Abstract
Background and Purpose
Niacin is the most effective medication in current clinical use for increasing high-density lipoprotein cholesterol (HDL-C). We tested the hypothesis that Niacin treatment of stroke promotes synaptic plasticity and axon growth in the ischemic brain.
Methods
Male Wistar rats were subjected to 2-hour of middle cerebral artery occlusion (MCAo) and treated with or without Niaspan (a prolonged release formulation of Niacin, 40 mg/kg) daily for 14 days starting 24 hours after MCAo. The expression of Synaptophysin, Nogo receptor (NgR), Bielschowsky silver, brain-derived neurotrophic factor (BDNF) and its receptor tropomyosin-related kinase B (TrkB) were measured by immunohistostaining and Western blot, respectively, in the ischemic brain. Complementing in vivo studies, primary cultured neurons (PCN) were employed to test the effect of Niacin and HDL on neurite outgrowth, and BDNF/TrkB expression.
Results
Niaspan treatment of stroke significantly increased Synaptophysin, Bielschowsky silver, BDNF/TrkB expression, and decreased NgR expression in the ischemic brain compared with MCAo control animals (p<0.05, n=8/group). Niacin and HDL treatment significantly increased neurite outgrowth, and BDNF/TrkB expression in PCNs. TrkB-inhibitor attenuated Niacin-induced neurite outgrowth (p<0.05, n=6/group).
Conclusions
Niacin treatment of stroke promotes synaptic plasticity and axon growth, which is mediated, at least partially, by the BDNF/TrkB pathways.
Keywords: Niacin, HDL-cholesterol, synaptic plasticity, axon growth, stroke
Synaptic plasticity and axon growth is related to behavioral change and functional recovery after brain and spinal cord injury.1 Functional alterations in motor cortex organization are accompanied by changes in dendritic and synaptic structure.2 Cortical stimulation-induced functional improvements after stroke are mediated by synaptic structural plasticity.3 Increases in dendritic arborization and spine density are potential morphological strategies that enable the brain to reorganize its neuronal circuits.4
High-density lipoprotein cholesterol (HDL-C) is critical in maintaining the homeostasis of cell membrane cholesterol and thus plays an essential role in the regulation of synaptic function and cell plasticity.5 The expression of brain-derived neurotrophic factor (BDNF) and its receptor tropomyosin-related kinase B (TrkB) supports neuron survival and axon growth after neuronal injury.6, 7 Voluntary exercise leads to an endogenous upregulation of BDNF and associated proteins involved in synaptic function, and enhances functional recovery after traumatic brain injury.8 Synaptic plasticity is also influenced by mutations in BDNF.9
Niacin (nicotinic acid) is the most effective medication in current clinical use for increasing HDL-C.10 Our previous study showed that Niaspan (a prolonged release formulation of niacin) treatment of stoke significantly increases serum HDL-C level and improves functional outcome in rats.11 However, whether Niacin increases synaptic plasticity and axon growth, and whether BDNF/TrkB play a role in Niacin-induced synaptic plasticity and axon growth has not been investigated. In this study, we tested a novel hypothesis, that Niacin treatment of stroke promotes synaptic plasticity and axon growth in the ischemic brain in rats. In addition, the contributions of BDNF/TrkB to Niacin-induced synaptic plasticity and axon growth were investigated.
Materials and Methods
Animal Middle Cerebral Artery Occlusion (MCAo) and Experimental Groups
Adult male Wistar rats weighing 270–300g were subjected to 2 hours of right MCAo.11 Rats were gavaged starting 24 hours after surgery with: 1) saline for vehicle control; 2) Niaspan (KOS Pharmaceuticals) 40 mg/kg daily for 14 days.11 Sham-operated rats underwent the same surgical procedure without suture insertion. These rats were sacrificed 14 days after MCAo for immunostaining (n=8/group). Additional sets of rats (n=4/group) were sacrificed 5 days after MCAo, and the brain tissues were prepared for Western blot assay.
Histological and Immunohistochemical Assessment
Rats were sacrificed 14 days after stroke. The brains were fixed by transcardial-perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde before being embedded in paraffin. A standard paraffin block was obtained from the center of the lesion (bregma −1 mm to +1 mm). A series of 6μm thick sections were cut from the block. Every 10th coronal section for a total 5 sections was used for immunohistochemical staining. Antibody against Synaptophysin (1:1000, Chemicon), Nogo receptor (NgR) (1:50, Santa Cruz), BDNF (1:300, Santa Cruz) and TrkB (1:500, Santa Cruz) immunostaining were performed. Bielschowsky silver staining was also employed as previously described.12 Control experiments consisted of staining brain coronal tissue sections as outlined above, but nonimmune serum was substituted for the primary antibody. The immunostaining analysis was performed by an investigator blinded to the experimental groups.
Immunostaining Quantification
As our previous description,11 Synaptophysin, NgR, BDNF, TrkB and Bielschowsky silver immunohistostained sections were digitized using a 40× objective (Olympus BX40) using a 3-CCD color video camera (Sony DXC-970MD) interfaced with the Micro Computer Imaging Device (MCID) computer imaging analysis system (Imaging Research). Synaptophysin-, BDNF- and TrkB-positive area was counted in the ischemic boundary zone (IBZ, which adjacent to the ischemic core) in each section.11 Five sections and eight views in each section were counted per rat. For semi-quantitative measurements of Bielschowsky Silver and NgR, the positive stained areas in the bundle of stratum in the IBZ were measured. Data were analyzed in a blinded manner and presented as percentage of positive area for Synaptophysin, Bielschowsky silver, NgR, BDNF and TrkB immunoreactive, respectively.
Primary Cultured Neuron (PCN) and Treatments
To test whether Niacin regulates dendrite outgrowth, PCN culture was employed. Embryonic day 17 (E17) cortical cells were isolated under the 10× microscope from embryonic brain of Wistar rats and cultured in 4-chamber slides with Neuralbasal-A medium (GIBCO) containing 2% B27 medium-supplement (GIBCO) and antibiotics for 7 days (6 chambers/group) in vitro.13 To mimic the ischemic condition in vivo, oxygen-glucose deprivation (OGD) was induced in vitro, as previously described.14 OGD was induced within an anaerobic chamber. Briefly, the PCN cultures were transferred to the anaerobic chamber (model 1025, Forma Scientific) and were incubated in 85% N2, 10% H2, and 5% CO2, at 37°C for 1 hour. The PCN cultures were removed from the anaerobic chamber, rinsed with PBS, and fed with the primary media, and the groups were divided into: 1) Control; 2) HDL (80μg/ml, Calbiochem, Cat# 437641); 3) Niacin 1mM; 4) Niacin 5mM; 5) TrkB-inhibitor (K252a, 200nM, Calbiochem, Cat# 480354); 6) TrkB-inhibitor + HDL; 7) TrkB-inhibitor + Niacin 1mM; 8) TrkB-inhibitor + Niacin 5mM for 24h. Western blot assay and real time-polymerase chain reaction (RT-PCR) were performed, respectively. The PCN cultures were also performed for Neuron-specific class III β-tubulin (TUJ1, a phenotypic marker of neural cells) immunofluorescent staining using a monoclonal anti-TUJ1 antibody (Covance, 1:1000) with Cy3 for PCN number counting and neurite outgrowth measurement.15
Quantification of PCN Numbers and Measurement of PCN Neurite Outgrowth
To count PCN numbers and trace the axonal arbors of fluorescently labeled neurons, the fluorescent photomicrographs were captured at 40× magnification with a digital camera, the TUJ1-positive PCN numbers and the length of TUJ1-positive dendrites were measured using MCID analysis system.16 The average number of TUJ1-positive PCNs per 40× field and the average length of total 20 neuronal dendrite outgrowth was presented.
Western Blot
Rats were sacrificed 5 days after MCAo and brain tissues were extracted from the IBZ tissue. Equal amounts of cell lysate were subjected to Western blot analysis, as previously described.17 PCN cells were harvested after 24h of treatment for Western blot. Total protein was isolated from treated cells with TRIzol (Invitrogen), following a standard protocol. Heat the protein samples in 1% SDS for about 20 min at 60°C to recover the protein activity. Specific proteins were visualized using a SuperSignal West Pico chemiluminescence kit (Pierce). The following primary antibodies were used: anti-BDNF (1:1000; Santa Cruz), anti-TrkB (1:1000; Santa Cruz), and anti-β-actin (1:2000; Sigma).
Real Time Polymerase Chain Reaction (RT-PCR)
PCN cultures were harvested after 24h of treatment and total RNA was isolated with TRIzol (Invitrogen). Quantitative PCR was performed using the SYBR Green RT-PCR method on an ABI 7000 PCR instrument (Applied Biosystems), as previously described.11 The following primers for RT-PCR were designed using Primer Express software (ABI). BDNF Fwd: TAC TTC GGT TGC ATG AAG GCG; Rev: GTC AGA CCT CTC GAA CCT GCC. TrkB Fwd: TCA TCA AGT CAG AGG TGA CAG G; Rev: ACT GGG TAC ACT CCT TCT CTC G. GAPDH: Fwd: AGA ACA TCA TCC CTG CAT CC; Rev: CAC ATT GGG GGT AGG AAC AC. Each sample was tested in triplicate, and samples were obtained from six independent experiments that were used for analysis of relative gene expression data using the 2− ΔΔCT method.
Statistical Analysis
Two-way ANOVA was performed on data of the percentage of positive area for Synaptophysin, NgR, Bielschowsky silver, BDNF and TrkB in the ischemic brain. If an overall treatment group effect was detected at p<0.05, Tukey test after Post Hoc Test was used for multiple comparison. Independent Samples T-Test was used for testing the expression of BDNF and TrkB by Western blot assay in the brain tissues between two groups. One-way ANOVA and Least Significant Difference analysis after Post Hoc Test was performed to assess data of BDNF and TrkB expression by Western blot and RT-PCR assay in the ischemic brain and PCN cultures, and the neurite outgrowth in vitro. All data are presented as mean ± Standard Error (SE).
Results
Niaspan Treatment of Stroke Increases Synaptic Plasticity and Axon growth in the ischemic brain
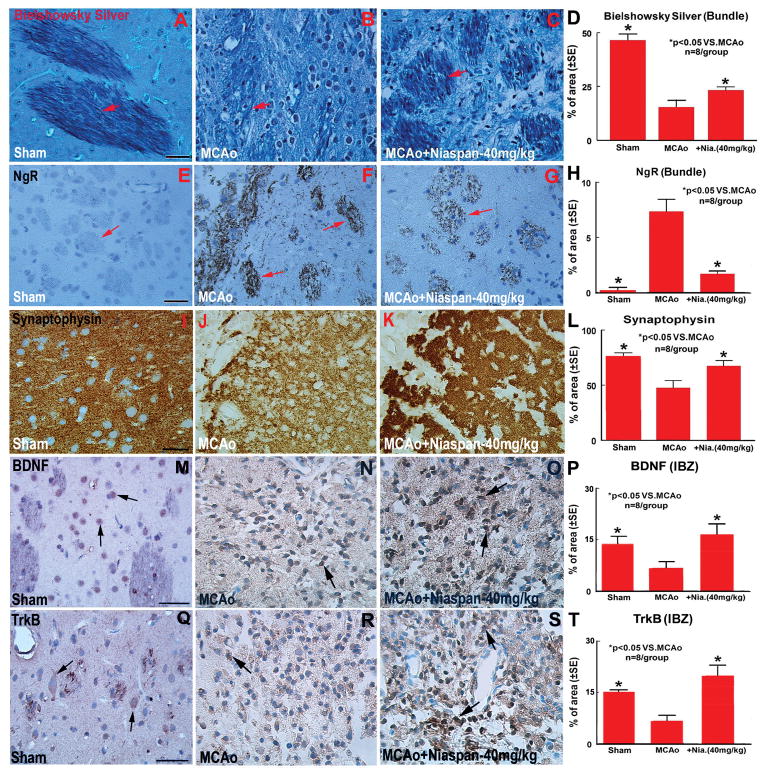
To test whether Niaspan treatment of stroke induces synaptic plasticity and axon growth, Synaptophysin, Bielschowsky silver and NgR immunostaining were performed. Synaptophysin is a marker for presynaptic plasticity and synaptogenesis.18 Bielschowsky silver is a marker for axons.19,20 NgR, a neurite outgrowth inhibitor, regulates axonal growth as well as axon regeneration after injury.12,21 Figure 1A-L shows that the expression of Bielschowsky sliver (A-D) and Synaptophysin (I-L) significantly increased in the IBZ, while, the expression of NgR (E-H) significantly decreased in the Niaspan treatment rats compared with MCAo control rats (P<0.05, n=8/group).
Figure 1.
Niaspan treatment of stroke increases Synaptophysin, Bielschowsky Silver, BDNF and TrkB expression, and decrease NgR expression in the ischemic brain. A–D: Bielschowsky Silver staining and quantitative data; E–H: NgR immunostaining and quantitative data; I–L: Synaptophysin immunostaining and quantitative data; M–P: BDNF immunostaining and quantitative data; Q–T: TrkB immunostaining and quantitative data. n=8/group. Scale bar in A and E = 40 μm, M and Q = 50 μm.
Niaspan Treatment of Stroke Increases BDNF and TrkB Expression in the Ischemic Brain
To elucidate the mechanism of Niaspan induced synaptic plasticity and axon growth, BDNF and TrkB expression in the ischemic brain was measured using immunostaining and Western blot assays. Figure 1M-T shows that Niaspan treatment of stroke significantly increased BDNF (M-P) and TrkB (Q-T) expression in the IBZ compared with MCAo alone control animals (P<0.05, n=8/group).
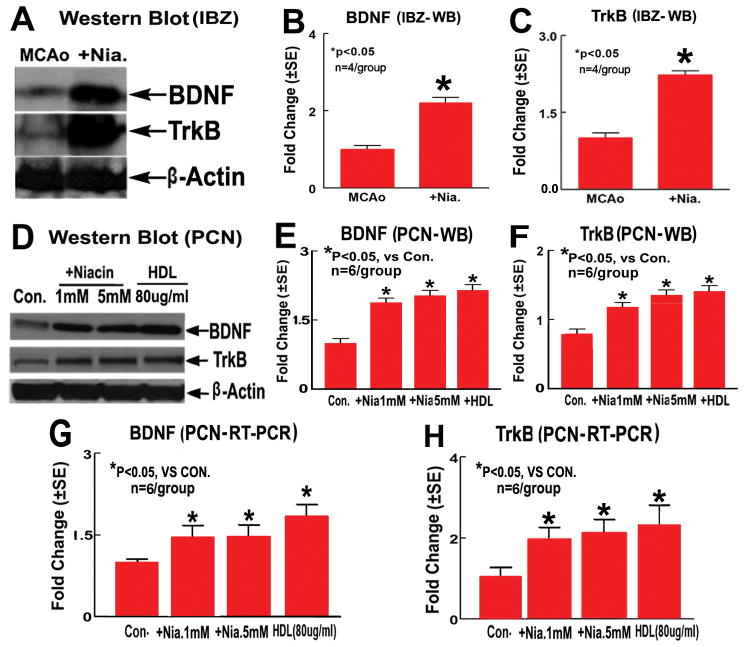
To verify the immunohistostaining data, rat brain tissues were extracted from Niaspan-treatment and MCAo control rats 5 days after MCAo, and Western blot assays were performed. Figure 2A–C show that BDNF and TrkB expression in the IBZ significantly increased in Niaspan-treatment rats compared with MCAo control rats (P<0.05, n=4/group).
Figure 2.
Niaspan treatment of stroke increases BDNF/TrkB expression in the ischemic brain, and Niacin/HDL increase BDNF/TrkB expression in PCN cultures. A–C: BDNF and TrkB Western blot assay (A) and quantitative data (B: BDNF, C: TrkB) in the IBZ (n=4/group); D–F: BDNF and TrkB Western blot assay and quantitative data (E: BDNF, F: TrkB) in PCNs (n=6/group); G and H: BDNF (G) and TrkB (H) mRNA expression measured by RT-PCR in PCNs (n=6/group).
Niacin Increases BDNF and TrkB Expression in PCNs
To further investigate the mechanism underlying BDNF/TrkB pathway mediates Niacin-induced synaptic plasticity and axon growth, and whether the increased BDNF/TrkB expression is mediated by Niacin-treatment-induced increase in HDL, the expression of BDNF and TrkB were also investigated using an in vitro PCN culture model. PCNs were subjected to OGD and treated with Niacin (1mM or 5mM) and HDL (80μg/ml) for 24 hours. Figure 2D-H show that Niacin (1mM or 5mM) and HDL (80μg/ml) treatment significantly increased BDNF and TrkB protein and mRNA expression measured by Western blot (WB) assay (2D–F) and RT-PCR (2G and H) in PCN cultures compared to non-treatment control (P<0.05, n=6/group).
Niacin Increases Neurite Outgrowth in PCNs
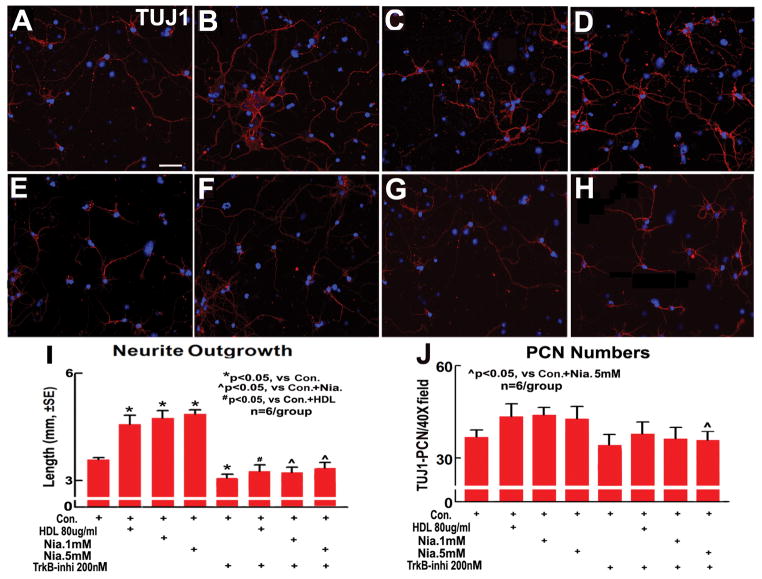
To test whether Niacin/HDL affect PCN cell number and increases neurite outgrowth, and whether the BDNF/TrkB pathway plays a role in Niacin/HDL induced neurite outgrowth, PCN cultures were treated with HDL, Niacin 1mM, Niacin 5mM and TrkB-inhibitor (K252a, 200nM) for 24 hours after OGD. TUJ1 immunofluorescent stainng, TUJ1-positive cell number and dendrite outgrowth measurements were performed. Figure 3A-I show that HDL (B), Niacin 1mM (C) and Niacin 5mM (D) significantly increased neurite outgrowth, TrkB-inhibitor (E), however, significantly decreased neurite outgrowth in PCNs compared to non-treatment control (A). The TrkB-inhibitor significantly decreased HDL- (F) and Niacin- (G and H) induced neurite outgrowth in PCNs compared to Niacin-treated alone group (p<0.05, n=6/group). Niacin and HDL do not affect PCN number, TrkB-inhibitor decreases PCN numbers in Niacin-treated PCN compared with Niacin alone treated PCN (p<0.05, n=6/group). These data indicate that Niacin treatment significantly increased neurite outgrowth in PCNs, and Niacin-induced neurite outgrowth is, at least partially, mediated by HDL-induced upregulation in BDNF/TrkB.
Figure 3.
Niacin increases neurite outgrowth in PCNs after OGD. A–H: Neurite outgrowth in the PCNs (A, Control; B, HDL 80μg/ml; C, Niacin 1mM; D, Niacin 5mM; E, TrkB 200nM; F, TrkB-inhibitor + HDL; G, TrKB-inhibitor + Niacin 1mM; H, TrKB-inhibitor + Niacin 5mM); I: Quantitative data of PCN neurite outgrowth (n=6/group). J: Quantitative data of PCN numbers (n=6/group). Scale bar in A = 40μm.
Discussion
Functional recovery following acute central nervous system (CNS) injury in humans, such as stroke, is exceptionally limited, leaving the affected individual with life-long neurological deficits. This lack of functional recovery, such as motor recovery, can at least in part be attributed to the restriction of axon growth and synaptic plasticity.22,23
Cellular cholesterol modulates axon and dendrite outgrowth and neuronal polarization under culture conditions.24,25 Astrocytes are a major source of HDL-C synthesis in CNS.26 Growing evidence strengthens the link between brain HDL-C metabolism and factors involved in synaptic plasticity. For example, the scavenger receptor, class B, type I (SR-BI) binds HDL and mediates the selective transfer of cholesteryl esters and alpha-tocopherol from circulating HDL to cells. Aged SR-BI knockout mice show deficient synaptic plasticity (long-term potentiation) in the CA1 region of the hippocampus. Very aged SR-BI knockout mice also display selective impairments in recognition memory and spatial memory.5 The cholesterol transporter ATP-binding cassette transporter A1 (ABCA1) also plays a critical role in brain cholesterol metabolism. Mice that specifically lacked ABCA1 in the CNS exhibit reduced plasma HDL-C levels and changes in synaptic ultrastructure, including reduced synapse and synaptic vesicle numbers. Disturbances in cholesterol transport in the CNS are associated with structural and functional deficits in neurons.27 Thus, agents that increase HDL level may increase synaptic plasticity and axon growth after stroke. Niacin is the most potent HDL-C increasing drug used in the clinic. Our previous study showed that Niaspan treatment of stroke significantly increases serum HDL-C level and promotes functional outcome in rats.11 In this study, we find that Niaspan treatment of stroke significantly decreases NgR expression, upregulates Synaptophysin and increases axon growth in the ischemic brain in rats. In addition, HDL and Niacin treatment significantly increase neurite outgrowth after OGD in PCN cultures. Therefore, Niaspan promotes functional outcome via increasing HDL-C, which act in concert to promote synaptic plasticity and axon growth after stroke.
Synaptic plasticity and axon growth induced by Niacin treatment of stroke may be mediated by HDL-induced upregulation in BDNF/TrkB axis. In the mature nervous system, BDNF/TrkB is crucial for regulating neuronal migration, morphological and biochemical differentiation, and controlling synaptic function and synaptic plasticity, while continuing to modulate neuronal survival.28, 29 After somatosensory cortex injury, NgR is down-regulated specifically in cortical areas deprived of sensory input and in adjacent cortex, while BDNF is up-regulated.30, 31 NgR mRNA is down-regulated in the dentate gyrus after delivery of BDNF into the rat hippocampus formation in rats subjected to kainic acid.32 In the present study, Niacin treatment of stroke significantly increases BDNF/TrkB expression both in the ischemic brain and in PCN cultures. HDL also significantly increases the expression in BDNF/TrkB and neurite outgrowth in PCN cultures. In addition, a TrkB-inhibitor significantly decreases HDL- and Niacin-induced neurite outgrowth, which indicates that the BDNF/TrkB axis may mediate, at least in part, Niacin/HDL-induced synaptic plasticity and axon growth.
Summary
We demonstrated that Niacin treatment of stroke promotes synaptic plasticity and axon growth in rats. The BDNF/TrkB pathways appear to contribute to Niacin/HDL-induced synaptic plasticity and axon growth after stroke.
Acknowledgments
The authors wish to thank Qinge Lu and Supata Santra for technical assistance.
Sources of Funding
This work was supported by National Institute on Aging RO1 AG031811 (JC), National Institute of Neurological Diseases and Stroke PO1 NS23393 (MC) and 1R41NS064708 (JC), and American Heart Association grant 09GRNT2300151 (JC).
Footnotes
Disclosures
None.
References
- 1.Kolb B. Synaptic plasticity and the organization of behaviour after early and late brain injury. Can J Exp Psychol. 1999;53:62–76. doi: 10.1037/h0087300. [DOI] [PubMed] [Google Scholar]
- 2.Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001;24:1000–1019. doi: 10.1002/mus.1104. [DOI] [PubMed] [Google Scholar]
- 3.Adkins DL, Hsu JE, Jones TA. Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions. Exp Neurol. 2008;212:14–28. doi: 10.1016/j.expneurol.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keyvani K, Schallert T. Plasticity-associated molecular and structural events in the injured brain. J Neuropathol Exp Neurol. 2002;61:831–840. doi: 10.1093/jnen/61.10.831. [DOI] [PubMed] [Google Scholar]
- 5.Chang EH, Rigotti A, Huerta PT. Age-related influence of the hdl receptor sr-bi on synaptic plasticity and cognition. Neurobiol Aging. 2009;30:407–419. doi: 10.1016/j.neurobiolaging.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li F, Li L, Song XY, Zhong JH, Luo XG, Xian CJ, Zhou XF. Preconditioning selective ventral root injury promotes plasticity of ascending sensory neurons in the injured spinal cord of adult rats--possible roles of brain-derived neurotrophic factor, trkb and p75 neurotrophin receptor. Eur J Neurosci. 2009;30:1280–1296. doi: 10.1111/j.1460-9568.2009.06920.x. [DOI] [PubMed] [Google Scholar]
- 7.Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. 2009;26:E3. doi: 10.3171/FOC.2009.26.2.E3. [DOI] [PubMed] [Google Scholar]
- 8.Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: Brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 9.Johnston MV. Plasticity in the developing brain: Implications for rehabilitation. Dev Disabil Res Rev. 2009;15:94–101. doi: 10.1002/ddrr.64. [DOI] [PubMed] [Google Scholar]
- 10.Elam MB, Hunninghake DB, Davis KB, Garg R, Johnson C, Egan D, Kostis JB, Sheps DS, Brinton EA. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: The admit study: A randomized trial. Arterial disease multiple intervention trial. Jama. 2000;284:1263–1270. doi: 10.1001/jama.284.10.1263. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Cui X, Zacharek A, Jiang H, Roberts C, Zhang C, Lu M, Kapke A, Feldkamp CS, Chopp M. Niaspan increases angiogenesis and improves functional recovery after stroke. Ann Neurol. 2007;62:49–58. doi: 10.1002/ana.21160. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Zacharek A, Cui X, Shehadah A, Jiang H, Roberts C, Lu M, Chopp M. Treatment of stroke with a synthetic liver x receptor agonist, to 901317, promotes synaptic plasticity and axonal regeneration in mice. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, Gould TD, Manji HK, Chen G. Mood stabilizer valproate promotes erk pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24:6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Q, Li Y, Chopp M. Bone marrow stromal cells increase astrocyte survival via upregulation of phosphoinositide 3-kinase/threonine protein kinase and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways and stimulate astrocyte trophic factor gene expression after anaerobic insult. Neuroscience. 2005;136:123–134. doi: 10.1016/j.neuroscience.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 15.Halterman MW, Giuliano R, Dejesus C, Schor NF. In-tube transfection improves the efficiency of gene transfer in primary neuronal cultures. J Neurosci Methods. 2009;177:348–354. doi: 10.1016/j.jneumeth.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markus A, Zhong J, Snider WD. Raf and akt mediate distinct aspects of sensory axon growth. Neuron. 2002;35:65–76. doi: 10.1016/s0896-6273(02)00752-3. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 18.Ujike H, Takaki M, Kodama M, Kuroda S. Gene expression related to synaptogenesis, neuritogenesis, and map kinase in behavioral sensitization to psychostimulants. Ann N Y Acad Sci. 2002;965:55–67. doi: 10.1111/j.1749-6632.2002.tb04151.x. [DOI] [PubMed] [Google Scholar]
- 19.Karnezis T, Mandemakers W, McQualter JL, Zheng B, Ho PP, Jordan KA, Murray BM, Barres B, Tessier-Lavigne M, Bernard CC. The neurite outgrowth inhibitor nogo a is involved in autoimmune-mediated demyelination. Nat Neurosci. 2004;7:736–744. doi: 10.1038/nn1261. [DOI] [PubMed] [Google Scholar]
- 20.Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 21.Peng X, Zhou Z, Hu J, Fink DJ, Mata M. Soluble nogo receptor down-regulates expression of neuronal nogo-a to enhance axonal regeneration. J Biol Chem. 2009 doi: 10.1074/jbc.M109.046425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walmsley AR, Mir AK. Targeting the nogo-a signalling pathway to promote recovery following acute cns injury. Curr Pharm Des. 2007;13:2470–2484. doi: 10.2174/138161207781368611. [DOI] [PubMed] [Google Scholar]
- 23.Ramic M, Emerick AJ, Bollnow MR, O’Brien TE, Tsai SY, Kartje GL. Axonal plasticity is associated with motor recovery following amphetamine treatment combined with rehabilitation after brain injury in the adult rat. Brain Res. 2006;1111:176–186. doi: 10.1016/j.brainres.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 24.Ko M, Zou K, Minagawa H, Yu W, Gong JS, Yanagisawa K, Michikawa M. Cholesterol-mediated neurite outgrowth is differently regulated between cortical and hippocampal neurons. J Biol Chem. 2005;280:42759–42765. doi: 10.1074/jbc.M509164200. [DOI] [PubMed] [Google Scholar]
- 25.Goodrum JF, Brown JC, Fowler KA, Bouldin TW. Axonal regeneration, but not myelination, is partially dependent on local cholesterol reutilization in regenerating nerve. J Neuropathol Exp Neurol. 2000;59:1002–1010. doi: 10.1093/jnen/59.11.1002. [DOI] [PubMed] [Google Scholar]
- 26.Mulder M. Sterols in the central nervous system. Curr Opin Clin Nutr Metab Care. 2009;12:152–158. doi: 10.1097/MCO.0b013e32832182da. [DOI] [PubMed] [Google Scholar]
- 27.Karasinska JM, Rinninger F, Lutjohann D, Ruddle P, Franciosi S, Kruit JK, Singaraja RR, Hirsch-Reinshagen V, Fan J, Brunham LR, Bissada N, Ramakrishnan R, Wellington CL, Parks JS, Hayden MR. Specific loss of brain abca1 increases brain cholesterol uptake and influences neuronal structure and function. J Neurosci. 2009;29:3579–3589. doi: 10.1523/JNEUROSCI.4741-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bibel M, Barde YA. Neurotrophins: Key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- 29.Huang EJ, Reichardt LF. Neurotrophins: Roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Endo T, Spenger C, Tominaga T, Brene S, Olson L. Cortical sensory map rearrangement after spinal cord injury: Fmri responses linked to nogo signalling. Brain. 2007;130:2951–2961. doi: 10.1093/brain/awm237. [DOI] [PubMed] [Google Scholar]
- 31.Josephson A, Trifunovski A, Scheele C, Widenfalk J, Wahlestedt C, Brene S, Olson L, Spenger C. Activity-induced and developmental downregulation of the nogo receptor. Cell Tissue Res. 2003;311:333–342. doi: 10.1007/s00441-002-0695-8. [DOI] [PubMed] [Google Scholar]
- 32.Trifunovski A, Josephson A, Ringman A, Brene S, Spenger C, Olson L. Neuronal activity-induced regulation of lingo-1. Neuroreport. 2004;15:2397–2400. doi: 10.1097/00001756-200410250-00019. [DOI] [PubMed] [Google Scholar]