Abstract
Recurrent respiratory papillomas are pre-malignant tumors of the airway caused by human papillomaviruses (HPVs), primarily types 6 and 11. We had reported that respiratory papillomas overexpress the epidermal growth factor receptor (EGFR), the small GTPase Rac1 and cyclooxygenase-2 (COX-2), and have enhanced Nuclear Factor-κB (NFκB) activation with decreased levels of IκB-β but not IκB-α. We also showed that EGFR-activated Rac1 mediates expression of COX-2 through activation of p38 mitogen activated protein kinase. We have now asked whether the p21-activated kinases Pak 1 or Pak2 mediate activation of p38 by Rac1 in papilloma cells. Pak1 and Pak2 were constitutively activated in vivo in papilloma tissue compared to normal epithelium, and Rac1 siRNA reduced the level of both phospho-Pak1 and phospho-Pak2 in cultured papilloma cells. Reduction in Pak1 and Pak2 with siRNA decreased COX-2 expression in papilloma cells, increased levels of IκB-Iβ and reduced nuclear localization of NF-κB, but had no effect on p38 phosphorylation. Our studies suggest that Rac1→ Pak1/Pak2→ NFκB is a separate pathway that contributes to the expression of COX-2 in HPV-induced papillomas independently of the previously described Rac1→ p38 → COX-2 pathway.
Keywords: COX-2, Pak1, Pak2, Rac 1, Respiratory Papillomas, HPV
Introduction
Recurrent respiratory papillomas (RRP) are benign tumors induced by human papillomaviruses (HPVs), primarily types 6 or 111,2 which are members of a large family of viruses that cause benign and malignant tumors.3 Respiratory papillomas cause significant morbidity and mortality because they block the airway. Standard therapy is surgical removal, and there is no currently-approved medical treatment. In approximately three percent of patients the papillomas convert to aggressive squamous-cell cancers.
Respiratory papillomas over-express high levels of the epidermal growth factor receptor (EGFR),4,5 have altered signal transduction pathways linked to the EGFR,6–9 and have increased activation of NF-κB p50.10 COX-2 is expressed in papillomas, requiring both EGFR and Phosphoinositol 3-kinase (PI3K) activity.11 COX-2 expression in papilloma cells is mediated in part through Rac1-dependent activation of the kinase p38, while the low basal level of COX-2 in normal cells is independent of p38 activation.9 Others have shown that RelA NF-κB activates COX-2 expression,12 while Thornburg et al.13 reported that NF-κB p50 can also activate gene expression when complexed with a co-activator. Thus, the increased activation of NF-κB p50 could contribute to COX-2 expression in papilloma cells. Inhibition of COX-2 suppresses proliferation of papilloma cells and enhances spontaneous apoptosis, suggesting that it plays an important role in the biology of the tumors.11 Moreover, Farley et al.14 reported that celecoxib, a selective COX-2 inhibitor, showed efficacy in the treatment of moderate to severe cervical dysplasia. Thus, understanding the pathway of COX-2 induction could potentially lead to targets for treatment of many HPV-induced diseases.
The p21-activated kinases (Paks) are a family of serine/threonine protein kinases that can be activated by multiple mechanisms, including the small GTPases Cdc42 and Rac1.15–17 Paks can be divided into Group I (Paks1 to 3) and Group II (Pak4 to 6) isoforms. The catalytic activity of the Group I Paks is induced upon binding to GTP-bound Rac or Cdc42. Pak1,15 Pak2,18 and Pak319 phosphorylate a number of substrates, regulating the cytoskeleton and both proliferative and anti-apoptotic signaling.20 Elevated expression and hyperactivity of Paks is linked to tumorigenesis, invasion and metastasis.16,17,21,22 In this study, we have asked whether Pak 1 and/or Pak2 might play a role in mediating Rac1 activation of p38, and the subsequent induction of COX-2, in papilloma cells.
Materials and Methods
Tissues and Cultured Cells
The use of human tissues and cultured cells was approved by the Institutional Review Board of the Feinstein Institute for Medical Research, North Shore- Long Island Jewish Health System, in accordance with an assurance filed with and approved by the Department of Health and Human Services. Informed consent for use of tissues for research was obtained from each subject or the subject’s guardian. Laryngeal papillomas and normal laryngeal epithelium were obtained from surgical biopsies. Biopsies were used to establish primary cell cultures as previously described,23 or were snap frozen in liquid nitrogen until used. Cells were sub-cultured in supplemented serum-free KGM (Clonetics, San Diego, CA, USA),11 and studies repeated at least three times with cells from different patients to control for variation among specimens.
Immunohistochemistry
Normal laryngeal and papilloma specimens were fixed in 10% buffered formalin, paraffin-embedded, and processed for immunohistochemical staining by conventional methods. Primary antibodies were anti-phospho (ser144 Pak1/ser141 Pak2/ser139 Pak3 (Invitrogen Life Sciences, Carlsbad, CA, USA), Pak1 or Pak2 (Cell Signaling Technology, Danvers, MA, USA), and anti-p50 NFκB and IκB-β (Santa Cruz Biotechnology, CA, USA). Secondary antibodies were horseradish peroxidase anti-mouse and anti-rabbit IgG (pierce, Rockford, IL, USA). Staining was detected by the avidin-biotin-complex (ABC) method with diaminobenzidine as label (Vectastain ABC Elite Kit, Vector Laboratories, Burlingame, CA, USA), and sections counterstained lightly with hematoxylin.
Western Blot Analysis
Pulverized frozen tissues and cultured cells were extracted as previously described,10,11 30 µg proteins separated on 10% SDS-PAGE, and electroblotted onto nitrocellulose membranes (Schleicher & Schuell, Keene, NH, USA), blocked with dried milk, incubated with primary antibody overnight at 4°C, washed and incubated with secondary antibody. Standard molecular weight markers (BIO-RAD, broad range, Hercules, CA, USA) were used for molecular weight estimation. After detection, blots were stripped and re-probed with antibodies to β-actin to confirm equivalence in loading and transfer. The immunoreactive species were detected with Super Signal West Pico chemiluminescent substrates (Pierce, Rockford, IL). Signal intensity was quantified by UN-SCAN-IT Program (Silk Scientific, Inc., Orem, UT, USA). Primary antibodies were as follows: anti-COX-2, anti-p50 NFκB, anti-IκBβ (C-20), and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-phospho-Pak1 (ser199/204)/Pak2 (ser192/197), anti-Pak1 and anti-Pak2 from Cell Signaling Technology, Inc. (Waltham, MA, USA), anti-Rac1 (Upstate Biotechnology, Temecula, CA, USA), anti-phospho-p38 and anti-p38 (BD Transduction Laboratories, San Diego, CA, USA). Secondary antibodies were horseradish peroxidase anti-mouse and anti-rabbit IgG (Pierce, Rockford, IL, USA).
Quantitative Analysis of Pak1 and Pak2 mRNA
Total RNA was extracted from powdered frozen tissue using the RNeasy Mini Kit (QIAGEN Inc., Chatsworth, CA, USA) and DNase treated to avoid genomic DNA contamination. TaqMan primers and probes were designed using Primer Express® software version 1.5 (Applied Biosystems, Foster City, CA, USA). Real-time PCR was performed as previously described.24 Data was analyzed using Sequence Detection System (SDS) software version 2.2.1. Relative expression of Pak1 or Pak2 mRNA in papilloma samples compared to normal samples was calculated by the delta delta Ct method (User Bulletin #2, Applied Biosystems Inc., USA). The Pak1 forward primers were 5’-CCCATTTCACCTACTGAAAATAACA-3’, which anneals between residues 1019 and 1044 and reverse primer 5’- CCCACACTCACTATGCTTCG -3’, which anneals between residues between 1150 and 1130. Probe sequences were: CCCATTTCACCTACTGAAAATAACA and CCCACACTCACTATGCTTCG. The Pak2 forward primer was 5’-CAGAAACAGCCAAAGAAGGAAC -3’ which anneals between residues 982 and 1004, the reverse primer 5’- AACGATGTTGGGATTTTTCAA-3’, which anneals between residues 1057 and 1036. The Pak2 probe sequences were CAGAAACAGCCAAAGAAGGAAC and AACGATGTTGGGATTTTTCAA. Human β-actin primers were 5’- CCTGGCACC CAGCACAAT-3’, which anneals between 1030 and 1047, and 5’- GCCGATCCACACGGAGTACT-3’, which anneals between residues 1099 and 1080, with probe sequence 5’-ATCAAGATCATTGCTCCTCCTGAGCGC-3’, which anneal between residues 1052 and 1078.
Transient Transfections of siRNAs
Cells cultured in KGM were transfected with specific siRNAs directed against Rac-1, Pak1 and Pak2 when they were 60–80% confluent. Two separate siRNAs were used to target Pak1 and Pak2. Luciferase siRNA was used as a control. Transfections were performed as previously described 9 and cells extracted for analysis 72 hr later. All siRNAs were purchased from Dharmacon (Chicago, IL, USA).
Rac1 siRNA: AAGGAGAUUGGUGCUGUAAAA.
Pak1 siRNA: GAAGAAAUAUACACGGUUU.
Pak 1′ siRNA: CAUCAAAUAUCACUAAGUCUU.
Pak2 siRNA: AGAAGGAACUGAUCAUUAA.
Pak2′ siRNA: GAAACUGGCCAAACCGUUAUU.
Inhibition of Group I Pak activity
Papilloma cells were cultured in supplemented KGM until sub-confluent, cultured overnight in KBM without growth factors, pre-treated with 50 µM IPA-3 (Tocris Bioscience, Missouri, USA) for one hour, then cultured in supplemented KGM for 24 hours, and cells extracted for western blot as described above. Control cultures were treated with solvent (DMSO).
Quantitation of Rac activity
Lysates of normal and papilloma tissues and primary cell cultures were analyzed for total Rac GTP levels using a G-LISA™ colorometric assay as instructed by the manufacturer (Cytoskeleton Inc., Denver CO, USA).
Statistical analysis
A Student’s t-test was used to determine statistical significance. Values were expressed as mean ± SD of multiple experiments using tissues or cells from different patients. A difference between groups of p<0.05 was considered significant.
Results
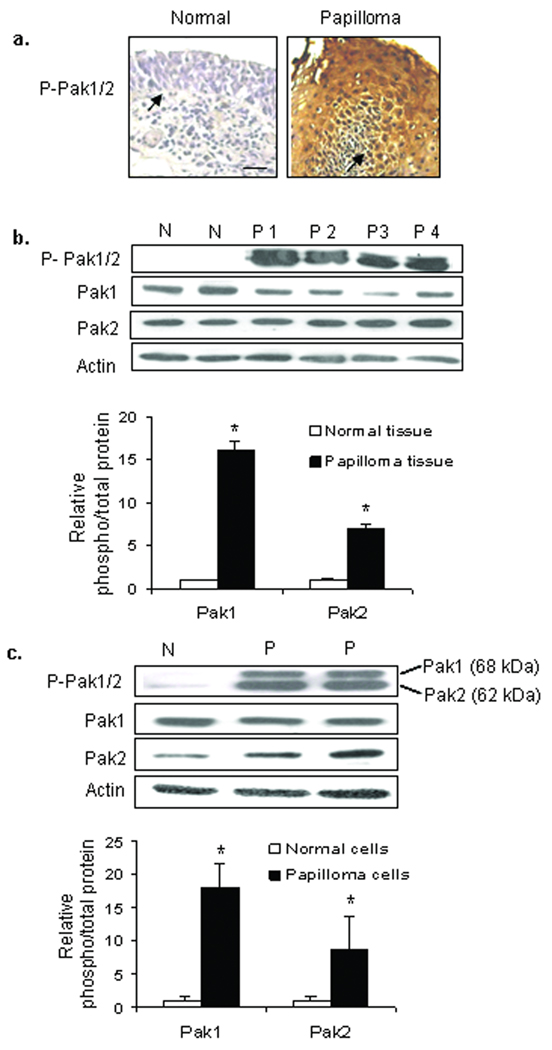
First, we asked whether Pak 1 and Pak2 were activated in papilloma tissues and cultured cells. Pak1/2 was clearly activated in biopsies of respiratory papillomas compared with normal laryngeal epithelium when evaluated by immunohistochemical staining using an antibody that recognized phosphorylation of Pak1 ser-144 or Pak2 ser-141,25 the initial activating sites for these two isoforms (Fig. 1a). Phospho-Pak1/2 staining was specially pronounced in the spinous layers of the papilloma tissue, where HPV expression is elevated.26 When analyzed by western blot (Fig. 1b) with an antibody that recognized phospho-ser-199/204 (Pak1) or ser- 192/197 (Pak2), activating sites that are targets for GTPase-induced or autophosphorylation,27 the ratio of phosphorylated to total Pak1 and Pak2 was elevated approximately 16 and 7 fold respectively in papilloma tissues compared with normal laryngeal epithelium (*: p< 0.001). Total Pak1 and Pak2 protein levels varied among biopsies, with the suggestion that Pak1 was reduced in papillomas, but the difference was not significant with this relatively small number of samples. There was no significant difference in levels of mRNA for either isoform (data not shown). Elevated steady-state levels of activated Pak1 and Pak2 were also seen in papilloma cells cultured in serum-free medium containing EGF and insulin (Fig. 1c) (*: p<0.01). Culturing either papilloma or normal cells with 10-fold higher concentrations of EGF (20 ng/ml) did not result in a significant change in phospho-Pak1 or phospho-Pak2 levels (data not shown).
Figure 1. Pak1 and Pak2 are highly phosphorylated in recurrent respiratory papilloma tissues and cultured cells.
a. Representative immunohistochemical staining of formalin-fixed, paraffin-embedded normal laryngeal and papilloma tissues, showing abundant phosphorylated Pak1/2. Arrows indicate location of the basement membrane, bar = 24 µm. Three papillomas and two normal tissues were analyzed. b. Western blot showing elevation of phosphorylated Pak1 and Pak2 in papilloma tissues compared to normal tissue. Bar graph shows the mean ± SD of the ratio of phosphorylated Pak1 and Pak2 to total Pak 1 and Pak2 in 5 papillomas, normalized to the mean ratio of 4 normal tissues (*: p<0.01). c. Representative western blot showing steady-state levels of phospho-Pak1 and Pak2 in papilloma cells and normal laryngeal cells cultured in serum-free medium containing EGF and insulin. The bar graph shows the mean ± SD of the ratio of phosphorylated to total protein, normalized to actin and expressed relative to normal cells, from 3 separate experiments (*: p<0.01).
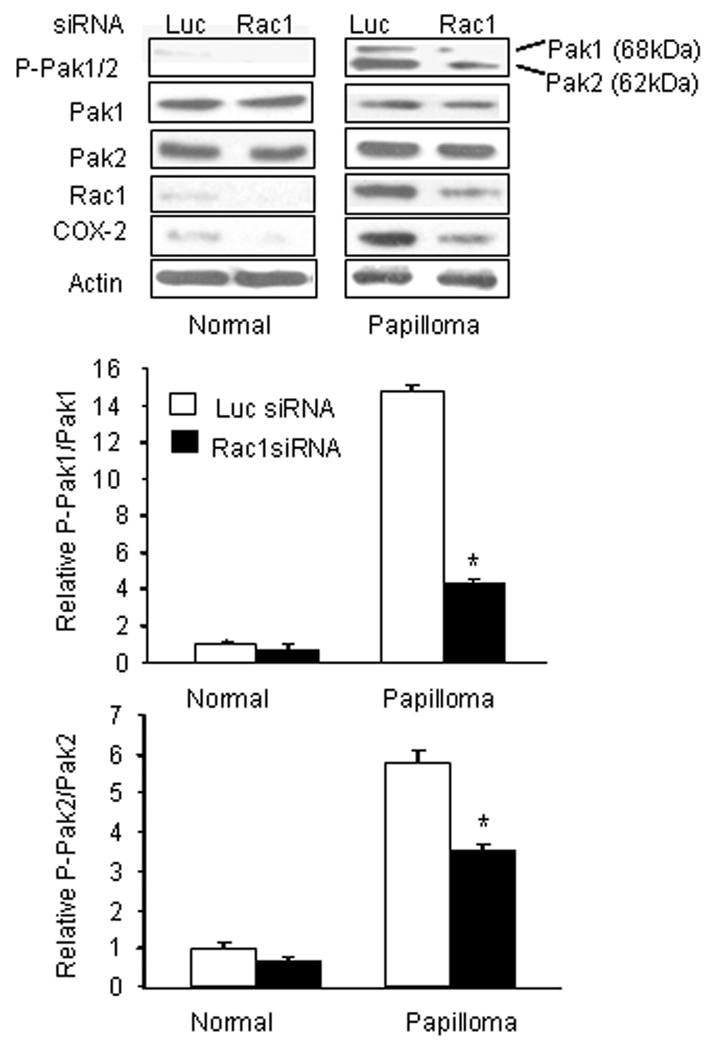
We had previously reported that Rac1 protein was overexpressed in papilloma tissues and cells.9 We have now determined that Rac activity is also elevated: 4.0 ± 0.02 fold in papilloma tissues and 3.1 ± 0.01 fold in cultured cells, relative to normal laryngeal tissue and cells (p=0.006, data not shown). Knockdown of Rac1 protein levels with specific siRNA (Fig. 2) significantly reduced activation of both Pak1 and Pak2 in papilloma cells (*: p <0.05), as well as levels of COX-2 as previously described. 9 This result confirmed that Rac1 contributed to the activation of Pak1 and Pak2 in papilloma cells, and suggested that the elevated Rac1 signaling in papilloma cells could mediate the hyper-phosphorylation of Pak1 and Pak2.
Figure 2. Activation of Pak1 and Pak2 requires Rac1.
Normal and papilloma cells were transfected with Rac1-specific siRNA and levels of phospho-Pak1, phospho-Pak2, Pak1, Pak2, Rac1, COX-2 and β-actin determined by western blot after 72 hrs. Luciferase (Luc) siRNA was used as a control. The bar graph shows the mean ± SD of the ratios of phosphorylated to total Pak1 and Pak2, normalized to actin and expressed relative to the ratios in normal cells treated with luciferase siRNA (*: p<0.01).
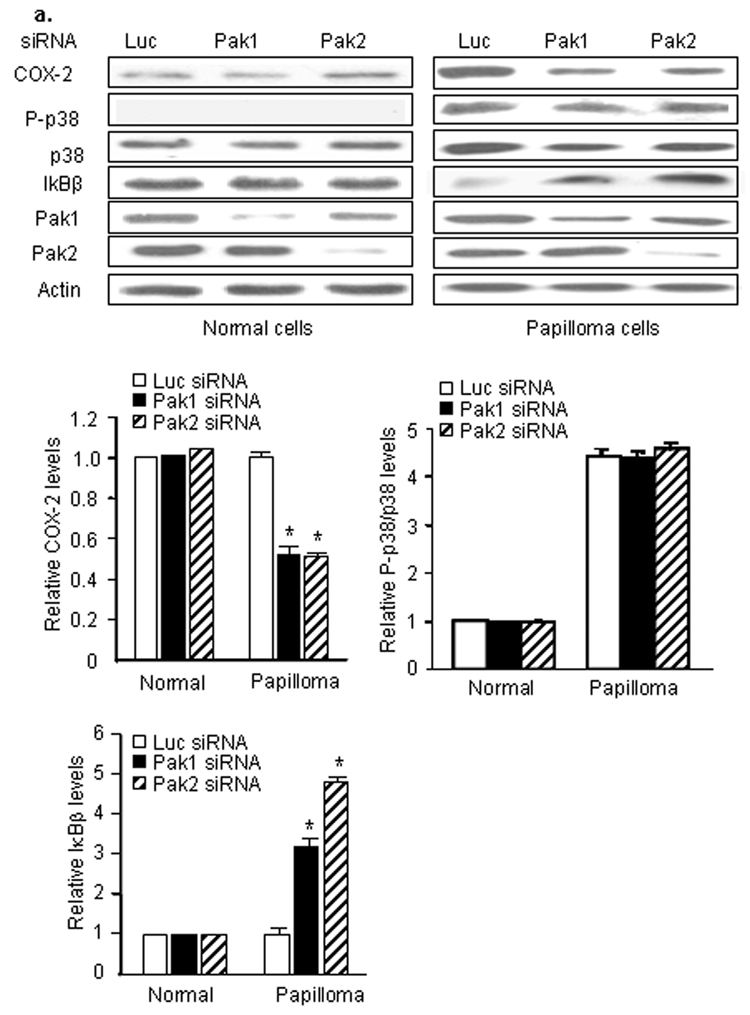
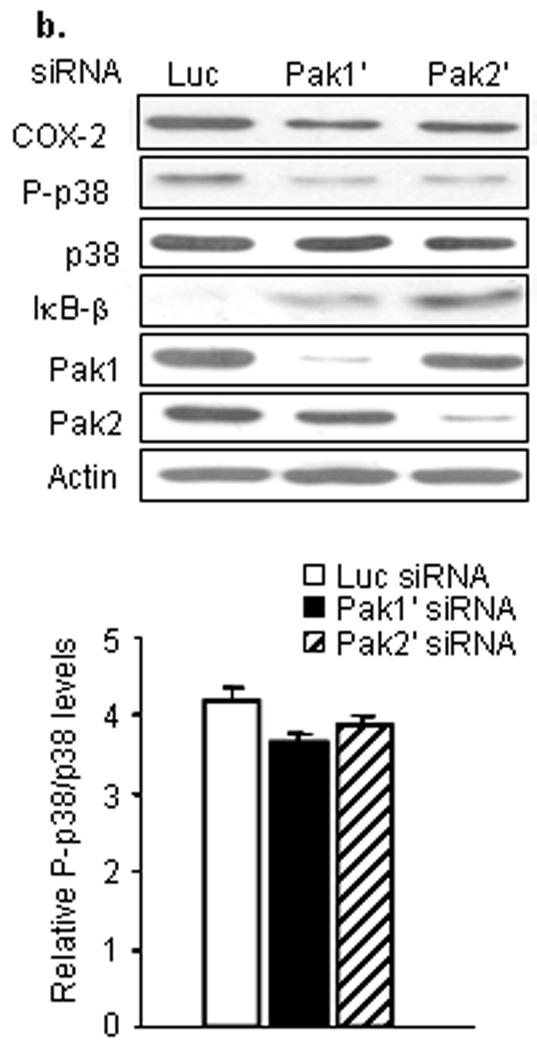
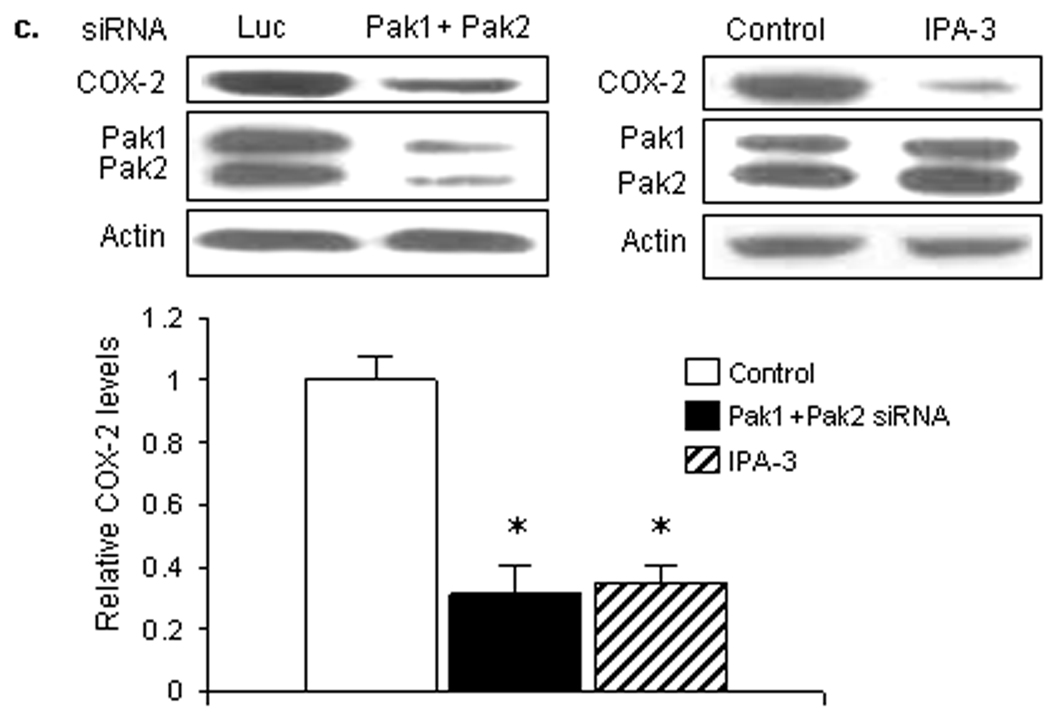
We then asked whether Pak1 and/or Pak2 contributed to the elevated level of COX-2 in HPV-infected papillomas (Fig. 3a). Reduction of either Pak1 or Pak2 with specific siRNAs reduced the steady-state level of COX-2 in papilloma cells (*: p<0.05), but not normal cells, confirming that both Pak1 and Pak2 specifically contributed to Rac1-mediated COX-2 expression in the tumor cells. Surprisingly, Pak1 and Pak 2 siRNAs had no effect on p38 phosphorylation. Rather, knockdown of Pak1 or Pak2 increased IκB-β in papilloma cells more than three-fold, to levels approaching those seen in uninfected cells, without affecting the levels of IκB-β in normal cells. We confirmed these results, treating papilloma cells with a second set of Pak1 and Pak2 siRNAs (Fig. 3b), to rule out off-target effects. Again, there was a reduction in COX-2 levels and elevation of IκB-β. In the second set of experiments, there was a modest, but not significant reduction in relative p38 phosphorylation, as seen by densitometry analysis. The blot shown in Figure 3 appears to show a greater reduction, but the total p38 protein level was also reduced slightly in those cells. We also determined that simultaneous knockdown of Pak1 and Pak2, or inhibition of both with the Group I-selective Pak inhibitor IPA-3,28 had a greater effect on COX-2 levels than targeting just one isoform (Fig. 3c), confirming that signaling through both Pak1 and Pak2 contributes to COX-2 induction in papilloma cells.
Figure 3. Pak1 and Pak2 signaling mediates COX-2 expression in papilloma cells via activation of IκBβ, not activation of p38.
Western blots showing effects of specific siRNAs targeting either Pak1 or Pak2 on levels of COX-2, phosphorylated and total p38 and IκBβ in normal and papilloma cells. Luciferase (Luc) siRNA was used as a negative control. Levels of Pak 1 and Pak2 were measured to assess knockdown, actin measured to confirm equal loading. a. Representative western blot and bar graphs showing mean ± SD of 3 experiments with papilloma and normal cells, with levels normalized to actin and expressed relative to control cells of each type treated with luciferase siRNA (* p<0.01). b. Western blot of parallel experiment on papilloma cells using a second set of Pak1 and Pak 2 siRNAs (Pak1' and Pak2'), with the associated bar graph showing confirmation of minimal effects on relative phosphorylation of p38. c. Western blots and associated bar graph of papilloma cells treated with combined Pak1 and Pak2 siRNAs, and cells treated with the GroupI Pak1 inhibitor IPA-3, to assess effects on COX-2 levels of simultaneously reducing both Pak1 and Pak2 signaling (* p<0.05).
The results in Fig. 3a and 3b suggested that Rac1-activated Pak1 and Pak2 contribute to induction of COX-2 in papilloma cells primarily through activation of the NFκB pathway, not through activation of p38. Others have reported that Pak1 or Pak2 mediate NFκB activation,29,30 but those studies have primarily used expression of constitutively activated or dominant negative constructs which can affect multiple pathways. To our knowledge this is the first study that used siRNAs to show that IκB-β is regulated by Pak1 or Pak2.
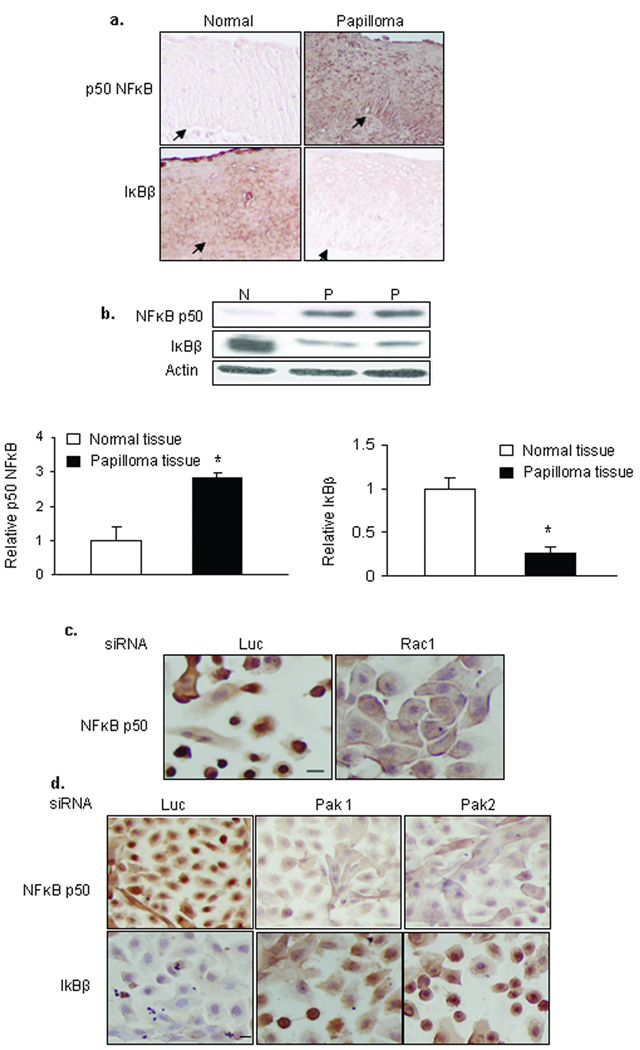
Because IκB-β was increased when Pak1 and Pak2 levels were reduced, we assessed the abundance and localization of NFκB p50 and IκB-β in papilloma tissues compared to normal laryngeal epithelium, and the effects of Rac1, Pak1 and Pak2 siRNAs on NFκB and IκB localization in papilloma cells (Fig. 4). Immunohistochemical staining showed heavy expression and significant nuclear localization of NFκB p50 in papilloma tissues, with very low levels of IκB-β (Fig. 4a). Quantitation of tissue extracts by western blot (Fig. 4b) showed that NFκB p50 protein levels were three-fold higher in papillomas than in normal tissue (*: p<0.05), and IκB-β levels 80% lower. Lower IκB-β levels are consistent with our earlier report.10 Treating papilloma cells with siRNAs to reduce levels of Rac1 (Fig. 4c) or Pak1 and Pak2 (Fig. 4d) shifted NFκB p50 from the nucleus to the cytoplasm, and Pak siRNAs appeared to markedly reduce the level of NFκB protein. Moreover, reducing levels of Pak1 or Pak2 increased both cytoplasmic and nuclear staining of IκB-β.
Figure 4. Reduction in Pak1 or Pak2 levels reduce nuclear NFκB and elevate cytoplasmic and nuclear IκBβ in HPV6/11-infected papilloma cells.
a. Immunohistochemistry of papilloma tissues and normal laryngeal epithelium, showing high levels of NFκB p50 and minimal IκBβ in papilloma tissues. b. Western blot of tissue extracts, confirming high levels of NFκB p50 protein and limited IκBβ in papillomas. Bar graph shows mean ± SD of multiple samples of each type, normalized to actin and expressed relative to normal tissue. c. Representative effects of Rac1 siRNA on localization of NFκB p50 in papilloma cells, assessed by immunohistochemistry. Luciferase siRNA was used as control. d. Representative effects of Pak1 and Pak 2 siRNAs on abundance and localization of NFκB p50 and IκBβ in papilloma cells. Luciferase siRNA (Luc) was used as a control. Bar is 24 µm. The experiments in 4c and 4d were repeated 3 times.
Discussion
In this paper, we have examined the role of Pak1 and Pak2 as Rac1 mediators in the over-expression of COX-2 in HPV6/11-infected papillomas. These studies indicate for the first time that activation of Pak1 and Pak2 are markedly increased in benign tumor tissues, and that they are activated in HPV-induced lesions. In addition, our data show that both Pak1 and Pak2 contribute to Rac1 mediated COX-2 expression in papillomas, but not through activation of p38. Rather, they appear to do so via activation of p50 NFκB, since reduction of Pak1/2 increased IκB-β levels and reduced levels of nuclear NFκB. NFκB can directly activate the COX-2 promoter.31,32 Of interest, the Pak1/2 → NFκB→COX-2 pathway does not appear to function in normal laryngeal epithelial cells that express low levels of COX-2 in culture.
Our results suggest that there are two parallel pathways downstream of Rac1 that regulate COX-2 expression in respiratory papillomas. One consists of Rac1→ Pak1/ Pak2→ NFκB→ COX-2, the other is the Rac1→ p38→ COX-2 pathway we previously described.9 Our previous studies had shown that chemical inhibitors of p38 could completely block over-expression of COX-2, 9 while the knockdown of either Pak1 or Pak2 only partially reduced COX-2 levels. However, simultaneously reducing signaling from both Paks further suppressed COX-2 levels. Thus, the Rac1→ Pak1/ Pak2→ NFκB pathway may be as critical as the Rac1→ p38 pathway in induction of COX-2. We propose that while the pathways appear parallel, both are required for efficient, sustained expression of COX-2. Drugs that inhibit components of either of these two pathways could potentially be effective therapies for this serious disease, and other HPV-induced tumors, with minimal effects on normal tissues. Future studies in animal models will determine whether drugs that preferentially target these pathway components33,34 are equally efficacious.
Acknowledgments
We thank Dr. Mark Shikowitz for providing tissues, Salvatore Coniglio for valuable discussion, and May Nouri and Ray Pica for their assistance with establishment of primary cultures. This study was supported in part by grants P50 DC00203 and R01 DC008579 from the National Institute on Deafness and Other Communication Disorders (NIDCD), by The Feinstein Institute for Medical Research General Clinical Research Center Grant #M01 RR018535 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and a grant from the Frankfort Family Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or the National Institutes of Health.
References
- 1.Mounts P, Shah KV, Kashima H. Viral etiology of juvenile- and adult-onset squamous papilloma of the larynx. Proc Natl Acad Sci U S A. 1982;79(17):5425–5429. doi: 10.1073/pnas.79.17.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gissman L, Diehl B, Schults-Coulon J, zur Hausen H. Molecular cloning and characterization of human papillomavirus DNA derived from a laryngeal papilloma. J Virol. 1982;44:393–399. doi: 10.1128/jvi.44.1.393-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monk BJ, Tewari KS. The spectrum and clinical sequelae of human papillomavirus infection. Gynecol Oncol. 2007;107:S6–S13. doi: 10.1016/j.ygyno.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 4.Vambutas A, Di Lorenzo TP, Steinberg BM. Laryngeal papilloma cells have high levels of epidermal growth factor receptor and respond to epidermal growth factor by a decrease in epithelial differentiation. Cancer Res. 1993;53:910–914. [PubMed] [Google Scholar]
- 5.Johnston D, Hall H, Dilorenzo TP, Steinberg BM. Elevation of the epidermal growth factor receptor and dependent signaling in human papillomavirus-infected laryngeal papillomas. Cancer Res. 1999;59:968–974. [PubMed] [Google Scholar]
- 6.Zhang P, Steinberg BM. Overexpression of PTEN/MMAC1 and decreased activation of Akt in human papillomavirus-infected laryngeal papillomas. Cancer Res. 2000;60(5):1457–1462. [PubMed] [Google Scholar]
- 7.Sun S, Steinberg BM. PTEN is a negative regulator of STAT3 activation in human papillomavirus-infected cells. J Gen Virol. 2002;83:1651–1658. doi: 10.1099/0022-1317-83-7-1651. [DOI] [PubMed] [Google Scholar]
- 8.Wu R, Sun S, Steinberg BM. Requirement of STAT3 activation for differentiation of mucosal stratified squamous epithelium. Mol Med. 2003;9:77–84. doi: 10.2119/2003-00001.wu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu R, Coniglio SJ, Chan A, Symons MH, Steinberg BM. Up-regulation of Rac1 by epidermal growth factor mediates COX-2 expression in recurrent respiratory papillomas. Mol Med. 2007;13(3–4):143–150. doi: 10.2119/2007-00005.Wu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vancurova I, Wu R, Miskolci V, Sun S. Increased p50/p50 NF-kappaB activation in human papillomavirus type 6- or type 11-induced laryngeal papilloma tissue. J of Virol. 2002;76:1533–1536. doi: 10.1128/JVI.76.3.1533-1536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu R, Abramson AL, Shikowitz MJ, Dannenberg AJ, Steinberg BM. Epidermal growth factor-induced cyclooxygenase-2 expression is mediated through phosphatidylinositol-3 kinase, not mitogen-activated protein/extracellular signal-regulated kinase kinase, in recurrent respiratory papillomas. Clin Cancer Res. 2005;11(17):6155–6161. doi: 10.1158/1078-0432.CCR-04-2664. [DOI] [PubMed] [Google Scholar]
- 12.Tanabe T, Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002;68–69:95–114. doi: 10.1016/s0090-6980(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 13.Thornburg NJ, Pathmanathan R, Raab-Traub N. Activation of nuclear factor-κB p50 homodimer/Bcl3 complexes in nasopharyngeal carcinoma. Cancer Res. 2003;63:8293–8301. [PubMed] [Google Scholar]
- 14.Farley JH, Truong V, Goo E, Uyehara C, Belnap C, Larsen WI. A randomized double-blind placebo-controlled phase II trial of the cyclooxygenase-2 inhibitor Celecoxib in the treatment of cervical dysplasia. Gynecol Oncol. 2006;103(2):425–430. doi: 10.1016/j.ygyno.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 15.Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367(6458):40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 16.Jaffer ZM, Chernoff J. p21-activated kinases: three more join the Pak. Int J Biochem Biol. 2002;34:713–717. doi: 10.1016/s1357-2725(01)00158-3. [DOI] [PubMed] [Google Scholar]
- 17.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 18.Teo M, Manser E, Lim L. Identification and molecular cloning of a p21cdc42/rac1-activated serine/threonine kinase that is rapidly activated by thrombin in platelets. J Biol Chem. 1995;270:26690–26697. doi: 10.1074/jbc.270.44.26690. [DOI] [PubMed] [Google Scholar]
- 19.Manser E, Chong C, Zhao ZS, Leung T, Michael G, Hall C, Lim L. Molecular cloning of a new member of the p21-Cdc42/Rac-activated kinase (PAK) family. J Biol Chem. 1995;270:25070–25078. doi: 10.1074/jbc.270.42.25070. [DOI] [PubMed] [Google Scholar]
- 20.Rennefahrt UE, Deacon SW, Parker SA, Devarajan K, Beeser A, Chernoff J, Knapp S, Turk BE, Peterson JR. Specificity profiling of Pak kinases allows identification of novel phophorylation sites. J Biol Chem. 2007;282(21):15667–15678. doi: 10.1074/jbc.M700253200. [DOI] [PubMed] [Google Scholar]
- 21.Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- 22.Coniglio SJ, Zavarella S, Symons MH. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol Cell Biol. 2008;28:4162–4172. doi: 10.1128/MCB.01532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinberg BM, Abramson AL, Meade RP. Culture of human laryngeal papilloma cells in vitro. Otolaryngol Head Neck Surg. 1982;90:728–735. doi: 10.1177/019459988209000610. [DOI] [PubMed] [Google Scholar]
- 24.Auborn KJ, Fan S, Rosen EM, Goodwin L, Chandraskaren A, Williams DE, Chen D, Carter TH. Indole-3-Carbinol is a negative regulator of Estrogen. J Nutr. 2003;133:2470–2475. doi: 10.1093/jn/133.7.2470s. [DOI] [PubMed] [Google Scholar]
- 25.Orr AW, Stockton R, Simmers MB, Sanders JM, Sarembock IJ, Blackman BR, Schwartz MA. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J Cell Biol. 2007 February 26;176(5):719–727. doi: 10.1083/jcb.200609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoler MH, Wolinsky SM, Whitbeck A, Broker TR, Chow LT. Differentiation-linked human papillomavirus types 6 and 11 transcription in genital condylomata revealed by in situ hybridization with message-specific RNA probes. Virology. 1989;172:331–340. doi: 10.1016/0042-6822(89)90135-9. [DOI] [PubMed] [Google Scholar]
- 27.Zhao ZS, Manser E. PAK and other Rho-associated kinases--effectors with surprisingly diverse mechanisms of regulation. Biochem J. 2005;386(Pt 2):201–214. doi: 10.1042/BJ20041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15(4):322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frost JA, Swantek JL, Stippec S, Yin MJ, Gaynor R, Cobb MH. Stimulation of NFkappa B activity by multiple signaling pathways requires PAK1. J Biol Chem. 2000;275:19693–19699. doi: 10.1074/jbc.M909860199. [DOI] [PubMed] [Google Scholar]
- 30.Dadke D, Fryer BH, Golemis EA, Field J. Activation of p21-activated kinase 1-nuclear factor kappaB signaling by Kaposi's sarcoma-associated herpes virus G protein-coupled receptor during cellular transformation. Cancer Res. 2003;63:8837–8847. [PubMed] [Google Scholar]
- 31.Schmedtje JF, Jr, Ji YS, Liu WL, DuBois RN, Runge MS. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997;272(1):601–608. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- 32.Inoue H, Tanabe T. Transcriptional role of the nuclear factor kappa B site in the induction by lipopolysaccharide and suppression by dexamethasone of cyclooxygenase-2 in U937 cells. Biochem Biophys Res Commun. 1998;244:143–148. doi: 10.1006/bbrc.1998.8222. [DOI] [PubMed] [Google Scholar]
- 33.Medicherla S, Reddy M, Ying J, Navas TA, Li L, Nguyen AN, Kerr I, Hanjarappa N, Protter AA, Higgins LS. p38alpha-selective MAP kinase inhibitor reduces tumor growth in mouse zenograft models of multiple myeloma. Anticancer Res. 2008;28(6A):3827–3833. [PubMed] [Google Scholar]
- 34.Hirokawa Y, Nakajima H, Hanemann CO, Kurtz A, Frahm S, Mautner V, Maruta H. Signal therapy of NF1-deficient tumor xenograft in mice by the anti-PAK1 drug FK228. Cancer Biol Ther. 2005;4(4):379–381. doi: 10.4161/cbt.4.4.1649. [DOI] [PubMed] [Google Scholar]