Abstract
Purpose
Several recent oral oncology drug labels were labeled to be administered in fasted states despite the fact that food increases their bioavailability. Since this was inconsistent with principles of oral drug delivery, we hypothesized that there were inconsistencies across therapeutic areas.
Experimental Design
Oral agents approved by US FDA from January 2000 to May 2009 were included in our study. Comparison of the food labeling patterns between oncology and non-oncology drugs was made using Fisher's exact test.
Results
Of 99 drugs evaluated, 34 showed significant food effects on bioavailability. When food markedly enhanced bioavailability, 8 out of 9 non-oncology drugs were labeled “fed” to take advantage of the food-drug interaction while all oncology drugs (n=3) were labeled to be administered in “fasted” states (Fisher's exact; p= 0.01).
Conclusions
Drug labeling pattern with respect to food-drug interactions observed with oncology drugs is in contradiction to fundamental pharmacological principles, as exemplified in the labeling of non-oncology drugs.
Keywords: Drug label, Food-drug interaction, Oral bioavailability, Bioavailability, Drug development, Oncology
Introduction
Many new antineoplastic agents are intended for daily administration requiring the availability of oral formulations. Oral therapies can improve a patient's quality of life by offering convenience and a sense of control, as well as avoiding the cost of administering parenteral agents.(1, 2). However, oral cancer drugs can present special challenges. With increased patient responsibility, non-adherence to potent agents is a major concern to oncologists.(3) In addition, oral agents generally have more complex pharmacokinetic challenges compared to the drugs administered intravenously.
Administration of oral drugs with meals can influence drug absorption and systemic exposure. The food effect on oral bioavailability is the result of a complex interplay of drug, formulation, intestinal physiology, and meals. As food can either increase or decrease bioavailability, the interaction should be studied early in drug development to provide rational dosing recommendations for the pivotal clinical trials.(4)
Questions were raised in the recent past regarding the labeling of lapatinib, a dual tyrosine kinase inhibitor used to treat advanced breast cancer.(5) Lapatinib is labeled to be taken fasting, despite the fact that food markedly improved bioavailability of the drug, exemplifying a missed opportunity to take advantage of pharmacologically favorable food-drug interactions.(6) We hypothesized that there might be a systematic difference between oncology and non-oncology products in terms of food labeling. The present work examines food labeling pattern of oncology and non-oncology drugs to highlight the inconsistency across the therapeutic areas and to suggest potential implications.
Materials and Methods
The present study is based on examination of all new molecular entities (NME) that were approved for oral administration by the United States Food and Drug Administration (FDA) between January 2000 and May 2009. The primary source of data was the FDA website (http://www.accessdata.fda.gov/scripts/cder/drugsatfda). This included both clinical pharmacology/biopharmaceutics reviews and labels. A search of published literature was conducted in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) to gather missing information from the FDA website.(7-19) Main search terms used were food effect, bioavailability, pharmacokinetics, and the agents' names.
Data captured for the purpose of the present analysis included drug approval date, drug name, therapeutic area, magnitude of food effect, interindividual variability of area under the curve (AUC) of fed and fasted states, dosing recommendation with regards to meals (fed, fasted, or either), and black box safety warning in drug labels.
We included all NMEs with food effect bioavailability study results available on the FDA website or via publication, and divided them into two categories (oncology versus non-oncology) based on their indications and divisions of US FDA involved in the approval processes. We used AUC as the reflection of the extent of drug exposure, and used the ratio of fed to fasted AUC to measure the food effect. In keeping with Guidance from FDA, food effect was determined to be significant if the AUC ratio (fed: fasted) was greater than 1.25 or less than 0.8.(20) We then sub-classified NMEs with the ratio greater than 1.5 and less than 0.5, a magnitude of food effects that will likely have clinical implications. Comparison of food effects was made between oncology and non-oncology drugs that met these criteria.
The dosing recommendations regarding meal intakes (fasted, fed, or either) available in dosage and administration section of package inserts were surveyed in order to study how food effect study results are applied. Comparison of the drug label patterns between oncology and non-oncology drugs with marked food effects were made by using Fisher's exact test.
In order to assess the impact of food intake on inter-individual variability (IIV) of AUC, we compared coefficient of variation (CV) of AUC between fasted and fed states of 23 drugs with significant food effects and available PK data. Black box safety warnings were available from package inserts and the frequency of black box warnings were compared between oncology and non-oncology drugs. We also surveyed package inserts of NMEs with black box warnings to identify warnings related to food intake and associated risks.
Results
We identified 104 oral NMEs that were approved by FDA between January 2000 and May 2009. This included 11 oncology drugs and 93 non-oncology drugs. Most of these NMEs (n=99) had food effect study results reported in the clinical pharmacology section of the package inserts.
Influence of meals on drug exposure
Of 99 NMEs with available food effect study results, about one-third (n=34) showed significant food effects on their bioavailability. Marked food effects were observed in nearly 20% of drugs (14 increasing and 4 decreasing AUC). Of the 18 NMEs with marked food effects, 3 were oncology agents and 15 were non-oncology agents.
Application of Food Effect Study to Drug Labeling
The vast majority (94%, n=93) of NMEs had specific recommendations with respect to food co-administration: 60% (n=56) without regard to meals, 24% (n=22) to be administered with food, and 16% (n=15) to be administered in fasted state. Only 6 NMEs had no specific recommendations regarding food intake.
Analysis of 14 NMEs with marked increase in AUC with food revealed that there are different food labeling patterns between oncology and non-oncology drugs. For the non-oncology drugs, the marked increase in bioavailability with food generally (with one exception,) led to recommendations to administer drugs in a fed state to take advantage of the favorable food effects. The opposite labeling pattern was observed for oncology drugs, as all three drugs with a marked increase in bioavailability with food were recommended to be administered in fasting states.
Food significantly decreased (by ≥ 20%) the bioavailability of 11 NMEs, and the majority were labeled to be administered in fasted state (7 fasted, 1 fed, and 3 either). Sorafenib was the only oncology NME in this group, which is labeled to be taken fasting. Four NMEs showed a marked decrease of bioavailability (by ≥ 50%) with food, and all of them were labeled to be administered in a fasted state to maximize their bioavailability. FIGURE1
FIGURE 1. Application of marked food effects on labels: Oncology VS Non-oncology.
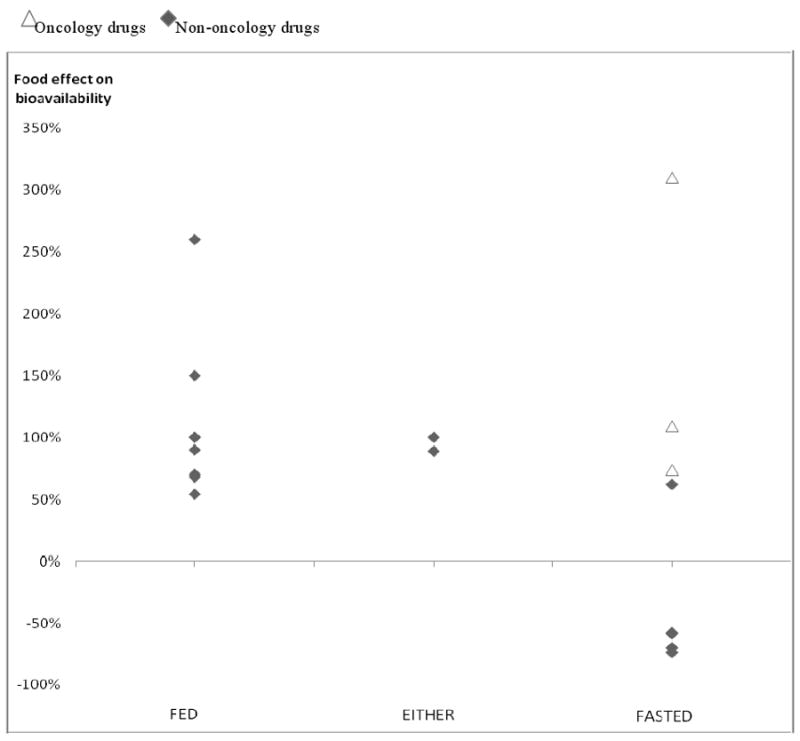
Food recommendations on 18 drugs with marked food effects (AUC ratio ≥1.5 or ≤0.5). Y-axis represents change of AUC from fasted states to fed states. Food recommendations in labels were to be administered with meals (fed), without meals (fasted), or without regards to meals (either). When food recommendation was made either fed or fasted on drugs with marked increase of AUC, oncology drugs were labeled as fed while most non-oncology drugs were labeled fed. (p=0.01, fisher exact test)
Influence of meals on IIV of AUC
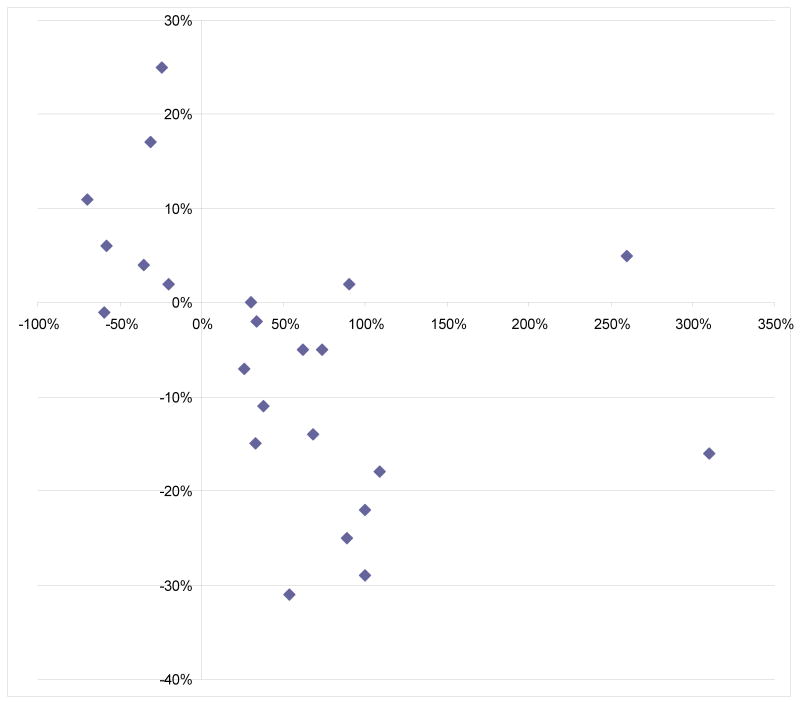
Of 34 NMEs with significant food effects, 23 had CV data available through the FDA website (Package insert or Clinical Pharmacology and Biopharmaceutics Review) or via publications. TABLE 1 In general, we observed an inverse relationship between food effects on bioavailability and IIV. In most cases, increase of bioavailability with food resulted in decrease of IIV of AUC. Figure 2 Of 16 drugs with significant increase of bioavailability with meals (and with available CV data), only one drug (posaconazole) showed an increase of bioavailability associated with a slight increase of IIV; the CV increase with food was merely 5%. The CVs of three oncology drugs with marked positive food effect were decreased by food intake, which indicate that co-administration of drugs with food did not add to the risk of unpredictable exposure, but in fact improved the exposure variability probably as a result of enhanced bioavailability. On the other hand, when food had a negative effect on a drug's bioavailability, administering the drug with food resulted in greater IIV compared to administering it without food. FIGURE 2
TABLE 1. Summary of food effects and labels of 23 new molecular entities that were approved by FDA from January 2000 to May 2009.
NMEs with both AUC and coefficient of variation (CV) data available were included.
| Name | AUC ratio (Fed/Fasted) | CV Fasted | CV Fed | CV Fed-CV Fasted | Label |
|---|---|---|---|---|---|
| lapatinib | 4.1 | 68% | 52% | -16% | Fasted |
| posaconazole | 3.6 | 36% | 41% | 5% | Fed |
| erlotinib | 2.09 | 36% | 18% | -18% | Fasted |
| alfuzosin HCL | 2 | 69% | 40% | -29% | Fed |
| ziprasidone HCL | 2 | 44% | 22% | -22% | Fed |
| nitazoxanide | 1.9 | 35% | 37% | 2% | Fed |
| rifaximin | 1.89 | 51% | 26% | -25% | Either |
| nilotinib | 1.74 | 35% | 30% | -5% | Fasted |
| cinacalcet | 1.68 | 60% | 46% | -14% | Fed |
| deferasirox | 1.62 | 36% | 31% | -5% | Fasted |
| etravirine | 1.54 | 111% | 80% | -31% | Fed |
| vorinostat | 1.38 | 43% | 32% | -11% | Fed |
| rufinamide | 1.34 | 30% | 28% | -2% | Fed |
| gefitinib | 1.33 | 55% | 40% | -15% | Either |
| darunavir | 1.3 | 15-35% | 15-35% | 0% | Fed |
| tapentadol | 1.26 | 36% | 29% | -7% | Either |
| rasagiline | 0.8 | 30% | 32% | 2% | Either |
| voriconazole | 0.76 | 125% | 150% | 25% | Fasted |
| sorafenib | 0.69 | 36% | 53% | 17% | Fasted |
| sodium oxybate | 0.65 | 38% | 42% | 4% | Fasted |
| tegaserod maleate | 0.42 | 26% | 32% | 6% | Fasted |
| eltrombopag | 0.41 | 28% | 27% | -1% | Fasted |
| aliskiren | 0.3 | 49% | 60% | 11% | Fasted |
FIGURE 2. Relationship between food effects on bioavailability and inter-individual variation of drug exposure.
Y-axis represents change in coefficient of variance of AUC from fasted to fed states. X-axis represents food effect on mean AUC.
Examination of 23 drugs with significant food effects (AUC ratio ≥1.25 or ≤0.8) and available coefficient of variation (CV) data revealed inverse relationship between change in BA and change in IIV of drug exposure from prandial states.
Safety Warning Label
We surveyed NMEs with a blackbox safety warning in order to determine whether marked food-drug interactions are incorporated in such warning. The frequencies of blackbox warnings were similar between oncology and non-oncology drugs (36% VS 32%). Of three drugs with marked food-drug interactions, only one NME (nilotinib) had its food effect on drug exposure clearly described in the blackbox safety warning. Lapatinib, which showed the greatest food effect on its AUC, did not have a blackbox warning regarding its food-drug interaction, even though QT prolongation is an acknowledged risk. TABLE 2
TABLE 2. Drugs with positive food effect* with meals and black box safety warnings.
| Drugs with positive food effects* and black box safety warnings | Therapeutic area | Black box warning | Risk of QT prolonga tion | AUC Ratio (Fed/Fasted) | Warning regarding food effect | Label |
|---|---|---|---|---|---|---|
| ziprasidone HCL | Non oncology | Elderly with dementia related psychosis with increased risk of death. | Yes | 2 | No | Fed |
| lapatinib | Oncology | Hepatoxicity | Yes | 4.1 | No | Fasted |
| nilotinib | Oncology | QT prolongation | Yes | 1.74 | Yes | Fasted |
positive food effect: increase of AUC by at least 25% with meals
Discussion
Dosing strategies to enhance bioavailability offer several advantages to delivery of oral drugs. They include reduced gastrointestinal toxicity (from unabsorbed drug) and decreased intra-individual and/or inter-individual variability in drug exposure.(4, 21) FIGURE 3 Increased drug absorption also reduces wasted drug product and improves pharmacoeconomic efficiency.
Unwarranted food restrictions may compromise practicality of drug administration for patients and result in decreased adherence. The complexity of the drug regimen is a major reason for non-adherence, and interventions such as reminder systems have been shown to improve adherence. (3, 22-26) Daily routines such as breakfast, can serve as great reminders to take medications consistently. Hence, a dosing schedule tied to routine meals will be easier for patients (particularly elderly cancer patients taking multiple oral medications) and can be a great way to improve adherence, which is recognized as a serious challenge in cancer treatments with oral agents.
The food labeling pattern of recently approved oral oncology drug products is inconsistent with fundamental principles of oral drug delivery. The labels of three agents (erlotinib, nilotinib, and lapatinib) minimize bioavailability through food restrictions, which is in contrast to labeling principles used for all other classes of oral agents. In the absence of a scientific basis for food restrictions, one can hypothesize that the atypical food labeling pattern of some oral oncology drug products may be a consequence of external pressures (corporate and regulatory pressures) in an era of immense competition in oncology. Phase II (and occasionally registration) studies are often initiated prior to completion of an appropriate food-effect study.(14, 27) Since the default position appears to be fasting, this has occasionally resulted in completion of all clinical studies with a fasting dosing regimen, in the absence of adequate supportive pharmacokinetic data. (14, 27, 28) Furthermore, there is little interest by industry in conducting such studies later if the result would be a lower labeled dose, since this would result in reduced revenues, unless accompanied by an increase in pricing. Thus, regulators should require such studies after approval, if the prior studies have not adequately addressed this issue.
A food effect study conducted in a timely fashion can facilitate pharmacologically rational drug dosing strategies. The optimal time for studying food-drug interactions would be at the end of the first phase I clinical trial: once the dose-toxicity relationship has been identified. At the very minimum, the appropriate prandial state(s) should be determined before undertaking pivotal trials, given the importance of such trials in drug labeling.
It should also be noted that food restriction in the first phase I clinical trial, before the characterization of food effect, is not ideal from standpoints of pharmacology and patient safety. Since a marked decrease of AUC with food is uncommon, it is probably most appropriate – in the absence of data – to begin studies of NMEs in a fed state. On the other hand, nearly 15% of recently approved NMEs showed a marked increase in bioavailability with food. Starting the first phase I trials in fed conditions will reduce the risks of severe adverse events due to inadvertent food-drug interactions.
We recognize the limitation of this retrospective analysis, particularly in regard to identifying the rationale for apparently irrational decisions given the complex nature of oncology drug development processes and decision-making. There are potential limitations in comparing drug labels of oncology and non-oncology drugs due to different potencies, indications, and targeted patients. Inability to study other factors relevant for food labeling, such as comparison of IIV of drug exposures between prandial states with differing food contents, due to lack of such data, is another limitation of this report. Despite these limitations, our analysis clearly illustrates a distinct food labeling pattern with oncology products that is inconsistent with fundamental principles routinely practiced in other disciplines. While there is understandable urgency that may be unique to oncology, it is important that regulatory agencies insist on uniform application of principles of clinical pharmacology, regardless of the impact on the sponsor's timelines.
Acknowledgments
Dr. Soonmo Peter Kang was supported by Clinical Therapeutics T32 GM007019 from NIH
Footnotes
Translational Relevance: Knowledge of food-drug interactions is critical in optimizing delivery of oral anticancer agents. We found a systematic difference between oncology and non-oncology drugs in how food-drug interactions are applied in their labels. When food enhanced bioavailability, non-oncology drugs were labeled “fed” to take advantage of the food-drug interaction while oncology drugs were labeled to be administered in “fasted” states. These oncology drug labeling decisions are in contradiction to fundamental pharmacological principle as exemplified in non-oncology drugs; therefore, they may lead to suboptimal dosing strategies and outcomes. While there is understandable urgency that may be unique to oncology, it is important that regulatory agencies insist on uniform application of principles of clinical pharmacology in oncology drug development.
Author Contributions:
Concept: Ratain.
Acquisition of data: Kang, Ratain.
Analysis and interpretation of data: Kang, Ratain.
Drafting of the manuscript: Kang, Ratain.
Statistical analysis: Kang, Ratain.
References
- 1.Parsad SD, Ratain MJ. BMJ. 7590. Vol. 334. 2007. Feb 24, Prescribing oral chemotherapy; p. 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeMario MD, Ratain MJ. Oral chemotherapy: rationale and future directions. J Clin Oncol. 1998 Jul;16(7):2557–67. doi: 10.1200/JCO.1998.16.7.2557. [DOI] [PubMed] [Google Scholar]
- 3.Partridge AH, Avorn J, Wang PS, Winer EP. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002 May 1;94(9):652–61. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 4.Singh BN, Malhotra BK. Effects of food on the clinical pharmacokinetics of anticancer agents: underlying mechanisms and implications for oral chemotherapy. Clin Pharmacokinet. 2004;43(15):1127–56. doi: 10.2165/00003088-200443150-00005. [DOI] [PubMed] [Google Scholar]
- 5.Ratain MJ, Cohen EE. The value meal: how to save $1,700 per month or more on lapatinib. J Clin Oncol. 2007 Aug 10;25(23):3397–8. doi: 10.1200/JCO.2007.12.0758. [DOI] [PubMed] [Google Scholar]
- 6.GlaxoSmithKline. TYKERB label. http://usgskcom/products/assets/us_tykerbpdf2007.
- 7.Williams DD, Peng B, Bailey CK, Wire MB, Deng Y, Park JW, et al. Effects of food and antacids on the pharmacokinetics of eltrombopag in healthy adult subjects: two single-dose, open-label, randomized-sequence, crossover studies. Clin Ther. 2009 Apr;31(4):764–76. doi: 10.1016/j.clinthera.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Scholler-Gyure M, Boffito M, Pozniak AL, Leemans R, Kakuda TN, Woodfall B, et al. Effects of different meal compositions and fasted state on the oral bioavailability of etravirine. Pharmacotherapy. 2008 Oct;28(10):1215–22. doi: 10.1592/phco.28.10.1215. [DOI] [PubMed] [Google Scholar]
- 9.Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer's disease. Clin Ther. 1998 Jul-Aug;20(4):634–47. doi: 10.1016/s0149-2918(98)80127-6. [DOI] [PubMed] [Google Scholar]
- 10.Koch KM, Reddy NJ, Cohen RB, Lewis NL, Whitehead B, Mackay K, et al. Effects of food on the relative bioavailability of lapatinib in cancer patients. J Clin Oncol. 2009 Mar 10;27(8):1191–6. doi: 10.1200/JCO.2008.18.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courtney R, Pai S, Laughlin M, Lim J, Batra V. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob Agents Chemother. 2003 Sep;47(9):2788–95. doi: 10.1128/AAC.47.9.2788-2795.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekar V, Kestens D, Spinosa-Guzman S, De Pauw M, De Paepe E, Vangeneugden T, et al. The effect of different meal types on the pharmacokinetics of darunavir (TMC114)/ritonavir in HIV-negative healthy volunteers. J Clin Pharmacol. 2007 Apr;47(4):479–84. doi: 10.1177/0091270006298603. [DOI] [PubMed] [Google Scholar]
- 13.Padhi D, Salfi M, Harris RZ. The pharmacokinetics of cinacalcet are unaffected following consumption of high- and low-fat meals. Am J Ther. 2007 May-Jun;14(3):235–40. doi: 10.1097/01.mjt.0000212703.71625.26. [DOI] [PubMed] [Google Scholar]
- 14.Ling J, Fettner S, Lum BL, Riek M, Rakhit A. Effect of food on the pharmacokinetics of erlotinib, an orally active epidermal growth factor receptor tyrosine-kinase inhibitor, in healthy individuals. Anticancer Drugs. 2008 Feb;19(2):209–16. doi: 10.1097/CAD.0b013e3282f2d8e4. [DOI] [PubMed] [Google Scholar]
- 15.Purkins L, Wood N, Kleinermans D, Greenhalgh K, Nichols D. Effect of food on the pharmacokinetics of multiple-dose oral voriconazole. Br J Clin Pharmacol. 2003 Dec;56 1:17–23. doi: 10.1046/j.1365-2125.2003.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin EH, Agrawal NG, Friedman EJ, Scott P, Mazina KE, Sun L, et al. A study to determine the effects of food and multiple dosing on the pharmacokinetics of vorinostat given orally to patients with advanced cancer. Clin Cancer Res. 2006 Dec 1;12(23):7039–45. doi: 10.1158/1078-0432.CCR-06-1802. [DOI] [PubMed] [Google Scholar]
- 17.Galanello R, Piga A, Cappellini MD, Forni GL, Zappu A, Origa R, et al. Effect of food, type of food, and time of food intake on deferasirox bioavailability: recommendations for an optimal deferasirox administration regimen. J Clin Pharmacol. 2008 Apr;48(4):428–35. doi: 10.1177/0091270007313327. [DOI] [PubMed] [Google Scholar]
- 18.Cardot JM, Lecaillon JB, Czendlik C, Godbillon J. The influence of food on the disposition of the antiepileptic rufinamide in healthy volunteers. Biopharm Drug Dispos. 1998 May;19(4):259–62. doi: 10.1002/(sici)1099-081x(199805)19:4<259::aid-bdd98>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Zhou H, Khalilieh S, Lau H, Guerret M, Osborne S, Alladina L, et al. Effect of meal timing not critical for the pharmacokinetics of tegaserod (HTF 919) J Clin Pharmacol. 1999 Sep;39(9):911–9. doi: 10.1177/00912709922008524. [DOI] [PubMed] [Google Scholar]
- 20.Services USDoHaH. Guidance for Industry: Food-Effect Bioavailability and Fed Bioequivalence Studies. Food and Drug Administration Center for Drug Evaluation and Research (CDER); Dec, 2002. [Google Scholar]
- 21.Hellriegel ET, Bjornsson TD, Hauck WW. Interpatient variability in bioavailability is related to the extent of absorption: implications for bioavailability and bioequivalence studies. Clin Pharmacol Ther. 1996 Dec;60(6):601–7. doi: 10.1016/S0009-9236(96)90208-8. [DOI] [PubMed] [Google Scholar]
- 22.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009 Jan-Feb;59(1):56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 23.Ruddy KJ, Partridge AH. Adherence with adjuvant hormonal therapy for breast cancer. Ann Oncol. 2009 Mar;20(3):401–2. doi: 10.1093/annonc/mdp039. [DOI] [PubMed] [Google Scholar]
- 24.Stone VE, Hogan JW, Schuman P, Rompalo AM, Howard AA, Korkontzelou C, et al. Antiretroviral regimen complexity, self-reported adherence, and HIV patients' understanding of their regimens: survey of women in the her study. J Acquir Immune Defic Syndr. 2001 Oct 1;28(2):124–31. doi: 10.1097/00042560-200110010-00003. [DOI] [PubMed] [Google Scholar]
- 25.Stone VE. Strategies for optimizing adherence to highly active antiretroviral therapy: lessons from research and clinical practice. Clin Infect Dis. 2001 Sep 15;33(6):865–72. doi: 10.1086/322698. [DOI] [PubMed] [Google Scholar]
- 26.Walsh JC, Horne R, Dalton M, Burgess AP, Gazzard BG. Reasons for non-adherence to antiretroviral therapy: patients' perspectives provide evidence of multiple causes. AIDS Care. 2001 Dec;13(6):709–20. doi: 10.1080/09540120120076878. [DOI] [PubMed] [Google Scholar]
- 27.Reigner B, Verweij J, Dirix L, Cassidy J, Twelves C, Allman D, et al. Effect of food on the pharmacokinetics of capecitabine and its metabolites following oral administration in cancer patients. Clin Cancer Res. 1998 Apr;4(4):941–8. [PubMed] [Google Scholar]
- 28.Novartis. Tasigna® (nilotinib) Capsules Package Insert. http://wwwaccessdatafdagov/drugsatfda_docs/label/2007/022068lblpdf2007.