In response to a clinical need for alternative treatments for fungal endophthalmitis, the toxicity of intravitreal injection of caspofungin was tested in mice at concentrations in the vitreous ranging from 0.1 to 100 times the 90th percentile inhibitory concentration (MIC90). No significant effects on ERG or retinal histology were observed at up to 10 times MIC90, suggesting that caspofungin may be safe at therapeutically effective doses, but at 100 times MIC90, reduced ERG amplitudes and loss of retinal ganglion cells were observed.
Abstract
Purpose.
Caspofungin is a synthetic echinocandin antifungal agent that inhibits the synthesis of β(1,3)-d-glucan, an essential component of the cell wall of susceptible Aspergillus and Candida species. In this study, retinal toxicity was determined after intravitreal injection of caspofungin in a mouse model to assess its safety profile for the treatment of fungal endophthalmitis.
Methods.
Caspofungin acetate was injected intravitreally in the left eyes of male C57BL/6 mice, with final vitreal concentrations corresponding to 0.41, 1.2, 2.5, 4.1, and 41 μM (five mice per cohort). A total of 25 age-matched male C57BL/6 mice injected with balanced salt solution were used as control subjects (five for each of the five different caspofungin acetate concentrations). Electroretinograms (ERGs) were recorded 7 weeks after the injections, and the injected eyes were examined histologically.
Results.
Mice injected with caspofungin at vitreal concentrations from 0.41 to 4.1 μM did not have significant alterations in their ERG waveforms, and their retinas had no detectable morphologic changes or loss of cells. At the vitreal concentration of 41 μM, caspofungin reduced the amplitudes of the a-waves, b-waves, and scotopic threshold responses of the ERG and also produced a decrease in the number of cells in the ganglion cell layer.
Conclusions.
Caspofungin is a safe antifungal agent at vitreal concentrations of 0.41 to 4.1 μM in mice and consequently shows promise for the treatment of fungal endophthalmitis in humans. Much higher doses produce toxicity and should not be used.
Infectious endophthalmitis is an inflammatory response of intraocular fluid or tissues to infection that represents one of the most serious and vision-threatening conditions in ophthalmology.1 According to past studies, mycotic or fungal endophthalmitis accounts for 8.6% to 18.6% of culture-positive endophthalmitis2–4 with Candida spp. and Aspergillus spp. being the most frequently isolated organisms.4–7 The prognosis of fungal endophthalmitis depends on the magnitude of intraocular involvement, the virulence of the organism, and the timing and mode of interventions. Because fungal endophthalmitis is a comparatively less common form of endophthalmitis of diverse etiology, it has been difficult to formulate an established treatment plan. Although systemic antifungals have been used in mild fungal endophthalmitis, intravitreal amphotericin B has traditionally been the drug of choice for moderate to severe cases of vitreous involvement or cases that are nonresponsive to systemic treatment.8 Many studies have been undertaken to find alternative antifungal pharmacologic agents for intravitreal injections in the treatment of fungal endophthalmitis.9–14 Alternative antifungals would be useful for several reasons: (1) Intravitreal amphotericin B is proinflammatory and can cause focal retinal necrosis, even at low doses (i.e., 4.1 or 8.3 μg/mL)10,15; (2) resistance to amphotericin B is an emerging threat,16–18 necessitating alternative therapy or combination therapy; (3) resistance to other systemically administered antifungals is also increasing; and (4) new antifungals are necessary to improve on the spectrum of fungicidal activity.
Caspofungin acetate (1-[(4R,5S)-5-[(2-aminoethyl)amino]-N2-(10,12-dimethyl-1-oxotetradecyl)-4-hydroxy-l-ornithine]-5-[(3R)-3-hydroxy-l-ornithine] pneumocandin B0 diacetate salt) is synthesized from a fermentation product of Glarea lozoyensis. It belongs to the echinocandin group of antifungals, and like other members of this group, it noncompetitively inhibits UDP-glucose β-(1,3)-d-glucan-β-(3)-d-glucosyltransferase (also referred to as 1,3-β-d glucan synthase), an enzyme that is necessary for the synthesis of an essential component of the cell wall of many fungal species, 1,3-β-d glucan.19–21 Inhibition of glucan synthase destabilizes the integrity of the fungal cell wall, ultimately resulting in cell lysis because of lack of rigidity and inability to resist osmotic pressure.22 We chose to study the retinal toxicity of caspofungin via intravitreal injections because 1 it is effective against a wide variety of Candida spp. (with the exception of C. parapsilosis and C. guilliermondii) and Aspergillus spp.,22 which are the most common causative organisms in fungal endophthalmitis; (2) it is less toxic than amphotericin B23; and (3) it is effective against a wide variety of fungal species, but has shown poor intravitreal penetration when administered systemically in both experimental and case studies,24,25 which necessitates direct intravitreal administration. The poor intraocular penetration of caspofungin is most likely due to its high molecular mass (1213 Da; the blood–eye barrier is thought to be impermeable to molecules >500 Da26). However, two other studies have shown that systemic caspofungin may be effective in fungal endophthalmitis.27,28 Although resistance to echinocandins can occur due to mutations in the FKS1 or FKS2 genes, which code for 1,3-β-d-glucan synthase, the presence of a drug efflux pump in the fungal cell wall, or the overexpression of cell wall transport proteins,20,29–31 the development of resistance to echinocandins has been sparsely documented in the literature.32–36
We used pigmented mice as a rodent model for our study, because, like rats, the mouse retina (i.e., its scotopic circuit and retinal vascular structure) is very similar to the human one. Moreover, the availability of transgenic and knockout animals with known defects opens the possibility of elucidating the exact mechanism of action and toxicity of intravitreally injected drugs.37 Similar to many other studies in the past, electroretinograms and retinal histology were used as methods to assay for retinal toxicity.9,10,12,13,15,37
Materials and Methods
Animals
Fifty male C57BL/6 mice, aged 7 to 8 weeks at the inception of the study were used as subjects. The animals were fed ad libitum with laboratory chow (Purina, Indianapolis, IN) and water and were reared in a room with a 12-hour light (<40 lux)/12-hour dark cycle. All animal procedures conformed to U.S. Public Health Service and the Institute for Laboratory Animal Research guidelines and were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. The experimental procedures were in accord with principles of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Caspofungin Intravitreal Injections
The mice were allowed food and water ad libitum before anesthesia. For each dose, the mice were divided into two cohorts of five mice each. One cohort was injected intravitreally with balanced salt solution and the other was injected with caspofungin acetate dissolved in balance salt solution (C+balanced salt solution). All injections were in the left eye. Before the administration of anesthesia, the pupils of the left eyes were fully dilated with a topical instillation of 1% tropicamide and 2.5% phenylephrine on the cornea. The mice were then anesthetized with intraperitoneal injection of ketamine (50 mg/kg) and xylazine (5 mg/kg). Under magnification of a zoom stereomicroscope (sm z800; Nikon Inc., Melville, NY), a pilot hole was made with a 30-gauge needle 0.5 mm behind the limbus, and 1.5 μL of the injectate was delivered slowly with a 35-gauge blunt needle (NF35BL-2; World Precision Instruments, Sarasota, FL) connected to a 100-μL syringe (Nanofil; World Precision Instruments) via tubing (SifFlex-2; World Precision Instruments). The syringe, which was controlled by a foot pedal–activated, microprocessor-based microsyringe pump controller (Micro 4; World Precision Instruments), was used to deliver the injectate at the rate of 170 nL/s into the intravitreal space of the eye. The eyes were lubricated with methylcellulose drops after injection, and an antibiotic eye ointment (Neosporin; Bausch & Lomb Inc., Tampa, FL) was administered topically after the procedure. The absence of vitreal hemorrhage in the injected eye was confirmed in all animals by planar ophthalmoscopy. All animals developed reversible bilateral cataracts while under anesthesia. The animals were warmed at 37°C until recovery. All animals were mobile and feeding well the day after the procedure. There was no conjunctival redness or posttraumatic cataract in the injected eye, nor was there any residual lens opacity in the contralateral eye of all animals studied.
Dose of Caspofungin
Caspofungin acetate (Cancidas; Merck & Co., Inc., Whitehouse Station, NJ) was obtained in vials of 50 mg lyophilized powder and serially diluted in balanced salt solution. The doses used in this study ranged from 0.41 μM (0.50 μg/mL) which is a dose that corresponds to the 90th percentile minimum inhibitory concentration (MIC90) for Aspergillus species,38 to 100 times that amount (41 μM, 50 μg/mL). Final concentrations were based on a mouse vitreal volume of 20 μL.39 Intravitreal concentrations of the five tested doses were: 41 μM (50 μg/mL, 100× MIC90), 4.1 μM (5.0 μg/mL, 10× MIC90), 2.5 μM (3.0 μg/mL, 6× MIC90), 1.2 μM (1.5 μg/mL, 3× MIC90), and 0.41 μM (0.50 μg/mL, 1× MIC90).
Electroretinographic Recording
Seven weeks after the intravitreal injections, electroretinographic (ERG) recordings were performed according to a protocol described elsewhere.39 Briefly, after overnight dark adaptation in a ventilated light-tight box, the animals were prepared for recording under red illumination (light-emitting diode [LED], >620 nm). To obtain consistent and maximum pupillary dilatation without causing ERG amplitude growth during the recording, the pupils were fully dilated with a single mydriatic, topical atropine (0.5%), before anesthesia.40 The mice were anesthetized with a single intraperitoneal injection of ketamine (70 mg/kg) and xylazine (7 mg/kg; both drugs from Vedco, Saint Joseph MO). Rectal temperature was maintained between 36°C and 37°C with an electrically heated blanket (CWE Inc., Ardmore, PA). Each animal was kept in an aluminum Faraday cage for the duration of the recording. The animal's head was held steady to reduce the noise originating from respiratory and other movements by using an aluminum head holder with a hole for the upper incisors to fix the upper jaw. This fixation ensured that the jaw remained open throughout the recording. Moist room air was pumped through a clear polyvinylchloride (PVC) pipe kept close to the open mouth. The head holder also served as the ground. All animals were kept warm at 37°C until they recovered from anesthesia. Recording sessions lasted up to 30 minutes. The animals were euthanatized soon after recording while still under the influence of anesthesia. Animals that died during anesthesia or during the ERGs were not included in the study for subsequent analysis. ERGs were recorded differentially between Dawson/Trick/Litzkow nylon/silver electrode fiber electrodes41 moistened with normal saline and placed on the two eyes. The eyes were covered with contact lenses that were pressure molded from 0.19 mm clear ACLAR film (Ted Pella Inc., Redding, CA) for the stimulated eye and 0.7 mm opaque PVC for the nonstimulated eye. Both lenses were placed over a cover of 1.2% methylcellulose in 1.2% saline. The signals were amplified (DC to 500 Hz), digitized at 2 kHz, and sent to the computer for averaging, display and storage, and subsequent analysis. A custom-made LED (λmax, 462 nm; −5.8–1.9 log scotopic Troland seconds; sc td s)–based stimulator controlled by a timer (AMD 9513 based; USB-4302; Measurement Computing, Norton, MA) provided the light stimuli.39 The intervals between flashes were adjusted so that the response returned to baseline before another stimulus was presented. A digital 60-Hz notch filter was applied off-line. The light-stimulus was calibrated with a photometer (IL1700; International Light Research, Peabody, MA) with a filter corrected for human scotopic vision based on the fact that spectral sensitivity of the mouse rods is very similar to the Commission Internationale de I'Eclairage scotopic spectral efficiency.42
Histology
Eye cup tissue was prepared for histologic analysis shortly after the ERG recordings. The details of tissue preparation and staining methods were similar to those published elsewhere.39,43–46 Each mouse was euthanatized by cervical dislocation after ERG recording, while the animal was still under anesthesia. The superior pole of the eye to be sectioned was cauterized for overall eye cup orientation. The eye was enucleated, punctured at the superior limbus with a 26-gauge needle eye, and immersed in fixative (4% paraformaldehyde and 0.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2) for 5 minutes on a shaker (Nutator; BD Diagnostics, Burlington, NC) at room temperature (RT). The cornea was then excised, and the enucleated eye was kept in fixative (at RT on the Nutator) for an additional 10 minutes. The lens was then removed and fixed for an additional 1.25 hours (at RT on the Nutator) with fresh fixative. The eye cup was rinsed three times for 10 minutes each in 0.1 M cacodylate buffer (RT) and subsequently infiltrated with 30% sucrose in 0.1 M cacodylate buffer for 15 to 17 hours (at 4°C on the Nutator). The sucrose was drained, and the eye cups were sectioned close to the optic nerve along the superior–inferior axis. The eye cup halves were then sequentially washed with OCT (Tissue Tek; Sakura Finetek USA. Inc., Torrance, CA) for 0.75 to 1.0 hours, transferred into a casting mold filled with fresh OCT, flash-frozen in liquid nitrogen, and stored at −80°C. Radial cryostat sections (10 μm) were made at −19°C, collected on glass slides (Superfrost plus; VWR international, West Chester, PA), and stored at −80°C.
The frozen sections were rinsed (Milli-Q purified water; Millipore, Billerica, MA) for 1 minute and then stained with 0.1% eosin solution for 2 minutes. The sections were washed in the purified water for 10 seconds, dehydrated, and coverslipped in a fade-retardant mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vectashield, Vector Laboratories, Burlingame, CA).
The eosin- and DAPI-stained frozen sections were viewed with a fluorescence microscope (Eclipse TE 2000-U; Nikon Inc.) under 4× and 20× magnification, and images were captured with a digital camera (CoolSNAP; Photometrics, Tucson, AZ) under software-controlled uniform conditions of exposure (MetaVue ver. 6.7r5; Molecular Devices, Downingtown, PA). Matching images in the different channels were overlaid by using image-management software (Photoshop 6.0; Adobe Systems, Inc., Mountain View, CA).
Results
In our experimental design the mice were separated into five groups. Each group was divided into age-matched cohorts of mice that received intravitreal injection of C+balanced salt solution and control animals that received intravitreal balanced salt solution alone. The caspofungin dose across each group was 1×, 3×, 6×, 10×, or 100× the MIC90 for Aspergillus spp. (0.41–41 μM). Each week for 5 weeks, injections were performed on a different dose group. Seven weeks after the injections, ERGs were recorded and histology samples collected from each group, thereby resulting in a total duration of 12 weeks for the entire study. The ERG a- and b-wave amplitudes for the balanced salt solution–injected control eyes for the different groups were not significantly different from one another (one-way ANOVA: a-wave, F(4,17) = 0.31, P = 0.86; scotopic b-wave Vmax [from Naka-Rushton fit, described later], F(4,17) = 0.10, P = 0.97; mixed b-wave amplitude [2.3 log sc td s], F(4,17) = 0.17, P = 0.94). These observations indicate that our ERG results across the total duration of the study had minimal confounders due to variabilities in the damage caused by the injection procedure, ERG recording settings, differing anesthesia depths, body temperature, and levels of dark-adaptation between sessions. Intravitreal injections did not produce observable histologic changes to the retina in the balanced salt solution–injected control eyes 7-weeks after injection, removing the injection process itself as a significant confounder in the interpretation of our results (see Fig. 4).
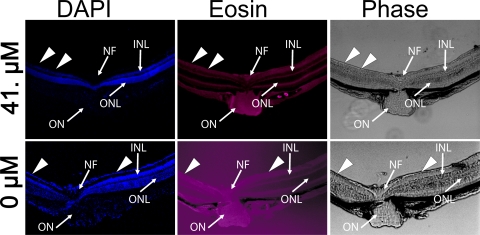
Figure 4.
Histopathologic examination of radial sections of mice eyes 7 weeks after intravitreal injection of balanced salt solution or incrementing concentrations of C+balanced salt solution. Superimposed on a phase contrast image are DAPI stained nuclei in various cell layers (blue) and eosin-stained cell membrane (red) prominently seen in the plexiform layers. No retinal abnormalities were noted in the retinas injected with balanced salt solution alone or 0.41 to 4.1 μM of caspofungin dissolved in balanced salt solution. Eyes injected with 41 μM of caspofungin showed loss of cells in the ganglion cell layer (arrowheads). OS, outer and inner segments of the photoreceptors; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar, 20 μm.
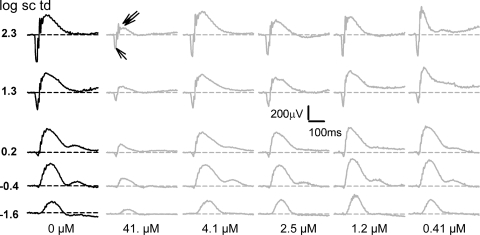
The raw ERGs recorded for those eyes injected intravitreally with 0.41, 1.2, 2.5, or 4.1 μM caspofungin were very similar to those of the mice injected with balanced salt solution alone. However, the mice that received a dose of 41 μM caspofungin showed reduced amplitudes (Fig. 1). The ERGs were analyzed for changes in the photoreceptor-derived, negatively going a-wave measured from the baseline to its trough and for changes in the ON-bipolar derived b-wave, with amplitudes measured from the a-wave trough to the b-wave peak.
Figure 1.
Effects of intravitreal caspofungin on the ERG flash response. Representative ERG responses subjects injected with intravitreal balanced salt solution or various doses of C+balanced salt solution to brief flashes of increasing stimulus energies, as indicated, for the fully dark-adapted condition. Black traces, left: representative intravitreal balanced salt solution–injected subject; gray traces, representative C+balanced salt solution–injected subjects; the intravitreal concentrations of caspofungin are indicated below each column of corresponding ERG traces. Unlabeled single and double arrows: reduced a- and b-waves, respectively, for a saturating flash of 2.3 log sc td s for the 41-μM concentration of caspofungin.
To interpret the ERG data in terms of alteration in retinal physiology after caspofungin injection, we plotted the mean b- and a-wave amplitudes of the C+balanced salt solution and balanced salt solution–injected eyes for each group as a function of stimulus energy, and interpreted their nonlinear monotonic relationship with a fitted Naka-Rushton function47 (Fig. 2) as in another study40:
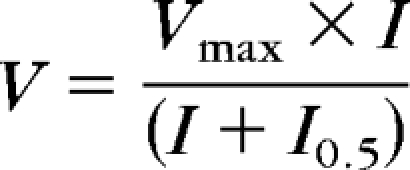 |
where, V is the ERG response amplitude, Vmax is the maximum amplitude of the response, I0.5 is the flash energy that elicits a half-maximum response, and I is the flash energy that elicits the response, V.
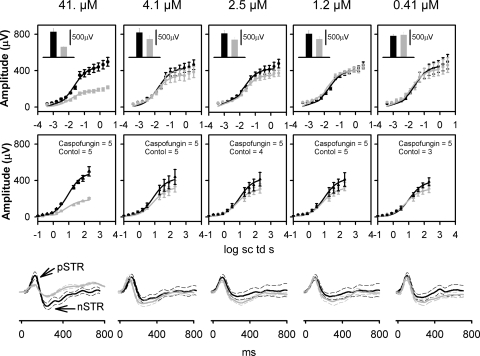
Figure 2.
Effects of intravitreal caspofungin on the mean ERG b- and a-wave stimulus-amplitude relationship and scotopic threshold responses. Caspofungin concentrations in micromolar are indicated at the top of each column. Top: Average dark-adapted electroretinogram (ERG) b-wave amplitudes (±SEM) for low scotopic energies plotted as a function of stimulus energy for the dark-adapted ERGs. Black circle: Intravitreal balanced salt solution–injected control subjects; gray circle: intravitreal C+balanced salt solution–injected subjects. Black and gray solid curves: best fit Naka-Rushton function for the balanced-saline control and C+balanced salt solution–injected eyes, respectively. Inset: average dark-adapted ERG b-wave amplitudes (±SEM) for a saturating flash of 2.3 log sc td s. (■) Balanced salt solution–injected control; ( ) C+balanced salt solution–injected eye. Middle: average dark-adapted electroretinogram (ERG) a-wave amplitudes (±SEM) plotted as a function of stimulus energy for the dark-adapted ERGs. Black circle: intravitreal balanced salt solution–injected controls; gray circle: intravitreal C+balanced salt solution–injected subjects. Black and gray solid curves: best fit Naka-Rushton function for the balanced salt solution control and C+balanced salt solution–injected eyes, respectively. Bottom: Averaged ERGs in response to low flash-energy (−3.7 log sc td s). Black solid lines: intravitreal balanced salt solution–injected controls; gray solid lines: intravitreal C+balanced salt solution–injected subjects. Dashed traces: ±1 SEM p-STR, positive scotopic threshold response; n-STR, negative scotopic threshold response.
) C+balanced salt solution–injected eye. Middle: average dark-adapted electroretinogram (ERG) a-wave amplitudes (±SEM) plotted as a function of stimulus energy for the dark-adapted ERGs. Black circle: intravitreal balanced salt solution–injected controls; gray circle: intravitreal C+balanced salt solution–injected subjects. Black and gray solid curves: best fit Naka-Rushton function for the balanced salt solution control and C+balanced salt solution–injected eyes, respectively. Bottom: Averaged ERGs in response to low flash-energy (−3.7 log sc td s). Black solid lines: intravitreal balanced salt solution–injected controls; gray solid lines: intravitreal C+balanced salt solution–injected subjects. Dashed traces: ±1 SEM p-STR, positive scotopic threshold response; n-STR, negative scotopic threshold response.
For examining the scotopic (rod-driven) b-wave, only those responses to flash energies between −3.5 and 0 log sc td s were used to produce the fit, to reduce the effects of the scotopic threshold responses (STRs) in the fit for low energies, and to minimize the influence of the cone-driven responses at higher energies40 (Fig. 2). The parameters of the fit for the C+balanced salt solution and balanced salt solution–injected cohorts for the scotopic b-wave for each group, along with the coefficient of determination (R2), are detailed in Table 1. For examining the mixed rod+cone–driven b-wave, the average amplitudes in response to a high energy stimulus of 2.3 log sc td s were examined (Fig. 2, top, inset; Table 1). The scotopic b-wave Vmax and I0.5 and the mixed rod+cone-driven b-wave amplitudes of the C+balanced salt solution–injected eyes were not significantly different from their balanced salt solution controls for caspofungin concentrations between 0.41 and 4.1 μM. Both the rod-driven b-wave Vmax and the mixed rod+cone driven b-wave amplitudes for the C+balanced salt solution–injected eyes that received the 41-μM dose showed a statistically significant reduction of ∼59% compared with the balanced salt solution control. The b-wave I0.5 for the C+balanced salt solution–injected eyes that received the 41-μM dose did not show a statistically significant difference compared with the balanced salt solution control. The amplitude–response relationship for the dark-adapted photoreceptor-driven a-wave is shown in Figure 2, middle. The parameters of the fit for the C+balanced salt solution and balanced salt solution–injected cohorts for the a-wave for each group, along with their coefficient of determination (R2), are detailed in Table 1. The scotopic a-wave Vmax and I0.5 of the C+balanced salt solution–injected eyes were not significantly different from their balanced salt solution controls for caspofungin concentrations between 0.41 and 4.1 μM. The a-wave Vmax for the C+balanced salt solution–injected eyes that received the 41-μM dose showed a statistically significant reduction of 61% compared with the balanced salt solution control. The a-wave I0.5 for the C+balanced salt solution–injected eyes that received a dose of 41 μM did not show a statistically significant difference from that of the balanced salt solution control. For both the rod-driven b-wave and a-waves, the I0.5 for the C+balanced salt solution–injected eye that received a dose of 41 μM was similar to that of the balanced salt solution–injected control eyes, indicating that caspofungin at this concentration must have caused damage to the retina without altering light transmission (for example, vitreal hemorrhage or cataracts) or altering photoreceptor sensitivity to light.37
Table 1.
Summary of ERG Results
|
Vmax |
I0.5 |
|||||||
|---|---|---|---|---|---|---|---|---|
| mV | SEM | n | P | log sc td s | SEM | n | P | |
| a-Wave | ||||||||
| Control (0 μM) | 393.18 | 44.04 | 3 | 0.2611 | 1.03 | 0.08 | 3 | 0.0655 |
| Caspofungin (0.41 μM) | 307.89 | 45.57 | 5 | 0.83 | 0.05 | 5 | ||
| Control (0 μM) | 401.23 | 74.66 | 5 | 0.3804 | 0.99 | 0.03 | 5 | 0.3667 |
| Caspofungin (1.2 μM) | 320.03 | 45.57 | 5 | 0.88 | 0.02 | 5 | ||
| Control (0 μM) | 403.06 | 74.75 | 4 | 0.3125 | 0.92 | 0.05 | 4 | 0.1284 |
| Caspofungin (2.5 μM) | 307.89 | 50.95 | 5 | 0.83 | 0.03 | 5 | ||
| Control (0 μM) | 430.37 | 81.76 | 5 | 0.3378 | 0.90 | 0.02 | 5 | 0.8852 |
| Caspofungin (4.1 μM) | 324.75 | 63.62 | 5 | 0.90 | 0.03 | 5 | ||
| Control (0 μM) | 488.37 | 51.94 | 5 | 0.0005* | 0.90 | 0.06 | 5 | 0.1061 |
| Caspofungin (41.0 μM) | 189.01 | 9.35 | 5 | 0.80 | 0.04 | 5 | ||
| b-Wave (scotopic intensities) | ||||||||
| Control (0 μM) | 441.49 | 79.70 | 3 | 0.7696 | −1.7 | 0.06 | 3 | 0.1976 |
| Caspofungin (0.41 μM) | 410.36 | 62.46 | 5 | −1.8 | 0.04 | 5 | ||
| Control (0 μM) | 403.36 | 26.05 | 5 | 0.9748 | −1.7 | 0.04 | 5 | 0.0805 |
| Caspofungin (1.2 μM) | 404.59 | 26.89 | 5 | −1.8 | 0.03 | 5 | ||
| Control (0 μM) | 418.93 | 43.71 | 4 | 0.2547 | −1.7 | 0.02 | 4 | 0.9 |
| Caspofungin (2.5 μM) | 352.68 | 32.73 | 5 | −1.7 | 0.03 | 5 | ||
| Control (0 μM) | 422.88 | 42.55 | 5 | 0.3504 | −1.9 | 0.06 | 5 | 0.2029 |
| Caspofungin (4.1 μM) | 350.73 | 59.01 | 5 | −1.8 | 0.04 | 5 | ||
| Control (0 μM) | 435.38 | 44.60 | 5 | 0.0007* | −1.7 | 0.03 | 5 | 0.1247 |
| Caspofungin (41.0 μM) | 180.18 | 16.11 | 5 | −1.8 | 0.05 | 5 | ||
| mV | SEM | n | P | |
|---|---|---|---|---|
| Mixed b-wave amplitude (2.3 log sc td s) | ||||
| Control (0 μM) | 747.63 | 61.19 | 3 | 0.8235 |
| Caspofungin (0.41 μM) | 780.58 | 100.64 | 5 | |
| Control (0 μM) | 817.76 | 109.78 | 5 | 0.2232 |
| Caspofungin (1.2 μM) | 635.12 | 84.13 | 5 | |
| Control (0 μM) | 834.59 | 98.91 | 4 | 0.2398 |
| Caspofungin (2.5 μM) | 615.05 | 92.13 | 5 | |
| Control (0 μM) | 865.43 | 141.52 | 5 | 0.294 |
| Caspofungin (4.1 μM) | 634.36 | 114.36 | 5 | |
| Control (0 μM) | 887.73 | 111.11 | 5 | 0.0017* |
| Caspofungin (41.0 μM) | 367.45 | 16.11 | 5 |
Data are the mean Vmax ± SEM, mean I0.5 ± SEM, and the coefficient of determination (R2) from the Naka-Rushton fit of the a-wave and scotopic b-wave amplitudes over incrementing energies, and mean rod+cone b-wave amplitude (±SEM) in response to a high-energy flash (2.3 log sc td s) for different doses of caspofungin.
P < 0.05 by Student's t-test was statistically significant.
For low flash energies the averaged scotopic threshold responses (STRs) for mice that received 100× caspofungin injection (but not those that received lower doses) showed a statistically significant difference compared with the balanced salt solution–injected controls at criterion times of 110 and 220 ms (t-test, P < 0.05), indicating that at this concentration there was a likelihood of toxicity to the inner retina, proximal to the bipolar cells.
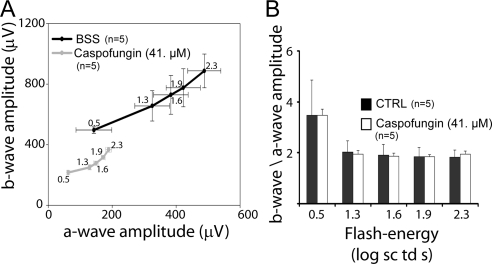
To investigate the probable cause of reduced a- and b-wave amplitudes for the C+balanced salt solution–injected eyes that received 100× caspofungin relative to the control injected only with balanced salt solution, we plotted the b-wave amplitudes for the four highest flash energies as a function of the a-wave amplitude (Fig. 3). Although both the b- and a-waves were reduced in the 100× injected eye, their relative ratios were indistinguishable from those of the control eyes, indicating that the cause of the reduced a- and b-waves was most likely due to the toxic effect of this drug on the photoreceptors.
Figure 3.
b-Wave as a function of a- and b-wave to a-wave ratios for the dark-adapted mixed rod-cone ERGs. (A) b-Wave plotted as a function of a-wave for those eyes that received C+balanced salt solution containing 41 μM caspofungin and for the balanced salt solution–only controls. The numbers alongside the data points indicate the flash-energy in log scotopic Trolands per second. (B) The b- to a-wave ratios as a function of the flash energy.
There were no signs of retinal hemorrhages or infection in any injected eye. Light microscopic histologic examination (Fig. 4) showed no observable retinal abnormality in eyes injected with balanced salt solution– or C+balanced salt solution–injected eyes that received a dose of 4.1 μM or less. The eyes that were injected with the 41-μM dose were conspicuous for a loss of nuclear staining in the ganglion cell layer. The loss of ganglion cells was seen in large areas of the retina (Fig. 5). We did not observe focal areas of necrosis, localized retinal detachment, or any observable changes in the photoreceptor outer and inner segments, outer nuclear layer, outer plexiform layer, the inner nuclear layer, or the inner plexiform layer.
Figure 5.
Histopathologic examination of radial sections through the optic nerve head of mice eyes 7 weeks after intravitreal injection of balanced salt solution or 41 μM (100× MIC90) of C+balanced salt solution. Blue: DAPI-stained nuclei in various cell layers; red: eosin-stained cell membranes prominent in the plexiform layers. No retinal abnormalities were noted in the retinas injected with balanced salt solution alone or 0.41 to 4.1 μM of C+balanced salt solution. Eyes injected with 41 μM of C+balanced salt solution showed loss of cells in the ganglion cell layer (arrowheads). ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer, NF, nerve fiber; ON, optic nerve. Scale bar, 20 μm.
Discussion
Caspofungin is known to cause local irritation at the site of injection, histamine release, phlebitis, and hemolysis,48 all of which may affect the retina. This study found that caspofungin did not cause statistically significant alterations in the electroretinogram or gross retinal histology for intravitreal concentrations of 4.1 μM or less. There were decreases in ERG amplitudes and a detectable loss of cells in the retinal ganglion cell layer for intravitreal concentrations of 41 μM (at 100× the MIC90). A caspofungin dose of 4.1 μM, which did not show signs of significant toxicity by our assay, was 10 times higher than the MIC90 of caspofungin for Aspergillus spp.38 or Candida spp.22 This dose of caspofungin may be sufficient for the treatment of fungal endophthalmitis, especially when used in combination therapy. Even when administered systemically in combination with voriconazole, the low intravitreal levels achieved by caspofungin may have contributed to the treatment of amphotericin-resistant Aspergillus fumigatus endophthalmitis27 and Candida endophthalmitis.49 Another advantage of caspofungin is its synergistic action with the azole group of antifungals and amphotericin B (for a review, see Ref. 23).
Intravitreal injection of amphotericin B has been the treatment of choice for severe fungal endophthalmitis, but it is known to produce retinal toxicity at low doses,15,50 making it the standard for comparing retinal toxicities of other candidate antifungals administered via intravitreal injection. The minimum dose of amphotericin B that produces retinal toxicity in the murine retina remains undetermined. In the rabbit eye, intravitreal concentration between 4.1 and 8.3 μg/mL of amphotericin B has been found to produce retinal toxicity,15 making this drug potentially toxic at concentrations that are only 1× its MIC90 for Aspergillus spp.(4 μg/mL38). Our study showed that a nontoxic dose of caspofungin is up to 10 times its MIC90 for Aspergillus spp. (0.50 μg/mL or 0.41 μM38), making it a much safer drug than amphotericin B. Our study reports a lower nontoxic dose of the drug compared with another study that found that in vitro concentrations up to 50 μg/mL (41 μM) did not show toxic effects of caspofungin on corneal endothelial cells, primary human trabecular meshwork cells, and primary human retinal pigment epithelial (RPE) cells.51 Another study conducted on a rabbit model of fungal endophthalmitis found no evidence of histologic damage to the retina 7 days after intravitreal injection of caspofungin at 500 μg/mL (1000× its MIC90).52 This nontoxic dose of caspofungin in the rabbit retina (as assayed by histology) is 10 times the concentration that produced toxic effects in the mouse, as assayed by ERG and histology 7 weeks after injection. The difference in the caspofungin concentrations that produced damage to the mouse retina, but not to the rabbit retina, could have been caused by either the sampling of the retinas in the two studies at different time points or the heightened toxicity of the mouse eye, with its limited intravitreal space (∼1% that of the rabbit). Our study establishes a safe range for retinal toxicity of intravitreally injected caspofungin in mice, which can help guide dosage in humans. The highest nontoxic intravitreal concentration in mice found in our study equates to an injected dose of 20 μg in a human eye, assuming a human intravitreal volume of 4 mL.
To the best of our knowledge, there is no information on the pharmacokinetics of intravitreal caspofungin in this animal model or others, and so it is difficult to predict how long this drug remains in ocular tissues in therapeutic concentrations. However, a single intravitreal dose of 100 μg of caspofungin injected into the rabbit vitreous in experimentally induced Candida endophthalmitis produced a greater improvement of clinical scores at the end of 3 days, and it reduced Candida colony-forming units/mL more at the end of 7 days than 50 μg voriconazole, 10 μg amphotericin B, or 10 μg itraconazole,52 indicating that caspofungin was retained in the ocular tissues for a sufficiently length of time to be therapeutically viable.
Intravitreal injection is likely to be the route of choice to deliver therapeutically effective doses of caspofungin in fungal endophthalmitis. After systemic administration, caspofungin has been reported only in low to undetectable levels in the vitreous, perhaps because of its high molecular weight. The maximum reported vitreal concentration in humans after systemic administration is 0.28 μg/mL (23. μM) in a case of fungal endophthalmitis that was culture-positive for Fusarium.28 It may not be feasible to administer sufficiently high concentrations of caspofungin systemically to achieve therapeutic intravitreal concentrations because of its hepatotoxicity (for review see Ref. 48). Even in cases of endophthalmitis with concomitant systemic fungal infection, direct vitreal injection (but not higher doses of systemic caspofungin) may be the preferred route of delivery for therapeutic doses of caspofungin to the retina because of the possibility of a paradoxically reduced efficacy of high doses of systemically administered caspofungin.53,54
Footnotes
Supported by Grants R01-EY11900 (TGW), EY11731 (JHW) and Core Grant EY02520 from the National Eye Institute, National Institutes of Health, and by the Welch Foundation (Q0035, TGW). FAC was supported by training Grant EY007001.
Disclosure: D.K. Mojumder, None; F.A. Concepcion, None; S.K. Patel, None; A.J. Barkmeier, None; P.E. Carvounis, None; J.H. Wilson, None; E.R. Holz, None; T.G. Wensel, None
References
- 1. Kresloff MS, Castellarin AA, Zarbin MA. Endophthalmitis. Surv Ophthalmol. 1998;43:193–224 [DOI] [PubMed] [Google Scholar]
- 2. Kunimoto DY, Das T, Sharma S, et al. Microbiologic spectrum and susceptibility of isolates: part I. Postoperative endophthalmitis. Endophthalmitis Research Group. Am J Ophthalmol. 1999;128:240–242 [DOI] [PubMed] [Google Scholar]
- 3. Nayak N. Fungal infections of the eye: laboratory diagnosis and treatment. Nepal Med Coll J. 2008;10:48–63 [PubMed] [Google Scholar]
- 4. Benz MS, Scott IU, Flynn HW, Jr, Unonius N, Miller D. Endophthalmitis isolates and antibiotic sensitivities: a 6-year review of culture-proven cases. Am J Ophthalmol. 2004;137:38–42 [DOI] [PubMed] [Google Scholar]
- 5. Narang S, Gupta A, Gupta V, et al. Fungal endophthalmitis following cataract surgery: clinical presentation, microbiological spectrum, and outcome. Am J Ophthalmol. 2001;132:609–617 [DOI] [PubMed] [Google Scholar]
- 6. Essman TF, Flynn HW, Jr, Smiddy WE, et al. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg Lasers. 1997;28:185–194 [PubMed] [Google Scholar]
- 7. Elder MJ, Morlet N. Endophthalmitis. Clin Exp Ophthalmol. 2002;30:394–398 [DOI] [PubMed] [Google Scholar]
- 8. Narendran N, Balasubramaniam B, Johnson E, Dick A, Mayer E. Five-year retrospective review of guideline-based management of fungal endophthalmitis. Acta Ophthalmol. 2008;86:525–532 [DOI] [PubMed] [Google Scholar]
- 9. Dunlap WA, Karacorlu M, Peyman GA, Nair MG, Rahimy M, Pedroza L. Retinal toxicity of intravitreally injected faeriefungin. Ophthalmic Surg. 1994;25:303–306 [PubMed] [Google Scholar]
- 10. Serracarbassa PD, Peyman GA, Liang C, Calixto N, Jr, Nair MG. Toxicity and efficacy of intravitreal injection of spartanamicin B in the treatment of Candida endophthalmitis. Int Ophthalmol. 1998;22:53–58 [DOI] [PubMed] [Google Scholar]
- 11. Shahsavari M, Peyman GA, Niesman MR. Retinal toxicity and in vitro efficacy study of cilofungin (LY121019). Ophthalmic Surg. 1990;21:726–728 [PubMed] [Google Scholar]
- 12. Gao H, Pennesi M, Shah K, et al. Safety of intravitreal voriconazole: electroretinographic and histopathologic studies. Trans Am Ophthalmol Soc. 2003;101:183–189, discussion 189 [PMC free article] [PubMed] [Google Scholar]
- 13. Gao H, Pennesi ME, Shah K, et al. Intravitreal voriconazole: an electroretinographic and histopathologic study. Arch Ophthalmol. 2004;122:1687–1692 [DOI] [PubMed] [Google Scholar]
- 14. Hariprasad SM, Mieler WF, Holz ER, et al. Determination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humans. Arch Ophthalmol. 2004;122:42–47 [DOI] [PubMed] [Google Scholar]
- 15. Axelrod AJ, Peyman GA, Apple DJ. Toxicity of intravitreal injection of amphotericin B. Am J Ophthalmol. 1973;76:578–583 [DOI] [PubMed] [Google Scholar]
- 16. Bruder-Nascimento A, Camargo CH, Sugizaki MF, et al. Species distribution and susceptibility profile of Candida species in a Brazilian public tertiary hospital. BMC Res Notes. 2010;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen PL, Lo HJ, Wu CJ, et al. Species distribution and antifungal susceptibility of blood Candida isolates at a tertiary hospital in southern Taiwan. 1999–2006. Mycoses. Published online December 17, 2009 [DOI] [PubMed] [Google Scholar]
- 18. van der Linden JW, Jansen RR, Bresters D, et al. Azole-resistant central nervous system aspergillosis. Clin Infect Dis. 2009;48:1111–1113 [DOI] [PubMed] [Google Scholar]
- 19. Denning DW. Echinocandins: a new class of antifungal. J Antimicrob Chemother. 2002;49:889–891 [DOI] [PubMed] [Google Scholar]
- 20. Kim R, Khachikian D, Reboli AC. A comparative evaluation of properties and clinical efficacy of the echinocandins. Expert Opin Pharmacother. 2007;8:1479–1492 [DOI] [PubMed] [Google Scholar]
- 21. Wagner C, Graninger W, Presterl E, Joukhadar C. The echinocandins: comparison of their pharmacokinetics, pharmacodynamics and clinical applications. Pharmacology. 2006;78:161–177 [DOI] [PubMed] [Google Scholar]
- 22. Bartizal K, Gill CJ, Abruzzo GK, et al. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743872). Antimicrob Agents Chemother. 1997;41:2326–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cappelletty D, Eiselstein-McKitrick K. The echinocandins. Pharmacotherapy. 2007;27:369–388 [DOI] [PubMed] [Google Scholar]
- 24. Goldblum D, Fausch K, Frueh BE, Theurillat R, Thormann W, Zimmerli S. Ocular penetration of caspofungin in a rabbit uveitis model. Graefes Arch Clin Exp Ophthalmol. 2007;245:825–833 [DOI] [PubMed] [Google Scholar]
- 25. Gauthier GM, Nork TM, Prince R, Andes D. Subtherapeutic ocular penetration of caspofungin and associated treatment failure in Candida albicans endophthalmitis. Clin Infect Dis. 2005;41:e27–e28 [DOI] [PubMed] [Google Scholar]
- 26. Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58:1131–1135 [DOI] [PubMed] [Google Scholar]
- 27. Durand ML, Kim IK, D'Amico DJ, et al. Successful treatment of Fusarium endophthalmitis with voriconazole and Aspergillus endophthalmitis with voriconazole plus caspofungin. Am J Ophthalmol. 2005;140:552–554 [DOI] [PubMed] [Google Scholar]
- 28. Spriet I, Delaere L, Lagrou K, Peetermans WE, Maertens J, Willems L. Intraocular penetration of voriconazole and caspofungin in a patient with fungal endophthalmitis. J Antimicrob Chemother. 2009;64:877–878 [DOI] [PubMed] [Google Scholar]
- 29. Park S, Kelly R, Kahn JN, et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother. 2005;49:3264–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perlin DS. Resistance to echinocandin-class antifungal drugs. Drug Resist Updat. 2007;10:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schuetzer-Muehlbauer M, Willinger B, Krapf G, Enzinger S, Presterl E, Kuchler K. The Candida albicans Cdr2p ATP-binding cassette (ABC) transporter confers resistance to caspofungin. Mol Microbiol. 2003;48:225–235 [DOI] [PubMed] [Google Scholar]
- 32. Laverdiere M, Lalonde RG, Baril JG, Sheppard DC, Park S, Perlin DS. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J Antimicrob Chemother. 2006;57:705–708 [DOI] [PubMed] [Google Scholar]
- 33. Krogh-Madsen M, Arendrup MC, Heslet L, Knudsen JD. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin Infect Dis. 2006;42:938–944 [DOI] [PubMed] [Google Scholar]
- 34. Miller CD, Lomaestro BW, Park S, Perlin DS. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy. 2006;26:877–880 [DOI] [PubMed] [Google Scholar]
- 35. Hakki M, Staab JF, Marr KA. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob Agents Chemother. 2006;50:2522–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moudgal V, Little T, Boikov D, Vazquez JA. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob Agents Chemother. 2005;49:767–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perlman I. Testing retinal toxicity of drugs in animal models using electrophysiological and morphological techniques. Doc Ophthalmol. 2009;118:3–28 [DOI] [PubMed] [Google Scholar]
- 38. Lalitha P, Shapiro BL, Srinivasan M, et al. Antimicrobial susceptibility of Fusarium, Aspergillus, and other filamentous fungi isolated from keratitis. Arch Ophthalmol. 2007;125:789–793 [DOI] [PubMed] [Google Scholar]
- 39. Mojumder DK, Qian Y, Wensel TG. Two R7 regulator of G-protein signaling proteins shape retinal bipolar cell signaling. J Neurosci. 2009;29:7753–7765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mojumder DK, Wensel TG. Topical mydriatics affect light-evoked retinal responses in anesthetized mice. Invest Ophthalmol Vis Sci. 2010;51:567–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dawson WW, Trick GL, Litzkow CA. Improved electrode for electroretinography. Invest Ophthalmol Vis Sci. 1979;18:988–991 [PubMed] [Google Scholar]
- 42. Lyubarsky AL, Falsini B, Pennesi ME, Valentini P, Pugh EN., Jr UV- and midwave-sensitive cone-driven retinal responses of the mouse: a possible phenotype for coexpression of cone photopigments. J Neurosci. 1999;19:442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Concepcion F, Mendez A, Chen J. The carboxyl-terminal domain is essential for rhodopsin transport in rod photoreceptors. Vision Res. 2002;42:417–426 [DOI] [PubMed] [Google Scholar]
- 44. Mojumder DK, Frishman LJ, Otteson DC, Sherry DM. Voltage-gated sodium channel alpha-subunits Na(v)1.1, Na(v)1.2, and Na(v)1.6 in the distal mammalian retina. Mol Vis. 2007;13:2163–2182 [PubMed] [Google Scholar]
- 45. Mojumder DK, Sherry DM, Frishman LJ. Contribution of voltage-gated sodium channels to the b-wave of the mammalian flash electroretinogram. J Physiol. 2008;586:2551–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mojumder DK, Wensel TG, Frishman LJ. Subcellular compartmentalization of two calcium binding proteins, calretinin and calbindin-28 kDa, in ganglion and amacrine cells of the rat retina. Mol Vis. 2008;14:1600–1613 [PMC free article] [PubMed] [Google Scholar]
- 47. Naka KI, Rushton WA. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966;185:536–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Denning DW. Echinocandin antifungal drugs. Lancet. 2003;362:1142–1151 [DOI] [PubMed] [Google Scholar]
- 49. Breit SM, Hariprasad SM, Mieler WF, Shah GK, Mills MD, Grand MG. Management of endogenous fungal endophthalmitis with voriconazole and caspofungin. Am J Ophthalmol. 2005;139:135–140 [DOI] [PubMed] [Google Scholar]
- 50. Baldinger J, Doft BH, Burns SA, Johnson B. Retinal toxicity of amphotericin B in vitrectomised versus non-vitrectomised eyes. Br J Ophthalmol. 1986;70:657–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kernt M, Kampik A. Intraocular caspofungin: in vitro safety profile for human ocular cells. Mycoses. Published online February 19, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Kusbeci T, Avci B, Cetinkaya Z, et al. The effects of caspofungin and voriconazole in experimental Candida endophthalmitis. Curr Eye Res. 2007;32:57–64 [DOI] [PubMed] [Google Scholar]
- 53. Stevens DA, Espiritu M, Parmar R. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob Agents Chemother. 2004;48:3407–3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stevens DA, White TC, Perlin DS, Selitrennikoff CP. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn Microbiol Infect Dis. 2005;51:173–178 [DOI] [PubMed] [Google Scholar]