Abstract
Cardiac calsequestrin (CSQ) is synthesized on rough endoplasmic reticulum (ER), but concentrates within the junctional sarcoplasmic reticulum (SR) lumen where it becomes part of the Ca2+-release protein complex. To investigate CSQ trafficking through biosynthetic/secretory compartments of adult cardiomyocytes, CSQ-DsRed was overexpressed in cultured cells and examined using confocal fluorescence microscopy. By 48 h of adenovirus treatment, CSQ-DsRed fluorescence had specifically accumulated in perinuclear cisternae, where it co-localized with markers of rough ER. From rough ER, CSQ-DsRed appeared to traffic directly to junctional SR along a transverse (Z-line) pathway along which sec 23-positive (ER-exit) sites were enriched. In contrast to DsRed direct fluorescence that presumably reflected DsRed tetramer formation, both anti-DsRed and anti-CSQ immunofluorescence did not detect the perinuclear CSQ-DsRed protein, but labeled only junctional SR puncta. These putative CSQ-DsRed monomers, but not the fluorescent tetramers, were observed to traffic anterogradely over the course of a 48 h overexpression from rough ER towards the cell periphery. We propose a new model of CSQ and junctional SR protein traffic in the adult cardiomyocyte, wherein CSQ traffics from perinuclear cisternae, along contiguous ER/SR lumens in cardiomyocytes as a mobile monomer, but is retained in junctional SR as a polymer.
Keywords: calsequestrin, DsRed, junctional sarcoplasmic reticulum, rough endoplasmic secretory pathway, cardiomyocytes, trafficking, polymerization, localization, fluorescence microscopy
Introduction
Calsequestrin (CSQ1) is a major protein of the junctional sarcoplasmic reticulum (SR) lumens in heart [1–3]. Its role in cardiomyocyte function is not yet clear, and may be multifaceted [4–6]. Overexpression studies in cultured myocytes support a role as a buffer of luminal Ca2+ [7], whereas data from CSQ knockout mice suggest a role in regulation of release of Ca2+ through the ryanodine receptor (RyR) Ca2+ channel [8]. Interestingly, transgenic CSQ overexpressing [9] and knockout mouse models [8], also exhibit unexplained changes in the levels of other junctional SR proteins, such as triadin, junctin, and RyR. These larger changes in junctional SR protein levels highlight our poor understanding of the biosynthetic/secretory system of the adult cardiomyocyte, of which endoplasmic reticulum (ER), free SR, and junctional SR are all a part [10].
Polymerization of CSQ is now thought to play a critical role in its biology, and is thought to occur in response to high luminal ER/SR Ca2+ [4, 5, 11]. Although difficult to study in myocytes, studies of CSQ overexpression in nonmuscle cells have clearly shown how polymerization can also determine CSQ localization [12–14]. In mammalian nonmuscle cells, each CSQ isoform (cardiac or skeletal muscle) undergoes polymerization that leads to its concentration in a specific cellular compartment. Cardiac CSQ concentrates exclusively in the ER of all nonmuscle cells, whereas skeletal muscle CSQ concentrates in the ER-Golgi intermediate compartment (ERGIC). The distinct sites of localization for the two CSQ isoforms were the result of their specific polymerization properties [13]. In this same way, polymerization of cardiac CSQ in adult cardiomyocytes might occur specifically in junctional SR puncta, brought about by the ionic milieu of junctional SR lumens.
Another biochemical property of cardiac CSQ that is likely to play a role in its biology is phosphorylation on two or more serines in the cardiac-specific tail [15–17]. These C-terminal protein kinase CK2 consensus sequences are present in all cardiac isoforms of CSQ and are exquisitely sensitive to CK2 in vitro. This modification is believed to occur at the rough ER of the cardiomyocyte where it may affect CSQ translocation or CSQ trafficking from rough ER to junctional SR [17]. CSQ glycans show trimming by cellular mannosidases with no other modification. Because N-acetyl glucosamine (GlcNAc) would be found on CSQ glycans were CSQ to traffic to early Golgi compartments [12, 18], it becomes likely that CSQ remains inside classical mammalian ER compartments.
In a canine tachycardia-induced model of hypertrophy that leads to heart failure, CSQ glycosylation and phosphorylation are very significantly altered [19]. CSQ glycan structures in the hypertrophic heart show a two-fold increase in ER-localized CSQ and a roughly two-fold increase in levels of phosphorylated CSQ in the ER [19]. The cellular processes underlying these changes have remained uncertain due to the lack of understanding of CSQ biosynthesis and trafficking in the adult cardiomyocyte.
In the present study, we used confocal imaging techniques to investigate the direct fluorescence of CSQ-DsRed along with a complementary indirect immunofluorescence, to outline ER and SR regions in adult cardiomyocytes that parallel putative CSQ compartmentation based upon on its biochemistry.
Materials and Methods
Heart cell preparation and culture
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). Animal research was approved by the Wayne State University Animal Investigation Committee (protocol # A 06-07-07). Excised Sprague-Dawley (Rattus norvegicus) rat hearts were perfused using a Langendorff apparatus for 5 min before enzymatic dissociation using a 40 ml solution consisting of Liberase Blendzyme (Roche) types 1 and 2 (3 mg/6 mg) in Hank’s buffer containing 5 mM pyruvic acid, 1.2 mM MgSO4, 5 mM creatine, 11 mM Glucose, 5 mM taurine, 5 mM carnitine and 0.1 mM CaCl2 (20 min). Cells were titurated for 10 min at 37°C, filtered, pelleted at 300 × gmax, then washed in phosphate buffered saline (PBS) containing gradually increasing CaCl2 during 3 consecutive washes, to a concentration of 500 µM CaCl2, after which cells were resuspended in Medium 199 containing 2% bovine serum albumin, 2 mM carnitine, 5 mM creatine, 5 mM taurine, 2 mM L-glutamine, 2 mM Glutamax-1 (Invitrogen), ITS mixture (Sigma I3146), 100 units/ml penicillin G, 0.1 mg/ml streptomycin and plated on laminin-coated dishes at 37°C with 5% CO2.
CSQ plasmid and adenoviral constructs
CSQ constructs were generated from wild type canine cardiac CSQ cDNA λgt10 clone IC3A [20]. Adenoviral CSQ-DsRed (Ad.CSQ-DsRed) (from Discosoma) was created using the AdEasy XL Adenoviral Vector System (Stratagene) after canine cardiac CSQ cDNA had been cloned into pDsRed2-N1 vector as previously described [13]. Adenoviral CSQ-hemagglutinin (Ad.CSQ-HA) was previously described [12].
Adenoviral-mediated overexpression
Recombinant adenoviral treatment was carried out in cultured adult rat primary cardiomyocytes as previously described [16]. Adenoviruses were added directly to dishes 2 h post-plating at a multiplicity of infection (MOI) of 100. Virus treatments were routinely carried out for 48 h,before harvesting for biochemical analysis or fixing for microscopy as described below. Individual dishes and coverslips were incubated for shorter or longer times as indicated.
Antibodies
Monoclonal antibodies specific to cardiac CSQ and RyR were the generous gift of Dr. Larry Jones, Indiana University School of Medicine. Rabbit polyclonal antibodies raised to canine CSQ2 were purchased from Abcam (ab3516). Rabbit polyclonal anti-HA antibody was purchased from Sigma-Aldrich. Rabbit polyclonal anti-sec23 antibody was obtained from Affinity BioReagents. Rabbit polyclonal anti-DsRed antibody was purchased from Clontech. Rabbit polyclonal antibodies specific to translocon-associated protein complex (TRAP) and translocating chain-associated membrane protein (TRAM) were the generous gift of Dr. Ramanujan Hegde, National Institute of Child Health and Human Development, NIH. Alexa Fluor 488-conjugated goat anti-rabbit IgG, Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Fluor 568-conjugated goat anti-mouse IgG secondary antibodies were purchased from Invitrogen.
Immunoblotting
SDS-PAGE was carried out according to Laemmli [21] on 7.5% acrylamide gels, and transferred to nitrocellulose membranes (0.45 µm, Bio-Rad Laboratories) and stained with Amido black (Sigma). Immunoblotting was carried out as previously described [22] using HRP-coupled secondary antibodies. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG and goat anti-mouse IgG secondary antibodies were obtained from Jackson ImmunoResearch Laboratories. A SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) electrochemiluminescence (ECL) kit was used according to the manufacturer’s protocol to detect immune complexes, which were then visualized by autoradiography using Amersham Hyperfilm ECL film (GE Healthcare). Protein concentrations were determined according to Lowry [23].
Fluorescence microscopy
Cells were fixed on coverslips with 4% paraformaldehyde in PBS for 10 min and then permeabilized for 5 min in 0.2% Triton X-100. Coverslips were blocked in PBS with 0.2% Tween-20 (PBS/Tween) and 2% goat serum at room temperature for 1 hour. Cells were then incubated in PBS/Tween with primary antibody (1:200) for 90 min at ambient temperatures, followed by washing in PBS/Tween, and incubation with goat anti-rabbit IgG or goat anti-mouse IgG antibodies conjugated to Alexa Fluor dyes (1:500 dilution in PBS/Tween for 60 min). Cells were counterstained with 100 µM 4′-6-diamidino-2-phenylindole (DAPI) for 2 min, rinsed, and mounted to microscope slides with ProLong Antifade Mounting Kit (Molecular Probes). Confocal microscopy was performed at the Microscopy and Imaging Resources Laboratory at Wayne State University, School of Medicine. Imaging was performed using a C-Apochromat 63×/1.2 WKorr water objective on either a Zeiss LSM 510 laser scanning microscope or an Axioplan2 Imaging fluorescent microscope with Apotome imaging, or using a 63×/1.4 oil objective on a Leica TCS SP5 laser scanning microscope. Laser scanning images were acquired on a 1024 × 1024 pixel canvas with 8-line averaging. Apotome images were obtained with a Zeiss Axiocam MRm B/W CCD camera. Confocal images were processed and optimized offline for publication using Photoshop (Adobe Systems Inc.). Z-stack images created by the Leica TCS SP5 were compiled and optimized in ImageJ (Wayne Rasband, National Institutes of Health). The ImageJ 3D Viewer plugin was used to create three-dimensional z-stack images.
Results
CSQ-DsRed is localized to perinuclear regions of the cardiomyocyte
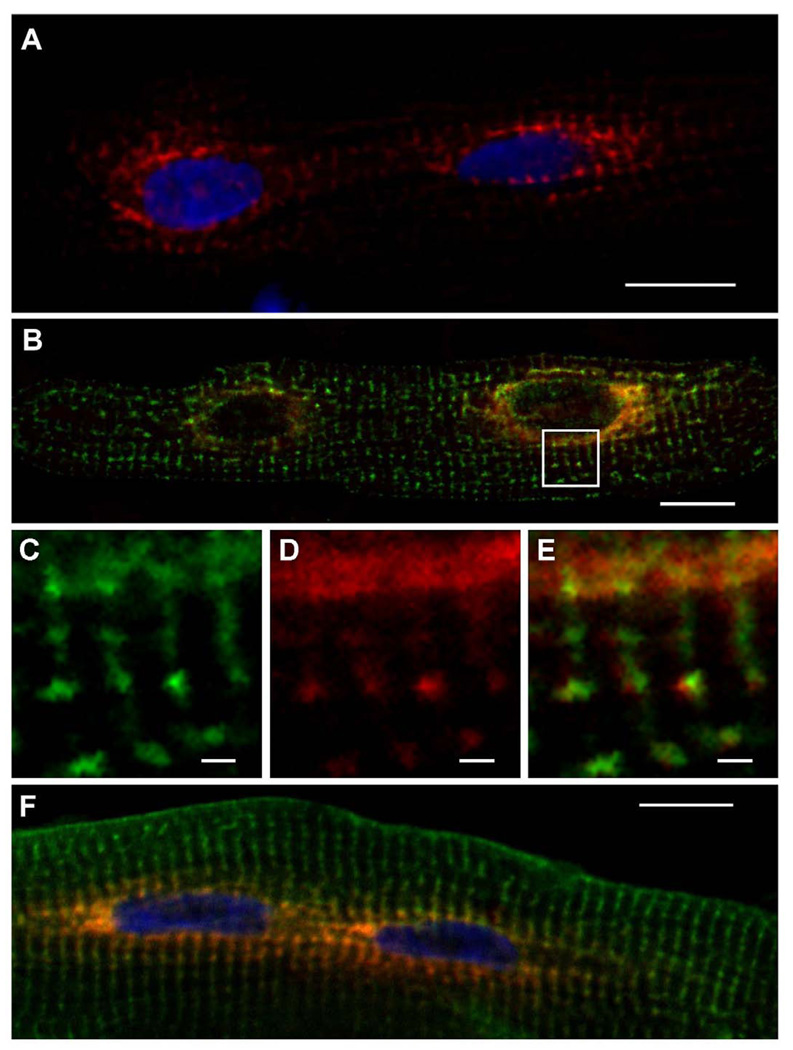
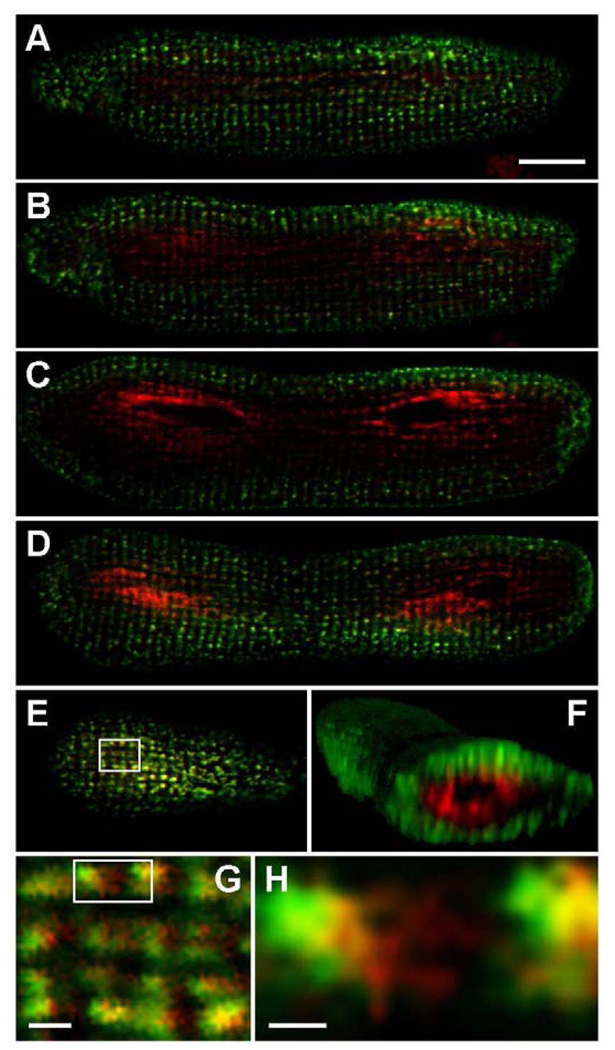
Following its overexpression in cultured adult rat cardiomyocytes, the fluorescent fusion protein CSQ-DsRed produced a unique pattern of subcellular localization in which both myonuclei were surrounded by intense red fluorescence (Fig. 1). Red fluorescence could be visualized in most cells after 32 h of virus treatment, consistent with levels of overexpression observed by immunoblotting (Fig. 2). CSQ-DsRed accumulated in cardiomyocytes to levels that were comparable to endogenous levels of CSQ after 48 h, by which time CSQ-DsRed prominently surrounded both nuclei producing a pattern that was qualitatively similar for hundreds of cells examined. We observed a very similar pattern of CSQ-DsRed localization in dishes of live (unfixed) cultured cardiomyocytes (data not shown). In addition to a very high level of red fluorescence that appeared in close apposition to the nucleus, CSQ-DsRed could be seen, albeit with much lower intensity, in perinuclear puncta. At these sites, the pattern of CSQ-DsRed fluorescence was similar (Fig. 1C,D) but not identical (Fig. 1E) to RyR-positive immunofluorescence. Peripheral CSQ-DsRed puncta became more apparent with increasing time of virus treatment in culture, or when images were brightened offline. In contrast to the peculiar CSQ-DsRed localization, the CSQ-fusion protein CSQ-HA was uniformly present in puncta throughout the cell, and never exhibited the perinuclear enrichment observed using CSQ-DsRed (Fig. 1F). This suggested that the concentration of CSQ-DsRed in perinuclear cisternae had resulted from the characteristic tetramerization of the DsRed portion of the fusion protein [24, 25].
Fig. 1. Perinuclear localization of CSQ-DsRed in cultured adult rat cardiomyocytes.
Cultured primary adult rat cardiomyocytes plated on glass coverslips were treated with Ad.CSQ-DsRed for 48 h, and DsRed fluorescence was analyzed by confocal fluorescence microscopy after fixation (A). Cells treated with Ad.CSQ-DsRed (red) were stained with anti-RyR antibodies (green) (B). Enlarged area (white box in panel B) shows RyR immunostaining (C) or CSQ-DsRed fluorescence (D). E, a merge of C and D, shows imperfect colocalization of CSQ-DsRed and RyR. Cells co-infected with Ad.CSQ-DsRed (red) and Ad.CSQ-HA (green) were stained with anti-HA antibodies (F). Bars, 20 µm (panels A,B,F) or 2 µm (panels C,D,E). Imaging, Apotome (panels A,F), Leica, (panels B–E).
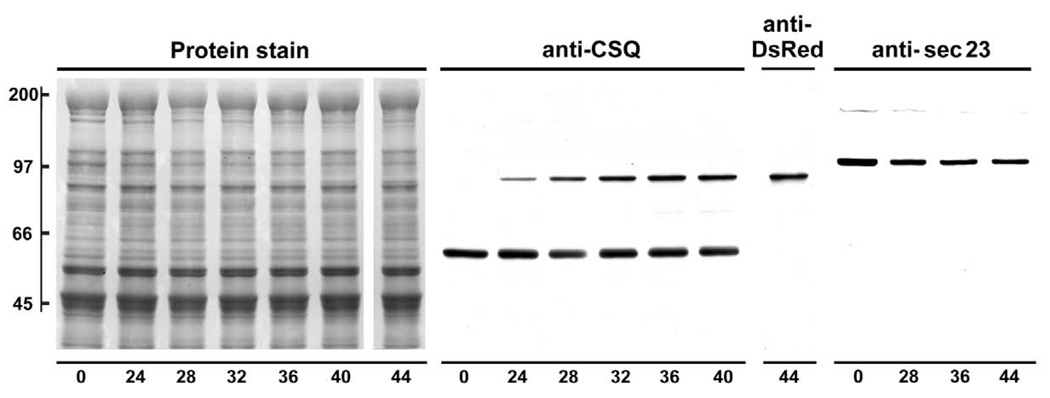
Fig. 2. Immunoreactivity and specificity of anti-CSQ, anti-DsRed, and anti-sec23 antibodies examined by immunoblotting as a function of CSQ-DsRed overexpression.
Following addition of Ad.CSQ-DsRed, cardiomyocytes were cultured for indicated times (24–44 h). Cells prior to adenovirus treatment are shown as t = 0. Cells were harvested in SDS-containing buffer and whole cell homogenates (equal volumes from each cardiomyocyte dish, roughly 80 µg) were analyzed by SDS-PAGE and immunoblotting. The nitrocelluose membrane was stained with amido black (Protein stain), then probed with either anti-CSQ (t = 0, 24–36 h), anti-DsRed (t = 44 h), or anti-sec23 antibodies. Mobilities of molecular weight standards in kDa, left margin.
CSQ-DsRed around the nucleus co-localizes with rough ER markers
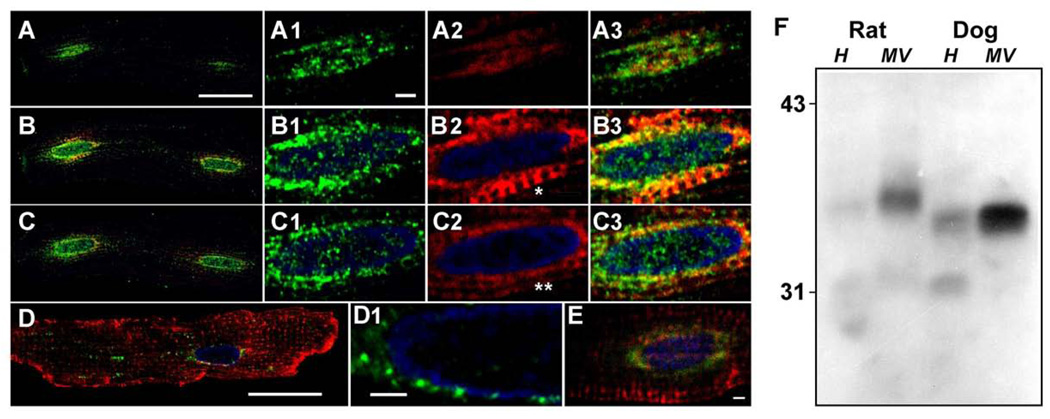
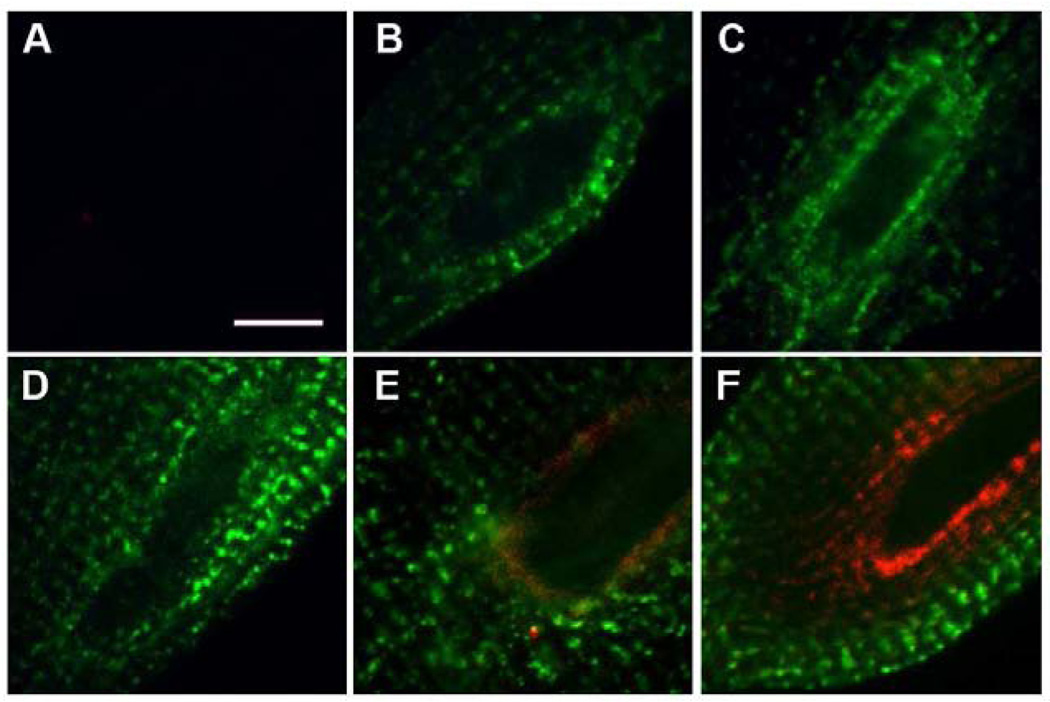
We hypothesized that the localization of CSQ-DsRed around the nucleus represented its site of biosynthesis, that is, cardiac rough ER. To determine whether markers of rough ER supported the localization of CSQ-DsRed to cardiac rough ER, we immunostained cultured cardiomyocytes with antibodies against components of the protein biosynthesis and translocation machinery. TRAP is a major protein component of the translocon in mammalian cells, where it is involved in nascent protein translocation across the rough ER membrane [26]. Indirect immunofluorescence of the subunit TRAPα in cultured cardiomyocytes expressing CSQ-DsRed co-localized with CSQ-DsRed fluorescence (Fig. 3A–C). Levels of anti-TRAPα immunofluorescence were highest in cisternae closely apposed to the nucleus, whereas CSQ-DsRed could be seen in selected optical slices to extend beyond the TRAPα–positive cisternae (panel C2, double asterisk). A single optical slice was sometimes able to capture transverse trafficking as a continuous CSQ-DsRed fluorescence between adjacent cisterna (panel B2, asterisk). The abundance of TRAPα in cardiomyocyte membranes was also evident by immunoblotting, where it appeared as a single immunoreactive protein band of roughly 34 kDa [27] enriched in microsomes prepared from either rat or canine heart tissue (Fig. 3F). Further support for the perinuclear localization of rough ER in cardiomyocytes was the localization of the translocon accessory protein TRAM [28] and the ribosomal S6 protein (Fig. 3D,E respectively) to these same areas surrounding the myonucleus.
Fig. 3. Labeling of the cardiomyocyte using rough ER markers.
Cardiomyocytes were treated with CSQ-DsRed adenovirus for 48 h (red, A–C,E). Anti-TRAPα images (green) were acquired from three optical planes starting from atop the nucleus (A), and 2.2 µm (B) or 4.4 µm (C) below (bar, 20 µm). Panels 1–3 (TRAPα, CSQ-DsRed, merge, respectively) are enlargements of the leftmost nucleus of each cell shown in A–C (bar, 2 µm). Transverse trafficking of CSQ-DsRed can be seen in the optical plane that dissects the nuclear midline (B2, asterisk) but appears as distinct cisternae in an adjacent optical slice (C2, double asterisk). Anti-TRAM immunofluorescence (green) was imaged in cardiomyocytes not treated with Ad.CSQ-DsRed, but immunostained with ant-RyR (red): D1, rightmost nucleus in panel D. Bar, 2 µm. E, anti-ribosomal S6 protein immunofluorescence (green) was imaged in cells overexpressing CSQ-DsRed (red). Bar, 2 µm. Imaging, Leica (panels A–D), Zeiss LSM (panel E). F, immunoblot analysis of rat and dog heart homogenates (H, 100 µg) and microsomal vesicles (MV, 50 µg) using anti-TRAPα antibodies. Mobilities of molecular weight standards in kDa, left margin.
Transport of proteins from the rough ER in mammalian cells is associated with the formation of ER exit sites that become COPII transport vesicles [29–31]. Because fluorescence microscopy of CSQ-DsRed suggested a relatively direct transport step between rough ER and adjacent junctional SR puncta, we asked whether ER exit site location might also reflect such transport in cardiomyocytes. Sec23 is a well-characterized marker of ER exit sites and COPII vesicles in mammalian cells [32]. In untreated cardiomyocytes, sec23 produced a pattern of immunostaining along Z-lines that was interspersed with RyR-positive staining (Fig. 4A and inset). This suggested that sec23 might be bound to membrane surfaces of cisternae along Z-lines. When sec23 localization was analyzed in cells overexpressing CSQ-DsRed, sec23 appeared to be bound to the perinuclear membrane compartment enriched in CSQ-DsRed (Fig. 4B). Because CSQ-DsRed is expected to be an intralumenal ER protein whereas sec23 is a cytosolic protein, such interactions could not theoretically be direct, and would presumably require a transmembrane signal resulting from enhanced levels of lumenal CSQ-DsRed. The reason for this unexplained translocation of sec23 was not further investigated, but could reflect the inefficient ER exit of CSQ-DsRed. Sec23 levels measured by immunoblotting and densitometry exhibited a roughly 25% decrease in cells treated with Ad.CSQ-DsRed, but showed no further changes with time of adenoviral treatment (Fig. 2, rightmost panel).
Fig. 4. Sec23 subcellular localization in cultured rat cardiomyocytes.
A, cultured rat cardiomyocytes were immunostained using anti-sec23 antibodies (green), and anti-RyR antibodies (red, shown only in inset). Inset, sec23, RyR, and merge (top to bottom) of representative Z-lines. B, cultured cardiomyocytes were treated with Ad.CSQ-DsRed (red) for 48 h. Red (CSQ-DsRed) and green (sec23 immunofluorescence) channels are shown superimposed. Bar, 20 µm. Images, Leica TCS SP5.
Direct CSQ-DsRed fluorescence is distinct from indirect CSQ-DsRed immunofluorescence
Although CSQ-DsRed was readily detected on immunoblots using either anti-DsRed or anti-CSQ antibodies (Fig. 2), we were surprised to find that after 48 h of DsRed overexpression, neither anti-DsRed nor anti-CSQ antibodies were able to detect CSQ-DsRed in the perinuclear compartments of fixed cardiomyocytes (Figs 5–7). Instead, anti-DsRed and anti-CSQ antibodies prominently labeled the cell periphery. This result was seen with three separate antibodies to the CSQ-DsRed fusion protein (Fig. 6F, 7C, and 7D).
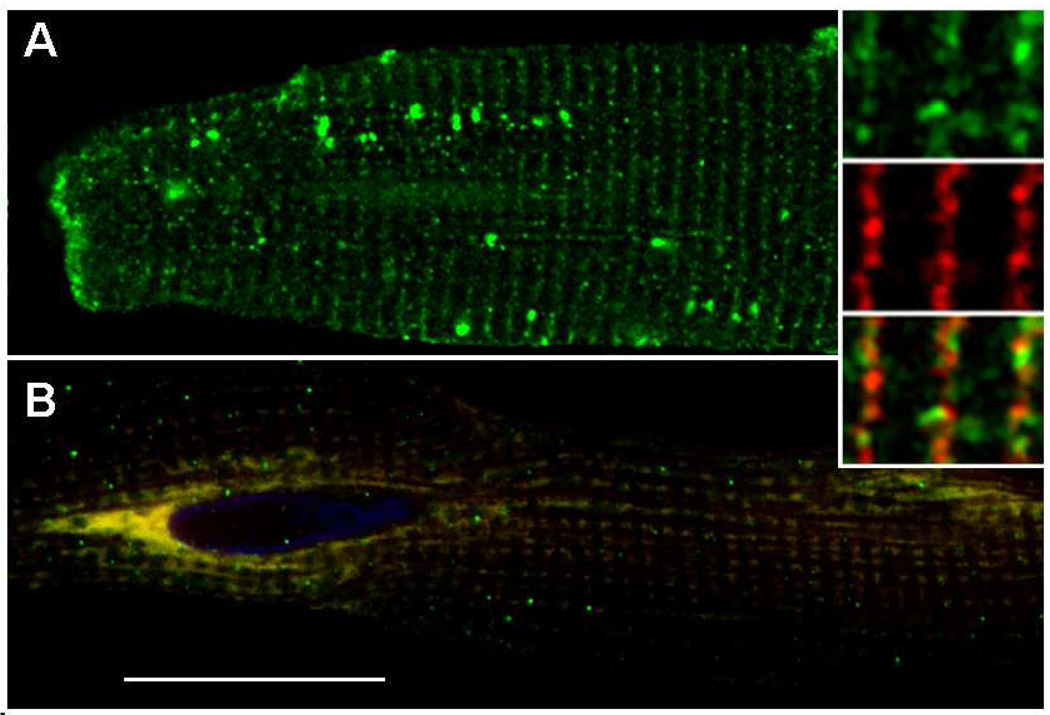
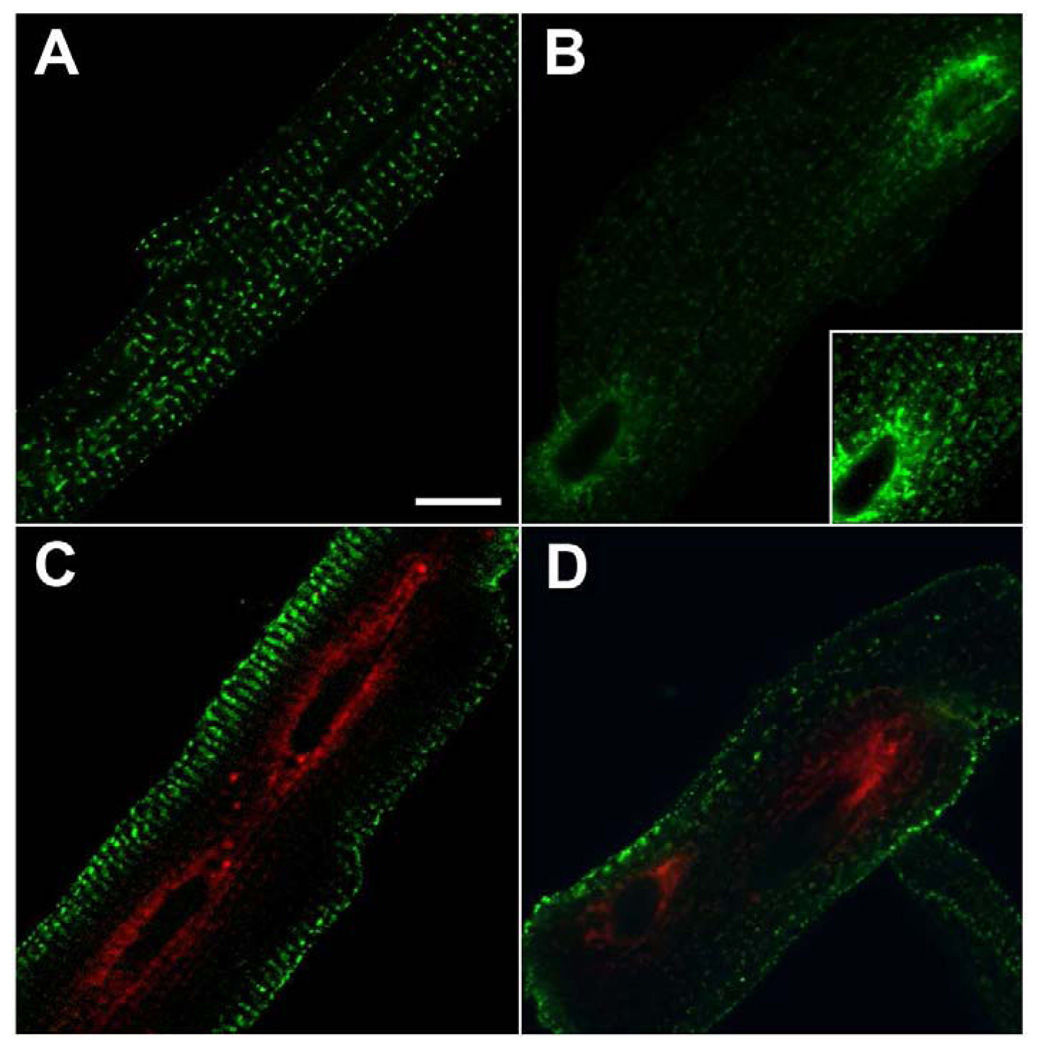
Fig. 5. CSQ-DsRed fluorescence versus anti-DsRed immunofluorescence.
Cardiomyocytes were treated with CSQ-DsRed adenovirus for 48 h, then fixed and stained with anti-DsRed antibodies. Confocal fluorescent images were obtained (z-stack consisting of 38 × 0.5 µm optical slices) on the Leica TCS SP5; selected slices are shown (z = 2.5, 3.5, 5.0, 8.0, 9.5 µm for panels A, B, C, D and E, respectively). CSQ-DsRed fluorescence (red) and indirect anti-DsRed (green) fluorescence are shown together in all panels (bar, 10 µm). The entire Z-stack was assembled into a three-dimensional image using the ImageJ 3D Viewer plugin in conjunction with ImageJ software, and cropped at the first nucleus in order to highlight the radial distribution and separation of CSQ-DsRed tetramers (red) and monomers (green) (panel F). An enlargement of the boxed area in E (panel G), representing a peripheral slice of the cardiomyocyte, shows the presence of CSQ-DsRed tetramer largely in compartments distinct from the monomer (bar, 1 µm). The boxed area in G was also enlarged (panel H) to show non-colocalization occurring even at the sarcomeric level, where it appears that CSQ-DsRed tetramers redirect into free-SR rete. Bar, 0.5 µm.
Fig. 7. Time-dependent changes in anti-CSQ immunofluorescence during CSQ-DsRed overexpression.
Cardiomyocytes were left untreated (A), or treated with CSQ-DsRed adenovirus for 20 h (B) or 48 h (C,D) before fixation and pemeabilization. Fixed cells were immunostained with monoclonal anti-CSQ2 antibodies (green) (A–C) or rabbit polyclonal anti-CSQ2 antibodies (D), and analyzed for red DsRed fluorescence and green immunofluorescence using laser confocal microscopy (Leica TCS SP5). The inset in panel B shows a portion of the cell which is brightened offline so that fluorescence of endogenous native rat CSQ is similar in panels A and B. Bar, 20 µm.
Fig. 6. Time-dependent changes in CSQ-DsRed fluorescence and anti-DsRed immunofluorescence.
Cardiomyocytes were left untreated (A), or treated with CSQ-DsRed adenovirus for 16 h (B), 20 h (C), 24 h (D), 36 h (E), or 48 h (F) before fixation and permeabilization. Fixed cells were immunostained with anti-DsRed antibodies (green), and analyzed for red DsRed direct fluorescence and green indirect immunofluorescence using laser confocal microscopy (Leica TCS SP5). Bar, 10 µm. Magnifications among panels are similar but may vary slightly to better illustrate structural differences.
As discussed below, a likely explanation for the loss of immunoreactivity was that interaction of antibodies was lost due to the (predicted) tetrameric structure of DsRed [24, 25]. This did lead, however, to a labeling of two separate pools of overexpressed CSQ-DsRed having alternate subcellular distributions. As supported in experiments below, and discussed in later sections, we interpreted the red and green labeling as tetrameric CSQ-DsRed and an immunoreactive CSQ-DsRed monomer, respectively. The perinuclear versus peripheral (tetrameric versus monomeric) staining patterns were seen in sequential optical slices (z-stack), validating a 3-dimensional distribution of the direct DsRed fluorescence near the nucleus (Fig. 5A–E). Indeed, the radial nature of the direct DsRed fluorescence was apparent in virtual transverse slices of the reconstructed z-stack along its longitudinal length (Fig. 5F). Closer examination of an optical slice (panel G) near the cell periphery (panel E) where green immunofluorescence (CSQ-DsRed monomers) dominates direct red fluorescence (CSQ-DsRed tetramer), revealed an imperfect co-localization of the two forms. Higher magnification of the two fluorescent signals confirmed minor differences in distribution at the level of individual sarcomeric SR structure (panel H). Even where indirect and direct CSQ-DsRed fluorescence signals occurred together, a distinct distribution was revealed for each, perhaps suggesting that CSQ-DsRed tetramers and monomers traffic differently.
To further analyze the relationship between CSQ-DsRed fluorescence and its immunoreactive forms, we examined a time course of overexpression by confocal fluorescence microscopy. Whereas CSQ-DsRed fluorescence did not develop until about 32 h of adenovirus treatment, anti-DsRed and anti-CSQ immunofluorescence were both detected after only 16–20 h, appearing around nuclei (Figs. 6,7). Time-dependent changes in CSQ-DsRed fluorescence and immunofluorescence were consistent with its translation and accumulation in rough ER after 16 h, followed by its increasing concentration as a red fluorescent polymer. Once polymerization occurred in rough ER, CSQ-DsRed, already in junctional SR, continued its anterograde movement towards the cell periphery where it appeared to concentrate by 48 h as (green) monomer. The fact that this movement of immunofluorescent CSQ-DsRed could be visualized suggested that additional CSQ-DsRed was not being generated along the rough ER-junctional SR pathway, perhaps because all CSQ-DsRed monomer was scavenged within perinuclear cisternae by the polymer.
The fluorescence staining pattern of native rat CSQ could be seen in untreated (control) heart cells exhibiting a steady-state distribution characteristic of junctional SR puncta (Fig. 7A). Visualization of native rat CSQ, however, required fluorescence acquisition times that produced overexposures of CSQ-DsRed immunofluorescence (Fig. 7B, inset), so was not visible with the exposure times used in subsequent figures. In contrast to the relatively unchanging pattern of native CSQ over a 48 h time course (not shown), overexpressed CSQ-DsRed immunofluorescence exhibited a time-dependent trafficking.
To summarize, our data suggest that the tetrameric form of CSQ-DsRed, while necessary to produce the intense red fluorescence, was relatively incapable of interaction with antibody molecules. Meanwhile, antibodies to either half of the fusion protein detected CSQ-DsRed monomers which exhibit highly reduced, if any, intrinsic (direct) fluorescence. The data indicate that once CSQ-DsRed tetramerizes within rough ER, its anterograde trafficking is very substantially restricted, whereas molecules that do not incorporate into the polymer, populate increasingly peripheral junctional SR sites. Based upon these new studies on CSQ trafficking from rough ER to junctional SR compartments, and our recent work on polymer-dependent trafficking of CSQ in nonmuscle cells [12, 13], we propose a new model of CSQ trafficking and junctional SR biology in the adult cardiomyocyte.
Discussion
CSQ-DsRed synthesis on rough ER and translocation into the rough ER lumen reflects the presence of the N-terminal signal sequence of CSQ, since DsRed is a cytosolic protein [25]. Once in the cardiac secretory pathway, CSQ-DsRed displays the properties expected for any luminal ER protein, moving with bulk flow unless retained, or retrieved by a downstream binding protein such as the KDEL receptor used for several luminal ER proteins [3]. Interestingly, CSQ and CSQ-DsRed are both retained, by virtue of their polymerization. The retention of each is site-specific – similar to what we recently reported for the two CSQ isoforms when examined in nonmuscle cells [12, 13]. In cultured heart cells, native CSQ polymerizes in junctional SR (Fig. 6A,B), while CSQ-DsRed polymerizes in rough ER (Figs. 1,5).
Anti-DsRed fluorescence and CSQ-DsRed immunofluorescence were mutually exclusive because DsRed tetramerization simultaneously produces a bright red fluorescence [25] while effectively turning off antibody detection. This generated separate assays for polymeric versus monomeric protein molecules that exhibited distinct patterns of intracellular trafficking. Time-dependent features of the overexpression of CSQ-DsRed were particularly revealing. CSQ-DsRed was initially detectable only by immunoreactivity, where it was localized to one or two perinuclear cisternae. At longer times, immunoreactive CSQ-DsRed was replaced with the bright red fluorescence expected for DsRed tetramers in the same compartment. However, whereas immunoreactive (monomeric) CSQ trafficked anterogradely away from the perinuclear region, albeit slowly (Figs. 6,7), the red fluorescent tetramer was too large to traffic to junctional SR puncta to any significant extent [13, 33, 34].
By the end of a 48 h incubation period, the entire immunoreactive pool of CSQ was concentrated at the cell periphery (Fig. 5F). If immunoreactive CSQ-DsRed represents monomeric CSQ-DsRed, then the shift of this pool of protein from perinuclear cisternae to the cell periphery suggests that the input of additional immunoreactive CSQ-DsRed protein molecules was no longer occurring. This may be directly due to the scavanging of all newly synthesized CSQ-DsRed within the fluorescent polymer in rough ER once formed. Thus, without further input of newly-synthesized CSQ-DsRed, the "pulse" of monomeric CSQ-DsRed that escaped polymerization in rough ER could be visualized as it moved through the full extent of the Z-line localized ER/SR pathway. The fate of this CSQ-DsRed that concentrated in the cell periphery after 48 h was not investigated in this study, but represents an interesting question for future studies.
Protein markers of the ER translocon complex, TRAPα and TRAM, co-localized with CSQ-DsRed in perinuclear cisterna, suggesting that this is a major site of rough ER localization in cardiomyocytes and of CSQ biosynthesis. Rough ER was previously visualized by electron microscopy in a continuum with the nuclear membrane, as one of a few morphologically-defined sites within adult rabbit cardiomyocytes [35]. Translation around myonuclei in cardiomyocytes for specific cardiac proteins has not been reported to our knowledge, but has been discussed as one of at least two such sites in skeletal muscle myocytes [36].
CSQ-DsRed fluorescence could be observed connecting the perinuclear cisternae directly to adjacent junctional SR (RyR-positive) puncta in optical sections that bisected the myonuclear midline (Figs 1E, 3B2, asterisk). A pathway for SR membrane protein trafficking along Z-lines has not previously been reported in cardiomyocytes, but may be similar to the exporting ER network described by Kaisto and Metsikkö in rat skeletal muscle myofibers [37]. We also observed apparent longitudinal trafficking of CSQ-DsRed, particularly when CSQ-DsRed-containing cisternae were viewed near the outer edges of the cell (Fig. 5E,G,H). A mechanism of longitudinal trafficking of CSQ is also not known, but our data for sec23, a microtubule-based trafficking protein of COPII vesicles [32], suggests that junctional SR to longitudinal SR might represent the normal direction of protein trafficking in the cardiac secretory pathway. Free SR is enriched in proteins that contain the C-terminal KDEL retrieval sequence [38–40]. Because KDEL proteins are thought to be enriched in both ER and ERGIC [12, 41], their presence is consistent with free SR being a subcellular compartment distal to junctional SR. The view of junctional SR as equivalent to ER, and longitudinal (or free) SR as being equivalent to the intermediate (ERGIC) compartment is consistent with studies from the Metsikko laboratory in skeletal muscle myofibers [37, 42]. Similar to the limited accumulation of CSQ-DsRed in longitudinal SR (Fig. 5H), native CSQ that escapes retention by polymerization should also traverse this part of the cardiac secretory pathway, as do the other muscle-specific lumenal ER/SR Ca2+-binding proteins sarcalumenin and histidine-rich Ca2+-binding protein [3].
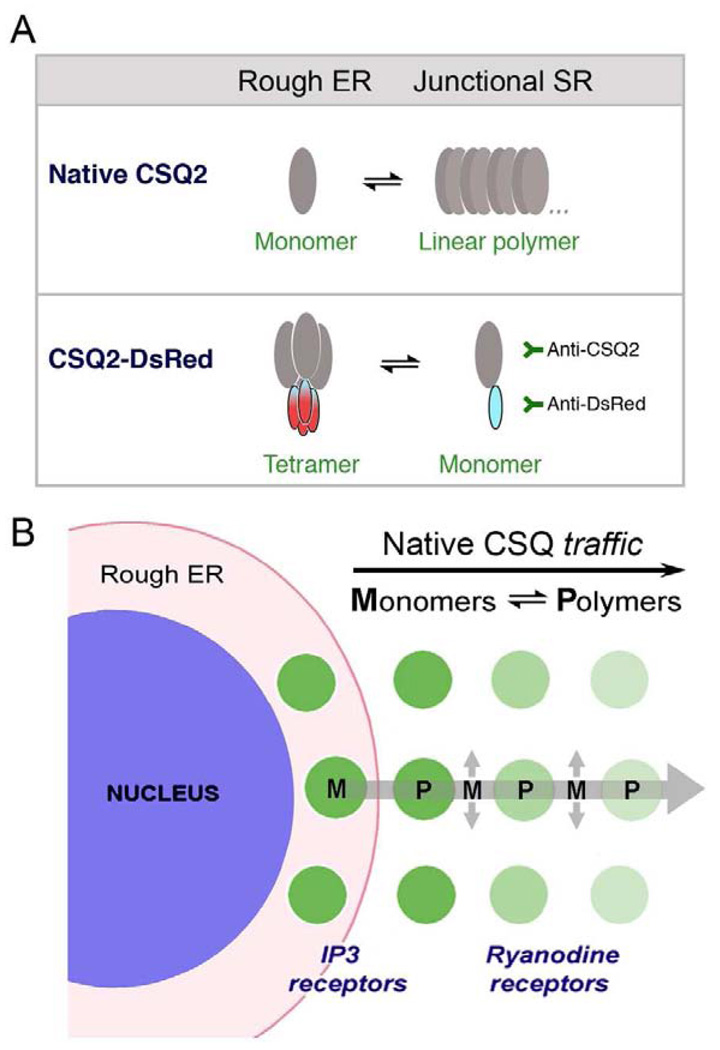
In a new model of CSQ biosynthesis and trafficking in cardiomyocytes (Fig. 8), we propose that CSQ is synthesized at sites close to the nuclear envelope, and then traffics directly to junctional SR in the direction of the cell surface. In the case of CSQ-DsRed, polymers (tetramers) become retained in perinuclear cisternae that form the rough ER in cardiomyocytes. As CSQ-DsRed tetramers dissociate to monomers, it traffics slowly towards distal junctional SR We infer from these data that CSQ normally polymerizes only within junctional SR puncta, a process that prevents or slows its anterograde trafficking, and presumably its secretion. We conclude therefore, that, as in the case of mammalian nonmuscle cells, CSQ polymerizes in junctional SR because it is the first intracellular compartment that satisfies the biophysical criteria for polymerization.
Fig. 8. Model of native CSQ trafficking from rough ER to junctional SR in cardiomyocytes.
A, Native CSQ exists as monomers or linear polymers in dynamic equilibrium inside the secretory pathway of cardiomyocytes. While monomeric CSQ traffics anterogradely, polymeric CSQ cannot, leading to its accumulation in junctional SR puncta. CSQ-DsRed, in contrast to native CSQ, polymerizes and accumulates in rough ER around nuclei. CSQ-DsRed monomers, like CSQ monomers, can traffic anterogradely where they can be specifically detected by antibodies against either half of the fusion protein pair. Not shown in this scheme are forms of CSQ in junctional SR that might contain CSQ-DsRed linear homopolymers and heteropolymers (with native CSQ). B, In cardiomyocytes, native CSQ is synthesized in perinuclear rough ER. Trafficking proceeds across a direct cellular transport pathway (large arrow) to perinuclear junctional SR, dependent on a dynamic polymer (P)-monomer (M) equilibrium. A predominance of native CSQ monomers in rough ER and along the rough ER-junctional SR trafficking pathway lead to anterograde trafficking. CSQ polymers form within junctional SR puncta due to its distinctive intralumenal ionic conditions, leading to its accumulation [13]. ER-exit sites along this same ER pathway (highlighted by sec23 immunostaining, cf. Fig. 4), may separate the basic cardiac ER (junctional SR) from an intermediate compartment (longitudinal SR) that traffics along myofibrils (smaller arrows). In the case of CSQ-DsRed overexpression in cardiomyocytes, tetrameric CSQ-DsRed accumulates in rough ER. Protein monomers of CSQ-DsRed predominantly traffic anterogradely to accumulate in peripheral junctional SR puncta. Puncta in close proximity to cardiomyocyte nuclei may contain the inositol trisphosphate (IP3) receptor in addition to, or instead of the more prominent ryanodine receptor contained in more peripheral junctional SR puncta [43].
The findings presented here may also help to explain previously reported CSQ protein structures in normal and failed hearts [19]. In normal heart tissue, CSQ glycans show extensive mannose trimming (Man3–5), indicative of junctional SR localization [16, 17], but in failed heart tissue, a portion of CSQ contain glycans having structures indicative of rough ER localization (Man8,9) [12, 16]. Based upon the current study, we would predict that this increase in newly-synthesized CSQ would preferentially reside in perinuclear cisternae. These ER forms of CSQ (Man9,8) in heart are fully phosphorylated, whereas junctional SR forms of CSQ (Man 3–6) are increasingly dephosphorylated [17]. Possible relationships between CSQ phosphorylation and its accumulation in perinuclear rough ER in heart failure is an interesting topic for research.
In summary, CSQ-DsRed fluorescence and immunofluorescence reveal several new aspects of CSQ biology by essentially slowing down its secretion, and by causing it to polymerize in a proximal compartment (rough ER). Our new findings and accompanying model include: 1) the identification of the subcellular site of CSQ biosynthesis (cardiac rough ER), 2) demonstration of a CSQ polymerization-dependent compartmentalization in cardiomyocytes, 3) the suggestion that CSQ-DsRed traffics directly from rough ER to junctional SR along a novel, albeit uncharacterized intracellular pathway, and 4) the characterization of rough ER and junctional SR as cardiac ER, and longitudinal SR as a compartment distal to ER exit sites possibly corresponding to ERGIC. These data and model may aid in understanding the changes in CSQ and SR biology associated with new transgenic mouse models and in diseases associated with inner membrane biology in adult cardiomyocytes.
Acknowledgements
This work was supported by grant HL062586 from the NIH/NHLBI. We thank Timothy D. Houle and Naama H. Sleiman for expert technical assistance. The Microscopy and Imaging Resources Laboratory (MIRL) is supported, in part, by NIH Center grants P30ES06639 and P30CA22453. We gratefully acknowledge expert assistance from the staff of the MIRL at Wayne State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The abbreviations used are: CSQ, calsequestrin; SR, sarcoplasmic reticulum; ER, endoplasmic reticulum; ERGIC, ER-Golgi intermediate compartment; RyR, ryanodine receptor; GlcNAc, N-acetyl-D-glucosamine; Man, mannose; Ad.CSQ-DsRed, adenoviral calsequestrin-DsRed; Ad.CSQ-HA, Adenoviral calsequestrin-hemagglutinin; MOI, multiplicity of infection; TRAP, translocon-associated protein complex; TRAM, translocating chain-associated membrane protein; HRP, horseradish peroxidase; ECL, electrochemiluminescence; DAPI, 4′-6-diamidino-2-phenylindole; IP3R, inositol trisphosphate receptor.
Disclosure statement
No conflicts of interest by any authors.
References
- 1.Campbell KP, MacLennan DH, Jorgensen AO, Mintzer MC. Purification and characterization of calsequestrin from canine cardiac sarcoplasmic reticulum and identification of the 53,000 dalton glycoprotein. J Biol Chem. 1983;258(2):1197–1204. [PubMed] [Google Scholar]
- 2.Jorgensen AO, Shen AC, Campbell KP. Ultrastructural localization of calsequestrin in adult rat atrial and ventricular muscle cells. J Cell Biol. 1985;101(1):257–268. doi: 10.1083/jcb.101.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cala SE, Scott BT, Jones LR. Intralumenal sarcoplasmic reticulum Ca(2+)-binding proteins. Semin Cell Biol. 1990;1(4):265–275. [PubMed] [Google Scholar]
- 4.Gyorke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008 Jan 15;77(2):245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- 5.Royer L, Rios E. Deconstructing calsequestrin. Complex buffering in the calcium store of skeletal muscle. J Physiol. 2009 Jul 1;587(Pt 13):3101–3111. doi: 10.1113/jphysiol.2009.171934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knollmann BC. New roles of calsequestrin and triadin in cardiac muscle. J Physiol. 2009 Jul 1;587(Pt 13):3081–3087. doi: 10.1113/jphysiol.2009.172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terentyev D, Viatchenko-Karpinski S, Gyorke I, Volpe P, Williams SC, Gyorke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc Natl Acad Sci U S A. 2003;100(20):11759–11764. doi: 10.1073/pnas.1932318100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006 Sep;116(9):2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones LR, Suzuki YJ, Wang W, Kobayashi YM, Ramesh V, Franzini-Armstrong C, et al. Regulation of Ca2+ signaling in transgenic mouse cardiac myocytes overexpressing calsequestrin. J Clin Invest. 1998;101(7):1385–1393. doi: 10.1172/JCI1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franzini-Armstrong C. Architecture and regulation of the Ca2+ delivery system in muscle cells. Appl Physiol Nutr Metab. 2009 Jun;34(3):323–327. doi: 10.1139/H09-017. [DOI] [PubMed] [Google Scholar]
- 11.Park H, Wu S, Dunker AK, Kang C. Polymerization of calsequestrin. Implications for Ca2+ regulation. J Biol Chem. 2003;278(18):16176–16182. doi: 10.1074/jbc.M300120200. Epub 2003 Feb 19. [DOI] [PubMed] [Google Scholar]
- 12.Houle TD, Ram ML, McMurray WJ, Cala SE. Different endoplasmic reticulum trafficking and processing pathways for calsequestrin (CSQ) and epitope-tagged CSQ. Exp Cell Res. 2006;312(20):4150–4161. doi: 10.1016/j.yexcr.2006.09.010. Epub 2006 Sep 20. [DOI] [PubMed] [Google Scholar]
- 13.Milstein ML, Houle TD, Cala SE. Calsequestrin isoforms localize to different ER subcompartments: evidence for polymer and heteropolymer-dependent localization. Exp Cell Res. 2009 Feb 1;315(3):523–534. doi: 10.1016/j.yexcr.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Cho JH, Ko KM, Singaruvelu G, Lee W, Kang GB, Rho SH, et al. Functional importance of polymerization and localization of calsequestrin in C. elegans. J Cell Sci. 2007 May 1;120(Pt 9):1551–1558. doi: 10.1242/jcs.001016. [DOI] [PubMed] [Google Scholar]
- 15.Cala SE, Jones LR. Phosphorylation of cardiac and skeletal muscle calsequestrin isoforms by casein kinase II. Demonstration of a cluster of unique rapidly phosphorylated sites in cardiac calsequestrin. J Biol Chem. 1991;266(1):391–398. [PubMed] [Google Scholar]
- 16.O'Brian JJ, Ram ML, Kiarash A, Cala SE. Mass spectrometry of cardiac calsequestrin characterizes microheterogeneity unique to heart and indicative of complex intracellular transit. J Biol Chem. 2002 September 27;277(40):37154–37160. doi: 10.1074/jbc.M204370200. 2002. [DOI] [PubMed] [Google Scholar]
- 17.Ram ML, Kiarash A, Marsh JD, Cala SE. Phosphorylation and dephosphorylation of calsequestrin on CK2-sensitive sites in heart. Mol Cell Biochem. 2004;266(1–2):209–217. doi: 10.1023/b:mcbi.0000049164.28580.56. [DOI] [PubMed] [Google Scholar]
- 18.Dunphy WG, Brands R, Rothman JE. Attachment of terminal N-acetylglucosamine to asparagine-linked oligosaccharides occurs in central cisternae of the Golgi stack. Cell. 1985;40(2):463–472. doi: 10.1016/0092-8674(85)90161-8. [DOI] [PubMed] [Google Scholar]
- 19.Kiarash A, Kelly C, Phinney B, Valdivia H, Abrams J, Cala S. Defective glycosylation of calsequestrin in heart failure. Cardiovasc Res. 2004;63:264–272. doi: 10.1016/j.cardiores.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Scott BT, Simmerman HK, Collins JH, Nadal-Ginard B, Jones LR. Complete amino acid sequence of canine cardiac calsequestrin deduced by cDNA cloning. J Biol Chem. 1988 Jun 25;263(18):8958–8964. [PubMed] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Cala SE, Miles K. Phosphorylation of the cardiac isoform of calsequestrin in cultured rat myotubes and rat skeletal muscle. Biochim Biophys Acta. 1992;1118(3):277–287. doi: 10.1016/0167-4838(92)90285-l. [DOI] [PubMed] [Google Scholar]
- 23.Lowry HO, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Wall MA, Socolich M, Ranganathan R. The structural basis for red fluorescence in the tetrameric GFP homolog DsRed. Nat Struct Biol. 2000 Dec;7(12):1133–1138. doi: 10.1038/81992. [DOI] [PubMed] [Google Scholar]
- 25.Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, et al. A monomeric red fluorescent protein. Proc Natl Acad Sci U S A. 2002 Jun 11;99(12):7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fons RD, Bogert BA, Hegde RS. Substrate-specific function of the translocon-associated protein complex during translocation across the ER membrane. J Cell Biol. 2003 Feb 17;160(4):529–539. doi: 10.1083/jcb.200210095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartmann E, Gorlich D, Kostka S, Otto A, Kraft R, Knespel S, et al. A tetrameric complex of membrane proteins in the endoplasmic reticulum. Eur J Biochem. 1993 Jun 1;214(2):375–381. doi: 10.1111/j.1432-1033.1993.tb17933.x. [DOI] [PubMed] [Google Scholar]
- 28.Gorlich D, Hartmann E, Prehn S, Rapoport TA. A protein of the endoplasmic reticulum involved early in polypeptide translocation. Nature. 1992 May 7;357(6373):47–52. doi: 10.1038/357047a0. [DOI] [PubMed] [Google Scholar]
- 29.Barlowe C. Traffic COPs of the early secretory pathway. Traffic. 2000 May;1(5):371–377. doi: 10.1034/j.1600-0854.2000.010501.x. [DOI] [PubMed] [Google Scholar]
- 30.Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994 Jun 17;77(6):895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 31.Tang BL, Ong YS, Huang B, Wei S, Wong ET, Qi R, et al. A membrane protein enriched in endoplasmic reticulum exit sites interacts with COPII. J Biol Chem. 2001 Oct 26;276(43):40008–40017. doi: 10.1074/jbc.M106189200. [DOI] [PubMed] [Google Scholar]
- 32.Orci L, Ravazzola M, Meda P, Holcomb C, Moore HP, Hicke L, et al. Mammalian Sec23p homologue is restricted to the endoplasmic reticulum transitional cytoplasm. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8611–8615. doi: 10.1073/pnas.88.19.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houle TD, Ram ML, Cala SE. Calsequestrin mutant D307H exhibits depressed binding to its protein targets and a depressed response to calcium. Cardiovasc Res. 2004;64(2):227–233. doi: 10.1016/j.cardiores.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Rivera VM, Wang X, Wardwell S, Courage NL, Volchuk A, Keenan T, et al. Regulation of protein secretion through controlled aggregation in the endoplasmic reticulum. Science. 2000;287(5454):826–830. doi: 10.1126/science.287.5454.826. [DOI] [PubMed] [Google Scholar]
- 35.Slade AM, Severs NJ. Rough endoplasmic reticulum in the adult mammalian cardiac muscle cell. J Submicrosc Cytol. 1985 Oct;17(4):531–536. [PubMed] [Google Scholar]
- 36.Antony C, Huchet M, Changeux JP, Cartaud J. Developmental regulation of membrane traffic organization during synaptogenesis in mouse diaphragm muscle. J Cell Biol. 1995 Aug;130(4):959–968. doi: 10.1083/jcb.130.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaisto T, Metsikko K. Distribution of the endoplasmic reticulum and its relationship with the sarcoplasmic reticulum in skeletal myofibers. Exp Cell Res. 2003 Sep 10;289(1):47–57. doi: 10.1016/s0014-4827(03)00231-3. [DOI] [PubMed] [Google Scholar]
- 38.Volpe P, Villa A, Podini P, Martini A, Nori A, Panzeri MC, et al. The endoplasmic reticulum-sarcoplasmic reticulum connection: distribution of endoplasmic reticulum markers in the sarcoplasmic reticulum of skeletal muscle fibers. Proc Natl Acad Sci U S A. 1992;89(13):6142–6146. doi: 10.1073/pnas.89.13.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cala SE, Jones LR. GRP94 resides within cardiac sarcoplasmic reticulum vesicles and is phosphorylated by casein kinase II. J Biol Chem. 1994;269(8):5926–5931. [PubMed] [Google Scholar]
- 40.Cala SE. Determination of a putative phosphate-containing peptide in calreticulin. Biochem Biophys Res Commun. 1999;259(2):233–238. doi: 10.1006/bbrc.1999.0760. [DOI] [PubMed] [Google Scholar]
- 41.Pelham HR. Sorting and retrieval between the endoplasmic reticulum and Golgi apparatus. Curr Opin Cell Biol. 1995 Aug;7(4):530–535. doi: 10.1016/0955-0674(95)80010-7. [DOI] [PubMed] [Google Scholar]
- 42.Rahkila P, Alakangas A, Vaananen K, Metsikko K. Transport pathway, maturation, and targetting of the vesicular stomatitis virus glycoprotein in skeletal muscle fibers. J Cell Sci. 1996 Jun;109(Pt 6):1585–1596. doi: 10.1242/jcs.109.6.1585. [DOI] [PubMed] [Google Scholar]
- 43.Higazi DR, Fearnley CJ, Drawnel FM, Talasila A, Corps EM, Ritter O, et al. Endothelin-1-stimulated InsP3-induced Ca2+ release is a nexus for hypertrophic signaling in cardiac myocytes. Mol Cell. 2009 Feb 27;33(4):472–482. doi: 10.1016/j.molcel.2009.02.005. [DOI] [PubMed] [Google Scholar]