Abstract
Stem cells reside in specialized microenvironments created by supporting stromal cells that orchestrate self-renewal and lineage-specific differentiation. However, the precise identity of the cellular and molecular pathways that support self-renewal of stem cells is not known. For example, long-term culture of prototypical stem cells, such as adult spermatogonial stem and progenitor cells (SPCs), in vitro has been impeded by the lack of an optimal stromal cell line that initiates and sustains proliferation of these cells. Indeed, current methods, including the use of mouse embryonic fibroblasts (MEFs), have not been efficient and have generally led to inconsistent results. Here, we report the establishment of a novel CD34-positive cell line, referred to as JK1, derived from mouse testicular stromal cells that not only facilitated long-term SPC culture but also allowed faithful generation of SPCs and multipotent stem cells. SPCs generated on JK1 maintained key features of germ line stem cells, including expression of PLZF, DAZL, and GCNA. Furthermore, these feeders also promoted the long-term cultivation of other types of primitive cells including multi-potent adult spermatogonial-derived stem cells, pluripotent murine embryonic stem cells, and embryonic germ cells derived from primordial germ cells. Stem cells could be passaged serially and still maintained expression of characteristic markers such as OCT4 and NANOG in vitro, as well as the ability to generate all three germ layers in vivo. These results indicate that the JK1 cell line is capable of promoting long-term culture of primitive cells. As such, this cell line allows for identification of stromal-derived factors that support long-term proliferation of various types of stem cells and constitutes a convenient alternative to other types of feeder layers.
Keywords: CD34, Spermatogonia, Germ cells, Stem cells, Adult stem cells, Embryonic stem cells, Multipotent stem cells, Pluripotent stem cells, OCT4
Introduction
The stem cell niche is essential in the maintenance and differentiation of stem and progenitor cells in vivo [1]. Supporting stromal cells within this microenvironment not only provide a structural scaffold but also communicate with stem cells to support a balance between long-term survival and differentiation. Organ-specific stem cells including intestinal stem cells and hematopoietic stem cells have been found to reside within such specific locations as the base of intestinal crypts and in the osteoblastic and perivascular niches, respectively [2–5]. Stem cell maintenance in the gonads also relies on germ cell-soma interactions that, through engagement of the BMP, Notch, and Jak/STAT signaling pathways, support stem cell self-renewal and lineage-specific differentiation [6]. Spermatogonial stem cells are localized to the basement membrane of seminiferous tubules, where they are in close contact with Sertoli cells and adjacent to peritubular myoid cells [7]. Indeed, the first demonstrated derivation of spermatogonial stem cells in vitro could be achieved only after the addition of certain growth factors, including glial cell line-derived neurotrophic factor (GDNF), which is produced by Sertoli cells [8], epidermal growth factor, and basic fibroblast growth factor (bFGF, FGF-2) [7]. Furthermore, the importance of Sertoli cell-derived cytokines in the maintenance of spermatogonial stem cells in vivo was confirmed by demonstrating the eventual depletion of spermatogonial stem cells in the seminiferous tubules of mice with a targeted deletion of ERM, a Sertoli cell factor that affects the expression of key spermatogonial stem cell genes [9]. In addition to growth factors, most culture systems also include feeder layers. However, because Sertoli cells also produce factors such as inhibin, which seems to prevent stem cell self-renewal, researchers have resorted to other common feeder cell types [10]. Many groups, including Kanatsu-Shinohara et al. [11], have used mitomycin-C-inactivated murine embryonic fibroblasts (MEFs), which produce leukemia inhibitory factor (LIF), whereas the Brinster group [12, 13] used STO cells and Seandel et al. [14] generated mouse testicular stroma (MTS).
Since successful cultivation of stem cells hinges upon achieving the proper balance of signals that maintain an undifferentiated state, cytokines and feeder cells must be selected carefully. One of the most extensively studied systems to date is the cultivation of embryonic stem cells (ESCs). ESCs are normally maintained on MEFs or STO cells, which are a subline of fibroblasts from SIM mice and are thioguanine and ouabain resistant [15, 16]. These feeder cells produce LIF, which keeps ESCs undifferentiated. LIF is also sufficient to keep murine ESCs pluripotent without the use of feeders [17, 18]; however most researchers continue to use MEFs or STO, because these cells may produce other factors that enhance survival and growth of ESCs. LIF is not sufficient to keep human ESCs undifferentiated [19, 20]. Thus, human ESCs require the use of MEFs or other feeder layers, such as human fetal-derived feeder layers, in conjunction with media containing a serum replacement [21–23]. Human ESCs can also be cultured feeder-free on Matrigel, laminin, or fibronectin in MEF-conditioned medium [19]. Notwithstanding, defining feeder-free culture conditions that are not supplemented with serum replacement or feeder-conditioned medium will provide a more effective means to identify as yet unrecognized factors that support expansion of stem cells. Although many improvements have been made, such as the defined medium for human ESC culture developed by Ludwig et al. [24] or the combination of high bFGF and Noggin used by Xu et al. [25], the use of MEFs is still standard procedure for most laboratories. Unfortunately, MEFs can be passaged only approximately five times before undergoing senescence. This requires the fresh derivation of MEFs in large quantities from mice and the preparation of a frozen stock, which can be thawed, growth inactivated, and used as feeder when needed. This process not only is cumbersome but also requires a significant devotion of time and reagents, most often resulting in the generation of a heterogenous population of stromal cells that varies in its capacity to support stem cell expansion.
Our group previously reported the use of murine testicular stroma (MTS) for the derivation and cultivation of spermatogonial progenitor cells (SPCs) in an effort to more faithfully recreate the testicular milieu [14]. MTS is a heterogeneous primary cell culture that expresses CD34 and α-smooth muscle actin (SMA) [14]. Recent in vivo analysis describing a potential vasculature-associated niche for SPCs provides further support for the use of SMA+CD34+ feeder cells in vitro [26]. Although MTS is sufficient for the generation and proliferation of SPCs, similar to MEFs, the MTS can be passaged only three to seven times before use and must constantly be freshly prepared from wild-type mouse testis, requiring preparation at least 1 week in advance.
During the course of generating SPC cultures, we established an immortalized cell line that originated from a spontaneous transformation of the CD34+ enriched testicular stromal cells. Continuous passaging of this unique cell line resulted in the generation of a heterogeneous SMA+ CD34+ cell line, referred to as JK1, which is endowed with the capacity to support long-term proliferation of numerous different types of stem cells, including germ line-derived stem cells, such as SPCs and multipotent adult spermatogonial-derived stem cells (MASCs), as well as pluripotent ESCs and primordial germ cell (PGC)-derived embryonic germ cells (EGCs). The JK1 feeder line has maintained its capacity for promoting stem cell self-renewal even after serial passaging during the course of more than one year. Here, we describe the use and provide the cellular and molecular characterization of JK1 as a feeder cell line for various stem and progenitor cells.
Materials and Methods
Mice
C57/Bl6 mice were purchased (Jackson Laboratory, Bar Harbor, ME, http://www.jax.org) for the production of MTS, which gave rise to JK1 cells. SPCs were derived from GPR125-βgal (VG156) homozygous mice as described previously [14]. In these mice, the intracellular domain of the G-coupled protein receptor GPR125 is replaced by the β-galactosidase gene and is fused inframe with the extracellular domain to allow for detection of GPR125 expression in tissues. OCT4-green fluorescent protein (GFP) mice (Jackson Laboratory) previously described by Szabó et al. were used to isolate PGCs, which convert to EGCs [27]. Mice were bred, manipulated, and sacrificed under the guidelines of the Institutional Animal Care and Use Committee.
Cell Culture
JK1 feeder cells were purified and separated from SPC cultures by serially passaging four times over the span of 2 weeks in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS). JK1 cells were then expanded by trypsinizing and splitting in a ratio of one to four into flasks that had been coated with 0.2% gelatin every 2 to 4 days and culturing in DMEM with 10% FBS. Before use as feeder cells, confluent JK1 cells were treated with 10 μg/ml mitomycin-C (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com) to prevent further cell division. For establishing the proliferation curves of SPCs cultured on JK1 clones, 1,000 SPCs were plated per well of a 24-well plate containing mitomycin-C-inactivated JK1 clones. SPCs were trypsinized, stained with trypan blue, and counted with a hemocytometer every week for 3 weeks. SPCs were distinguished from JK1 cells based on cell size and morphology. Cell proliferation data were analyzed using the Student’s t test.
SPCs were generated and expanded as described in Seandel et al. [14]. In brief, tubules from mouse testis were extracted and minced on ice, washed in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) (Sigma-Aldrich), and dissociated at 37°C with agitation in a buffer containing equal parts of trypsin/EDTA, 0.1% collagenase, and DMEM supplemented with 0.5% BSA and 100 ng/ml DNase (Sigma-Aldrich). The dissociated cells were cultured on growth-inactivated JK1 cells in media consisting of StemPro (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) with modifications described by Kanatsu-Shinohara et al. [28]. SPC colonies were triturated and passaged onto fresh JK1 cells every 3 to 4 days to separate them from endogenous stromal cells [11, 29].
C57/Bl6 murine ESCs were cultivated on mitomycin-C-inactivated JK1 cells for more than 25 passages, without the addition of LIF, in knockout (KO)-DMEM (Invitrogen) containing 10% FBS and 55 μM β-mercaptoethanol (Sigma-Aldrich). The ESCs were trypsinized and split 1:10 every 2 days onto fresh JK1 cells.
EGC cultures were derived from primordial germ cells of 11.5 days postcoitus (dpc) embryos of mice expressing GFP driven by the OCT4 promoter [27]. Gonads were isolated by microdissection and digested for 10 minutes in trypsin. Dissociated cells were plated on mitomycin-C-inactivated JK1 cells or mitomycin-C-inactivated CF1 MEFs (Chemicon, Temecula, CA, http://www.chemicon.com) in KO-DMEM supplemented with 20% FBS, forskolin (10 μM), bFGF (10 ng/ml), and LIF (103 U/ml). Resulting GFP-positive colonies were manually dissected using a Pasteur pipette, dissociated with trypsin, and replated onto fresh JK1 feeders. These cells were subsequently expanded by trypsinizing and splitting one to five onto fresh JK1 cells every 2 days. The frequency of colony formation on JK1 cells or MEFs was analyzed using the Fisher’s exact test.
Clonal Derivation of JK1 Cells
Heterogeneous JK1 cells were stably transfected with lentivirus containing GFP driven by the Pgk promoter. After multiple rounds of passaging, these JK1 cells were trypsinized to a single-cell suspension. Limiting dilution was carried out in 96-well plates coated with 0.2% gelatin. One hundred cells per well were plated in a row of 12 wells and serially diluted one to two until fewer than one cell per well was achieved. Wells containing one GFP-positive cell were confirmed by fluorescence microscopy and expanded.
Immunohistochemistry and Immunofluorescence
Cells or cryosections were fixed for 10 minutes with 4% paraformaldehyde (Alfa Aesar, Ward Hill, MA, http://www.alfa.com). After washing, permeabilization was carried out with 0.2% Triton X-100 and 10% normal donkey serum in PBS for 30 minutes. The primary antibodies rat monoclonal anti-GCNA (courtesy of Dr. G. Enders), mouse anti-DAZL (Abcam, Cambridge, U.K., http://www.abcam.com), hamster anti-PLZF (courtesy of Drs. R. Hobbs and P.P. Pandolfi), mouse anti-α-smooth muscle actin (DakoCytomation, Glostrup, Denmark, http://www.dakocytomation.com), rat anti-mouse CD34 (Abcam), rabbit anti-NANOG (Abcam), mouse anti-OCT4 (R&D Systems Inc., Minneapolis, http://www.rndsystems.com), anti-mouse HNF3β (Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com), anti-mouse GFAP (DakoCytomation), rat anti-CD31 (RDI), mouse anti-nestin (Rat 401; Santa Cruz Biotechnology Inc.), goat anti-vascular endothelial (VE)-cadherin (R&D Systems Inc.), and mouse anti-mucin 5AC (clone 45M1; Lab Vision, Fremont, CA, http://www.labvision.com) were incubated overnight at 4°C. The next day, cells were washed with PBS before incubating for 2 hours at room temperature with directly conjugated secondary antibodies or biotinylated secondary antibodies (Jackson Laboratory). Biotinylated secondary antibodies were detected with either streptavidin-Alexa488 or streptavidin conjugated to horseradish peroxidase (HRP) (Jackson Laboratory). HRP was visualized with 3-amino-9-ethylcarbazole (AEC) (Dako-Cytomation). For coimmunostained sections, two primary antibodies were incubated simultaneously: rat-anti-mouse CD34 (eBioscience, San Diego, http://www.ebioscience.com) and either goat-anti-VE-cadherin (R&D Systems Inc.) or anti-mouse αSMA (DakoCytomation). Slides were washed before incubating with secondary antibodies biotin anti-rat and either cyanin 3 (Cy3)-anti-goat or Cy3-anti-mouse (all from Jackson Laboratory). Slides were washed again before a final incubation with streptavidin-Alexa488. Sections were washed and mounted with Topro-3 (Invitrogen).
Flow Cytometry
NIH3T3 and JK1 cells were trypsinized to a single-cell suspension and counted. Cells were fixed with 4% paraformaldehyde (Alfa Aesar) for 10 minutes at room temperature. Fixed cells were either washed with PBS or permeabilized with ice-cold methanol at a final concentration of 90% for 30 minutes on ice. Permeabilized cells were washed twice with PBS containing 5% BSA. All cells underwent a protein-blocking step with PBS containing 5% BSA for 10 minutes at room temperature before incubation with primary antibodies against CD34 (eBioscience) or α-smooth muscle actin (Jackson Laboratory) for 30 minutes at room temperature. These cells were then washed twice with PBS/5% BSA and incubated with secondary antibodies conjugated to Cy2 (Jackson Laboratory) for 30 minutes at room temperature. Cells were then washed twice in PBS/5% BSA before flow cytometric analysis (Beckman Coulter, Fullerton, CA, http://www.beckmancoulter.com).
Teratoma Formation in Nonobese Diabetic/Severe Combined Immunodeficient Mice
MASCs, ESCs, and EGCs were expanded on JK1 cells for more than 2 weeks before harvesting. One million feeder-subtracted stem cells were injected subcutaneously into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. Tumors were allowed to grow for 3 to 4 weeks. Mice were then sacrificed, and tumors were rinsed in PBS before embedding in O.C.T. freezing compound (Tissuetek; Sakura, Torrance, CA, http://www.sakuraus.com). These tissues were cryosectioned into 8-μm slices onto glass slides. Cryosections were then fixed with 4% paraformaldehyde (PFA) for 10 minutes before proceeding to immunofluorescence analysis for all markers.
Results
CD34+ α-Smooth Muscle Actin+ JK1 Cells Arose from a Spontaneous Transformation of MTS
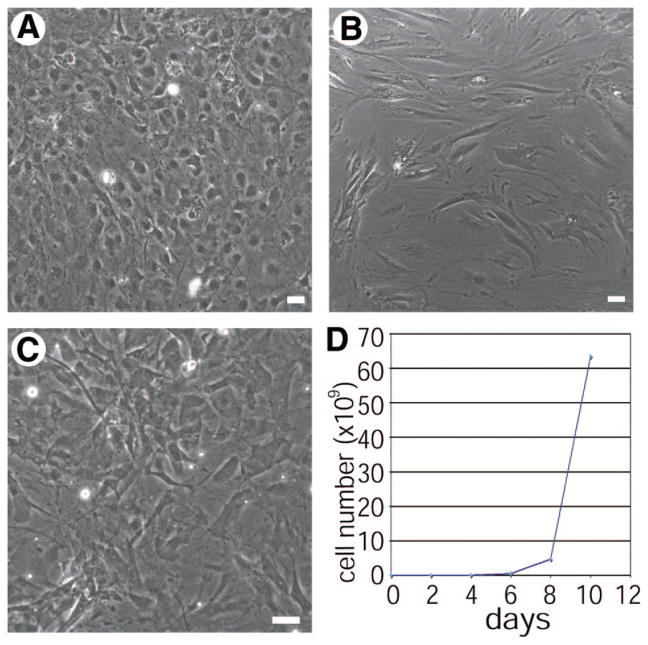
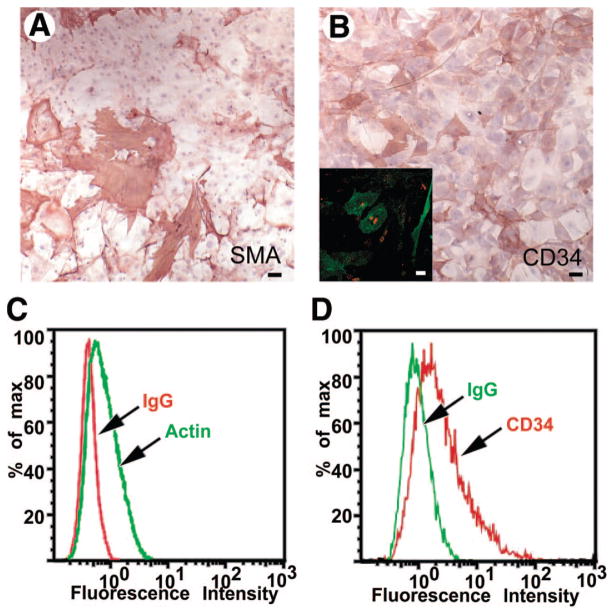
Spermatogonial stem cells are typically cultured on feeder layers such as MEFs or MTS that provide key signals regulating stem cell maintenance [30]. MTS is composed of CD34+ stromal cell-enriched primary testicular cells generated by dissociating mouse testicular tissue and culturing in high serum-containing medium [14]. These cells are usually only viable for three to seven passages and have to be freshly derived each time they are depleted. However, after multiple passages with SPCs in a low serum containing medium, a heterogeneous population of adherent cells expanded from one of these preparations. This outgrowth, subsequently referred to as JK1, was purified from the coculture with SPCs by serial passaging over the course of several weeks in medium containing high serum (Fig. 1A) because SPCs do not survive in a high serum environment [28]. To confirm that this stromal cell line originated from the wild-type feeder layer and that the cells had not differentiated from the β-galactosidase-expressing SPCs, the JK1 cells were genotyped as well as stained with X-gal. The JK1 cells neither expressed β-galactosidase nor matched the genotype analysis of the SPCs, confirming their independent origin (data not shown). Stromal cells such as MEFs (Fig. 1C) and MTS (Fig. 1B) are adherent and are usually used as a confluent monolayer to support stem cells. JK1 cells were found to be adherent but exhibited more cobblestone-like morphology than MTS and MEFs, which contain more spindle-shaped cells (Fig. 1A–1C). Most importantly, JK1 cells exhibited exponential overall growth (Fig. 1D) and, in contrast to MEFs and MTS, could be passaged more than 30 times over the course of many months. Approximately 50% of live JK1 cells stained positively for α-smooth muscle actin (Fig. 2A, 2C) and exhibited varying levels of staining for CD34 (Fig. 2B, 2D), both of which are also expressed in MTS (Fig. 2B inset). These observations suggested that JK1 cells were similar to other testicular stromal cells, which could explain their capacity to support SPCs.
Figure 1.
JK1 cells exhibit distinct morphological differences from mouse testis stroma (MTS) and mouse embryonic fibroblasts (MEFs). (A–C): Phase-contrast images of a confluent monolayer of JK1 (A), MTS (B), and MEFs (C) showed that JK1 cells are adherent cells containing one or multiple nuclei, whereas MTS and MEFs contain more spindle-shaped cells (size bars = 50 μm). (D): JK1 cells were stained with trypan blue and counted using a hemocytometer over the course of 1 week, exhibiting exponential growth.
Figure 2.
JK1 cells exhibit markers associated with mouse testis stroma. (A–B): Immunohistochemistry revealed that JK1 cells expressed (A) α-smooth muscle actin (red) and (B) CD34 (red). (Nuclear counterstain is blue.) (B inset): CD34 (green) was also found in mouse testicular stroma (nuclear counterstain is red). (C–D): This immunostaining was confirmed by flow cytometry for (C) α-smooth muscle actin (green; isotype control is red) and (D) CD34 (red; isotype control is green) in JK1 cells. All size bars = 50 μm. Abbreviations: MTS, mouse testis stroma; SMA, α-smooth muscle actin.
JK1 Cells Support Derivation and Expansion of Spermatogonial Stem and Progenitor Cells
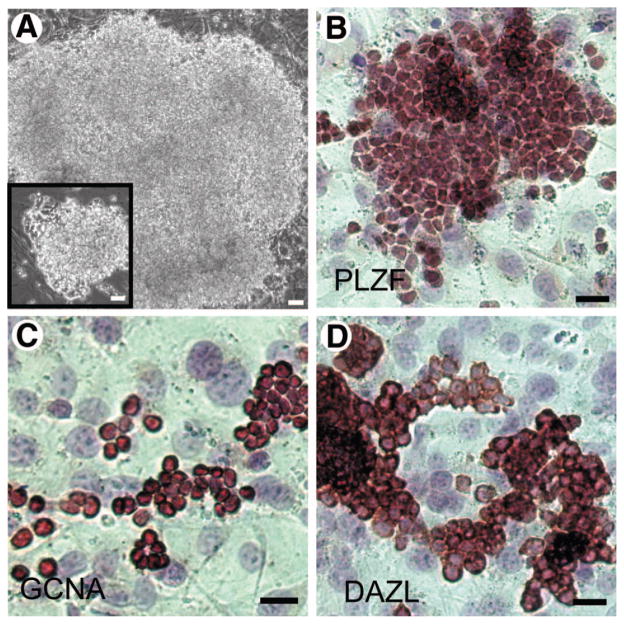
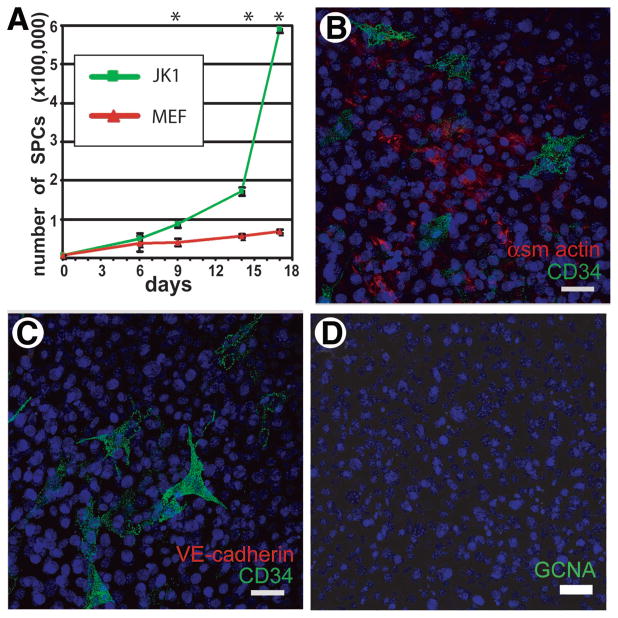
Feeder cells must be able to maintain the functional characteristics of stem cells, as well as promote their proliferation. To assess the ability of JK1 to enhance the growth of spermatogonial progenitors, equal numbers of SPCs were seeded on mitomycin-C-treated JK1 cells and MTS and allowed to expand in culture for 1 week before passaging onto fresh feeders. JK1 cells allowed for exponential expansion of SPCs, supporting the development of very large colonies (Fig. 3A) similar to the colonies seen on MTS (Fig. 3A inset). These feeders also supported the derivation of new adult SPC lines in nine of ten attempts. Like the SPC lines generated and maintained on MTS [13], the SPC lines generated and maintained on JK1 cells expressed the undifferentiated spermatogonial marker PLZF (Fig. 3B) and the germ cell markers GCNA (Fig. 3C) and DAZL (Fig. 3D) [31]. These SPCs were functional in vivo, because they could also repopulate the seminiferous tubules of busulfan-treated mice (data not shown). Notably, we also found a significantly more rapid expansion of SPCs plated on JK1 cells compared with MEFs in cell proliferation assays (Fig. 4A).
Figure 3.
JK1 cells support spermatogonial progenitor cell (SPC) proliferation without loss of germ cell markers. (A): Bright-field images (original magnification, ×100) of a large SPC colony on JK1 and (A inset) mouse testis stroma (MTS) demonstrated similarities in size and shape of SPC colonies grown on these two feeders. (B–D): Immunohistochemistry for (B) PLZF, (C) GCNA, and (D) DAZL revealed that the expression of these spermatogonial stem cell markers was maintained in SPCs cultured on JK1 cells for multiple passages. Antibody staining is red; nuclear counterstain is blue. All size bars = 50 μm.
Figure 4.
JK1 cells enhance proliferation of spermatogonial progenitor cells (SPCs) and express markers associated with peritubular myoid cells. (A): The growth rates (mean ± SD) of SPCs on JK1 cells and MEFs were compared over the course of 2.5 weeks (n = 3; * indicates p < .05 by Student’s t test). (B–C): Coimmunostaining for CD34 (green) and either α-smooth muscle actin (red, B) or VE-cadherin (red, C) indicated that the JK1 cells contained cells positive for either αSMA or CD34 but not both, whereas VE-cadherin staining was completely negative. (D): JK1 cells also did not contain GCNA (red)-positive cells, indicating that they probably did not originate from GCNA-positive germ cells or VE-cadherin-positive endothelial cells in the testis. Nuclear counterstain is blue. All size bars = 50 μm. Abbreviations: MEF, mouse embryonic fibroblast.
Because JK1 cells appeared heterogeneous, it is plausible that a particular type of cell within the population is responsible for supporting stem cells. Limiting dilution analysis has been previously demonstrated to produce clonal derivatives of heterogeneous populations. We used this technique with GFP-expressing JK1 cells to generate clones from single cells. Furthermore, analysis of three clones showed varying efficiencies relative to each other and to MEFs in the support of SPC proliferation (supplemental online Fig. 1). Although all three clones were capable of supporting SPC proliferation, clone 6 appeared to be optimal. When plated at the same densities, growth arrested, and seeded with the same number of SPCs, clone 6 exhibited the largest colonies and the greatest magnitude of proliferation (supplemental online Fig. 1). These results imply that the different cells constituting the parental JK1 cells may have distinct roles in supporting stem cell growth.
Such distinct populations in the parent JK1 cell line may reflect the origin of JK1 cells from somatic testicular cells. Indeed, assessment of SMA expression in JK1 revealed a subset of positive cells, distinct from the CD34+ population, whereas staining for endothelial cells (with VE-cadherin) and germ cells (with GCNA) was negative (Fig. 4B–4D). In parallel, immunostaining of mouse testis sections showed that there are putative peritubular cells positive for CD34 and SMA, but CD34 did not colocalize with VE-cadherin+ blood vessels (supplemental online Fig. 2A–2C). These data are consistent with the possibility that JK1 cells could have arisen from peritubular myoid cells, as opposed to endothelial or germ cells.
JK1 Cells Support In Vitro Cultivation of MASCs
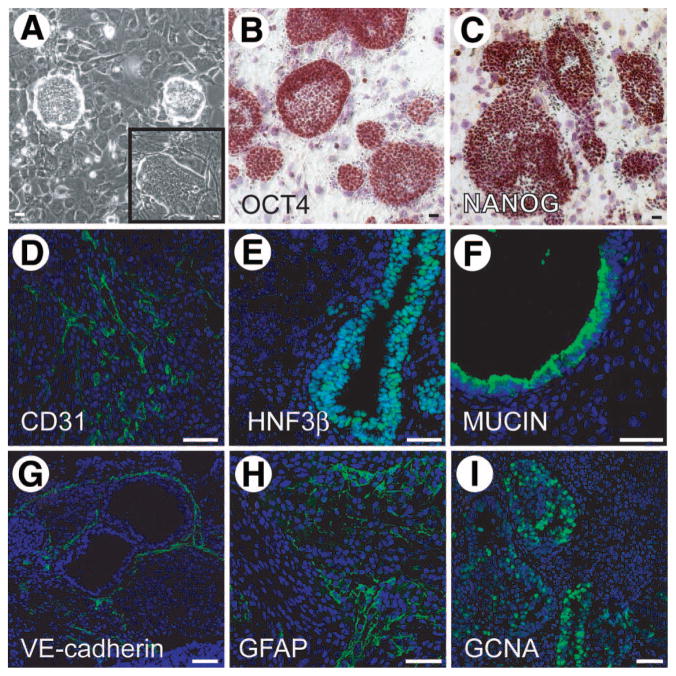
We previously reported the generation of multipotent adult spermatogonial-derived stem cells from spermatogonial progenitors [14, 30]. MASCs exhibited a number of embryonic stem cell-like characteristics, including the expression of OCT4 and NANOG, as well as the ability to form chimeras when injected into mouse blastocysts. Because of the origin of MASCs and the ability of JK1 cells to support SPCs, we tested the ability of JK1 cells to sustain MASCs. MASCs and ESCs are usually cultured with LIF, which prevents differentiation on MEFs; however, mESCs can be cultured on MEFs without the addition of LIF, because MEFs produce LIF. To ascertain whether JK1 cells are also sufficient to maintain multipotency in the absence of LIF, MASCs were passaged on JK1 cells without the addition of LIF for greater than five passages for more than 2 weeks. The resultant colonies were morphologically similar to those grown on MEFs (Fig. 5A and A inset) and expressed the multipotency markers OCT4 (Fig. 5B) and NANOG (Fig. 5C). These MASCs were capable of generating teratomas in NOD/SCID mice at 100% efficiency (6/6 attempts). The teratomas contained cells from all three germ layers, indicating that the MASCs maintained multipotency. The differentiated elements expressed CD31 (mesoderm, Fig. 5D), HNF3β (endoderm, Fig. 5E), mucin (endoderm, Fig. 5F), VE-cadherin (mesoderm, Fig. 5G), GFAP (ectoderm, Fig. 5H), and GCNA (germ line, Fig. 5I). Thus, JK1 not only supported proliferation of MASCs, but also preserved multipotency.
Figure 5.
Multipotent adult spermatogonial-derived stem cells (MASCs) cultured on JK1 cells maintain multipotency. (A): Phase-contrast images of MASC colonies on JK1 cells (A inset) and mouse embryonic fibroblasts (original magnification, ×100). (B–C): Immunohistochemistry revealed (B) OCT4 and (C) NANOG expression was preserved in MASCs passaged on JK1 cells for more than 2 weeks. (D–I): MASC-derived teratomas expressed markers for all three germ layers by immunofluorescence. (D) CD31, (E) HNF3β, (F) mucin, (G) VE-cadherin, (H) GFAP, and (I) GCNA. Antibody staining is green. Nuclear counterstain is blue. All size bars = 50 μm.
JK1 Cells Support In Vitro Cultivation of Murine Embryonic Stem Cells
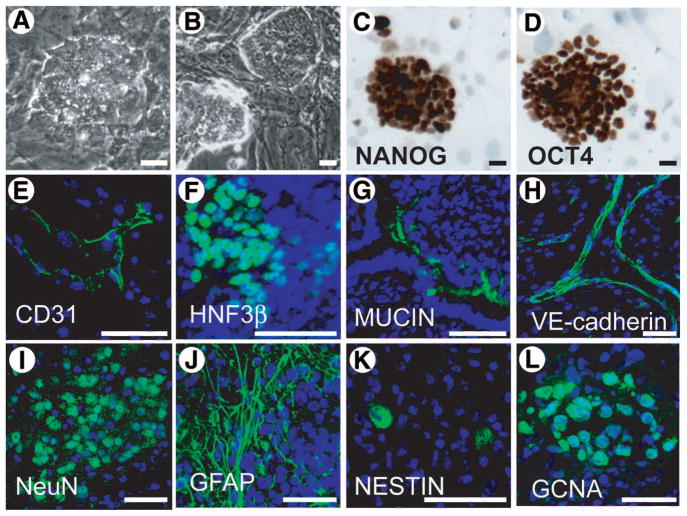
MASCs were first isolated because of their similarity to embryonic stem cells [14]. Because JK1 cells are capable of supporting MASCs, we next assessed the ability of JK1 cells to support the expansion of murine ESCs in vitro. When seeded at a similar density to MEFs, JK1 cells permitted the long-term cultivation of ESCs in the absence of LIF (Fig. 6A). The resultant ES colonies were morphologically similar to those grown on MEFs (Fig. 6B) and maintained OCT4 (Fig. 6D) and NANOG (Fig. 6C) expression after more than 20 passages on JK1 (approximately 2 months). Furthermore, these ESCs were capable of forming teratomas with 100% efficiency (4/4 attempts) when injected subcutaneously into NOD/SCID mice. The teratomas contained cells that were positive for markers of all three germ layers, including CD31, HNF3β, mucin, VE-cadherin, NeuN (neuroectoderm), GFAP, nestin, and GCNA, indicating that these ESCs maintain pluripotency (Fig. 6). These data provided evidence that JK1 cells are not only useful for germ line-derived stem cells, but also for stem cells of different origin.
Figure 6.
JK1 cells support the maintenance of murine embryonic stem cells. (AB): Phase-contrast images (original magnification, ×200) of murine ESC colonies on (A) JK1 cells and (B) mouse embryonic fibroblasts (MEFs) showed a similar, characteristic ESC morphology on both feeders: tight, round colonies of cells with a high nucleus to cytoplasm ratio. (C–D): Immunohistochemistry (brown staining) showed expression of (C) NANOG, and (D) OCT4 in ESCs was maintained after culturing on JK1 for more than 1 month. (E–L): Immunofluorescence demonstrated that mESC-derived teratomas expressed markers of all three germ layers: (E) CD31, (F) HNF3beta, (G) mucin, (H) VE-cadherin, (I) NeuN, (J) GFAP, (K) Nestin, and (L) GCNA. Antibody staining is green. Nuclear counterstain is blue. All size bars = 50 μm.
JK1 Cells Support the Derivation and Expansion of Embryonic Germ Cell Colonies
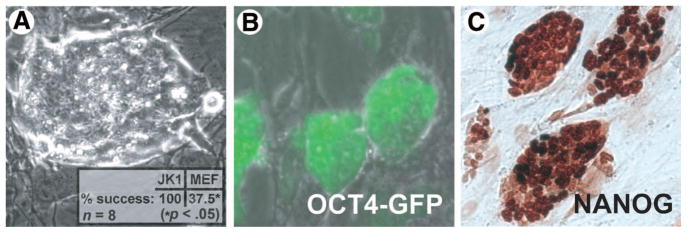
Embryonic germ cell colonies have previously been isolated from primordial germ cells cultivated in vitro using KO-DMEM containing forskolin, bFGF (FGF-2), and LIF [32–34]. In an effort to determine whether JK1 can be used as a general feeder cell line for support of other types of stem cells in addition to SPCs, MASCs, and ESCs, PGCs from the gonads of 11.5-dpc mice expressing GFP under the control of the OCT4 promoter were plated on growth-arrested JK1 and MEFs. Like ESCs, the EGCs formed compact, refractile colonies with a high nuclear to cytoplasmic ratio (Fig. 7A) and expressed stem cell markers OCT4 (Fig. 7B) and NANOG (Fig. 7C) after cultivation on JK1 for one month. Like the other pluripotent stem cells described above, EGCs derived in this manner formed teratoma, containing cells positive for markers of all three germ layers, including CD31, HNF3β, mucin, VE-cadherin, NeuN (neuroectoderm), and nestin (supplemental online Fig. 3). EGC colonies were successfully established in eight of eight attempts on JK1 cells but in only three of eight attempts on MEFs (p < .05 by Fisher’s exact test). It should be noted, however, that only the aforementioned EGC colonies produced on JK1 cells and not those on MEFs yielded long-term cell lines. The increased efficiency of JK1 cells compared with MEFs at generating EGCs suggests that this feeder line expresses certain factors that enhance PGC survival and/or subsequent conversion to EGCs.
Figure 7.
JK1 cells support the derivation and establishment of embryonic germ cell (EGC) colonies from day 11.5 (d11.5) primordial germ cells (PGCs). (A): Phase-contrast image (original magnification, ×100) of a typical EGC colony composed of compact, highly refractile cells that have a high nucleus to cytoplasm ratio. (A inset): The success rate of EGC derivation on JK1 cells was higher than that achieved on MEFs (p < .05 by Fisher’s exact test). (B): All EGC colonies were GFP positive, indicating that they expressed OCT4. (C): All EGCs showed nuclear staining for NANOG by immunohistochemistry (brown). All size bars = 50 μm. Abbreviations: GFP, green fluorescent protein; MEF, mouse embryonic fibroblast.
Discussion
In vitro culture of stem cells is supported by a complex network of known and unidentified soluble and membrane cytokines, as well as extracellular matrix provided by stromal feeder cells. Current protocols require the constant generation of fresh murine embryonic fibroblasts or mouse testicular stroma for the maintenance of stem cells, because these feeders can lose their stem cell supportive properties and undergo senescence after several passages [11, 13, 14]. Feeder cell lines such as MEFs or MTS produce factors, including LIF or GDNF, that promote expansion of stem cell colonies. In this study, we isolated a novel feeder cell line that can be passaged serially without losing its capacity to support stem cell self-renewal and survival. The JK1 cell line and its derivative subclones were the result of an outgrowth of cells from a mouse testicular stromal preparation used during the culture of spermatogonial stem cells. JK1 cells could have originated from a CD34+ peritubular myoid cell or another type of somatic cell in the testis. Subsequently, JK1 cells were found to be capable of supporting the expansion of SPCs, as well as maintaining SPC characteristics. This cell line may not only provide an efficient means to derive organ-specific stem cells but may also be used to identify the yet unrecognized factors that support stem cell self-renewal and differentiation.
Although MEFs have been shown to support spermatogonial stem cells, MASCs, and ESCs, stromal cells originating from testicular preparations would not necessarily be expected to behave in the same manner. In this study, we demonstrate the use of JK1 cells originating from testicular stroma in the expansion not only of male germ line-derived stem cells but also of embryonic germ cells as well as embryonic stem cells. Indeed, in the case of EGCs, JK1 cells were more successful at initiating new cell lines. This discovery seems to imply that similarities exist between MEFs and JK1 cells that allow them to support various stem cells. However, differences in the transcriptional regulation of certain genes, including growth factors, such as fibroblast growth factor could explain the higher efficiency of JK1 cells. Yet, whereas MEF-conditioned medium is sufficient to maintain murine ESCs in culture, JK1-conditioned medium does not prevent the rapid differentiation of ESCs. This indicates first that the crucial factor(s) produced by MEFs, such as LIF, is different than those produced by JK1 cells. In addition, unlike the MEF-derived factors that may be secreted, the factors produced by JK1 cells that are actually responsible for maintaining stem cells are either bound to the JK1 cell membrane or deposited in the extracellular matrix. Further comparative analysis of all feeder cells used is required to ascertain the precise alternative mechanisms of stem cell maintenance.
Also noteworthy is the possibility that JK1 cells could be used as a generalized stem cell feeder line. Preliminary data indicate the potential for using these cells in the maintenance of Sca1+Lin−CD45+ hematopoietic stem and progenitor cells. It is feasible that JK1 cells create a pan-stem cell permissive niche, allowing the cultivation of all these different types of stem cells. With the establishment and characterization of the JK1 cell line provided herein, the in vitro niche can be analyzed in the future for factors that promote the genetic and epigenetic status of stem cell self-renewal, while serving as a cellular platform for the expansion of stem cells that could potentially be exploited for therapeutic organ regeneration.
Supplementary Material
Acknowledgments
We are grateful to Robin Hobbs and Pier-Paolo Pandolfi for graciously providing anti-PLZF antibody and to George Enders for graciously providing anti-GCNA antibody. Marco Seandel is a New York Stem Cell Foundation Fellow. This work was also supported by Memorial Sloan Kettering Cancer Center T32 grant (M.S.) and an American Society of Clinical Oncology Young Investigator Award (M.S.).
Footnotes
Author contributions: J.K. and M.S.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; I.F.: collection and assembly of data, data analysis and interpretation, manuscript writing; D.W.: collection and assembly of data; S.R.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
See www.StemCells.com for supplemental material available online.
References
- 1.Morrison SJ, Spradling AC. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 3.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 4.Avecilla ST, Hattori K, Heissig B, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 5.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 6.Cinalli RM, Rangan P, Lehmann R. Germ cells are forever. Cell. 2008;132:559–562. doi: 10.1016/j.cell.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Aponte PM, van Bragt MP, de Rooij DG, et al. Spermatogonial stem cells: Characteristics and experimental possibilities. Apmis. 2005;113:727–742. doi: 10.1111/j.1600-0463.2005.apm_302.x. [DOI] [PubMed] [Google Scholar]
- 8.Meng X, Lindahl M, Hyvonen ME, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Ouyang W, Grigura V, et al. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–1034. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hess RA, Cooke PS, Hofmann MC, et al. Mechanistic insights into the regulation of the spermatogonial stem cell niche. Cell Cycle. 2006;5:1164–1170. doi: 10.4161/cc.5.11.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanatsu-Shinohara M, Inoue K, Lee J, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Kubota H, Avarbock MR, Brinster RL. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod. 2004;71:722–731. doi: 10.1095/biolreprod.104.029207. [DOI] [PubMed] [Google Scholar]
- 13.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seandel M, James D, Shmelkov SV, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 16.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith AG, Heath JK, Donaldson DD, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 18.Williams RL, Hilton DJ, Pease S, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 19.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 20.Reubinoff BE, Pera MF, Fong CY, et al. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 21.Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 22.Richards M, Fong CY, Chan WK, et al. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 23.Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 24.Ludwig TE, Levenstein ME, Jones JM, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 25.Xu RH, Peck RM, Li DS, et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 27.Szabó PE, Hubner K, Scholer H, et al. Allele-specific expression of imprinted genes in mouse migratory primordial germ cells. Mech Dev. 2002;115:157–160. doi: 10.1016/s0925-4773(02)00087-4. [DOI] [PubMed] [Google Scholar]
- 28.Kanatsu-Shinohara M, Miki H, Inoue K, et al. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72:985–991. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 29.Kanatsu-Shinohara M, Ogonuki N, Inoue K, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 30.Seandel M, Falciatori I, Shmelkov SV, et al. Niche players: Spermatogonial progenitors marked by GPR125. Cell Cycle. 2008;7:135–140. doi: 10.4161/cc.7.2.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enders GC, May JJ., 2nd Developmentally regulated expression of a mouse germ cell nuclear antigen examined from embryonic day 11 to adult in male and female mice. Dev Biol. 1994;163:331–340. doi: 10.1006/dbio.1994.1152. [DOI] [PubMed] [Google Scholar]
- 32.Resnick JL, Bixler LS, Cheng L, et al. Long-term proliferation of mouse primordial germ cells in culture. Nature. 1992;359:550–551. doi: 10.1038/359550a0. [DOI] [PubMed] [Google Scholar]
- 33.Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- 34.Durcova-Hills G, Tokunaga T, Kurosaka S, et al. Immunomagnetic isolation of primordial germ cells and the establishment of embryonic germ cell lines in the mouse. Cloning. 1999–2000;1:217–224. doi: 10.1089/15204559950019852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.