Abstract
The EphA2 receptor tyrosine kinase has emerged as a promising new therapeutic target in cancer due to its high expression in tumors. EphA2-specific antibodies have been used to deliver drugs and toxins to tumor cells, leading to inhibition of tumor growth and metastatic dissemination. We previously identified two related peptides, YSA and SWL, that selectively bind to the ligand-binding domain of EphA2 but not other Eph receptors, and could therefore be useful as selective targeting agents. Here we characterize the two peptides and a series of derivatives. Based on systematic amino acid replacements, only 5 YSA residues appear to be critical for high affinity receptor binding. Furthermore, a peptide comprising only the first 5 residues of YSA retains selectivity for EphA2. Similar to ephrin-A1, the physiological ligand for EphA2, both YSA and SWL activate EphA2 and inhibit downstream oncogenic signaling pathways in PC3 cancer cells. The two peptides and derivatives are quite stable in conditioned cell culture medium and show promise for delivering drugs and imaging agents to EphA2-expressing tumors.
The EphA2 receptor tyrosine kinase, a member of the large Eph receptor family, is a promising therapeutic target in cancer because it is widely overexpressed in many cancer types, including breast, ovarian, prostate, pancreatic and lung cancer (1-3). EphA2 is present not only in the tumor cells but also in the tumor vasculature, while it is undetectable in normal quiescent vasculature (4, 5). Furthermore, high EphA2 levels have been associated with a poor clinical prognosis (1-3) and with the more malignant, basal type of breast and prostate cancers (6, 7). EphA2 overexpression has indeed been shown to induce oncogenic transformation and invasiveness of cultured mammary epithelial cells (8), and EphA2 downregulation with siRNA or anti-sense oligonucleotides has a negative impact on tumor growth and metastasis in mouse cancer models (9, 10). Interestingly, EphA2 is tyrosine phosphorylated (activated) at low to undetectable levels in most tumors, suggesting an oncogenic role that is independent of ligand-mediated activation (11-13).
Five glycosylphosphatidylinositol (GPI)-linked ligands (ephrin-A1 to -A5) can induce EphA2 tyrosine phosphorylation and activation in mammalian cells (14). A number of studies have shown that ephrin-induced EphA2 activation inhibits major oncogenic signaling pathways, such as the Ras-MAP kinase pathway and the PI3 kinase/Akt pathway, as well as cell transformation (11, 13, 15). Therefore, EphA2 functions as a tumor suppressor when its signaling ability is activated by ephrin ligands, whereas its tumor promoting effects may be ligand-independent (3, 12, 13, 16).
A number of EphA2-targeting agents have been developed. Several agonistic monoclonal antibodies and ephrin-A1 Fc, a soluble form of ephrin-A1, have been shown to decrease tumor growth and metastasis in mouse models (17-22). Their mechanism of action is not entirely understood, and may involve a combination of several factors, including: (1) activation of EphA2 downstream signaling pathways with tumor suppressor activity, (2) receptor internalization and degradation, possibly accompanied by presentation of EphA2-derived peptides recognized by effector T cells, and (3) antibody-dependent immune cell-mediated cytotoxicity. A bispecific antibody engineered to simultanously bind EphA2 and the T cell receptor/CD3 complex has also been shown to effectively promote destruction of EphA2-expressing tumor cells (23). Furthermore, gold-coated silica nanoshells conjugated to ephrin-A1 have been used for targeted photothermal ablation of cultured PC3 prostate cancer cells, which express high levels of EphA2 (24).
Ephrin ligands and agonistic antibodies that cause EphA2 internalization can also be used to deliver drugs or toxins to tumors. Ephrin-A1 conjugated to Pseudomonas aeruginosa exotoxin A has been shown to kill EphA2-expressing cancer cells in culture (25). Furthermore, an EphA2-specific antibody conjugated to a derivative of auristatin, a drug that disrupts microtubules, dramatically inhibits tumor growth in animal models (26, 27). Finally, EphA2 antibodies coupled to imaging agents have been successfully used for tumor visualization in mouse xenograft models (28). This could be useful for cancer diagnosis, particularly because EphA2 appears to be overexpressed starting from early stages of cancer (4).
As an alternative to the use of ephrins or antibodies, we have identified two related EphA2-targeting peptides by using phage display (29). These 12-mer peptides, designated YSA and SWL, selectively bind to the ephrin-binding domain of EphA2 but not other Eph receptors, and thus may be used as selective targeting agents. Both peptides also inhibit ephrin binding to EphA2. In addition, the YSA peptide, which has been more extensively characterized, was shown to induce EphA2 tyrosine phosphorylation and inhibition of Erk MAP kinases in endothelial cells (29). Therefore, the YSA peptide is an agonist capable of activating EphA2 signaling. We also previously showed that the YSA peptide, fused to the pIII coat protein of M13 phage, can target the phage to EphA2-expressing cancer cells in culture (29). Other groups have demonstrated the usefulness of YSA, conjugated to magnetic nanoparticles, for targeting and removal of cancer cells from the ascites fluids of mice and ovarian cancer patients (30, 31). The YSA peptide has also been used for targeted delivery of siRNA-loaded nanogels used to chemosensitize cancer cells expressing EphA2 (32, 33). In addition, adenoviral vectors engineered to include the YSA peptide in the HI loop of their fiber cell-binding protein have been successfully targeted to EphA2-expressing pancreatic cancer cells in vitro (34). In vivo targeting of EphA2-expressing cells with the engineered adenovirus however was not successful, presumably because of insufficient binding affinity of the peptide inserted in the adenoviral protein (34).
All of these studies have been carried out with the original YSA peptide identified by phage display. However, structural-activity analysis of the YSA peptide, and also the SWL peptide, could provide useful information for further optimization to generate more effective EphA2-targeting agents. Here we identify the residues of the YSA peptide that are critical for EphA2 binding, and characterize additional derivatives of the YSA and SWL peptides. We also demonstrate that the YSA and SWL peptides are quite stable in conditioned cell culture medium. Furthermore, both peptides can activate the EphA2 receptor and inhibit phosphorylation of Akt and Erk1/Erk2 MAP kinases in PC3 prostate cancer cells. These peptides, and derivatives with improved binding affinity, represent promising candidates as EphA2-targeting agents in cancer.
Experimental Procedures
Peptide Synthesis
Some peptides were purchased from Neobioscience (Boston, MA) and others were synthesized automatically on an Applied Biosystems 433A (Applied Biosystems, Foster City, CA) peptide synthesizer by using Fmoc (N-(9-fluorenyl)methoxycarbonyle) chemistry. Low loading Tenta Gel S RAM amide resin (loading: 0.24 mmol/g, Fluka) was used for the in house synthesis. 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) and N-hydroxybenzotriazole (HOBt) in N,N-dimethylformamide (DMF) were used as coupling and activating reagents in the presence of diisopropylethylamine (DIEA). Fmoc group deprotection at each step was carried out using piperidine in NMP (20%). Side chain deprotection and cleavage was performed by treatment with a mixture of trifluoroacetic acid TFA/H2O/thioanisole/phenol/ethanedithiol (82.5%:5%:5%:5%:2.5% v/v/v/v, 5 mL) at room temperature for 2.5 hours. TFA was removed by evaporation, and the crude peptides were precipitated with cooled tert-butylmethyl ether, washed repeatedly in cold ether, centrifuged and lyophilized. The peptides were characterized by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry. The purity of the crude peptides was 82% to 94% as measured by HPLC, and HPLC traces revealed in each case only one major peak and some minor impurities. The peptides used for the experiments in Figures 1 and 4C,D were synthesized with a biotinylated GSGSK tail and purified as previously described (29).
Figure 1.
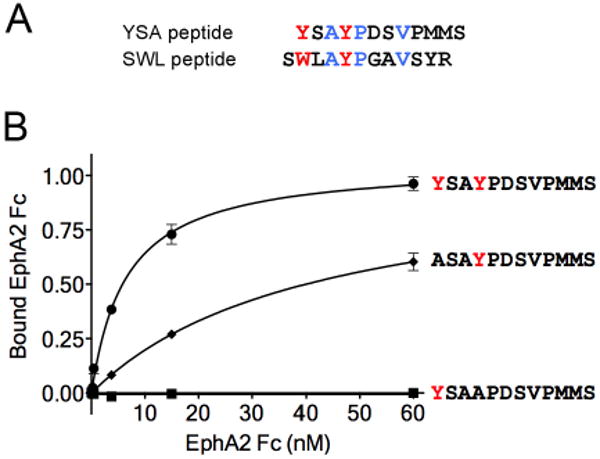
(A) Alignment of the sequences of the YSA and SWL 12mer peptides. The aromatic amino acids are in red; other residues conserved in the two peptides are in blue. (B) The two tyrosines in the YSA peptide are critical for binding to EphA2. The curves show the binding of EphA2 Fc to immobilized biotinylated YSA peptide, or peptide in which Y1 or Y4 is mutated to alanine. Similar amounts of the three peptides were immobilized on streptavidin-coated ELISA wells.
Figure 4.
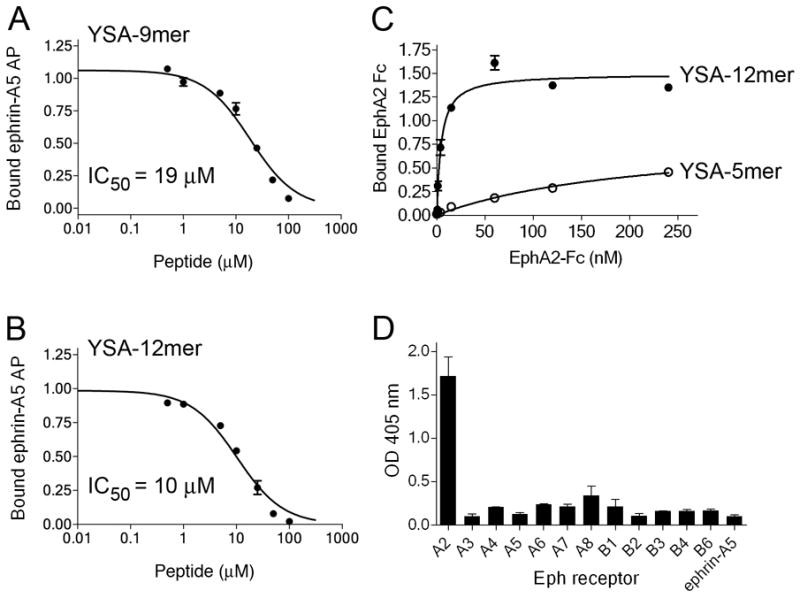
The YSA-9mer peptide retains substantially high potency and the YSA-5mer peptide retains high selectively for EphA2. (A, B) Inhibition of ephrin-A5 AP binding to immobilized EphA2 Fc by YSA-9mer and YSA-12mer peptides. (C) Binding of EphA2 Fc to immobilized biotinylated YSA-5mer peptide and YSA-12mer peptides. Similar amounts of the two peptides were immobilized on streptavidin-coated ELISA wells. (D) Binding of the indicated Eph receptor Fc fusion proteins, and ephrin-A5 Fc as a negative control, to the immobilized YSA-5mer peptide.
The crude YSA-C-10mer peptide was dissolved in 80/20% CH3CN/H2O and purified using semi preparative reverse-phase-high performance liquid chromatography (RP-HPLC). Fractions containing the peptide were pooled together and lyophilized. Dimerization of the purified peptide was performed in 1 M guanidinium hydrochloride and 0.1 M Trisma base at pH 8.5 (1 mg peptide/mL buffer), and was monitored by analytical RP-HPLC using a Vydac C-18 column 5μ, 150mm × 4.6mm (Length and ID) with a flow rate of 1 ml/min. The solvents used were: A, water with 0.1% TFA; and B, 20% water in CH3CN with 0.1% TFA in a linear 10%–65% gradient over 30 min. The purity of the dimerized YSA-C-10mer was 99%. For DTT treatment of the YSA-C10mer peptide (containing an unpaired cysteine), 1 mM solution of the peptide was incubated with 2 mM DTT (20 uL total volume) for 20 min at room temperature, to ensure that no intermolecular disulfide bonds were formed. The 1 mM stock was then diluted to 0.5 μM-50 μM for the ELISA assay.
ELISA Assays
To determine EphA2 Fc binding to immobilized peptides, streptavidin-coated wells were incubated with 1 μM biotinylated peptides for 1 hour at room temperature, rinsed with Tris buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.5) containing 0.1% Tween and 1 mM CaCl2, and incubated with 2 μg/ml EphA2 Fc (R&D Systems, Minneapolis, MN) for 1 hour at room temperature. After washing away the unbound EphA2 Fc with the same buffer, the plate was incubated with anti-Fc antibody conjugated to AP (anti-Fc AP) (Promega, Madison, WI) for 1 hour to measure bound EphA2 Fc. Bound anti-Fc AP was detected using 5.4 mM of para-nitrophenol phosphate (pNPP) substrate in SEAP buffer (0.22 M diethanolamine, 1 mM MgCl2, pH 9.8) and measuring absorbance at OD 405 nm.
To determine the Eph receptor selectivity of the YSA-5mer peptide, streptavidin-coated wells were incubated with 5 μM biotinylated peptide for 1 hour at room temperature, followed by incubation with 20 μg/mL Eph receptor Fc fusion proteins (R&D Systems, Minneapolis, MN) for 1 hour. Bound Eph Fc receptors were then detected using the anti-Fc AP antibody as described above.
To measure the IC50 values of the peptides, protein A-coated wells (Pierce Biotechnology, Rockford, IL) were incubated with a 50 μL solution of 1 μg/mL EphA2 Fc in Tris buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.5) containing 0.02 mM Tween-20 for 1 hour. EphA2 Fc-coated wells were then rinsed with Tris buffer, 0.01% Tween 20, and incubated with different peptide concentrations and 0.6 nM ephrin-A5 AP (0.94 OD min-1 mL-1) in a total volume of 50 μL for 2 hour. After washing away the unbound peptide and ephrin, bound ephrin-A5 AP was detected using 1 mM pNPP substrate. Data were fitted using non linear regression and IC50 values were calculated using the program Prism (GraphPad Software Inc.).
Molecular Docking
Computer docking of the YSA peptide to the EphA2 ligand-binding domain (PDB 3HEI) was performed using the ZDOCK module of DiscoveryStudio 2.1 (Accelrys, Inc., San Diego). Residues defined as important for the interaction of the peptide with the ephrin-binding channel of EphA2 in order to guide the docking were Y1, Y4, P5, and D6 in YSA and R103 in EphA2 (35). Docked poses with high ZDOCK score, high density, and low cluster number were typed with CHARMm Polar H forcefield and refined by RDOCK. The docking pose with the lowest RDOCK energy was chosen for detailed analysis and display.
Determination of Peptide Stability
For determination of stability in PC3 cell culture medium, 1 mM solution of the peptides in PC3 conditioned medium was incubated for 5 days in a 37°C incubator. The peptides were then used in the ELISA assay described above for determination of IC50 values, at concentrations calculated based on the original concentration before incubation.
Measurements of EphA2 Activation and Downstream Signaling
PC3 prostate cancer cells were grown in RPMI 1640 medium (Mediatech, Inc. Herndon, VA) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin and 1% sodium pyruvate, and then serum-starved overnight in medium without FBS. For measurements of EphA2 tyrosine phosphorylation, the cells were then treated for 20 min with 0.1 μg/mL human Fc (as a negative control), 0.1 μg/mL ephrin-A1 Fc (as a positive control), or 50 μM peptides. The cells were then rinsed once with ice-cold PBS, lysed in modified RIPA lysis buffer (150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 20 mM Tris, pH 8.0) containing protease inhibitors and 1 mM sodium orthovanadate and centrifuged at 16,000 g for 10 min. For immunoprecipitations, 25 μL GammaBind G sepharose beads (GE Health Care Life Sciences, Piscataway, NJ) were rinsed twice with modified RIPA buffer and incubated for 3 hours at 4°C with 3 μg anti-EphA2 antibody (Millipore-Upstate, Inc. Temecula, CA) and the cell lysates. The immunoprecipitates were resolved by gel electrophoresis on 4-20 % Tris-glycine gels (Invitrogen, Carlsbad, CA) and transferred to Immobilon-P transfer membranes (Millipore Corp., Bedford, MA), which were subsequently probed with horseradish peroxidase (HRP)-conjugated anti-phosphotyrosine antibody (PY20; BD Transduction Laboratories, Inc), and reprobed with an anti-EphA2 antibody (Zymed/Invitrogen, Carlsbad, CA).
To measure the effects of the peptides on Akt and Erk1/Erk2 phosphorylation, PC3 cells were serum-starved overnight, pre-treated with 50 μM peptides for 10 min, and then treated with 10 % FBS in the continued presence of the peptides for 10 min. Controls in the absence of the peptides included cells stimulated with 1 μg/mL ephrin-A1 Fc along with FBS for 10 min and cells not stimulated with FBS. Cells were then lysed in RIPA lysis buffer as described above, and proteins were resolved by gel elctrophoresis and probed by immunoblotting as described above using antibodies to phosphoAkt (Thr308), total Akt, phospho-p44/42 MAPK (Erk1/2) and total Erk (Cell Signaling Technology, Inc. Danvers, MA).
Cell Retraction Assay
PC3 cells were grown for 48 hours on glass coverslips, and serum starved for 4 hours. The cells were then treated with 100 μM YSA-12mer or SWL-12mer for 30 min, 1 μg/mL ephrin-A1 Fc for 20 min or left untreated as a control. The coverslips were then washed with cold PBS, fixed in 4% formaldehyde and 4% sucrose for 10 min, and permeabilized with 0.5 % Triton-X100 for 3 min. The coverslips were then washed with 1× PBS, blocked in 2 % normal goat serum (NGS) and 2% BSA in PBS for 1 hour at room temperature, and incubated with rhodamine/ phalloidin (1:300 in PBS containing 2% non-immune goat serum) for 1 hour at room temperature. After washing with PBS, the coverslips were mounted on microscope slides with Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA). Images were collected using an Inverted TE300 Nikon wide-field fluorescence microscope and processed using Adobe Photoshop.
Results and Discussion
Two Tyrosines in the YSA Peptide Are Critical for EphA2 Binding
The YSA and SWL peptides share a (Y/W)XAYPXXV motif (where X represents any amino acid; Figure 1A). Because tyrosine and tryptophan are known to be critical residues for mediating molecular contacts in protein-protein interfaces (36), we examined the importance of the two tyrosine residues in the YSA peptide by replacing them with alanine. Replacement of the first tyrosine (Y1) resulted in a marked decrease in binding of the EphA2 ligand-binding domain fused to human Fc (EphA2 Fc) to the immobilized peptide in ELISA assays (Figure 1B). Binding of EphA2 Fc to the YSA peptide was no longer detectable when the second tyrosine (Y4) was changed to alanine (Figure 1B). These results illustrate the critical importance of the two tyrosine residues at positions 1 and 4 of the YSA peptide.
Only 5 of the 12 Residues in the YSA Peptide Are Critical for EphA2 Binding
Due to the lack of information available about the pharmacophoric elements involved in the binding of the YSA peptide to the EphA2 receptor, we utilized a systematic alanine scanning approach to identify residues important for potency. Eleven YSA peptide derivatives were synthesized with alanine at each position (Figure 2A). The YSA peptide was denoted as Ala-3 because it already has an alanine at that position. To determine the inhibitory concentrations of the peptides, EphA2 Fc immobilized on protein A-coated plates was incubated with ephrin-A5 alkaline phosphatase (AP) ligand in the presence of varying peptide concentrations, and the amount of ephrin-A5 AP bound to EphA2 was determined. The results confirm the importance of Y1 and Y4, since the Ala-1 and Ala-4 peptides lost the ability to inhibit ephrin-A5 AP binding to EphA2. The Ala-5 peptide also did not detectably inhibit EphA2-ephrin-A5 binding, indicating that P5 is also critical for high binding affinity of the YSA peptide to EphA2 (Figure 2). This suggests that the proline, which is also present in the SWL peptide (Figure 1A), may help bend the peptide to fit in the EphA2 ephrin-binding channel. Aspartic acid at position 6 (D6) was also found to be important for binding, since the Ala-6 peptide had barely measurable inhibitory activity. The Ala-8 and Ala-9 peptides had a 2-3 fold decreased potency, demonstrating a minor contribution of V8 (which is conserved in SWL) and P9 in receptor binding. The alanine scanning approach did not provide information on the importance of alanine at position 3 (A3), which is also present in the SWL peptide (Figure 1A). However, its replacement in the YSA peptide with a serine dramatically decreased potency (YSS-12mer in Table 1), suggesting a critical role also for A3. Changing each of the remaining 5 residues of YSA to alanine did not substantially affect the inhibitory potency of the peptide, suggesting that these residues may be modified (for example to further increase solubility and/or stability). The identification of amino acids that are not critical for receptor binding will also greatly simplify preparation of the peptide if a modified YSA peptide containing alanines at several positions simultaneously retains high binding affinity. The 12 peptides used for alanine scanning were also examined for their ability to inhibit ephrin-A5 AP binding to the immobilized EphA3 through EphA7 receptors in ELISA assays. None of the peptides showed detectable inhibitory activity for these other EphA receptors at a concentration of 100 μM (data not shown), suggesting that the single alanine replacements do not generate less selective peptides able to efficiently target other EphA receptors.
Figure 2.
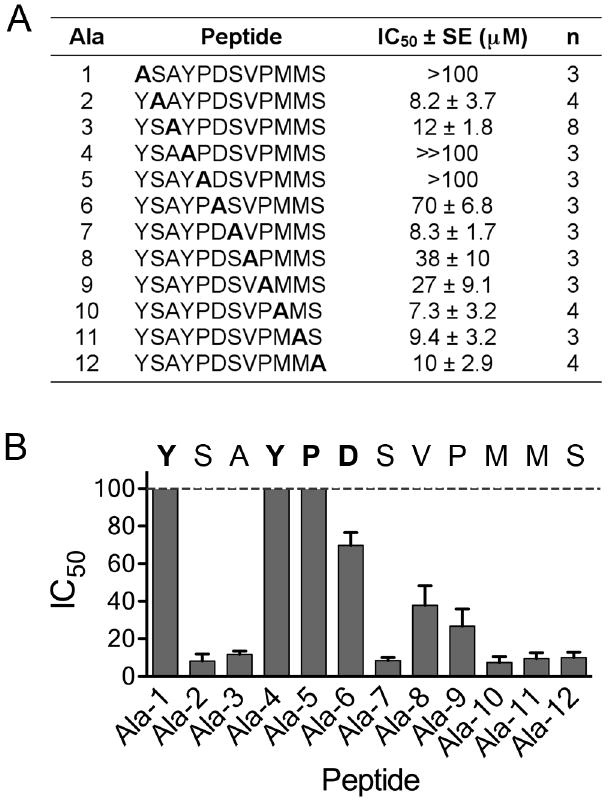
Alanine scan for the YSA peptide. (A) IC50 values for the indicated modified forms of the YSA peptide were calculated from curves of inhibition of ephrin-A5 AP binding to immobilized EphA2 Fc. The table shows average IC50 values, calculated from the indicated number of experiments (n). Ala-1 through Ala-12 are the peptides, where alanine replaces the indicated residue. Ala-3 is the original YSA peptide. For peptides Ala-1 and Ala-5 the IC50 values were too high to measure accurately (>100 μM); for the Ala-4 peptide no inhibition was detectable at 100 μM, which is the highest peptide concentration tested (≫100 μM). (B) The histogram shows the IC50 values ± SE. The sequence of the YSA peptide is shown, with the residues identified as critical for binding to EphA2 in bold.
Table 1. SAR for EphA2-targeting peptides.
| Name | Peptidea | IC50 ± SE (μM) |
nb |
|---|---|---|---|
| YSA-12mer | 13 ± 1.0 | 15 | |
| YSS-12mer | >100 | 3 | |
| YSAF-12mer | 47 ± 4.7 | 3 | |
| YSA-9mer | 17 ± 1.2 | 3 | |
| YSA-C-10mer | 5.2 ± 0.9 | 5 | |
| dimerized YSA-C-10mer | 1.8 ± 0.6 | 4 | |
| SWLA-13mer | 6.5 ± 1.2 | 7 | |
| SWLA-10mer | 39 ± 2.3 | 4 | |
| SWLA-C-11mer | 3.7 ± 0.4 | 10 | |
| SWL-12mer | 1.5 ± 0.2 | 5 |
Residues found only in the YSA peptide are in black, residues found only in the SWL peptide are in blue, residues present in both peptides are in red, residues not present in either of the original peptides are in green;
n, number of experiments.
Molecular Docking of the YSA Peptide in the Ephrin-Binding Channel of the EphA2 Receptor
The structure of the EphA2 ligand-binding domain in complex with ephrin-A1 has been recently solved by X-ray crystallography, revealing the molecular interactions in the high affinity interface between the ephrin-A1 G-H loop and the EphA2 hydrophobic channel (35). We took advantage of the information obtained from the alanine scan to construct a model of the YSA peptide in complex with the EphA2 ligand-binding domain by using the ZDOCK and RDOCK programs (37). We defined residues Y1, Y4, P5, and D6 of the YSA peptide as important for receptor binding, based on the results of the alanine scanning experiments. We also defined R103 of EphA2 as important for peptide binding because this residue was previously shown to be critical for ephrin-A1 ligand binding (35).
The lowest energy docking poses after refinement by RDOCK all adopted a similar orientation and conformation in the ephrin-binding channel of EphA2 (Figure 3A). In the conformation with the lowest RDOCK energy, the Y1, Y4, and D6 residues of the YSA peptide show strong interactions with EphA2 (Figure 3B). Backbone atoms of YSA amino-terminal amino acid Y1 interact with EphA2 R132 and E157 through a hydrogen bond and a salt bridge, respectively. The side-chain hydroxyl group of Y4 in the YSA peptide forms two hydrogen bonds with the backbone of EphA2 V102 and K162. Supporting the importance of the hydroxyl group, changing Y4 to phenylalanine substantially reduced the inhibitory potency of the peptide (YSAF in Table 1). However, the peptide with alanine rather than phenylalanine at position 4 did not have any detectable activity (Figure 2A), suggesting additional interactions mediated by the phenyl ring. In the crystal structure of the EphA2-ephrin-A1 complex, ephrin-A1 E119 forms a salt bridge with EphA2 R103, which is essential for the binding of ephrin-A1 to EphA2 (35). Interestingly, in the model YSA D6 also interacts with EphA2 R103 through a hydrogen bond and two salt bridges. Thus, overall the model is in good agreement with our experimental evidence. However, it does not completely explain the substantially higher binding affinity of the YSA peptide compared to the Ala-1 peptide, where the backbone interactions should be mostly preserved. This may be due to the fact that ZDOCK is a rigid docking algorithm while EphA2 may assume a slightly different conformation when bound to ephrin-A1 or the YSA peptide.
Figure 3.
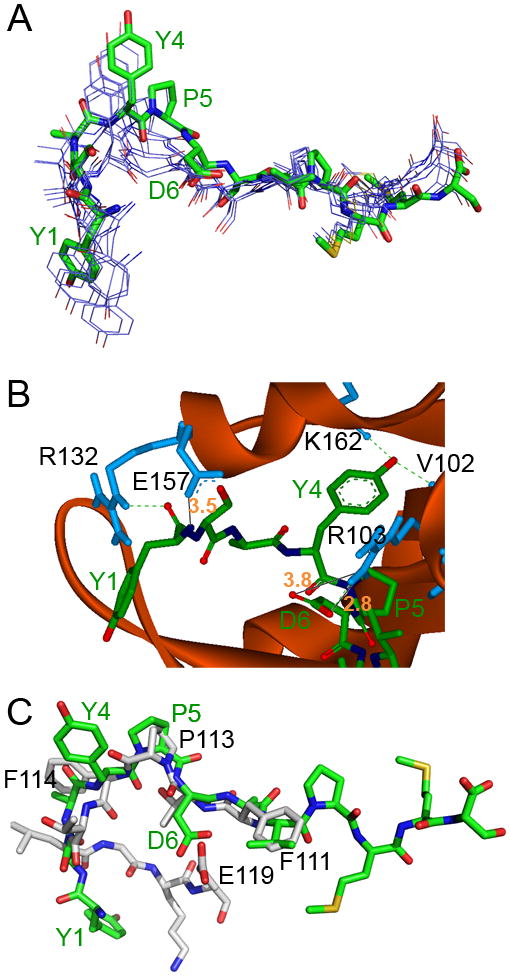
Docking of the YSA peptide in the EphA2 ephrin-binding channel. (A) Top 9 docking poses of the YSA peptide after refinement by RDOCK. All top 9 poses adopt a similar conformation in the ephrin-binding channel of EphA2. The pose with lowest RDOCK energy is presented as a stick structure (C colored in green, O colored in red, N colored in blue, S colored in yellow) and the others as line structures (C colored in purple, O colored in red, N colored in blue, S colored in yellow). The 4 amino acids found to be most critical for EphA2 binding based on the alanine scan (Y1, Y4, P5, and D6) are labeled in green. (B) View of the portion of the YSA peptide (stick representation, colored as in A) that is buried in the EphA2 channel (presented as a solid red ribbon with selected residues that are predicted to interact with the peptide shown in blue and labeled in black). Hydrogen bonds are represented by green dashed lines and salt bridges by black lines (distances in angstroms and indicated by orange numbers). (C) Comparison of the best docked conformation of the YSA peptide (stick representation, colored as in A) with the conformation of the G-H loop of ephrin-A1 in complex with EphA2 as determined by X-ray crystallography (35) (C colored in grey, N colored in blue, and O colored in red).
A structural alignment between the YSA peptide docked to EphA2 and the ephrin-A1 G-H loop in the crystal structure of the ephrinA1-EphA2 complex (Figure 3C) suggests that YSA P5 and ephrin-A1 P113 overlap and contribute to the formation of a β-turn in the peptide and the ephrin G-H loop, respectively. Thus P5, which is also present in the SWL peptide (Figure 1A), may help bend the peptide to fit in the EphA2 ephrin-binding channel. The P5-Y4 motif of YSA may therefore serve a similar function as the P113-F114 motif of the ephrin-A1 G-H loop, which is conserved in all ephrin-A ligands except ephrin-A3 (38). Interestingly, this motif also appears to be present in the KYL peptide, which targets the EphA4 receptor, and the KHL and WASH peptides, which target the EphA7 receptor (38). The side chains of YSA D6 and ephrin-A1 E119 are also close to each other in spatial position, and they may interact with the same EphA2 residues. YSA V8 overlaps with ephrin-A1 Phe111, and they may also form similar hydrophobic interactions with EphA2 residues.
Notably, the YSA peptide and the ephrin-A1 G-H loop are in opposite amino- to carboxy-terminal orientations. Interestingly, an opposite orientation was also previously observed for the FSPN motif found in both the G-H loop of ephrin-B2 and the TNYL-RAW peptide, both of which bind to the ligand-binding channel of EphB4 and have been crystallized in complex with this receptor (39, 40). Overall, our model of YSA in complex with EphA2 supports the idea that the YSA peptide binds in the ephrin-binding channel of EphA2. Furthermore, calculated docking energies may be useful to prioritize new YSA and SWL peptide derivatives to be synthesized for further optimization.
YSA-9mer and YSA-5mer Peptides Retain Some of the Binding Features of the YSA-12mer Peptide
The last three amino acids of the YSA peptide did not seem to be critical for binding to EphA2, based on their individual replacement with alanine residues. This is consistent with the docking model of the EphA2-YSA complex, where they are outside the ephrin-binding pocket (Figure 3). We therefore synthesized a YSA-9mer peptide lacking these amino acids. Besides being smaller, this peptide also has the advantage that it does not contain methionine residues, which can be easily oxidized (Table 1). The YSA-9mer peptide exibited a 1.5 to 2 fold higher IC50 value for inhibition of ephrin-A5-EphA2 binding compared to the YSA-12mer peptide in ELISA assays (Figure 4A and B, Table 1). We also synthesized a “minimal” YSA-5mer peptide comprising the 5 amino-terminal residues of YSA and containing 4 of the 5 most critical receptor binding determinants of the peptide. We found that the 5mer peptide retains the ability to bind to EphA2, albeit with much lower affinity than the YSA-12mer (Figure 4C). Interestingly, despite its much reduced size the YSA-5mer peptide still retained high selectivity for EphA2 (Figure 4D). This suggests that the (Y/W)XAYP motif, which is also conserved in the SWL peptide but not in the ephrins, is not only critical for binding but may also confer selectivity for EphA2.
Ephrin-A Fc fusion proteins have substantially increased apparent affinity for EphA receptors because each molecule has two EphA receptor binding sites (41). Dimeric peptides would be expected to similarly bind with increased avidity and thus exhibit higher potency. We therefore synthesized a peptide corresponding to the YSA-9mer with an additional carboxy-terminal cysteine (YSA-C-10mer, Table1). The monomeric peptide had a ∼2-fold higher potency than the YSA-12mer, which was not substantially affected by treatment with DTT to eliminate the possible intermolecular disulfide bond. Although unlikely under the conditions of our experiment, we cannot exclude that the unpaired cysteine in some of the peptide molecules may form a covalent bond with a cysteine in the receptor. Nevertheless, when dimerized via a disulfide bond between the carboxy-terminal cysteines and purified by HPLC, the dimeric peptide showed a further 3-fold increase in potency (Table 1). The small effect of dimerization may in large part reflect the 2-fold higher concentration of peptide moieties due to the dimeric nature of the molecules, suggesting that longer linkers may be needed to avoid steric hindrance. It will be interesting in future experiments to examine the ability of longer and non-reversible linkers to increase the avidity of the YSA-9mer peptide through dimerization.
Potency and Stability the YSA and SWL Peptides and Their Derivatives
Because a number of residues are conserved between the YSA-12mer and the SWL-12mer peptides (Figure 1A), we generated several hybrid peptides and characterized their ability to inhibit EphA2-ephrin-A5 interaction. For example, we replaced the “YS” amino-terminal sequence of YSA with the “SWL” sequence, to generate the SWLA-13mer hybrid peptide. This resulted in a ∼2-fold increase in inhibitory activity (Table 1). Similar to the YSA-12mer peptide, removal of the three most carboxy-terminal amino acids of the SWLA-13mer peptide (to generate the SWLA-10mer peptide) decreased potency, while further addition of a carboxy-terminal cysteine increased potency even when the peptide was treated with DTT to eliminate the possible intermolecular disulfide bond (Table 1). However, the SWL-12mer peptide was the most potent of the peptides tested (Table 1). This is in contrast to our previous results with YSA and SWL peptides that contained a carboxy-terminal biotinylated GSGSK linker, which had KD values of ∼200 and ∼700 nM respectively (29). It appears that the linker increased the inhibitory potency of YSA but not SWL, based on inhibition of ephrin-A5 AP binding to EphA2 Fc (compare Figure 3A in ref. (29) with Figure 4B and Table 1). Notably, in our phage display screens we isolated many more phage clones displaying the SWL peptide than the YSA peptide (29). In summary, our structure-activity relationship analysis reveals that the SWL-12mer and dimerized YSA-C-10mer peptides are the most potent EphA2-targeting peptides in the series examined so far, followed by the SWLA-C-11mer peptide. However, the unpaired cysteine in this peptide may result in some unwanted reactivity (see above).
We also examined the stability of the YSA-12mer, SWL-12mer, SWLA-13mer and SWLA-C-11mer peptides in conditioned cell culture medium from PC3 prostate cancer cells. We found that these peptides are remarkably stable, and are only slightly less effective at inhibiting ephrin-A5 binding to EphA2 even after a 5 day incubation in the conditioned medium (Figure 5). In comparison, a control peptide examined in parallel was completely inactivated within a few hours (data not shown). This apparent resistance to proteases is consistent with the recently reported ability of the YSA peptide to remain functional in culture medium and ascites fluids (30-33).
Figure 5.
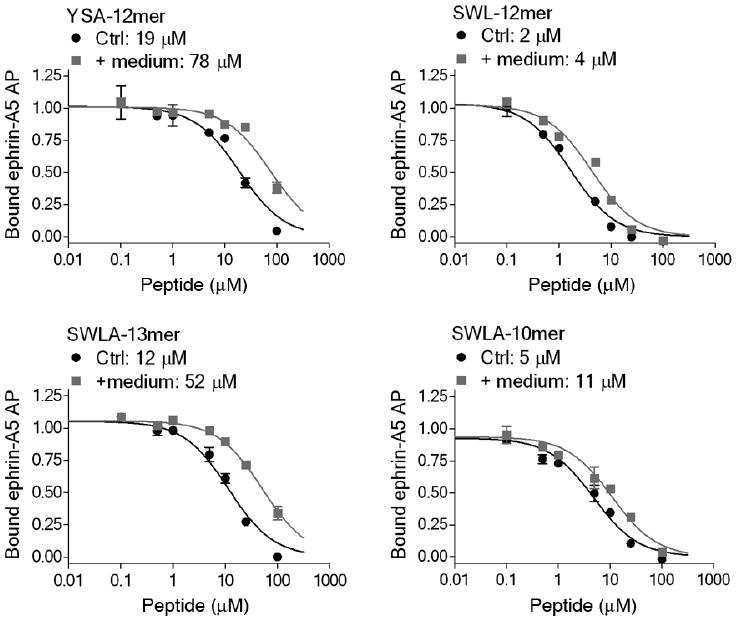
Stability of EphA2 targeting peptides. The indicated peptides were incubated in PC3 conditioned medium for 5 days at 37 °C (+ medium), or not incubated for comparison (Ctrl), and used for inhibition curves of ephrin-A5 AP binding to immobilized EphA2 Fc. The IC50 values are indicated. The increased IC50 values due to incubation of the peptides in medium were statistically significant (P<0.01 for SWL-13mer and SWLA-10mer; P<0.001 for YSA-12mer and SWL-12mer by Student's t test). However, the effects are small and thus may have little practical consequences.
The EphA2-Targeting Peptides Induce Receptor Phosphorylation and Inhibit Oncogenic Pathways in Cancer Cells
We have previously shown that the YSA peptide with a GSGSK-biotin tail can promote EphA2 tyrosine phosphorylation (which is indicative of activation) and induce similar biological responses as ephrin-A1 ligand stimulation in HUVE endothelial cells and COS cells (29, 42). We report here that the SWL-12mer and SWLA-C-11mer peptides are more effective than YSA-12mer in stimulating EphA2 tyrosine phosphorylation in PC3 prostate cancer cells, consistent with their higher potency (Figure 6A and Table 1). YSA-C-10-mer also induced EphA2 phosphorylation (data not shown). Furthermore the SWL-12mer peptide, and to some extent the YSA-12mer peptide, inhibit serum-induced phosphorylation of Erk1/Erk2 MAP kinases and Akt, which are indicative of Erk and Akt activation. This effect is similar to that of ephrin-A1 Fc (13, 43) and suggests that the peptides can inhibit two major oncogenic signaling pathways – the PI3 kinase-Akt and Ras-MAP kinase pathways – through activation of EphA2 (Figure 6B). Cell contraction, involving retraction of the cell periphery and cell rounding, is another consequence of ligand-dependent EphA2 activation (42-44). Both YSA-12mer and SWL-12mer peptides induce PC3 cell contraction (Figure 6C). This “repulsive” effect of EphA2 has been shown to involve decreased β1-integrin-dependent cell substrate adhesion and RhoA-dependent contraction of actin filaments (44). Integrin inactivation can in turn reduce cancer cell migratory and invasive ability as well as tumor growth.(13, 45) The fact that these peptides, which appear to be monomeric, can function as agonists is consistent with the fact that monomeric ephrin-A ligands have also been shown to activate EphA2 (46, 47). It will be interesting to determine whether ephrins and peptides cause EphA2 activation by disrupting homophilic interactions that involve the ephrin-binding channel. Such homophilic interactions have been recently hypothesized from the crystal structure of the EphA2 ephrin-binding domain (35). Regardless of the precise mechanism, peptides of the YSA/SWL series may have some intrinsic tumor suppressor activities, in addition to being possible tumor-targeting agents.
Figure 6.
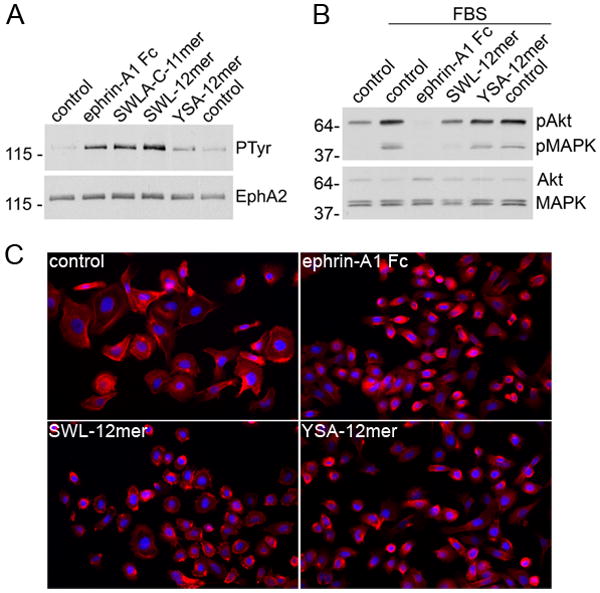
Biological effects of EphA2-targeting peptides. (A) PC3 cells were treated for 20 min with 0.1 μg/ml ephrin-A1 Fc, 50 μM of the indicated peptides, or left untreated as a control (in duplicate samples). EphA2 immunoprecipitates were probed with anti-phosphotyrosine (PTyr) antibodies and reprobed with anti-EphA2 antibodies. (B) Serum-starved PC3 cells were either left untreated (control), treated with 10% FBS (in duplicate samples), or treated with FBS together with 1μg/ml ephrin-A1 Fc or 50 μM of the indicated peptides. Cell lysates were probed with antibodies specific for Akt phosphorylated at T308 or phosphoErk1/Erk2 MAP kinases (pMAPK) and reprobed for total Akt and MAP kinases. (C) Cells were treated for 20 min with 1 μg/ml ephrin-A1 Fc, for 30 min with 100 μM SWL-12mer peptide or 100 μM YSA-12mer peptide, or left untreated as a control. The cells were then fixed in formaldehyde and labeled with phalloidin to stain actin filaments (red) and with DAPI to label nuclei (blue).
In conclusion, we have characterized a series of related peptides that selectively bind to the EphA2 receptor in order to identify features that are important for receptor binding. The peptides include the YSA and SWL peptides identified by phage display, derivatives of the YSA peptide with single amino acid substitutions, shortened forms of the peptides, a dimerized form, and hybrids of the two peptides. We found that the previously less characterized SWL peptide is a viable alternative to the YSA peptide, and in fact appears to have higher potency in its unmodified form and lacks potentially problematic methionines. The amino-terminal portion of the two peptides appears to be the most important for receptor binding and selectivity, and shorter peptides containing this region can be dimerized to improve potency. In contrast, several amino acids are dispensable for high potency and selectivity, particularly those in the carboxy-terminal part of the peptides. Optimized peptides of the YSA/SWL series could be used as starting points for further development of conjugates with drugs, toxins, or imaging agents. An advantage of these peptides is that it will be straightforward to prepare homogeneous peptide conjugates by introducing a desired reactive amino acid that can be used for conjugation, and our results show that the carboxy terminus is the region more amenable to modification without affecting potency. In contrast, antibodies contain many reactive groups, and conjugation yields many different molecular species that may have different targeting and pharmacokinetic properties. In addition, peptides can be easily synthesized and purified, and tend to be non-immunogenic. As an added benefit, peptides of the YSA/SWL series also demonstrate an intrinsic ability to activate the EphA2 receptor and inhibit several oncogenic pathways, including the Ras-MAP kinase and the PI3 kinase/Akt pathways, and integrin activity. These results are significant because peptides of the YSA/SWL series could be used in cancer therapeutic strategies to target EphA2, a receptor widely expressed not only in cancer cells but also the tumor vasculature.
Acknowledgments
The authors thank Fernando Ferrer for the synthesis of the biotinylated Ala-1, Ala-4, YSA-5mer and YSA-12mer peptides.
Abbreviations
- CD3
cluster of differentiation 3
- DAPI
4′,6-diamidino-2-phenylindole
- DTT
dithiothreitol
- ELISA
enzyme-linked immunosorbent assay
- GPI
glycosylphosphatidylinositol
- HUVE cells
human umbilical vein endothelial cells
- MAP kinase
mitogen-activated protein kinase
- PI3 kinase
phosphatidylinositol-3 kinase
Footnotes
This work was supported by NIH grant CA82713 (to EBP), a grant from MedImmune (to EBP), and Department of Defense Breast Cancer Research Program grant W81XWH-07-1-0462 (to ZH and EBP), Prostate Cancer Research Program grant W81XWH-06-1-0077 (to EBP) and Postdoctoral Fellowship DAAMD17-01-1-0168 (to MK).
References
- 1.Ireton RC, Chen J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr Cancer Drug Targets. 2005;5:149–157. doi: 10.2174/1568009053765780. [DOI] [PubMed] [Google Scholar]
- 2.Landen CN, Kinch MS, Sood AK. EphA2 as a target for ovarian cancer therapy. Expert opinion on therapeutic targets. 2005;9:1179–1187. doi: 10.1517/14728222.9.6.1179. [DOI] [PubMed] [Google Scholar]
- 3.Wykosky J, Debinski W. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res. 2008;6:1795–1806. doi: 10.1158/1541-7786.MCR-08-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19:6043–6052. doi: 10.1038/sj.onc.1204004. [DOI] [PubMed] [Google Scholar]
- 5.Brantley-Sieders DM, Chen J. Eph receptor tyrosine kinases in angiogenesis: from development to disease. Angiogenesis. 2004;7:17–28. doi: 10.1023/B:AGEN.0000037340.33788.87. [DOI] [PubMed] [Google Scholar]
- 6.Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, Lee F, Shaw P, Clark E. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–2238. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 7.Wang XD, Reeves K, Luo FR, Xu LA, Lee F, Clark E, Huang F. Identification of candidate predictive and surrogate molecular markers for dasatinib in prostate cancer: rationale for patient selection and efficacy monitoring. Genome Biol. 2007;8:R255. doi: 10.1186/gb-2007-8-11-r255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 Overexpression Causes Tumorigenesis of Mammary Epithelial Cells. Cancer Res. 2001;61:2301–2306. [PubMed] [Google Scholar]
- 9.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene. 2004;23:1448–1456. doi: 10.1038/sj.onc.1207247. [DOI] [PubMed] [Google Scholar]
- 10.Landen CN, Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, Sood AK. Therapeutic EphA2 Gene Targeting In vivo Using Neutral Liposomal Small Interfering RNA Delivery. Cancer Res. 2005;65:6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 11.Miao H, Wei BR, Peehl DM, Li Q, Alexandrou T, Schelling JR, Rhim JS, Sedor JR, Burnett E, Wang BC. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nature Cell Biology. 2001;3:527–530. doi: 10.1038/35074604. [DOI] [PubMed] [Google Scholar]
- 12.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D, Sloan AE, Cohen ML, Wang B. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell. 2009;16:9–20. doi: 10.1016/j.ccr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 15.Menges CW, McCance DJ. Constitutive activation of the Raf-MAPK pathway causes negative feedback inhibition of Ras-PI3K-AKT and cellular arrest through the EphA2 receptor. Oncogene. 2008;27:2934–2940. doi: 10.1038/sj.onc.1210957. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Zhuang G, Frieden L, Debinski W. Eph receptors and Ephrins in cancer: common themes and controversies. Cancer Res. 2008;68:10031–10033. doi: 10.1158/0008-5472.CAN-08-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coffman KT, Hu M, Carles-Kinch K, Tice D, Donacki N, Munyon K, Kifle G, Woods R, Langermann S, Kiener PA, Kinch MS. Differential EphA2 Epitope Display on Normal versus Malignant Cells. Cancer Res. 2003;63:7907–7912. [PubMed] [Google Scholar]
- 18.Noblitt LW, Bangari DS, Shukla S, Knapp DW, Mohammed S, Kinch MS, Mittal SK. Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1. Cancer Gene Ther. 2004;11:757–766. doi: 10.1038/sj.cgt.7700761. [DOI] [PubMed] [Google Scholar]
- 19.Landen CN, Jr, Lu C, Han LY, Coffman KT, Bruckheimer E, Halder J, Mangala LS, Merritt WM, Lin YG, Gao C, Schmandt R, Kamat AA, Li Y, Thaker P, Gershenson DM, Parikh NU, Gallick GE, Kinch MS, Sood AK. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. Journal of the National Cancer Institute. 2006;98:1558–1570. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]
- 20.Wesa AK, Herrem CJ, Mandic M, Taylor JL, Vasquez C, Kawabe M, Tatsumi T, Leibowitz MS, Finke JH, Bukowski RM, Bruckheimer E, Kinch MS, Storkus WJ. Enhancement in specific CD8+ T cell recognition of EphA2+ tumors in vitro and in vivo after treatment with ligand agonists. J Immunol. 2008;181:7721–7727. doi: 10.4049/jimmunol.181.11.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruckheimer EM, Fazenbaker CA, Gallagher S, Mulgrew K, Fuhrmann S, Coffman KT, Walsh W, Ready S, Cook K, Damschroder M, Kinch M, Kiener PA, Woods R, Gao C, Dall'Acqua W, Wu H, Coats S. Antibody-dependent cell-mediated cytotoxicity effector-enhanced EphA2 agonist monoclonal antibody demonstrates potent activity against human tumors. Neoplasia. 2009;11:509–517. doi: 10.1593/neo.81578. 502 p following 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signaling and beyond. Nat Rev Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammond SA, Lutterbuese R, Roff S, Lutterbuese P, Schlereth B, Bruckheimer E, Kinch MS, Coats S, Baeuerle PA, Kufer P, Kiener PA. Selective targeting and potent control of tumor growth using an EphA2/CD3-Bispecific single-chain antibody construct. Cancer Res. 2007;67:3927–3935. doi: 10.1158/0008-5472.CAN-06-2760. [DOI] [PubMed] [Google Scholar]
- 24.Gobin AM, Moon JJ, West JL. EphrinA I-targeted nanoshells for photothermal ablation of prostate cancer cells. Int J Nanomedicine. 2008;3:351–358. [PMC free article] [PubMed] [Google Scholar]
- 25.Wykosky J, Gibo DM, Debinski W. A novel, potent, and specific ephrinA1-based cytotoxin against EphA2 receptor–expressing tumor cells. Molecular Cancer Therapeutics. 2007;6:3208–3218. doi: 10.1158/1535-7163.MCT-07-0200. [DOI] [PubMed] [Google Scholar]
- 26.Jackson D, Gooya J, Mao S, Kinneer K, Xu L, Camara M, Fazenbaker C, Fleming R, Swamynathan S, Meyer D, Senter PD, Gao C, Wu H, Kinch M, Coats S, Kiener PA, Tice DA. A Human Antibody-Drug Conjugate Targeting EphA2 Inhibits Tumor Growth In vivo. Cancer Res. 2008;68:9367–9374. doi: 10.1158/0008-5472.CAN-08-1933. [DOI] [PubMed] [Google Scholar]
- 27.Lee JW, Han HD, Shahzad MM, Kim SW, Mangala LS, Nick AM, Lu C, Langley RR, Schmandt R, Kim HS, Mao S, Gooya J, Fazenbaker C, Jackson D, Tice DA, Landen CN, Coleman RL, Sood AK. EphA2 immunoconjugate as molecularly targeted chemotherapy for ovarian carcinoma. Journal of the National Cancer Institute. 2009;101:1193–1205. doi: 10.1093/jnci/djp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai W, Ebrahimnejad A, Chen K, Cao Q, Li ZB, Tice DA, Chen X. Quantitative radioimmunoPET imaging of EphA2 in tumor-bearing mice. Eur J Nucl Med Mol Imaging. 2007;34:2024–2036. doi: 10.1007/s00259-007-0503-5. [DOI] [PubMed] [Google Scholar]
- 29.Koolpe M, Dail M, Pasquale EB. An Ephrin Mimetic Peptide That Selectively Targets the EphA2 Receptor. J Biol Chem. 2002;277:46974–46979. doi: 10.1074/jbc.M208495200. [DOI] [PubMed] [Google Scholar]
- 30.Scarberry KE, Dickerson EB, McDonald JF, Zhang ZJ. Magnetic nanoparticle-peptide conjugates for in vitro and in vivo targeting and extraction of cancer cells. J Am Chem Soc. 2008;130:10258–10262. doi: 10.1021/ja801969b. [DOI] [PubMed] [Google Scholar]
- 31.Scarberry KE, Dickerson EB, Zhang ZJ, Benigno BB, McDonald JF. Selective removal of ovarian cancer cells from human ascites fluid using magnetic nanoparticles. Nanomedicine. 2010;6:399–408. doi: 10.1016/j.nano.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Dickerson EB, Blackburn WH, Smith MH, Kapa LB, Lyon LA, McDonald JF. Chemosensitization of cancer cells by siRNA using targeted nanogel delivery. BMC Cancer. 2010;10:10. doi: 10.1186/1471-2407-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackburn WH, Dickerson EB, Smith MH, McDonald JF, Lyon LA. Peptide-Functionalized Nanogels for Targeted siRNA Delivery. Bioconjugate chemistry. 2009;20:960–968. doi: 10.1021/bc800547c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Geer MA, Bakker CT, Koizumi N, Mizuguchi H, Wesseling JG, Oude Elferink RP, Bosma PJ. Ephrin A2 receptor targeting does not increase adenoviral pancreatic cancer transduction in vivo. World J Gastroenterol. 2009;15:2754–2762. doi: 10.3748/wjg.15.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Himanen JP, Goldgur Y, Miao H, Myshkin E, Guo H, Buck M, Nguyen M, Rajashankar KR, Wang B, Nikolov DB. Ligand recognition by A-class Eph receptors: crystal structures of the EphA2 ligand-binding domain and the EphA2/ephrin-A1 complex. EMBO Rep. 2009;10:722–728. doi: 10.1038/embor.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koide S, Sidhu SS. The importance of being tyrosine: lessons in molecular recognition from minimalist synthetic binding proteins. ACS Chem Biol. 2009;4:325–334. doi: 10.1021/cb800314v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Chen R, Weng Z. RDOCK: refinement of rigid-body protein docking predictions. Proteins. 2003;53:693–707. doi: 10.1002/prot.10460. [DOI] [PubMed] [Google Scholar]
- 38.Murai KK, Nguyen LN, Koolpe M, McLennan R, Krull CE, Pasquale EB. Targeting the EphA4 receptor in the nervous system with biologically active peptides. Mol Cell Neurosci. 2003;24:1000–1011. doi: 10.1016/j.mcn.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Chrencik JE, Brooun A, Recht MI, Kraus ML, Koolpe M, Kolatkar AR, Bruce RH, Martiny-Baron G, Widmer H, Pasquale EB, Kuhn P. Structure and thermodynamic characterization of the EphB4/Ephrin-B2 antagonist peptide complex reveals the determinants for receptor specificity. Structure. 2006;14:321–330. doi: 10.1016/j.str.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Koolpe M, Burgess R, Dail M, Pasquale EB. EphB receptor-binding peptides identified by phage display enable design of an antagonist with ephrin-like affinity. J Biol Chem. 2005;280:17301–17311. doi: 10.1074/jbc.M500363200. [DOI] [PubMed] [Google Scholar]
- 41.Lackmann M, Mann RJ, Kravets L, Smith FM, Bucci TA, Maxwell KF, Howlett GJ, Olsson JE, Vanden Bos T, Cerretti DP, Boyd AW. Ligand for EPH-related kinase (LERK) 7 is the preferred high affinity ligand for the HEK receptor. J Biol Chem. 1997;272:16521–16530. doi: 10.1074/jbc.272.26.16521. [DOI] [PubMed] [Google Scholar]
- 42.Dail M, Richter M, Godement P, Pasquale EB. Eph receptors inactivate R-Ras through different mechanisms to achieve cell repulsion. J Cell Sci. 2006;119:1244–1254. doi: 10.1242/jcs.02842. [DOI] [PubMed] [Google Scholar]
- 43.Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- 44.Huang X, Wu D, Jin H, Stupack D, Wang JY. Induction of cell retraction by the combined actions of Abl-CrkII and Rho-ROCK1 signaling. J Cell Biol. 2008;183:711–723. doi: 10.1083/jcb.200801192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, Muller WJ. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 46.Bartley TD, Hunt RW, Welcher AA, Boyle WJ, Parker VP, Lindberg RA, Lu HS, Colombero AM, Elliott RL, Guthrie BA. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature. 1994;368:558–560. doi: 10.1038/368558a0. [DOI] [PubMed] [Google Scholar]
- 47.Wykosky J, Palma E, Gibo DM, Ringler S, Turner CP, Debinski W. Soluble monomeric EphrinA1 is released from tumor cells and is a functional ligand for the EphA2 receptor. Oncogene. 2008;27:7260–7273. doi: 10.1038/onc.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]


