Abstract
The expression of many bacterial phenotypes is regulated according to the concentration of chemical cues that they or other bacteria produce, a process often termed quorum sensing. Many aspects of the environment can affect cue concentration. Thus these molecules might be indirect proxies for any one or combination of environmental factors. Recent research suggests that the adaptive significance of quorum sensing varies depending on its evolutionary and ecological context. Consequently, some researchers have proposed new terms, each emphasizing different adaptive functions, for the quorum sensing process. However, these new terms generate potential for a semantic quagmire and perpetuate the questionable notion that we can identify a single, dominant environmental feature to which the microbes respond. In fact, the ecological context of quorum sensing regulation, like the process itself, is complex and impacted by multiple aspects of natural environments.
Keywords: cell-cell signaling, communication, diffusion-sensing, confinement-induced quorum sensing, compartment-sensing, chemical gradient, cue
Quorum sensing gene regulation
Challenged by the ever-changing world in which they reside, bacterial cells have evolved a variety of means by which their behavior is modified based on their biotic and abiotic environment. Often, this simply involves responding to direct environmental cues. This is typified by the classic example of the lac operon of Escherichia coli, wherein the availability of lactose in the environment (monitored as cytoplasmic levels of the isomer allolactose) leads to repressor inactivation and thereby induction of genes involved in the catabolism of lactose [1]. However, bacteria also employ more sophisticated mechanisms of genetic regulation, sometimes modifying their behavior based on cues that only indirectly convey information about the local environment. Bacterial quorum sensing is the phenomenon by which a bacterium regulates its gene expression in response to the concentration of indirect, diffusible cues produced and released into the local environment by itself or other bacteria, either of the same or different species [2–4]. The diffusible compounds employed in quorum sensing regulation have often been referred to as signal molecules. However, the term ‘signal’ has a very important meaning in evolutionary biology, indicating chemical compounds produced explicitly to invoke a response from other organisms to coordinate activities between the signal producer and the responder (see Ref. [5]). This might not apply to all cases of quorum sensing regulation, and for this discussion we have elected to describe these chemical compounds less restrictively as quorum sensing cues.
The quorum sensing process was historically known as autoinduction (genetic induction in response to self-produced cues) or less frequently as alloinduction (response to cues produced by other bacterial species) [6–9]. More recently the term quorum sensing was proposed to embody the concept that bacteria can utilize this mechanism to respond to bacterial population density [2]. Over the years since its introduction, the usage of the term quorum sensing has evolved to be a general descriptor of the process of cue production and response at the level of gene expression and is now broadly used with limited regard for the ecological context of the behavior. For consistency and ease of comparison with this large body of prior research, we will utilize the term quorum sensing here in its broadest sense, and inclusive to most of these prior studies.
A wide array of bacterial phenotypes are regulated by quorum sensing across many bacterial species—including antibiotic resistance, plasmid conjugation, mutualistic associations, pathogenesis functions, and motility [3]. Quorum sensing mechanisms are diverse but two of the most common forms rely on acylhomoserine lactones (AHLs) and oligopeptides, in Gram-negative and Gram-positive bacteria, respectively (Box 1). A third intensively studied form is found in both Gram-negative and Gram-positive bacteria and employs a family of related molecules broadly termed autoinducer-2 (AI-2) (Box 1). The control of quorum sensing target functions depends on accumulation of the cue to concentrations at which cells can perceive and respond to it. Many biotic and abiotic factors can influence the chemical gradients of these molecules (see Ref. [10] for a comprehensive recent review). These factors include the spatial distribution of cells producing the cue, the rates at which the cue is produced and diffuses, and the stability of the cue itself. It is plausible that bacteria could be using quorum sensing cues as indirect proxies for any of the factors influencing the concentration and biological perception of these cues (Figure 1). This has led several researchers to introduce new terms including diffusion sensing, confinement-induced quorum sensing, and efficiency sensing, to describe these genetic and biochemical processes (Table 1). These new names emphasize different hypothesized adaptive functions for the quorum sensing regulation related to a specific sub-set of factors influencing cue concentrations. Here we discuss the importance of recognizing that the adaptive function of quorum sensing likely varies from system to system, depending on the ecological and evolutionary context of the organism and the regulated trait. However, we argue that using a different term for each possible adaptive function does more to confuse our understanding of the quorum sensing process, than it does to clarify it. Instead, we argue that quorum sensing regulation is best viewed broadly with full appreciation of the many environmental factors that influence it and thereby determine its ecological and adaptive significance.
Box 1. Common mechanisms of quorum sensing
The mechanisms of quorum sensing are varied but generally involve the production and detection of one or several molecules that, at sufficiently high concentrations, influence the expression of target functions. Diverse quorum sensing cues have now been identified. In Gram-negative bacteria acylated homoserine lactones (AHLs) are common quorum sensing cues. These molecules are typically synthesized by LuxI-type AHL synthases from acyl-acyl carrier proteins and S-adenosylmethionine [38]. In the most common examples, AHLs cross cell membranes via passive diffusion, though in some cases they are assisted by efflux systems. Above a threshold intracellular concentration AHLs bind a LuxR family transcriptional regulator, which once associated with the AHL signal can bind DNA and promote the expression of downstream target genes. A subset of LuxR-type proteins bind DNA in the absence of AHLs and are deactivated by AHLs.
In contrast to the AHL-based systems, the quorum sensing signals of Gram-positive bacteria are typically small peptides that are often post-translationally processed and exported out of the cell. When these signal peptides are sufficiently concentrated they either bind to a membrane-bound histidine sensor kinase which triggers a two-component phosphorylation cascade, or are transported into the cell where they can act directly on the response pathway, which ultimately influences transcription of target genes.
A third type of quorum sensing system has been attributed to both Gram-negative and Gram-positive bacteria. The quorum sensing cues of these systems are derived from 4, 5-dihydroxy-2,3-pentanedione and were originally described for the regulation of Vibrio harveyi bioluminescence [39] and are broadly termed autoinducer-2 (AI-2). LuxS catalyzes the production of AI-2 from S-ribosylhomocysteine (SRH) as a side reaction of homocysteine synthesis. LuxS mediated conversion of SRH into homocysteine, rather than AI-2, is an important step within the activated methyl cycle [30, 40]. The observation that luxS is very broadly distributed among bacteria was initially interpreted to suggest that AI-2 quorum sensing was also widespread and may facilitate interspecies interactions. However, the important metabolic role of LuxS in the activated methyl cycle offers an alternative reason that luxS may be broadly distributed. Indeed, how often LuxS and by extension AI-2 function in quorum sensing remains an open question for future research [31–35, 40].
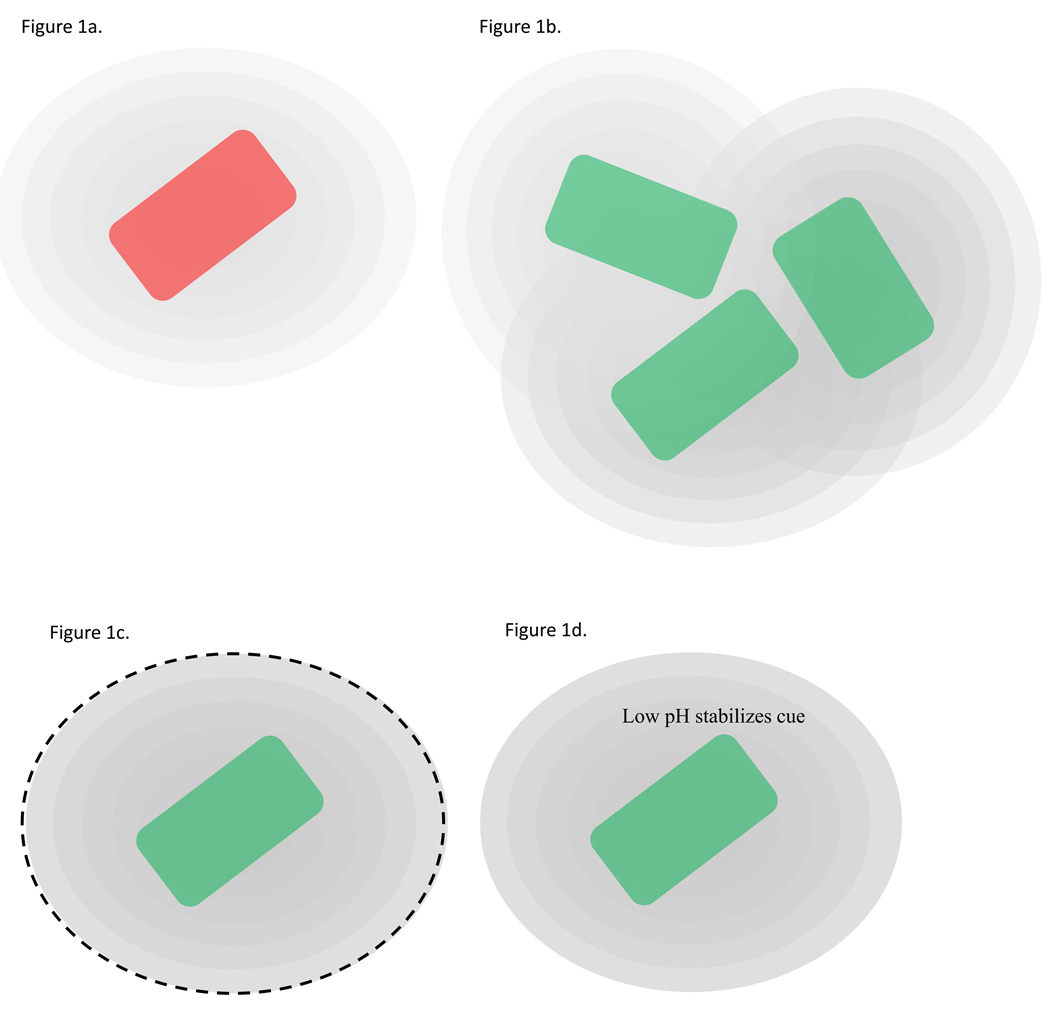
Figure 1.
Biotic and abiotic factors that bacterial cells might indirectly monitor using quorum sensing cues. The adaptive significance of quorum sensing regulation could relate to any one or a combination of these factors. (a) A cell at low local population density without barriers to diffusion or under environmental conditions under which quorum sensing cues are relatively unstable, experiences low intracellular concentrations of the quorum sensing cue. Consequently this cell is not active for quorum sensing regulated functions (cell is red). (b) When cue producing cells are at high local density the concentration of the cue that each cell experiences is higher due to the cumulative effects of neighboring cells. Thus high local population density is likely to be associated with elevated cue concentrations and thereby activation of quorum sensing regulated target functions (cell is green). (c) Limited diffusion or confinement results in high concentrations of quorum sensing cues in and around cells, leading to active quorum sensing regulated target functions (cell is green). (d) Environmental conditions under which quorum sensing cues are relatively stable or produced at high rates. A cell is more likely to experience high intracellular concentrations of the quorum sensing cue, and hence activate target functions (cell is green).
Table 1.
New terms emphasizing different factors influencing the concentration of quorum sensing cues
| Proposed name | Factor emphasized | Refs |
|---|---|---|
| Relative spatial location of cells | ||
| Quorum sensing | Local population density | [2] |
| Positional sensing | Spatial organization of cells | [16] |
| Cluster sensing | Spatial organization and distribution of cells in clumps |
[16] |
| Diffusion processes | ||
| Diffusion sensing | Diffusion rates | [18] |
| Compartment sensing | Limited diffusion due to enclosure | [41] |
| Confinement-induced quorum sensing | Limited diffusion due to enclosure | [13, 17] |
| Both spatial positioning of cells and diffusion processes | ||
| Efficiency sensing | Local cell density and diffusion rates | [14] |
| Cumulative gradient sensing | Accumulated cue as determined by cell density, production rate, and diffusion rate |
[16] |
| Environmental conditions | ||
| Diel sensing | Cue stability due to pH conditions | [20] |
The adaptive significance of quorum sensing
The name ‘quorum sensing’ highlights the idea that high local bacterial population densities are likely to be associated with elevated concentrations of the cue molecule and thus the expression of quorum sensing regulated functions. This, together with the observation that the target functions of many quorum sensing systems require the action of multiple cells, suggests that certain bacteria might be using quorum sensing systems to coordinate their behavior according to a minimum local population density, that is to say the presence of a ‘quorum’ of cells. This analogy to a quorum has often proven useful, since local population density frequently features prominently in quorum sensing regulated functions. Indeed, many quorum sensing regulated phenotypes require groups of cells to function. These include the multicellular swarming motility of several bacterial species [11] and the mutualistic bioluminescence of Vibrio fisheri [12].
However the name ‘quorum sensing’ also has shortcomings. The emphasis of the term on the importance of local population density leads to oversimplification and neglect of other factors that can influence the concentration of the cues that cells produce and perceive. Factors influencing the chemical gradients of quorum sensing cues, such as local flow and diffusion rates, can also determine their accumulation and concentration and thereby the expression of quorum sensing regulated phenotypes. Accordingly, the framework in which researchers examine quorum sensing ideally should account for a range of potential influences from a variety of environmental factors.
Several recent papers examine the importance of diffusion rates to quorum sensing— thereby highlighting some of its physical and chemical complexities, and the difficulties generated by an overly simplified view of the process [13–17]. An insightful review from Redfield [18], pointed out that bacteria might be employing cues to monitor diffusion, and proposed the term ‘diffusion sensing’. For most systems, it is challenging and arguably impossible to completely separate the influence of diffusion and local population density on the process of quorum sensing. Any given environment has intrinsic levels of diffusion, that might temporally vary to lesser or greater extents, and that impact the residence time and accumulation of diffusible cues. The relevant question is whether the bacteria are employing cues primarily to monitor the diffusion state of their environment or instead to monitor bacterial population density? It would seem likely that bacteria which inhabit a niche with highly variable diffusion rates (e.g. biofilm communities on a rock in a tide pool) might greatly benefit from timing the release of exoenzymes and other externalized factors to occasions when the flow and diffusion were limited, and thus the benefits will remain proximal to the producing cells. Conversely, bacteria that inhabit a niche within a diffusively static environment might utilize cue accumulation to respond to other factors, including but not restricted to local population density.
Bacteria might similarly benefit by cueing into other fluctuations in the environment. Short-chain AHLs quorum sensing cues are less stable at alkaline pH such that microbial mat communities that reside in environments with variable pH could be able to cue into these fluctuations and coordinate some behaviors with appropriate environmental pH. In these mat communities the pH fluctuations exhibit a daily cycle due to photosynthetic activity, and thus the process has been termed ‘diel sensing’ [19–20] (Figure 1d).
In actuality, most bacteria simultaneously experience multiple environmental factors influencing quorum sensing regulation. In certain cases, it might be very difficult to clearly identify the predominant adaptive significance of quorum sensing regulation in its natural ecological context. The well-studied mutualistic bioluminescence and colonization of marine animals by V. fischeri provides an illustrative example. The transition of V. fischeri cells from a free-living state in the water column to mutualistic residence inside the light organ of squid partners is associated with the quorum sensing regulated bioluminescence [12] and repression of motility [21]. The light organ environment is more diffusively constrained than the open ocean, but the host animal also supports the accumulation of high population densities and eventual induction of bioluminescence, such that both diffusion and population density simultaneously influence the quorum sensing process.
Dulla and Lindow [22] found that in Pseudomonas syringae quorum sensing induction of traits associated with virulence and the epiphytic environment was more common and required fewer cells on plant leaves with limited water availability. The limited hydration creates greater barriers to diffusion than in wet environments wherein signals could more readily diffuse away from the producing cells. In this case, the process remains responsive to population density, but the activating density varies substantially depending on the level of hydration. It seems unlikely that these bacteria are responding exclusively to their population density, the saturation level, or the diffusion environment, but rather a combination of all of these, and perhaps other factors encountered in its natural environment. Two revealing recent studies cleverly trap and monitor the quorum sensing induction of small numbers of cells, demonstrating that in a very confined space even a single cell can constitute a quorum, and they describe this as confinement-induced quorum sensing [13, 17]. The observation that quorum sizes can be very small—even as small as one cell—deftly illustrates the limitations imposed by over-simplistic interpretation of the name ‘quorum sensing’ as synonymous with high population density [13, 22–23].
A remarkable recent study revealed that Rhodopseudomonas palustris regulates multiple attributes by a quorum sensing mechanism involving LuxR and LuxI type proteins (RpaR and RpaI), but instead of employing an AHL, R. palustris produces a para-coumaroyl homoserine lactone (pC-HSL) [24]. Synthesis of pC-HSL requires the presence of coumaric acid from the environment, a metabolite often released by plants into the soil, which is directly incorporated into the quorum sensing cue. In the absence of coumaric acid R. palustris does not synthesize the quorum sensing cue, thereby precluding quorum sensing induction. Is this quorum sensing, or might it be described it as ‘precursor sensing’? Though coumaric acid is a prerequisite for pC-HSL synthesis, monitoring coumaric acid levels is unlikely to be the primary role for release of the pC-HSL cue.
Quorum sensing and bacterial social behavior
The importance of microbial interactions to the behavior, ecology, evolution, and molecular genetics of bacterial populations has brought these topics to the forefront of microbiology, and has revealed that bacterial populations can be remarkably social. Whereas bacteria have traditionally been thought of as simple, single-celled organisms, we now know that bacterial populations and communities commonly exhibit complex behaviors such as intra- and interspecific communication, kin discrimination, and cooperation [25].
Quorum sensing is often discussed in terms of representing examples of bacterial communication, signaling, and cooperation. In many cases, quorum sensing regulates phenotypes that appear to involve the coordinated action of many cells with group benefits such as bioluminescence, swarming motility, and virulence functions. Yet in general there have been few studies examining the individual and group fitness consequences of quorum sensing regulated phenotypes. A notable exception to this is the quorum sensing of Pseudomonas aeruginosa, for which there is empirical evidence that quorum sensing does mediate cooperative functions that are vulnerable to cheating [26–29]. However, quorum sensing and its regulated target functions may or may not always be social—that is, dependent on interactions between individuals. The potential for these molecules to mediate signaling, communication, and cooperation among bacterial cells does not necessarily indicate that they actually function in this capacity. Indeed simply determining whether or not an extracellular cue even functions in quorum sensing processes can be difficult. This is well illustrated by the case of AI-2 quorum sensing where this cue’s role in quorum sensing remains somewhat controversial. LuxS, the AI-2 synthase, has an important role in the activated methyl cycle, therefore complicating interpretations of deficiencies in the LuxS pathway with respect to the role of AI-2 as a quorum sensing cue (Box 1) [30–35].
Moreover, not all quorum sensing cues are signals involved in communication between individuals [5]. For example, several quorum sensing systems might regulate the expression of target functions according to the concentration of quorum sensing cues as a proxy for the enclosure of the bacterial cell in a eukaryotic endosome [17, 23]. In this case, communication and signaling between bacterial cells could be completely irrelevant to the adaptive function of quorum sensing regulation. Even in a closed system such as the endosome, however, there could remain opportunities for cooperation among cells that are enclosed together. Yet, the production of a cue is not in itself a demonstration of social behavior. Establishing that a trait is cooperative requires identifying a costly action that benefits a group [36]. Thus, future research into the fitness costs and benefits associated with quorum sensing behaviors is necessary to evaluate whether or not quorum sensing involves social interactions and phenotypes.
Conclusions and future directions
Identification and consideration of the environmental conditions and processes that can impact quorum sensing cue production, concentration, perception and gene expression response, are worthwhile and interesting endeavors. We argue however, that the introduction of a new term each time a different factor is found to influence the signaling process is more likely to introduce semantic discordance, rather than to improve understanding. It would seem that irrespective of which encapsulating term is used to describe these processes, quorum sensing or diffusion sensing or diel sensing or the others, that researchers are best served by viewing these processes broadly, and recognizing the many different levels and the diverse situations by which they could be impacted.
The widespread use of the term ‘quorum sensing’ in published work should not be so strictly interpreted as to suggest that the sole adaptive significance for all of these systems is to cue into the presence of a quorum (i.e. the local density of cells). Indeed quorum sensing molecules might also provide information as indirect cues for several possible environmental factors that influence their accumulation and perception (Figure 1). As has been pointed out previously [37], autoinduction and alloinduction are arguably the most functionally neutral terms that one might apply to these processes. At this juncture however, with wide recognition and frequent over-generalization of the term quorum sensing, it would seem prudent to promote a fully nuanced view of the quorum sensing process, including the many different environmental factors by which it could be influenced. Characterizing the adaptive function of quorum sensing regulation of gene expression requires assessing its evolutionary and ecological context, which can vary significantly depending on the bacterial species and target phenotype. Pursuing these issues for specific bacterial systems remains a challenge for future research. In the end the value of a name such as quorum sensing might be more what it has evolved to represent, rather than its literal interpretation.
Acknowledgements
We would like to thank Yaniv Brandvain, Mike Hibbing, Anna Larimer, Peter Merritt, and Elise Morton for helpful conversations. TGP was supported by the Genetics, Cellular and Molecular Sciences Training Grant (T32GM007757) and quorum sensing research in the Fuqua lab is funded by the NSF (MCB-0703467, DEB-0608155) and the NIH (GM092660).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Müller-Hill B. The lac operon: a short history of a genetic paradigm. de Gruyter; 1996. [Google Scholar]
- 2.Fuqua WC, et al. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. Journal of Bacteriology. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams P, et al. Look who's talking: communication and quorum sensing in the bacterial world. Philosophical Transactions of the Royal Society, Series B. 2007;362:1119–1134. doi: 10.1098/rstb.2007.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuqua C, et al. Regulation of gene expression by cell-to-cell communication: acylhomoserine lactone quorum sensing. Annual Review of Genetics. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 5.Keller L, Surette MG. Communication in bacteria: an ecological and evolutionary perspective. Nature Reviews Microbiology. 2006;4:249–258. doi: 10.1038/nrmicro1383. [DOI] [PubMed] [Google Scholar]
- 6.Nealson KH, et al. Cellular control of synthesis and activity of bacterial luminescent system. Journal of Bacteriology. 1970;104:313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg EP, et al. Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Archives of Microbiology. 1979;120:87–91. [Google Scholar]
- 8.Tomasz A, Hotchkiss RD. Regulation of transformability of pneumococcal cultures by macromolecular cell products. Proceedings of the National Academy of Sciences, USA. 1964;51:480–487. doi: 10.1073/pnas.51.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pakula R, Walczak W. On the nature of competence of transformable streptococci. Journal of General Microbiology. 1963;31:125–133. doi: 10.1099/00221287-31-1-125. [DOI] [PubMed] [Google Scholar]
- 10.Boyer M, Wisniewski-Dye F. Cell-cell signalling in bacteria: not simply a matter of quorum. FEMS Microbiology Ecology. 2009;70:1–19. doi: 10.1111/j.1574-6941.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 11.Daniels R, et al. Quorum sensing and swarming migration in bacteria. FEMS Microbiology Reviews. 2004;28:261–289. doi: 10.1016/j.femsre.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Stevens AM, Greenberg EP. Quorum sensing in Vibrio fischeri: Essential elements for activation of the luminescence genes. Journal of Bacteriology. 1997;179:557–562. doi: 10.1128/jb.179.2.557-562.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boedicker JQ, et al. Microfluidic confinement of single cells of bacteria in small volumes initiates high-density behavior of quorum sensing and growth and reveals its variability. Angewandte Chemie-International Edition. 2009;48:5908–5911. doi: 10.1002/anie.200901550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hense BA, et al. Does efficiency sensing unify diffusion and quorum sensing? Nature Reviews Microbiology. 2007;5:230–239. doi: 10.1038/nrmicro1600. [DOI] [PubMed] [Google Scholar]
- 15.Gantner S, et al. In situ quantitation of the spatial scale of calling distances and population density-independent N-acylhomoserine lactone-mediated communication by rhizobacteria colonized on plant roots. FEMS Microbiology Ecology. 2006;56:188–194. doi: 10.1111/j.1574-6941.2005.00037.x. [DOI] [PubMed] [Google Scholar]
- 16.Alberghini S, et al. Consequences of relative cellular positioning on quorum sensing and bacterial cell-to-cell communication. FEMS Microbiology Letters. 2009;292:149–161. doi: 10.1111/j.1574-6968.2008.01478.x. [DOI] [PubMed] [Google Scholar]
- 17.Carnes EC, et al. Confinement-induced quorum sensing of individual Staphylococcus aureus bacteria. Nature Chemical Biology. 2010;6:41–45. doi: 10.1038/nchembio.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redfield RJ. Is quorum sensing a side effect of diffusion sensing? Trends in Microbiology. 2002;10:365–370. doi: 10.1016/s0966-842x(02)02400-9. [DOI] [PubMed] [Google Scholar]
- 19.Decho AW, et al. Quorum sensing in natural environments: emerging views from microbial mats. Trends in Microbiology. 2010;18:73–80. doi: 10.1016/j.tim.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Decho AW, et al. Autoinducers extracted from microbial mats reveal a surprising diversity of N-acylhomoserine lactones (AHLs) and abundance changes that may relate to diel pH. Environmental Microbiology. 2009;11:409–420. doi: 10.1111/j.1462-2920.2008.01780.x. [DOI] [PubMed] [Google Scholar]
- 21.Lupp C, Ruby EG. Vibrio fischeri uses two quorum-sensing systems for the regulation of early and late colonization factors. Journal of Bacteriology. 2005;187:3620–3629. doi: 10.1128/JB.187.11.3620-3629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dulla G, Lindow SE. Quorum size of Pseudomonas syringae is small and dictated by water availability on the leaf surface. Proceedings of the National Academy of Sciences, USA. 2008;105:3082–3087. doi: 10.1073/pnas.0711723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qazi SNA, et al. agr expression precedes escape of internalized Staphylococcus aureus from the host endosome. Infection and Immunity. 2001;69:7074–7082. doi: 10.1128/IAI.69.11.7074-7082.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaefer AL, et al. A new class of homoserine lactone quorum-sensing signals. Nature. 2008;454:U595–U596. doi: 10.1038/nature07088. [DOI] [PubMed] [Google Scholar]
- 25.West SA, et al. The social lives of microbes. Annual Review of Ecology Evolution and Systematics. 2007;38:53–77. [Google Scholar]
- 26.Kohler T, et al. Cooperation and virulence of clinical Pseudomonas aeruginosa populations. Proceedings of the National Academy of Sciences, USA. 2009;106:6339–6344. doi: 10.1073/pnas.0811741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rumbaugh KP, et al. Quorum sensing and the social evolution of bacterial virulence. Current Biology. 2009;19:341–345. doi: 10.1016/j.cub.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 28.Diggle SP, et al. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450:U411–U417. doi: 10.1038/nature06279. [DOI] [PubMed] [Google Scholar]
- 29.Sandoz KM, et al. Social cheating in Pseudomonas aeruginosa quorum sensing. Proceedings of the National Academy of Sciences, USA. 2007;104:15876–15881. doi: 10.1073/pnas.0705653104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vendeville A, et al. Making 'sense' of metabolism: Autoinducer-2, LuxS and pathogenic bacteria. Nature Reviews Microbiology. 2005;3:383–396. doi: 10.1038/nrmicro1146. [DOI] [PubMed] [Google Scholar]
- 31.Winzer K, et al. LuxS: its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology. 2002;148:909–922. doi: 10.1099/00221287-148-4-909. [DOI] [PubMed] [Google Scholar]
- 32.Rezzonico F, Duffy B. Lack of genomic evidence of AI-2 receptors suggests a non-quorum sensing role for luxS in most bacteria. BMC Microbiology. 2008;8:154. doi: 10.1186/1471-2180-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nadell CD, et al. The sociobiology of biofilms. FEMS Microbiology Reviews. 2009;33:206–224. doi: 10.1111/j.1574-6976.2008.00150.x. [DOI] [PubMed] [Google Scholar]
- 34.Heurlier K, et al. Growth deficiencies of Neisseria meningitidis pfs and luxS mutants are not due to inactivation of quorum sensing. Journal of Bacteriology. 2009;191:1293–1302. doi: 10.1128/JB.01170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardie KR, Heurlier K. Establishing bacterial communities by 'word of mouth': LuxS and autoinducer 2 in biofilm development. Nature Reviews Microbiology. 2008;6:635–643. doi: 10.1038/nrmicro1916. [DOI] [PubMed] [Google Scholar]
- 36.Platt TG, Bever JD. Kin competition and the evolution of cooperation. Trends In Ecology & Evolution. 2009;24:370–377. doi: 10.1016/j.tree.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunny GM, Winans SC, editors. Cell-cell signaling in bacteria. American Society for Microbiology; 1999. [Google Scholar]
- 38.Parsek MR, et al. Acyl homoserine-lactone quorum-sensing signal generation. Proceedings of the National Academy of Sciences, USA. 1999;96:4360–4365. doi: 10.1073/pnas.96.8.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bassler BL, et al. Intercellular signaling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Molecular Microbiology. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 40.De Keersmaecker SCJ, et al. Let LuxS speak up in AI-2 signaling. Trends in Microbiology. 2006;14:114–119. doi: 10.1016/j.tim.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Winzer K, et al. Bacterial cell-to-cell communication: Sorry, can't talk now - gone to lunch! Current Opinion In Microbiology. 2002;5:216–222. doi: 10.1016/s1369-5274(02)00304-1. [DOI] [PubMed] [Google Scholar]