Abstract
Obesity in peripubertal girls is associated with hyperandrogenemia (HA), which can represent a forerunner of polycystic ovary syndrome. However, not all obese girls demonstrate HA, and determinants of HA in obese girls remain unclear. We hypothesized that insulin and luteinizing hormone (LH) are independent predictors of free T concentration in obese girls. To assess this further, fasting morning blood samples were collected from 92 obese (body mass index-for-age percentile [BMI%] ≥ 95) girls in various stages of puberty. A multivariate regression model was then constructed using free T (dependent variable), LH, insulin, pubertal group (early, mid-, or late puberty), BMI z-score, and age. Free T concentrations were highly variable among obese girls in each pubertal group. The regression model accounted for roughly half of the variability of free T in obese girls (adjusted R2 = 0.53, P < 0.001). LH was found to have the greatest independent ability to predict free T, followed by insulin, then age and BMI z-score. Pubertal group was not an independent predictor of free T. We conclude that morning LH and fasting insulin are significant predictors of free T in obese girls, even after adjusting for potential confounders (age, pubertal group, adiposity). We suggest that abnormal LH secretion and hyperinsulinemia can promote HA in some peripubertal girls with obesity.
Introduction
Polycystic ovary syndrome (PCOS) may be the most common endocrine disorder of young women, affecting approximately 7% of women in their reproductive years (1). Hallmarks of PCOS include ovarian hyperandrogenemia and ovulatory dysfunction (2, 3). The etiology of PCOS remains unclear, but hyperinsulinemia, neuroendocrine abnormalities (e.g., exaggerated luteinizing hormone [LH] secretion), and primary abnormalities of ovarian steroidogenesis have all been proposed as causes (4–6).
Manifestations of PCOS often begin soon after menarche, and hyperandrogenemia (HA) during adolescence can be a precursor of adult PCOS (7, 8). However, the cause(s) of adolescent HA is (are) unclear. Obesity is a well-recognized factor in the HA of adult PCOS (9); and some (10–12) but not all (13) studies suggest that obesity is linked to HA in peripubertal girls. Therefore, obesity during the pubertal transition may be an important factor contributing to adolescent and adult PCOS (14). Although mechanisms underlying the relationship between peripubertal obesity and HA remain uncertain, early data suggest that differences of insulin and luteinizing hormone (LH) contribute to free testosterone (T) differences between obese and non-obese girls (11, 12).
Of special interest, not all peripubertal obese girls demonstrate elevated free T (12, 13), suggesting that obesity per se is not sufficient to produce HA. Herein we present data that demonstrates marked variability of free T among obese girls. Furthermore, to assess potential determinants of free T elevations in obese girls, we evaluated the relationship between free T and both fasting insulin and morning LH, while simultaneously adjusting for differences in age, pubertal development, and gender-specific body mass index (BMI) z-score. Our primary hypotheses were that insulin and LH would be independent predictors of free T levels in obese girls.
Methods and Procedures
Subjects
Our collaborative group collected hormonal and anthropometric data from obese girls across the pubertal spectrum. Volunteers were recruited from Endocrinology Clinics and via local advertisements and evaluated at the University of Virginia (n= 61), the University of California at San Diego (n=8), and Yale (n=23). Gender-specific BMI-for-age percentile (BMI%) and BMI z-score was calculated for each participant using a SAS program incorporating normative data from the National Health and Nutrition Examination Surveys (15) (available at http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm). Subjects were considered obese if their BMI% was 95 or greater (16). Since girls classified as Tanner 1 can have variable physiology, we only included Tanner 1 subjects who demonstrated hormonal evidence of puberty, as defined by an estradiol concentration ≥ 73.4 pmol/liter (≥ 20 pg/ml). None of the girls was considered sexually precocious or had premature adrenarche. All participants had age-appropriate 17-hydroxyprogesterone and dehydroepiandrosterone sulfate (DHEAS) concentrations; undetectable β-human chorionic gonadotropin; normal thyroid stimulating hormone and prolactin levels; and unremarkable chemistry, liver, and hematology lab results (data not shown). All fasting glucose values were ≤ 105 mg/dl. Girls were not included or excluded based on the presence or absence of clinical hyperandrogenism (e.g., hirsutism, abnormal menses). Raw data from a portion of these subjects were included in previous analyses (11, 12).
Study Procedures
All study procedures were approved by the Institutional Review Board at each institution. Written informed assent and consent were obtained from participants and custodial parents, respectively. Subjects had not used hormonal medications in the 90 days preceding study, and none was taking mediations known to affect the reproductive axis.
Volunteers had a detailed history and physical exam, which included assessment of breast development (Tanner breast stage) via both inspection and palpation. Blood samples were obtained in the morning (approximately 0500–1000 h, after at least 8 hours of fasting) for later determination of LH, follicle stimulating hormone (FSH), total T, sex hormone binding globulin (SHBG), progesterone, and estradiol. In 77 subjects, insulin-like growth factor-I (IGF-I) was also measured. Premenarcheal girls were studied on a random day. Postmenarcheal girls were studied on cycle day 8–10 or at least 60 days after menses if oligomenorrheic. Sampling during the luteal phase was excluded in all subjects by a progesterone concentration < 6.36 pmol/liter (< 2 ng/ml).
Hormonal Measurements
All assays were performed in the Center for Research in Reproduction Ligand Core Laboratory (University of Virginia). All samples from an individual were analyzed in duplicate. Those samples with values below the assay sensitivity were assigned the value of the assay’s sensitivity.
Total T, estradiol, and progesterone were measured by RIA (Diagnostic Products Corporation [DPC], Los Angeles, CA; sensitivities 347, 36.7, and 0.3 pmol/liter (10 ng/dl, 10 pg/ml, and 0.1 ng/ml); intraassay coefficient of variation [CV] 3.5–6.8%; interassay CV 5.8–15.8%). FSH, LH, SHBG, and DHEAS were measured by chemiluminescence (DPC; sensitivities 0.05 IU/liter, 0.1 IU/liter, 0.2 nmol/liter, and 190 nmol/liter; intraassay CV 1.9–6.2%; interassay CV 4.8–7.8%). Insulin was measured by chemiluminescence (DPC) or RIA (Diagnostic Systems Laboratories, Inc., Webster, TX): sensitivity 9.3–18.7 pmol/liter, intraassay CV 3.0–8.5%, interassay CV 8.3–21%). IGF-I was measured in 77 subjects by chemiluminescence (Siemens Medical Solutions Diagnostics, Los Angeles, CA; sensitivity 3.3 nmol/liter; intra- and interassay CV 3.5% and 5.3%).
Free T was calculated from SHBG and total T using the following formula: FT = ([T − (N)(FT)]/[(KT)(SHBG) − (KT)(T) + (N)(KT)(FT)]), where FT is free T concentration (pmol/liter), KT is the association constant for T (1.0 × 109), T is the total T concentration in ng/dl, SHBG is in nmol/liter and N=(KA)(CA)+1, where KA is the association constant of albumin for T (3.6 × 104), and CA is the albumin concentration (assumed to be 4.3 g/dl) (17).
To convert from SI to conventional units: total and free T (pmol/liter) × 0.2884 (pg/ml); progesterone (pmol/liter) × 0.315 (ng/ml); estradiol (pmol/liter) × 0.272 (pg/ml); DHEAS (nmol/liter) × 0.0368 (μg/dl); insulin (pmol/liter) × 0.1394 (μIU/ml); IGF-I (nmol/liter) × 7.649 (ng/ml); SHBG (nmol/liter) × 0.0288 (μg/dl).
Data Analysis
For the purposes of this analysis, we divided girls into three groups based on pubertal maturation. Girls with physical exam evidence of early puberty (Tanner 2 breast development) and those with biochemical evidence of early puberty (estradiol ≥ 73.4 pmol/liter despite absence of palpable breast tissue) were combined into an early pubertal group. Tanner 3 girls comprised a mid-pubertal group. Lastly, since girls in Tanner stages 4 and 5 are very similar physiologically, we combined these girls into a late pubertal group.
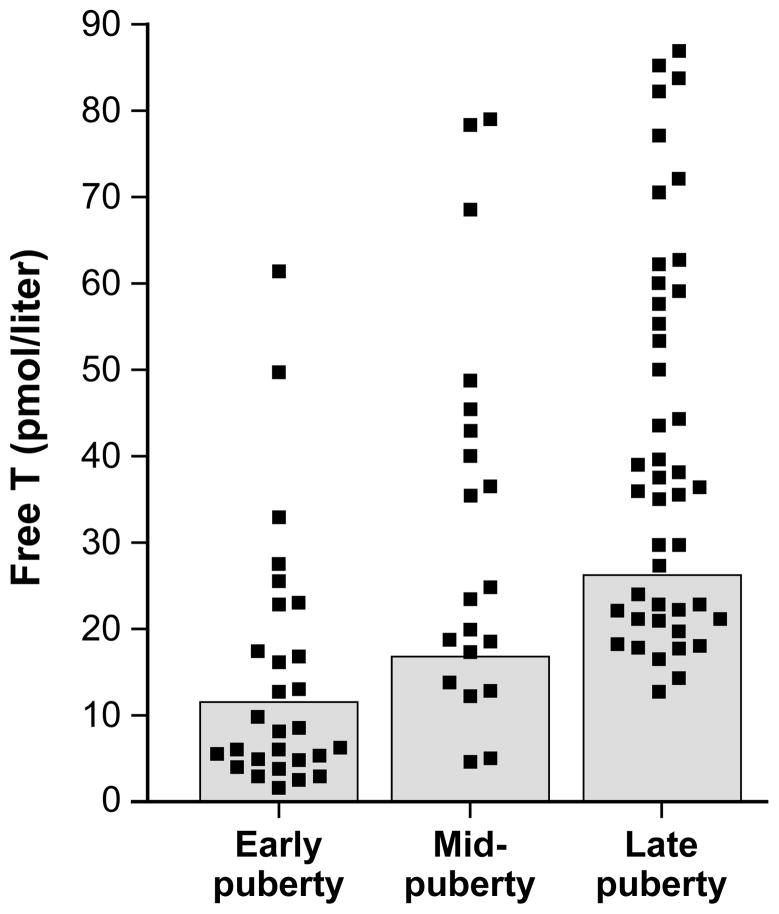
A puberty group-specific upper limit of normal for morning free T—based on data from normal controls studied by our group (data not shown)—was defined as the mean free T plus 2 standard deviations for normal weight (BMI% < 85) girls with neither clinical hyperandrogenism (e.g., hirsutism) nor abnormal menses (i.e., average intermenstrual length > 45 days in girls at least 2 years postmenarche). Cutoff values for normal free T were 11.98, 17.23, and 25.66 pmol/liter for early pubertal, mid-pubertal, and late pubertal girls, respectively.
Statistical Methods
Our a priori hypotheses were that morning LH and fasting insulin would be unique determinants of free T concentration. We used ordinary least-squares (OLS) regression analysis to test these hypotheses.
The utilized OLS regression model was defined a priori (i.e., before analyzing the data). LH and fasting insulin were considered the two primary predictor variables of interest, while age, BMI z-score, and pubertal stage (early pubertal, mid-pubertal, late pubertal) were considered potential confounders (i.e., adjustment factors). Restricted cubic spline functions of the LH and fasting insulin values were incorporated into the model so that linear and nonlinear associations could be examined simultaneously. The aforementioned regression model is symbolically displayed in equation 1:
| (1) |
In equation 1, E (free T | X) denotes the expected free T concentration given X, where X is the matrix of predictor variables;β 0 … β8 denote the regression model coefficients; and f (L)(LH), f (NL)(LH), f (L)(insulin), f (NL)(insulin) denote the linear (L) and the non-linear (NL) restricted cubic spline functions of LH and fasting insulin, respectively.
Hypothesis testing was carried out via a set of extra-sum of squares F-tests. The hypothesis testing procedure was conducted in the hierarchical manner, with global hypothesis tests of the overall fit of the model superseding hypothesis tests related to the individual predictors. A p<0.05 decision rule was specified a priori as the criterion for rejecting the null hypothesis of no association.
The accuracy of the free T predictions from the original regression model (equation 1) was compared via an extra-sum of squares F-test to the accuracy of the free T predictions from a reduced model—comprised of a subset of the original predictors—to determine the most parsimonious model for predicting free T.
We also preformed several separate secondary analyses. Firstly, to investigate to what degree (if any) the results were influenced by inclusion of girls with clinical findings potentially related to hyperandrogenemia (i.e., to assess the potential influence of recruitment bias), we performed the primary analysis after excluding all girls with hirsutism, abnormal menses, or both (n = 37). Secondly, we added to the model a set of interaction terms that would allow the linear and nonlinear associations between (a) LH and free T and (b) insulin and free T to change from one pubertal group to the next. Lastly, since FSH and IGF-I have been postulated to influence androgen levels, we added each variable to the model (one variable at a time). Extra-sum of squares F tests were conducted to determine if additional variability in free T was explained by any of these model modifications.
For all of the aforementioned models, goodness of fit was evaluated via traditional residual diagnostics. The regression analyses were carried out with the statistical software S-PLUS 7.0 (Insightful, Inc., Seattle, WA).
Results
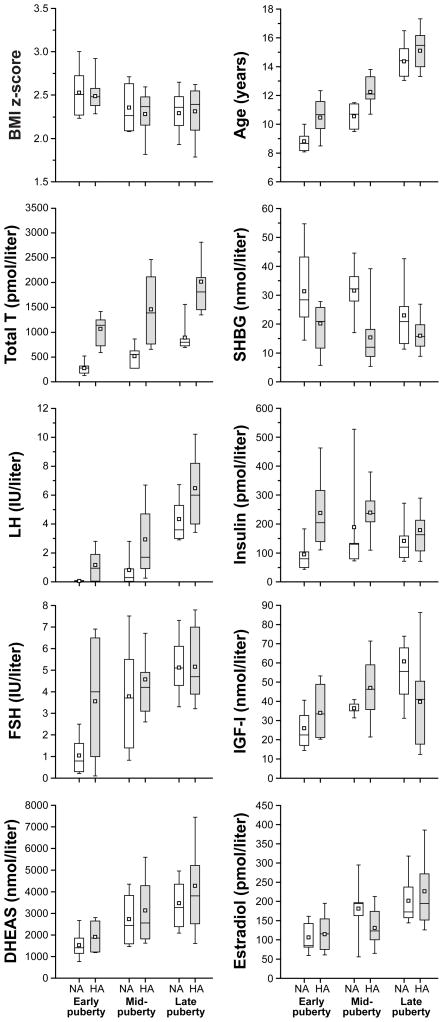
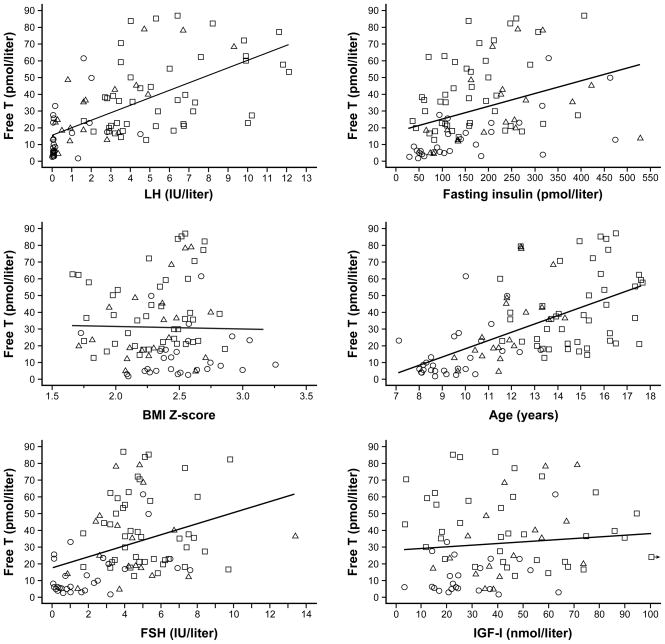
Free T values—and the marked variability thereof—for each pubertal group are shown in Figure 1. Summary data by pubertal group are presented in Table 1. In Figure 2, data are graphically represented for subjects categorized by pubertal group and according to the presence or absence of pubertal-group-specific HA. Simple relationships between free T (the dependent variable) and other independent variables used in the regression models are shown in Figure 3.
Figure 1. Free testosterone values in obese peripubertal girls.
Shaded boxes represent the normal range of free testosterone (defined in text) for each pubertal group. Early, mid-, and late puberty are defined in the text.
Table 1. Summary data by pubertal group.
| Early puberty (n=28) | Mid-puberty (n=20) | Late puberty (n=44) | |
|---|---|---|---|
| Age (y) | 9.5 ± 1.5 (9.3, 8.4–10.3) | 11.8 ± 1.3 (11.8, 11.0–12.5) | 14.9 ± 1.7 (15.0, 13.5–16.1) |
| BMI (kg/m2) | 31.4 ± 5.2 (30.9, 27.4–35.1) | 32.4 ± 4.9 (31.0, 28.6–36.9) | 37.3 ± 5.8 (36.3, 33.1–41.5) |
| BMI% | 99.2 ± 0.8 (99.4, 99.1–99.7) | 98.7 ± 1.1 (99.0, 98.2–99.4) | 98.6 ± 1.2 (99.1, 98.3–99.5) |
| BMI z-score | 2.51 ± 0.32 (2.49, 2.30–2.70) | 2.30 ± 0.29 (2.33, 2.10–2.51) | 2.30 ± 0.32 (2.36, 2.06–2.55) |
| Race | 17 W, 10 B, 1 O | 13 W, 6 B, 1 O | 25 W, 10 B, 1 A, 8 NR |
| Hispanic ethinicity | 6 of 28 (21%) | 3 of 20 (15%) | 7 of 44 (16%) |
| Tanner stage | 6 Tanner 1, 22 Tanner 2 | All Tanner 3 | 11 Tanner 4, 33 Tanner 5 |
| Postmenarcheal | 0 of 28 (0%) | 4 of 20 (20%) | 40 of 44 (91%) |
| Gynecological age * | NA | All < 1 y | 2.6 ± 1.7 (2.5, 2–3) |
| Irregular menses * | NA | NA | 25 of 40 (63%) |
| Hirsutism | 1 of 28 (4%) | 2 of 20 (10%) | 26 of 44 (59%) |
| Total testosterone (pmol/liter) | 60.7 ± 47.6 (41.6, 24.1–105.7) | 122.7 ± 75.2 (100.5, 64.1–174.8) | 161.1 ± 81.3 (147.4, 86.5–199.3) |
| SHBG (nmol/liter) | 26.6 ± 15.0 (24.1, 14.7–35.5) | 19.4 ± 12.9 (16.7, 9.1–30.1) | 18.7 ± 9.9 (16.6, 12.0–22.2) |
| Free testosterone (pmol/liter) | 14.3 ± 14.5 (8.3, 4.9–20.1) | 32.3 ± 22.7 (24.1, 15.6–44.2) | 40.9 ± 22.2 (36.2, 21.6–58.4) |
| Luteinizing hormone (IU/liter) | 0.6 ± 1.1 (0.1, 0.1–0.6) | 2.4 ± 2.5 (1.6, 0.4–3.9) | 5.7 ± 2.9 (5.2, 3.5–7.3) |
| Fasting insulin (pmol/liter) | 157 ± 124 (112, 58–205) | 227 ± 120 (228, 133–271) | 166 ± 90 (157, 102–215) |
| FSH (IU/liter) | 2.1 ± 2.3 (1.0, 0.3–3.2) | 4.4 ± 2.7 (4.0, 2.7–5.0) | 5.2 ± 1.9 (4.8, 3.9–6.3) |
| IGF-I (nmol/liter) | 29.3 ± 15.8 (23.5, 20.1–37.3) (n=23) | 44.4 ± 17.2 (40.9, 35.0–57.5) (n=17) | 45.9 ± 31.2 (42.2, 22.4–59.9) (n=37) |
| Estradiol (pmol/liter) | 110 ± 50 (86, 78–150) | 144 ± 66 (134, 87–190) | 217 ± 97 (188, 152–283) |
| DHEAS (nmol/liter) | 1683 ± 747 (1445, 1200–2269) | 3038 ± 1599 (2499, 1702–4068) | 3989 ± 2083 (3735, 2408–4891) |
BMI = body mass index; BMI% = BMI-for-age percentile; SHBG = sex hormone binding globulin; FSH = follicle stimulating hormone; IGF-I = insulin-like growth factor-I; DHEAS = dehydroepiandrosterone sulfate.
Figure 2. Selected characteristics of obese girls with normal (NA) and elevated (HA) pubertal group-specific free testosterone.
Data are presented as box and whisker plots, which show 25th and 75th percentiles (bottom and top of box); median (line within box); mean (open square); 10th and 90th percentiles (bottom and top whiskers). Numbers per subgroup are as follows: 16 NA, 12 HA (early puberty); 5 NA, 15 HA (mid-puberty); 16 NA, 28 HA (late puberty). Early, mid-, and late puberty are defined in the text. BMI = body mass index; T = testosterone; SHBG = sex hormone binding globulin; LH = luteinizing hormone; FSH = follicle stimulating hormone; IGF-I = insulin-like growth factor-I; DHEAS = dehydroepiandrosterone sulfate.
Figure 3. Simple relationships between free testosterone (T) and other predictor variables.
Data from early, mid-, and late pubertal girls are represented by circles, triangles, and squares, respectively. Regression lines were calculated using data from all subjects. LH = luteinizing hormone; BMI = body mass index; FSH = follicle stimulating hormone; IGF-I = insulin-like growth factor-I.
Primary analysis
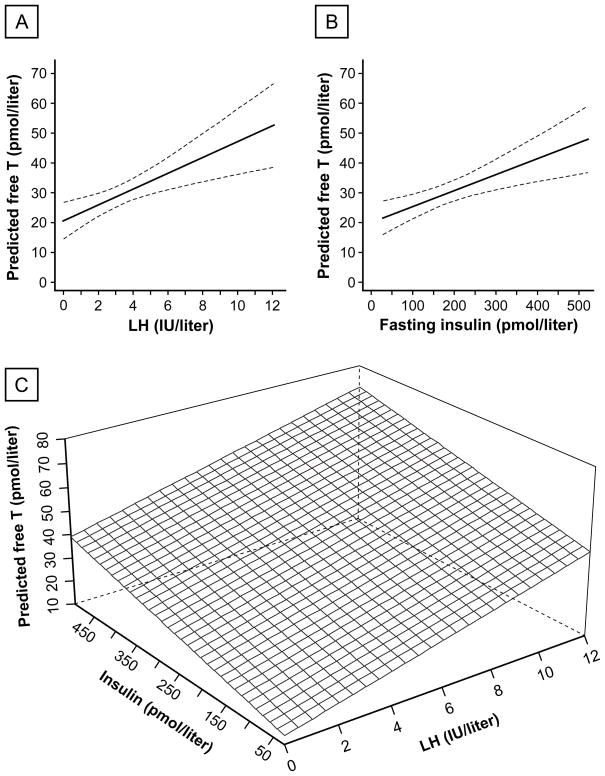
The primary OLS regression model (equation 1) was highly statistically significant (P < 0.001), with specified independent variables accounting for approximately half of observed free T variability (R2 = 0.57; adjusted R2 = 0.53). Independent predictors of free T included (in order of independent contribution to R2) LH, fasting insulin, age, and BMI z-score (P = 0.003, 0.023, 0.016, and 0.024 respectively). Predicted relationships among free T, LH, and insulin are shown in Figure 4. Pubertal group and the nonlinear components of LH and insulin were not independently associated with free T.
Figure 4. Predicted morning free testosterone (T) as a function of morning luteinizing hormone (LH) and fasting insulin concentrations in obese peripubertal girls.
A. Predicted free T as a function of morning LH concentration, with insulin, age, and BMI z-score held fixed at median values of 157 pmol/liter (21.9 μIU/ml), 12.4 years, and 2.4, respectively. The dashed lines enclose the 95% confidence band for the regression line. B. Predicted free T as a function of fasting insulin concentration, with LH, age, and BMI z-score held fixed at 2.9 IU/liter, 12.4 years, and 2.4, respectively. C. Predicted free T as a function of morning LH and fasting insulin concentrations (trivariate association), with age and BMI z-score held fixed at 12.4 years and 2.4, respectively.
A reduced model, which included only the significant and independent predictors of free T (i.e., LH, insulin, age, BMI z-score), was not statistically different from the original model (equation 1) in its ability to predict free T.
Secondary analyses
When girls with hirsutism and/or abnormal menses (n = 37) were excluded from analysis, the overall regression model remained highly statistically significant (P < 0.001, adjusted R2 = 0.53). Independent predictors of free T included LH (P = 0.004) and fasting insulin (P = 0.03), but not age, pubertal group, or BMI z-score. Similar results were obtained after excluding only girls with hirsutism (n = 29).
As discussed in Statistical Methods, we added a set of interaction terms to the model (equation 1) that would allow the associations between (a) LH and free T and (b) insulin and free T to change from one puberty group to the next. These interaction terms were not statistically significant.
Two additional models were constructed, one in which linear and nonlinear terms for FSH were added, and another in which linear and nonlinear terms for IGF-I were added (the latter variable being available for 77 subjects). Both models were highly statistically significant (adjusted R2 = 0.53; P < 0.001); and LH, insulin, age, and BMI z-score remained significant predictors of free T, while puberty group was not. However, neither FSH nor IGF-I were independent predictors of free T in these analyses (P= 0.255 and 0.154, respectively).
Discussion
As a group, obese peripubertal girls exhibit higher androgen levels compared to their normal weight counterparts (10, 12). However, the current data demonstrate that free T concentrations are highly variable among obese girls. In this large cohort of peripubertal obese girls, morning LH had the greatest ability to independently predict free T, followed by fasting insulin. Importantly, these associations were significant even after adjusting for differences in the potentially confounding variables age, pubertal group, and BMI z-score. These relationships were similar across pubertal development, and they remained significant even after excluding girls with clinical evidence of HA.
LH concentrations appear to be lower in obese girls compared to non-obese girls, especially when measured overnight during early puberty (13, 18). Nonetheless, morning LH was strongly and independently associated with free T in this cohort of obese girls. This association is reminiscent of findings in adult PCOS and adolescent HA, both of whom exhibit increased LH secretion and elevated androgen concentrations (19–21). This relationship in obese girls may reflect an ability of LH to promote androgen excess, as LH is the proximate stimulus for androgen production by the ovarian theca cell compartment. Conversely, HA may reduce GnRH pulse generator sensitivity to sex steroid negative feedback, thereby leading to persistently rapid GnRH pulse frequency and increased LH secretion (22–25).
The second best predictor of free T in our model was fasting insulin concentration. Insulin can act synergistically with LH to stimulate androgen production by the ovaries, and hyperinsulinemia also decreases SHBG concentrations, both of which increase free T (26). Additionally, therapies that reduce hyperinsulinemia ameliorate the HA of PCOS (1, 4, 26). Taken as a whole, these findings support a role of hyperinsulinemia in producing HA in obese girls. However, it remains possible that androgens contribute to insulin resistance and hyperinsulinemia during puberty (27).
Age and BMI z-score remained significant, albeit relatively weak, predictors of free T in our analysis. This may relate to unmeasured factors associated with age and/or BMI z-score that also influence free T concentrations. For example, girls with similar BMI z-scores may have important differences in both (a) percentage body fat and (b) fat distribution among visceral and subcutaneous compartments; and these (unmeasured) parameters may predict free T better than BMI z-score does. However, it is also possible that age and BMI z-score would not have been significant predictors of free T if we had obtained more detailed measurements of LH secretion and hyperinsulinemia. Specifically, puberty is marked by sleep-associated changes of LH pulse frequency and amplitude (28, 29). Thus, sampling for LH in the morning may not accurately reflect 24-hour LH secretion, particularly in early pubertal girls. Moreover, single measurements of LH do not capture the pulsatile nature of LH secretion. Likewise, fasting insulin is an imprecise measure of both insulin resistance and 24-hour insulin exposure.
While free T levels were generally higher with advancing pubertal maturation, pubertal group did not independently predict free T levels. This is partly related to the marked variability of free T observed within pubertal groups. Overall, it appears that certain variables that increase across pubertal development (e.g., LH) are more closely associated with free T than pubertal stage per se. Moreover, there was no demonsrable interaction between either (a) pubertal group and LH or (b) pubertal group and insulin, suggesting that the relationships between free T and LH and between free T and insulin are similar across pubertal development.
Our cohort of subjects included 37 girls with clinical findings potentially related to hyperandrogenemia such as hirsutism or abnormal menses. In a study of androgen excess, we believe that systematic exclusion of girls with clinical evidence of HA can be problematic. However, results after exclusion of such girls were not materially different from those of the primary analysis. Specifically, LH and insulin appear to be significant and independent predictors of free T in girls without clinical evidence of androgen excess. Nonetheless, the possibility of recruitment bias is important in other respects. For example, obese girls with clinical hyperandrogenism may have been more likely to participate in our studies compared to obese girls without clinical hyperandrogenism; thus, these data cannot be used to estimate the prevalence of HA in obese girls.
Secondary analyses explored the relationships between free T and both FSH and IGF-I, as these hormones have been postulated to influence ovarian androgen production. Many women with PCOS have a relative deficiency of FSH; this may contribute to HA via impaired follicular development and relative reductions of granulosa cell aromatase activity, which is responsible for estradiol synthesis from androgen precursors (6). IGF-I levels are highest during the pubertal growth spurt; IGF-I largely accounts for the so-called insulin resistance of puberty; and IGF-I can stimulate ovarian androgen synthesis via binding insulin and IGF-I receptors (26, 30). However, neither FSH nor IGF-I were independently predictive of free T in this cohort of obese peripubertal girls.
Based on these and earlier data, we propose the following working hypothesis concerning obestity-related HA and its potential relationship to the development of PCOS. Peripubertal obesity is associated with variable degrees of insulin resistance. Compensatory hyperinsulinemia can then increase ovarian and/or adrenal androgen production and lower SHBG, both of which increase free T concentrations. And in susceptible girls, HA impairs the sensitivity of the GnRH pulse generator to negative feedback, leading to persistently rapid GnRH pulses, elevated LH, and impaired FSH secretion. These neuroendocrine abnormalities maintain or worsen HA, leading to a vicious cycle that supports a progression toward the PCOS phenotype.
In conclusion, the current results suggest that LH and fasting insulin are significant and independent predictors of free T levels in obese girls, even after adjusting for potential confounders (age, pubertal group, BMI%). Although these strong associations do not prove causality, they point to a possible etiologic role for both LH excess and hyperinsulinemia in the development of HA in some obese girls. We recognize that morning LH and fasting insulin values are imprecise measures of pulsatile LH secretion and hyperinsulinemia/insulin resistance, respectively. Therefore, additional studies are needed to assess further the potential causative roles of LH and insulin in obesity-associated HA in peripubertal girls, in addition to mechanisms by which peripubertal obesity may predispose to the development of PCOS.
Acknowledgments
We gratefully acknowledge Lauren Lockhart, M.P.H., Quirine Lamberts Okonkwo, M.D., Amy Bellows, Ph.D., and Chandan Chopra for subject recruitment, study scheduling, and assistance with data management; Alan D. Rogol, M.D., William L. Clarke, M.D., Milagros G. Huerta, M.D., and Susan B. Cluett, CRNP, for assisting with subject recruitment; Christine A. Eagleson M.D., Kathleen A. Prendergast M.D., Kristin D. Helm, M.D., Sandhya Chhabra, M.D., and Richard Yoo, M.D., for assistance with protocol implementation; Mark D. DeBoer, M.D., for thoughtful editorial comments; the nurses and staff of the General Clinical Research Centers (University of Virginia, University of California San Diego, and Yale) for implementation of these study protocols; and the Center for Research in Reproduction Ligand Core Laboratory (University of Virginia) for performance of all assays.
This work was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54 HD 28934 (University of Virginia) and U54 HD 12303 (University of California, San Diego) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research; General Clinical Research Center Grants M01 RR00847 (University of Virginia), M01 RR00827 (University of California, San Diego), and M01 RR00125 (Yale); K23 HD044742 (C.R.M.); R01 HD058671 (C.R.M.); F32 HD055014 (S.K.B.) and T32 HD007382 (K.L.K.).
Footnotes
Disclosure statement
None of the authors has any potential conflicts of interest to declare.
References
- 1.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 2.Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, editors. Polycystic Ovary Syndrome. Blackwell Scientific; Boston: 1992. pp. 377–384. [Google Scholar]
- 3.Azziz R, Carmina E, Dewailly D, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 4.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 5.Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update. 2006;12:351–361. doi: 10.1093/humupd/dml017. [DOI] [PubMed] [Google Scholar]
- 6.Ehrmann DA, Barnes RB, Rosenfield RL. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev. 1995;16:322–353. doi: 10.1210/edrv-16-3-322. [DOI] [PubMed] [Google Scholar]
- 7.Franks S. Adult polycystic ovary syndrome begins in childhood. Best Pract Res Clin Endocrinol Metab. 2002;16:263–272. doi: 10.1053/beem.2002.0203. [DOI] [PubMed] [Google Scholar]
- 8.Witchel SF. Puberty and polycystic ovary syndrome. Mol Cell Endocrinol. 2006;254–255:146–153. doi: 10.1016/j.mce.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 9.Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2002;26:883–896. doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- 10.Reinehr T, de Sousa G, Roth CL, Andler W. Androgens before and after weight loss in obese children. J Clin Endocrinol Metab. 2005;90:5588–5595. doi: 10.1210/jc.2005-0438. [DOI] [PubMed] [Google Scholar]
- 11.McCartney CR, Prendergast KA, Chhabra S, et al. The Association of Obesity and Hyperandrogenemia during the Pubertal Transition in Girls: Obesity as a Potential Factor in the Genesis of Postpubertal Hyperandrogenism. J Clin Endocrinol Metab. 2006;91:1714–1722. doi: 10.1210/jc.2005-1852. [DOI] [PubMed] [Google Scholar]
- 12.McCartney CR, Blank SK, Prendergast KA, et al. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab. 2007;92:430–436. doi: 10.1210/jc.2006-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bordini B, Littlejohn E, Rosenfield RL. Blunted sleep-related luteinizing hormone rise in healthy premenarcheal pubertal girls with elevated body mass index. J Clin Endocrinol Metab. 2009;94:1168–1175. doi: 10.1210/jc.2008-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenfield RL. Clinical review: Identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:787–796. doi: 10.1210/jc.2006-2012. [DOI] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 16.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120 (Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 17.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 18.McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab. 2009;94:56–66. doi: 10.1210/jc.2008-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venturoli S, Porcu E, Fabbri R, et al. Postmenarchal evolution of endocrine pattern and ovarian aspects in adolescents with menstrual irregularities. Fertil Steril. 1987;48:78–85. doi: 10.1016/s0015-0282(16)59294-2. [DOI] [PubMed] [Google Scholar]
- 20.Waldstreicher J, Santoro NF, Hall JE, Filicori M, Crowley WF., Jr Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab. 1988;66:165–172. doi: 10.1210/jcem-66-1-165. [DOI] [PubMed] [Google Scholar]
- 21.Apter D, Butzow T, Laughlin GA, Yen SS. Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab. 1994;79:119–125. doi: 10.1210/jcem.79.1.8027216. [DOI] [PubMed] [Google Scholar]
- 22.Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 1998;83:582–590. doi: 10.1210/jcem.83.2.4604. [DOI] [PubMed] [Google Scholar]
- 23.Eagleson CA, Gingrich MB, Pastor CL, et al. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85:4047–4052. doi: 10.1210/jcem.85.11.6992. [DOI] [PubMed] [Google Scholar]
- 24.Chhabra S, McCartney CR, Yoo RY, Eagleson CA, Chang RJ, Marshall JC. Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: evidence for varied effects in hyperandrogenemic adolescent girls. J Clin Endocrinol Metab. 2005;90:2810–2815. doi: 10.1210/jc.2004-2359. [DOI] [PubMed] [Google Scholar]
- 25.Blank SK, McCartney CR, Chhabra S, et al. Modulation of GnRH pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls - Implications for regulation of pubertal maturation. J Clin Endocrinol Metab. 2009;94:2360–2366. doi: 10.1210/jc.2008-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20(4):535–82. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- 27.Corbould A. Effects of androgens on insulin action in women: is androgen excess a component of female metabolic syndrome? Diabetes Metab Res Rev. 2008;24:520–532. doi: 10.1002/dmrr.872. [DOI] [PubMed] [Google Scholar]
- 28.Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, Hellman L. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med. 1972;287:582–586. doi: 10.1056/NEJM197209212871203. [DOI] [PubMed] [Google Scholar]
- 29.Apter D, Butzow TL, Laughlin GA, Yen SS. Gonadotropin-releasing hormone pulse generator activity during pubertal transition in girls: pulsatile and diurnal patterns of circulating gonadotropins. J Clin Endocrinol Metab. 1993;76:940–949. doi: 10.1210/jcem.76.4.8473410. [DOI] [PubMed] [Google Scholar]
- 30.Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res. 2006;60:759–763. doi: 10.1203/01.pdr.0000246097.73031.27. [DOI] [PubMed] [Google Scholar]