantified by measuring the distance between the scratch edges and found to be statistically significant at 48 h after the scratch (Figure 1A; middle). Western blot analysis confirmed PTEN knockdown in HCT116 cells (Figure 1A; right). Next, a non-metastatic cell line, SW480, was transfected with NTC or PTEN siRNA and scratch assays were performed. SW480 PTEN siRNA cells demonstrated complete closure of the scratch after 48 h when compared to control (Figure 1B). Together, these findings demonstrate increased CRC migration associated with PTEN reduction.
Figure 1. PTEN knockdown increases migration of HCT116 and SW480 cells.
A, PTEN siRNA and NTC transfected cell lines were grown to confluency, scratched, and photographed 0 and 48 h. A significant increase in migration was observed after PTEN knockdown in HCT116 cells (* = p < 0.05). Western blot analysis of protein expression demonstrated knockdown of PTEN in HCT116 cells. B, A significant increase in migration was observed after PTEN knockdown in SW480 cells (* = p < 0.05). Western blot analysis of protein expression demonstrated knockdown of PTEN in SW480 cells.
MCM further increases CRC migration after PTEN knockdown
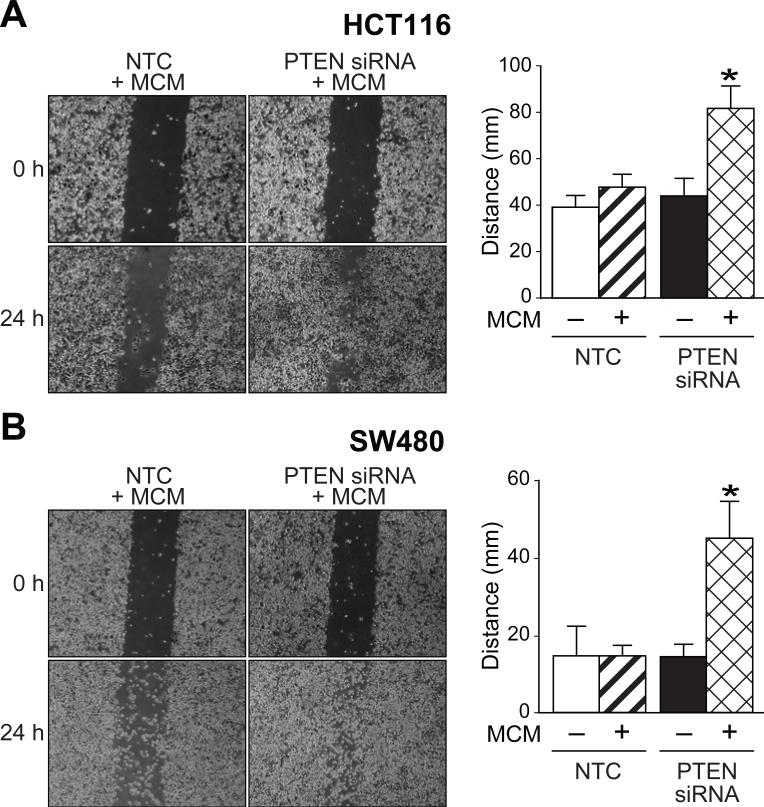
MCM is known to contain a variety of growth factors and cytokines, most importantly TNF-α (24, 25). To simulate the tumor microenvironment, MCM (containing 992 pg/ml TNF-α as measured by enzyme-linked immunosorbent assay), was added to HCT116 and SW480 cells after PTEN siRNA or NTC transfection and scratch assays were performed. The addition of MCM to HCT116 (Figure 2A) and SW480 (Figure 2B) PTEN siRNA cells resulted in near complete closure of both cell lines by only 24 h after the initial scratch compared with our results with PTEN siRNA alone which resulted in near complete closure of the wound by 48 h. The synergistic increase of migration that was observed after PTEN knockdown and addition of MCM containing TNF-α suggests that factors produced by the tumor microenvironment act in concert to enhance CRC migration.
Figure 2. MCM with TNF-α increases the migration of HCT116 and SW480 cells.
A, PTEN siRNA and NTC transfected cell lines were grown to confluency, scratched and photographed at 0 and 24 h. At 0 h, MCM (125 μl) was added to HCT116 PTEN siRNA cells and NTC. MCM significantly increased the migration of HCT116 PTEN siRNA at 24 h (* = p < 0.05). B, At 0 h, MCM (200μl) was added to SW480 cells PTEN siRNA cells and NTC. MCM significantly increased the rate of closure at 24 h (* = p < 0.05).
Stimulation by TNF-α increases CRC invasion after PTEN knockdown
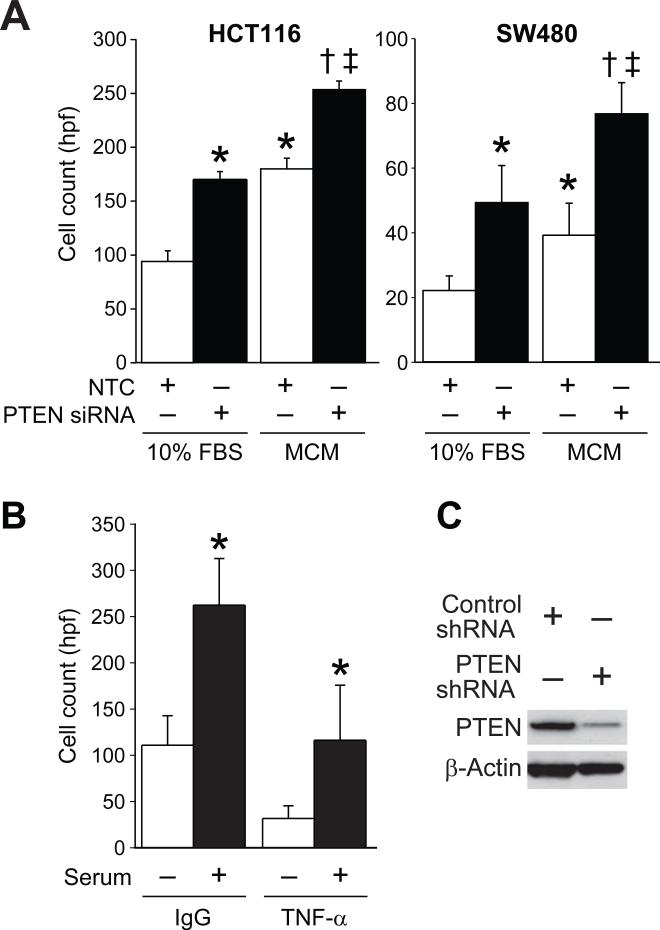
Invasion is a critical step in EMT and is characterized by the loss of epithelial adhesion molecules, cell dissociation, and penetration through the basement membrane (26). To determine if an inflammatory microenvironment is a hallmark of increased invasion of SW480 and HCT116 cell lines after PTEN knockdown, a modified Boyden chamber invasion assay with thin-layer Matrigel-coated Transwell chambers was performed. Cells were transfected with PTEN siRNA or NTC, plated onto Matrigel-coated chambers and, after 72 h, fixed and stained with Dapi. There was a significant increase in the invasion of HCT116 and SW480 cells in chambers with MCM compared to control media (Figure 3A). There was also a significant increase when compared to serum free media (data not shown). MCM further enhanced the invasion of metastatic HCT116 cells and increased the invasiveness of the non-metastatic SW480 cell line.
Figure 3. MCM increases the invasion of HCT116 and SW480 cells transfected with PTEN siRNA and TNF-α antibody reduces invasion.
A, A modified Boyden chamber invasion assay with thin-layered Matrigel-coated Transwell chambers was performed with HCT116 and SW480 cell lines. Cells were transfected with PTEN siRNA or NTC then plated onto Matrigel coated chambers. MCM, control media (10% FBS), or serum free media was used as the chemoattractant in the bottom well. MCM as a chemoattractant significantly increased invasion of both cell lines after the targeted inhibition of PTEN (* = p < 0.05). B, A modified Boyden chamber invasion assay with thin-layered Matrigel-coated chambers was performed with SW480 PTEN shRNA cells. RAW macrophages were co-cultured and SW480 cells and isotype control IgG or TNF-α antibody was added every 24 h to the control media (10% FBS), or serum free media. C, Western blot analysis confirmed the down-regulation of PTEN in SW480 PTEN shRNA cells when compared with control; β-Actin expression shows equal loading of protein.
To further assess the role of TNF-α in the enhanced invasion noted above, a co-culture assay was performed with SW480 PTEN shRNA cells. Isotype control antibody or TNF-α blocking antibody was added to the bottom chamber; a significant decrease in invasion was observed when TNF-α blocking antibody was used (Figure 3B; left).Western blot analysis showed knockdown of PTEN in the SW480 stable cell line (Figure 3B; right). The increased invasion of CRC cells toward TNF-α containing MCM further supports the importance of the tumor microenvironment in tumor invasion. These results, coupled with the significant decrease in invasion of CRC cells with TNF-α blocking antibody, suggests that TNF-α is a critical cytokine regulating the increased invasion noted with the addition of MCM.
Nuclear β-catenin is up-regulated after PTEN knockdown
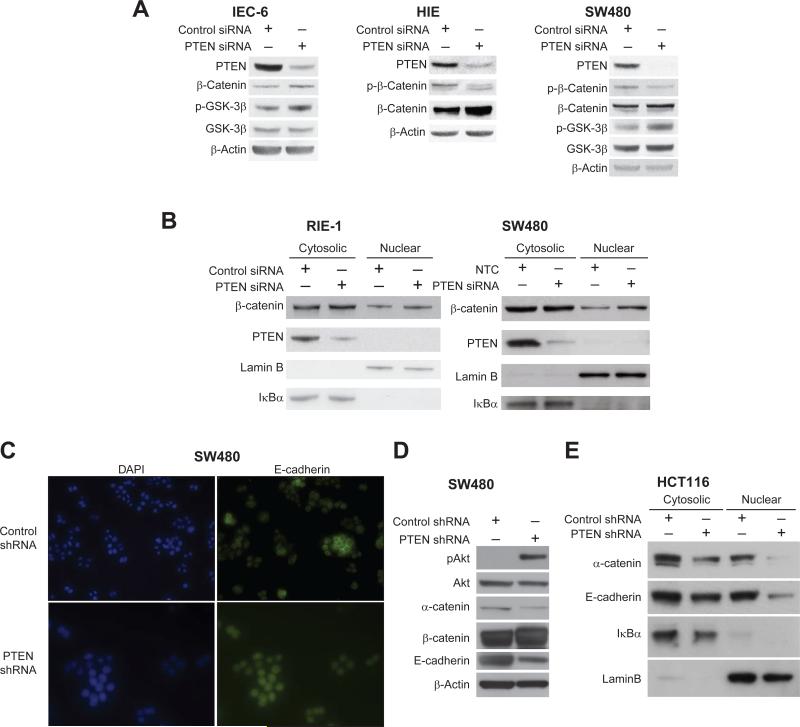
Activation of the Wnt / β-catenin pathway and the subsequent nuclear accumulation of β-catenin is a common feature of CRC (27). The nuclear accumulation of β-catenin at the invasive front has also been associated with EMT (28, 29). To determine whether an increase in total β-catenin and nuclear β-catenin occurs after the targeted knockdown of PTEN, intestinal epithelial cell lines RIE-1, IEC-6, and HIE and the CRC cell line SW480 were analyzed. Cells were transfected with PTEN siRNA or NTC and cell lysates were analyzed by Western blot for nuclear and cytoplasmic β-catenin expression. PTEN knockdown increased β-catenin expression levels in IEC-6 and HIE cells with a slight increase noted in SW480 cells, suggesting a general regulation of β-catenin expression by PTEN (Fig 4A).
Figure 4. PTEN knockdown increases nuclear β-catenin in RIE-1 and SW480 cell lines and decreases E-cadherin in SW480 and HCT116 cells.
A, PTEN siRNA and NTC IEC-6, HIE, RIE-1, and SW480 were transfected, cell lysates were analyzed by Western blot analysis for total protein levels of β-catenin. There was increased total β-catenin in the intestinal cell lines IEC-6 and HIE. B, Western blot analysis demonstrated increased nuclear β-catenin with PTEN knockdown in SW480 cells and RIE-1 cells. C, Immunofluorescence demonstrated a decreased amount of E-cadherin junctional staining in SW480 PTEN shRNA cells when compared with control. D-E, Western blot analysis confirmed the down-regulation of E-cadherin and α-catenin in SW480 and HCT116 PTEN shRNA cells when compared with control.
To detect whether β-catenin levels in the nucleus were increased, SW480 and RIE cells were transfected with PTEN or NTC siRNA and nuclear and cytosolic protein was isolated after 48 h. The knockdown of PTEN increased cytosolic as well as nuclear β-catenin expression levels as noted by Western blot analysis (Figure 4B). Lamin B, an integral protein of the nuclear envelope, and IκBα, located in the cytoplasm, were used as controls for the nuclear and cytosolic fractions, respectively. To assess whether the increased β-catenin altered targeted protein expression, cyclin D1 and c-Myc expression was determined. Inhibition of PI3K using wortmannin or LY294002 decreased c-Myc and cyclin D1 expression, and knockdown of PTEN increased the expression of these proteins in RIE-1 cells; the silencing of PTEN also increased c-Myc and cyclin D1 protein expression in IEC-6 and HIE cells (data not shown).
Epithelial markers are a consistent hallmark of EMT. For example, loss of E-cadherin is highly indicative of EMT (30). Because PTEN knockdown increased migration and invasion of two CRC cell lines we hypothesized that these cells would demonstrate a decrease in epithelial characteristics. Previous reports have shown that transient knockdown with siRNA does not correlate with a change in epithelial phenotype, thus a PTEN non-inducible vector system was used to create SW480 and HCT116 stable transfectants. As shown in Figure 4C, SW480 PTEN shRNA cells lacked the epithelial characteristics of E-cadherin when compared to control cells; Western blot confirmed the down-regulation of E-cadherin and α-catenin and the up-regulation of phosphorylated-Akt(Ser 473), which confirmed a decrease in PTEN activity (Figure 4D). The down-regulation of E-cadherin and α-catenin was also noted in HCT 116 cells transfected with PTEN shRNA (Figure 4E). α-catenin, an actin-binding protein, forms an important physical connection at the adherens junction that is present at adhesional complexes and attaches the microfilaments and associated proteins to cadherins via β-catenin (31). These findings are consistent with the cells undergoing a change from an epithelial to a mesenchymal phenotype after PTEN knockdown and further establishes a connection between EMT and the PI3K pathway in CRC.
Increased invasive phenotype noted in metastatic cells
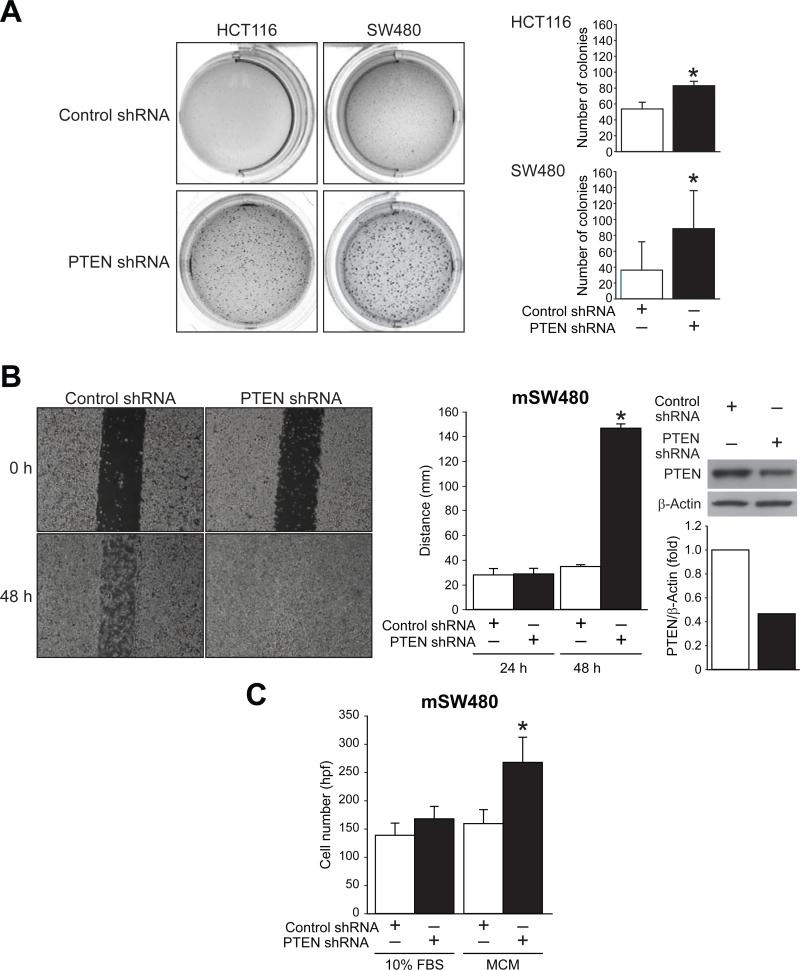
The ability of cells to invade and disseminate is yet another characteristic of EMT (32). Anchorage independent growth in soft agar is typically used to determine if cells will colonize in vivo (33). HCT116 and SW480 PTEN shRNA cells were plated into soft agar and analyzed after 2 wks. When compared to controls both PTEN shRNA cell lines demonstrated significantly more colony formation (Figure 5A).
Figure 5. PTEN knockdown increases invasion in soft agar and mSW480PTENshRNA demonstrates increased migration and invasion.
A, HCT116 and SW480 PTEN shRNA and control cells were grown in 0.35% Seaplaque agarose gel. After 2 wks there were significantly more colonies noted with PTEN knockdown compared with control (* = p < 0.05). B, mSW480 PTEN shRNA and vector control shRNA cells were plated to confluent monolayers, scratched, and photographed. A significant increase in the migration of mSW480 PTEN shRNA was noted at 48 h after the initial scratch (* = p < 0.05). Western blot analysis of protein expression demonstrated effective knockdown of PTEN in mSW480 PTEN shRNA. C, mSW480 PTEN shRNA and vector control shRNA were plated onto a modified Boyden chamber invasion assay with thin-layered Matrigel-coated Transwell chambers with MCM, control media (10% FBS), or serum free media. MCM significantly increased invasion when compared to control media (* = p < 0.05).
To further evaluate the role of the PI3K/Akt pathway in migration, a metastatic cell line isolated from a liver metastasis after stable PTEN shRNA knockdown (mSW480 PTEN shRNA) was used (15). A monolayer scratch assay was performed using this cell line and compared to control cells; increased migration of mSW480 PTEN shRNA cells was clearly noted compared to the non-metastatic control shRNA-transfected cells (Figure 5B; left and middle). These findings are consistent with our study using transient siRNA knockdown. Western blot analysis demonstrated effective knockdown of PTEN in the mSW480 PTEN shRNA cell line (Figure 5B; right). Increased invasion toward MCM was also noted using the mSW480 PTEN shRNA cells (Figure 5C). The increased migration and invasion that was observed in the metastatic PTEN shRNA cell line with the addition of MCM suggests that the tumor microenvironment plays a role in metastatic tumor formation as well. Taken together, these experiments corroborate our findings that PTEN is involved in EMT-induced metastasis.
Discussion
To our knowledge, this is the first report demonstrating that the knockdown of PTEN leads to the induction of EMT in CRC cells. The ability of CRC cells to migrate toward the basement membrane and then invade through its dense connective tissue matrix are key steps in the dissemination of these cells to distant sites (34). Our results demonstrate that the observed increases in migration and invasion are mediated in a PI3K/Akt-dependent manner. Furthermore, we show that, through PTEN down-regulation and a concomitant decrease in epithelial markers, the PI3K pathway mediates EMT in CRC.
Mutations of the PI3K pathway are a common occurrence in various cancers (10, 35). PTEN is an important regulator of the cell cycle, most notably cell division, and mutations of the Pten gene are found in high frequency among several cancers (36, 37). Loss of PTEN appears to influence metastasis by promoting cell proliferation while suppressing apoptosis at the metastatic site (38). Decreased PTEN expression occurs with relatively high frequency in metastatic CRC (15, 39, 40). Recently, PTEN was found to be weakly expressed in primary CRCs in patients with liver metastasis; decreased PTEN expression was also noted with advanced stage disease and lymph node metastasis (39). Rychahou et al. (15) found that Akt2 overexpression in wild-type PTEN CRC cells led to the formation of micrometastases. However, to observe sustained metastases, PTEN loss was required. The down-regulation of PTEN was also observed in advanced stage hepatocellular carcinoma, prostate carcinoma, and melanoma (41, 42). Taken together, this suggests that PTEN suppression or loss in advanced stage disease contributes to tumor invasion and metastasis.
Alterations of the PI3K pathway have been associated with EMT in a variety of cancers and activation of the PI3K effector protein Akt has been observed in squamous cell, renal, and bladder carcinomas (43-45). Transfection with constitutively active Akt in squamous cell carcinoma lines resulted in decreased cell-cell adhesion, increased motility, and increased invasiveness in vivo (43). Activation of Akt in rat kidney epithelial cells was found to be important for TGF-β1-induced EMT in vitro and in vivo which suggests that Akt may act as an important downstream mediator of TGF-β1 (44). In bladder cancer cell lines, N-cadherin expression was found to contribute to invasion by increasing phospho-Akt levels and decreasing E-cadherin expression (45). These studies suggest that therapeutic manipulation of the PI3K pathway may control tumor cell invasion and metastasis. Currently, little is known regarding the relationship of EMT to CRC and the PI3K pathway. Previously, we showed that induction of oncogenic K-Ras in CRC cells resulted in EMT; treatment with the PI3K inhibitor, LY294002, produced a spindle cell morphology, reduced expression of E-cadherin, and increased invasiveness of CRC cells, suggesting that inhibition of PI3K may enhance EMT in CRC (46). Recently, a correlation was reported between over-expressed phosphatase of regenerating liver 3, an upstream regulator of PTEN, and the down-regulation of PTEN which resulted in changes consistent with EMT (37). In normal cells, PTEN modulates migration of mesoderm cells in the chick embryo by controlling EMT and the directional motility (47).
Macrophages are a key source of TNF-α production which is thought to promote tumor invasion and metastasis (48, 49). This hypothesis is further supported by our data showing increased invasion and metastasis with the addition of TNF-α containing MCM and, importantly, a dramatic decrease in invasion after the addition of TNF-α neutralizing antibody. Recently, TNF-α was found to stabilize Snail, a transcription factor and critical regulator of EMT in breast cancer (50). The TNF-α-induced stabilization of Snail resulted in inflammation-induced EMT, migration, invasion, and metastasis of breast cancer cells. Increases in TNF-α also promotes tumor growth and vascularity in mouse melanoma, lung cancer, and mammary tumors (51). Clinical observations have shown an association between TNF-α expression and Dukes’ stages in CRC (52, 53). Furthermore, TNF-α stimulation represses E-cadherin and augments EMT induced invasion of renal cancer cells. These findings support the important role of inflammatory mediators, specifically TNF-α, in the tumor microenvironment.
The subsequent nuclear accumulation of β-catenin, that is responsible for the activation of Wnt genes, and loss of function APC mutations are found in almost all cases of malignant CRC transformation. The nuclear accumulation of β-catenin and the lack of nuclear β-catenin in the central tumor has provided insight into the reversible changes that occur at the invasive front of CRCs (54). Nuclear accumulation of β-catenin, occurring at the invasive front, is an important factor in EMT, and β-catenin regulation by Wnt signaling activation is implicated in the tumor microenvironment. Recently, it was noted that TNF-α from murine macrophages promoted Wnt/β-catenin signaling through inhibition of GSK3β in gastric tumor cells, yet providing another mechanistic link between inflammation and β-catenin signaling (55). Our results are consistent with the increase in nuclear β-catenin that is observed in the invasive front of CRCs (56). We noted an increase in the nuclear accumulation of β-catenin after PTEN knockdown in normal and neoplastic epithelial-derived cell lines. The nuclear accumulation of β-catenin at the invasive front of CRCs is an indication of EMT and the up-regulation of nuclear β-catenin after PTEN knockdown suggests that PTEN plays an integral role in EMT. Our data suggests that β-catenin and its mediated gene transcription are regulated by PTEN which may represent a possible mechanism by which PTEN enhances intestinal cell differentiation and suppresses CRC progression.
In conclusion, we show that knockdown of PTEN increases the migration and invasion of CRC cells which is associated with cellular changes consistent with EMT. These findings further corroborate recent in vivo and clinical studies demonstrating a correlation between PTEN reduction or loss and late stage cancers (15, 39, 40). The combination of PTEN knockdown and MCM containing TNF-α further enhanced CRC cell migration and invasion indicating that factors from the surrounding inflammatory tumor microenvironment act in concert with genetic alterations to stimulate CRC progression.
Acknowledgements
The authors thank Karen Martin for manuscript preparation, and Tatsuo Uchida for statistical analysis. This work was supported by grants RO1 CA104748, RO1 DK48498, PO1DK35608, R01 CA125454 (to BPZ) and T32 DK07639 from the National Institutes of Health. KB is a recipient of a Jeane B. Kempner Scholar Award.
REFERENCES
- 1.Baker CV. The evolution and elaboration of vertebrate neural crest cells. Curr Opin Genet Dev. 2008;18:536–543. doi: 10.1016/j.gde.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Diep CB, Kleivi K, Ribeiro FR, Teixeira MR, Lindgjaerde OC, Lothe RA. The order of genetic events associated with colorectal cancer progression inferred from meta-analysis of copy number changes. Genes Chromosomes Cancer. 2006;45:31–41. doi: 10.1002/gcc.20261. [DOI] [PubMed] [Google Scholar]
- 3.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 4.Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki H, Masuda N, Shimura T, Araki K, Kobayashi T, Tsutsumi S, Asao T, Kuwano H. Nuclear beta-catenin expression at the invasive front and in the vessels predicts liver metastasis in colorectal carcinoma. Anticancer Res. 2008;28:1821–1830. [PubMed] [Google Scholar]
- 6.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 7.Tsianos EV, Katsanos K. Do we really understand what the immunological disturbances in inflammatory bowel disease mean? World J Gastroenterol. 2009;15:521–525. doi: 10.3748/wjg.15.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 9.Rychahou PG, Jackson LN, Silva SR, Rajaraman S, Evers BM. Targeted molecular therapy of the PI3K pathway: therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann Surg. 2006;243:833–842. doi: 10.1097/01.sla.0000220040.66012.a9. discussion 843-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellacosa A, de Feo D, Godwin AK, Bell DW, Cheng JQ, Altomare DA, Wan M, Dubeau L, Scambia G, Masciullo V, Ferrandina G, Benedetti Panici P, Mancuso S, Neri G, Testa JR. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 11.Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci U S A. 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I, Ho A, Wakeham A, Itie A, Khoo W, Fukumoto M, Mak TW. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8:1169–1178. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- 13.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 14.Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci U S A. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rychahou PG, Kang J, Gulhati P, Doan HQ, Chen LA, Xiao SY, Chung DH, Evers BM. Akt2 overexpression plays a critical role in the establishment of colorectal cancer metastasis. Proc Natl Acad Sci U S A. 2008;105:20315–20320. doi: 10.1073/pnas.0810715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nassif NT, Lobo GP, Wu X, Henderson CJ, Morrison CD, Eng C, Jalaludin B, Segelov E. PTEN mutations are common in sporadic microsatellite stable colorectal cancer. Oncogene. 2004;23:617–628. doi: 10.1038/sj.onc.1207059. [DOI] [PubMed] [Google Scholar]
- 17.Abubaker J, Bavi P, Al-Harbi S, Ibrahim M, Siraj AK, Al-Sanea N, Abduljabbar A, Ashari LH, Alhomoud S, Al-Dayel F, Uddin S, Al-Kuraya KS. Clinicopathological analysis of colorectal cancers with PIK3CA mutations in Middle Eastern population. Oncogene. 2008;27:3539–3545. doi: 10.1038/sj.onc.1211013. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, Domon-Dell C, Kang J, Chung DH, Freund JN, Evers BM. Down-regulation of the tumor suppressor PTEN by the tumor necrosis factor-alpha/nuclear factor-kappaB (NF-kappaB)-inducing kinase/NF-kappaB pathway is linked to a default IkappaB-alpha autoregulatory loop. J Biol Chem. 2004;279:4285–4291. doi: 10.1074/jbc.M308383200. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: The force journey of a tumor cell. Cancer Metastasis Rev. 2009 doi: 10.1007/s10555-008-9173-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mbeunkui F, Johann DJ., Jr. Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol. 2009;63:571–582. doi: 10.1007/s00280-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson LN, Li J, Chen LA, Townsend CM, Evers BM. Overexpression of wild-type PKD2 leads to increased proliferation and invasion of BON endocrine cells. Biochem Biophys Res Commun. 2006;348:945–949. doi: 10.1016/j.bbrc.2006.07.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etienne-Manneville S. Polarity proteins in migration and invasion. Oncogene. 2008;27:6970–6980. doi: 10.1038/onc.2008.347. [DOI] [PubMed] [Google Scholar]
- 23.Rajput A, Dominguez San Martin I, Rose R, Beko A, Levea C, Sharratt E, Mazurchuk R, Hoffman RM, Brattain MG, Wang J. Characterization of HCT116 human colon cancer cells in an orthotopic model. J Surg Res. 2008;147:276–281. doi: 10.1016/j.jss.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Granger GA, Shacks SJ, Williams TW, Kolb WP. Lymphocyte in vitro cytotoxicity: specific release of lymphotoxin-like materials from tuberculin-sensitive lymphoid cells. Nature. 1969;221:1155–1157. doi: 10.1038/2211155a0. [DOI] [PubMed] [Google Scholar]
- 25.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009 doi: 10.1007/s10555-008-9169-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Gavert N, Ben-Ze'ev A. beta-Catenin signaling in biological control and cancer. J Cell Biochem. 2007;102:820–828. doi: 10.1002/jcb.21505. [DOI] [PubMed] [Google Scholar]
- 28.Huang D, Du X. Crosstalk between tumor cells and microenvironment via Wnt pathway in colorectal cancer dissemination. World J Gastroenterol. 2008;14:1823–1827. doi: 10.3748/wjg.14.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brabletz S, Schmalhofer O, Brabletz T. Gastrointestinal stem cells in development and cancer. J Pathol. 2009;217:307–317. doi: 10.1002/path.2475. [DOI] [PubMed] [Google Scholar]
- 30.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 31.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 32.Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305–318. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 33.Fan D, Morgan LR, Schneider C, Blank H, Fan S. Cooperative evaluation of human tumor chemosensitivity in the soft-agar assay and its clinical correlations. J Cancer Res Clin Oncol. 1985;109:23–28. doi: 10.1007/BF01884250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks SA, Lomax-Browne HJ, Carter TM, Kinch CE, Hall DM. Molecular interactions in cancer cell metastasis. Acta Histochem. 2009 doi: 10.1016/j.acthis.2008.11.022. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Brugge J, Hung MC, Mills GB. A new mutational AKTivation in the PI3K pathway. Cancer Cell. 2007;12:104–107. doi: 10.1016/j.ccr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27:5477–5485. doi: 10.1038/onc.2008.248. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Quah SY, Dong JM, Manser E, Tang JP, Zeng Q. PRL-3 down-regulates PTEN expression and signals through PI3K to promote epithelial-mesenchymal transition. Cancer Res. 2007;67:2922–2926. doi: 10.1158/0008-5472.CAN-06-3598. [DOI] [PubMed] [Google Scholar]
- 38.Simpson L, Parsons R. PTEN: life as a tumor suppressor. Exp Cell Res. 2001;264:29–41. doi: 10.1006/excr.2000.5130. [DOI] [PubMed] [Google Scholar]
- 39.Sawai H, Yasuda A, Ochi N, Ma J, Matsuo Y, Wakasugi T, Takahashi H, Funahashi H, Sato M, Takeyama H. Loss of PTEN expression is associated with colorectal cancer liver metastasis and poor patient survival. BMC Gastroenterol. 2008;8:56. doi: 10.1186/1471-230X-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parsons DW, Wang TL, Samuels Y, Bardelli A, Cummins JM, DeLong L, Silliman N, Ptak J, Szabo S, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Lengauer C, Velculescu VE. Colorectal cancer: mutations in a signalling pathway. Nature. 2005;436:792. doi: 10.1038/436792a. [DOI] [PubMed] [Google Scholar]
- 41.Hu TH, Wang CC, Huang CC, Chen CL, Hung CH, Chen CH, Wang JH, Lu SN, Lee CM, Changchien CS, Tai MH. Down-regulation of tumor suppressor gene PTEN, overexpression of p53, plus high proliferating cell nuclear antigen index predict poor patient outcome of hepatocellular carcinoma after resection. Oncol Rep. 2007;18:1417–1426. [PubMed] [Google Scholar]
- 42.Maehama T, Dixon JE. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol. 1999;9:125–128. doi: 10.1016/s0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- 43.Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN, Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–2178. [PubMed] [Google Scholar]
- 44.Kattla JJ, Carew RM, Heljic M, Godson C, Brazil DP. Protein kinase B/Akt activity is involved in renal TGF-beta1-driven epithelial-mesenchymal transition in vitro and in vivo. Am J Physiol Renal Physiol. 2008;295:F215–225. doi: 10.1152/ajprenal.00548.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallerand H, Cai Y, Wainberg ZA, Garraway I, Lascombe I, Nicolle G, Thiery JP, Bittard H, Radvanyi F, Reiter RR. Phospho-Akt pathway activation and inhibition depends on N-cadherin or phospho-EGFR expression in invasive human bladder cancer cell lines. Urol Oncol. 2008 doi: 10.1016/j.urolonc.2008.09.041. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Shao J, Evers BM, Sheng H. Roles of phosphatidylinositol 3'-kinase and mammalian target of rapamycin/p70 ribosomal protein S6 kinase in K-Ras-mediated transformation of intestinal epithelial cells. Cancer Res. 2004;64:229–235. doi: 10.1158/0008-5472.can-03-1859. [DOI] [PubMed] [Google Scholar]
- 47.Leslie NR, Yang X, Downes CP, Weijer CJ. PtdIns(3,4,5)P(3)-dependent and -independent roles for PTEN in the control of cell migration. Curr Biol. 2007;17:115–125. doi: 10.1016/j.cub.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 49.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 50.Wu YDJ, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-κB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2008 doi: 10.1016/j.ccr.2009.03.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li B, Vincent A, Cates J, Brantley-Sieders DM, Polk DB, Young PP. Low levels of tumor necrosis factor alpha increase tumor growth by inducing an endothelial phenotype of monocytes recruited to the tumor site. Cancer Res. 2009;69:338–348. doi: 10.1158/0008-5472.CAN-08-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Csiszar A, Szentes T, Haraszti B, Zou W, Emilie D, Petranyi G, Pocsik E. Characterisation of cytokine mRNA expression in tumour-infiltrating mononuclear cells and tumour cells freshly isolated from human colorectal carcinomas. Eur Cytokine Netw. 2001;12:87–96. [PubMed] [Google Scholar]
- 53.Chuang MJ, Sun KH, Tang SJ, Deng MW, Wu YH, Sung JS, Cha TL, Sun GH. Tumor-derived tumor necrosis factor-alpha promotes progression and epithelial-mesenchymal transition in renal cell carcinoma cells. Cancer Sci. 2008;99:905–913. doi: 10.1111/j.1349-7006.2008.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brabletz T, Jung A, Dag S, Reu S, Kirchner T. [beta-Catenin induces invasive growth by activating matrix metalloproteinases in colorectal carcinoma]. Verh Dtsch Ges Pathol. 2000;84:175–181. [PubMed] [Google Scholar]
- 55.Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo MM, Oshima M. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. Embo J. 2008;27:1671–1681. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]