Abstract
Background
The risk of pneumococcal disease persists and antibody responses to revaccination with the 23-valent polysaccharide vaccine (PPV) are low among HIV-infected adults. We determined whether revaccination with the 7-valent pneumococcal conjugate vaccine (PCV) would enhance these responses.
Methods
In a randomized clinical trial, we compared the immunogenicity of revaccination with PCV (n=131) or PPV (n=73) among HIV-infected adults (median CD4 count 533 cells/mm3) vaccinated with PPV 3–8 years earlier. HIV-uninfected adults (n=25) without prior pneumococcal vaccination received one dose of PCV. A positive response was defined as a ≥2-fold rise (baseline to day 60) in capsule-specific IgG with a post-vaccination level value ≥1000 ng/ml for at least 2 of the 4 serotypes.
Results
HIV-infected persons demonstrated a higher frequency of positive antibody responses to PCV vs. PPV (57% vs. 36%, p=0.004) and greater IgG concentration mean changes from baseline to day 60 for serotypes 4, 9V, and 19F (all p<0.05), but not for serotype 14. However by day 180 both outcomes were similar. Responses to PCV were greater in frequency and magnitude for all serotypes in HIV-uninfected compared with those in HIV-infected adults.
Conclusions
Among persons with HIV infection, revaccination with PCV was only transiently more immunogenic than PPV, and responses were inferior to those in HIV-uninfected subjects with primary vaccination. Pneumococcal vaccines with more robust and sustained immunogenicity are needed for HIV-infected adults.
Introduction
Streptococcus pneumoniae infections are a common cause of morbidity and mortality among persons infected with human immunodeficiency virus (HIV) [1–5]. Highly active antiretroviral therapy (HAART) has reduced the incidence of pneumococcal disease among HIV-infected persons by half. However, the incidence remains significantly greater than that of the general population [2, 6]. Despite administration of the 23-valent pneumococcal polysaccharide vaccine (PPV) to HIV-infected adults [7], their risk for S. pneumoniae infections persists [2, 5].
The 7-valent pneumococcal conjugate vaccine (PCV), which contained 70–80% of pediatric serotypes that cause invasive pneumococcal infections in North America at the time of its release [8], effectively prevents invasive pneumococcal disease in HIV-uninfected infants and children [9–12]. Compared with PPV, PCV elicits increased antibody responses among those with immature or compromised immune systems, including transplant recipients [13–16] and HIV-infected children [17, 18]. Studies among HIV-infected adults have mainly focused on comparing strategies for primary vaccination using varying sequences of two doses of PCV and PPV, which have shown variable results [19–21].
Most persons diagnosed with HIV infection receive primary PPV vaccination based on current guidelines [7]. A critical issue is to determine the most effective strategy for revaccination among this prevaccinated group. Earlier results revealed that the immunogenicity of PPV revaccination five or more years after the initial dose was very limited [22]. Therefore, we performed a prospective, randomized study to determine whether the immunogenicity of revaccination with PCV exceeded that of PPV to guide recommendations on revaccination of HIV-infected adults.
Methods
Study Population
HIV-infected adults previously vaccinated with PPV 3–8 years earlier were randomized 2:1 to be revaccinated with PCV (Prevnar; Wyeth Pharmaceuticals) or PPV (Pneumovax, Merck & Co., Inc.). A block randomization strategy coordinated at a central location was utilized to attain an overall 2:1 vaccine ratio for the PCV and PPV randomization arms. A group of HIV-uninfected subjects (n=25) without prior pneumococcal vaccination were enrolled and received a single injection of PCV. Study participants were enrolled at five sites: Naval Medical Center San Diego, National Naval Medical Center, Naval Medical Center Portsmouth, Brooke Army Medical Center, and Walter Reed Army Medical Center. All subjects provided written informed consent, the study was approved by both central and local military institutional review boards (IRB) and the University of Colorado Multi-institutional IRB, and was registered with the Clinical Trials network (registration ID# NCT00622843).
All study participants were 18–60 years old. Participants with HIV infection had documented evidence of HIV infection (positive ELISA and Western Blot tests). Subjects without HIV infection had a negative HIV ELISA result at or within one year of enrollment. Exclusion criteria included documented pregnancy or lactation, chronic active viral hepatitis, splenectomy, current temperature of ≥ 38°C, poor performance status (inability to ambulate >1000 meters), contraindications to an intramuscular injection, ongoing illicit drug use or alcohol abuse, current use of immunosuppressive or cancer chemotherapeutic agents, AIDS-related wasting, and a current plasma HIV RNA level of >50,000 copies/ml.
Study and Laboratory Procedures
Pneumococcal vaccines were administered intramuscularly (0.5 ml) in the deltoid muscle using a 23-gauge, 1-inch needle. Vaccines were stored in temperature-controlled and monitored refrigerators, and transportation was in accordance with manufacturers’ guidelines. Adverse events (AE) temporally related (within seven days) to revaccination were graded based on their impact on participants’ daily activities [23]. Serious reactions, possibly related to vaccination resulting in hospitalization, disability, or death, were reported to the Vaccine Adverse Event Reporting System.
Serum samples for pneumococcal capsule-specific IgG responses were collected at baseline (1–21 days prior to revaccination) and days 14, 60 and 180 after revaccination. CD4+ T cell counts (flow cytometry) and plasma HIV RNA levels (Roche Amplicor) were determined locally at each time point. We measured IgG reactive with each of four pneumococcal serotypes (4, 9V, 14, and 19F) by ELISA, as described [22]. The four serotypes evaluated were chosen as they were common to both PPV and PCV and represent a range of frequencies of infection. In brief, sera were preadsorbed with cell wall polysaccharide and type 22F capsular polysaccharide overnight to eliminate non-capsule-specific antibodies, capsular polysaccharides were adhered to 96 well microtiter plates, and capsule-specific IgG was detected with affinity-purified horseradish peroxidase-conjugated goat anti-human IgG label and appropriate substrates. Samples were tested in triplicate. Antibody concentrations were extrapolated from standards (Sample 89-SF; Food and Drug Administration) on each plate. Coefficients of variation based on control samples on each plate were 16.3%, 17.3%, 15.9%, and 15.1% for serotypes 4, 9V, 14, and 19F, respectively.
Study Design
The primary endpoint was defined a priori as the proportion of subjects in each arm with positive antibody response to at least two of four serotypes at day 60. A positive response was defined as a ≥2 rise in IgG level with a post-vaccination level value ≥1000 ng/ml. Secondary outcomes included positive IgG responses and change in IgG concentrations for each serotype at each time point.
The randomized study was designed assuming that 60% of the PCV arm and 40% of the PPV arm would achieve a positive antibody response. With a PCV to PPV allocation ratio of 2:1, alpha=0.05, and 90% power, the original sample size estimate was 320 HIV-infected participants (210 in the PCV arm and 110 in the PPV arm). The study was designed for a Data Safety and Monitoring Board (DSMB) to evaluate the first 150 study participants to assess safety and adequacy of the sample size with pre-established stopping rules. The DSMB revealed no safety concerns, but response rates at the time of the DSMB review projected less than 50% power to find a significant difference between the HIV-infected vaccine arms for the primary endpoint. In addition, study enrollment was slower than anticipated, and the number of remaining eligible participants was limited. Thus, the study was closed to enrollment, and all active participants continued study visits through 180 days after revaccination.
Statistical Methods
Descriptive statistics are presented as means with standard deviations (SD) and medians with interquartile ranges (IQR). Differences in the proportions of responders between groups were compared using chi-squared tests. Medians were compared with Kruskal-Wallis tests. For each analysis there were two comparisons: a randomized comparison of the HIV-infected PCV and PPV arms, and a non-randomized comparison of the HIV-infected PCV arm to the HIV-uninfected PCV group.
Geometric mean concentrations (GMC) were calculated by arm for each serotype at each time point by determining the average on the log10 scale and then back transforming the results. Logistic regression models were used to examine the odds of a positive antibody response for the primary endpoint (a 2-fold risk in IgG for at least two of the four serotypes at day 60 with capsule-specific IgG ≥1000 ng/mL) and for each serotype separately. To calculate changes in IgG concentrations from baseline, the capsule-specific IgG measurements were log10 transformed. At each follow-up visit, the difference between the arms for change in IgG concentrations was estimated with generalized linear fixed effects models. Unless otherwise noted, all regression models were adjusted for age (≤ 40 or > 40 years), ethnicity (Caucasian or other), and prior pneumonia; regression models comparing the HIV-infected arms were also adjusted for CD4+ T cell counts (<500 cells/mm3 or ≥500 cells/mm3), HIV RNA level (≤50 copies/ml or >50 copies/ml), and HAART use at baseline. Models considering each serotype separately were further adjusted for baseline level after log10 transformation. Odds ratios and estimates of change in IgG concentrations are given with 95% confidence intervals (CI).
To determine whether baseline demographics and HIV-related factors differentially affected responses within each vaccine arm, the heterogeneity of the treatment effect for subgroups was assessed with additional models by including an interaction term between randomized arm (PCV or PPV) and subgroup. Finally, for the HIV-infected arms separately and pooled together, exploratory logistic regression models were used to examine possible predictors for a positive antibody response. All p-values are two-sided. Analyses were conducted using SAS (version 9.1, Cary, NC).
Results
Study Population Characteristics
From February 2006 through September 2008, a total of 204 HIV-infected subjects (131 in the PCV arm and 73 in the PPV arm) and 25 HIV-uninfected persons were enrolled. All participants met eligibility criteria, except one HIV-infected person in the PCV arm who had received the initial PPV vaccination 8 years and 15 days prior to randomization. The median time from last PPV to enrollment among HIV-infected subjects was 4.6 years (IQR 3.6–6.0). The median time from HIV diagnosis to study enrollment was 9.5 years (IQR 4.8–15.4); median CD4+ T cell count at baseline was 533 (IQR 391–701) cells/mm3; 68% had an HIV RNA level <50 copies/ml; and 82% were currently receiving HAART (Table 1). The HIV-infected subjects in each arm were well-matched by baseline clinical, immunologic, and virologic parameters. Compared with the HIV-infected subjects receiving PCV, the HIV-uninfected group had a higher proportion of females and Caucasians (Table 1).
Table 1.
Baseline Characteristics of the Study Participants
| HIV-Infected PCV Arm (n=131) | HIV-Infected PPV Arm (n=73) | HIV-Infected Overall (n=204) | p-value1 | HIV-Uninfected Arm (n=25) | p-value2 | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, median (IQR) years | 42 (36 – 47) | 42 (35 – 46) | 42 (36–47) | 0.81 | 37 (29 – 46) | 0.16 |
| Gender, N (%) male | 120 (91.6) | 70 (95.9) | 190 (93.1) | 0.25 | 18 (72.0) | 0.005 |
| Ethnicity, N (%) | 0.98 | 0.01 | ||||
| Caucasian | 71 (54.2) | 40 (54.8) | 111 (54.4) | 21 (84.0) | ||
| African American | 50 (38.2) | 27 (37.0) | 77 (37.7) | 2 (8.0) | ||
| Other | 10 (7.6) | 6 (8.2) | 16 (7.8) | 2 (8.0) | ||
| Clinical History | ||||||
| History of Pneumonia, N (%) | 22 (16.8) | 13 (17.8) | 35 (17.2) | 0.85 | 1 (4.0) | 0.10 |
| Time since last PPV, median (IQR) years | 4.6 (3.7 – 6.1) | 4.7 (3.6 – 5.9) | 4.6 (3.6 – 6.0) | 0.55 | --- | --- |
| HIV History | ||||||
| Time from HIV diagnosis, median (IQR) years | 10.2 (5.0–16.3) | 8.9 (4.4–13.5) | 9.5 (4.8–15.4) | 0.20 | --- | --- |
| CDC Stage, N (%) | 0.46 | --- | --- | |||
| A | 91 (69.5) | 55 (75.3) | 146 (71.6) | |||
| B | 25 (19.1) | 9 (12.3) | 34 (16.7) | |||
| C | 15 (11.5) | 9 (12.3) | 24 (11.8) | |||
| CD4+ T cell count, median (IQR) | 533 (396 – 700) | 513 (388 – 714) | 533 (391 – 701) | 0.85 | --- | --- |
| cells/mm3 CD4+ T cell categories, N (%) | 0.52 | --- | --- | |||
| <200 | 4 (3.1) | 3 (4.1) | 7 (3.4) | |||
| 200–350 | 17 (13.0) | 6 (8.2) | 23 (11.3) | |||
| 351–500 | 35 (26.7) | 26 (35.6) | 61 (29.9) | |||
| 500–750 | 53 (40.5) | 24 (32.9) | 77 (37.7) | |||
| >750 | 22 (16.8) | 14 (19.2) | 36 (17.6) | |||
| HIV RNA, median (IQR) log10 copies/ml | 1.7 (1.7 – 2.2) | 1.7 (1.7 – 3.1) | 1.7 (1.7 – 2.3) | 0.39 | --- | --- |
| HIV RNA <50 copies/ml, N (%) | 91 (69.5) | 47 (64.3) | 138 (68.0) | 0.54 | --- | --- |
| Current HAART, N (%) | 111 (84.7) | 56 (76.7) | 167 (81.9) | 0.15 | --- | --- |
| Baseline Serology Data, Median (IQR) ng/ml | ||||||
| Serotype 4 | 293 (108–793) | 254 (105–580) | 278 (106–726) | 0.50 | 264 (108–595) | 0.47 |
| Serotype 9V | 556 (258–1389) | 425 (237–1269) | 502 (246–1277) | 0.26 | 746 (488–1437) | 0.32 |
| Serotype 14 | 860 (321–5039) | 771 (218–3083) | 817 (315–3733) | 0.31 | 254 (99–564) | <0.001 |
| Serotype 19F | 723 (329–1971) | 480 (239–1154) | 604 (302–1697) | 0.08 | 737 (182–1127) | 0.45 |
Comparison of the HIV-infected PCV and PPV arms
Comparison of the HIV-infected PCV arm with the HIV-uninfected PCV arm
Median baseline levels of untransformed capsule-specific IgG were similar in the three groups, although levels to serotype 14 were higher among previously vaccinated HIV-infected subjects compared to HIV-uninfected subjects receiving PCV (860 vs. 254 ng/ml, p<0.001; Table 1). Among all HIV-infected subjects, time since last PPV did not correlate with baseline antibody level to any serotype, nor did baseline antibody levels vary by ethnicity (data not shown).
Antibody Responses to PCV and PPV among HIV-Infected Participants
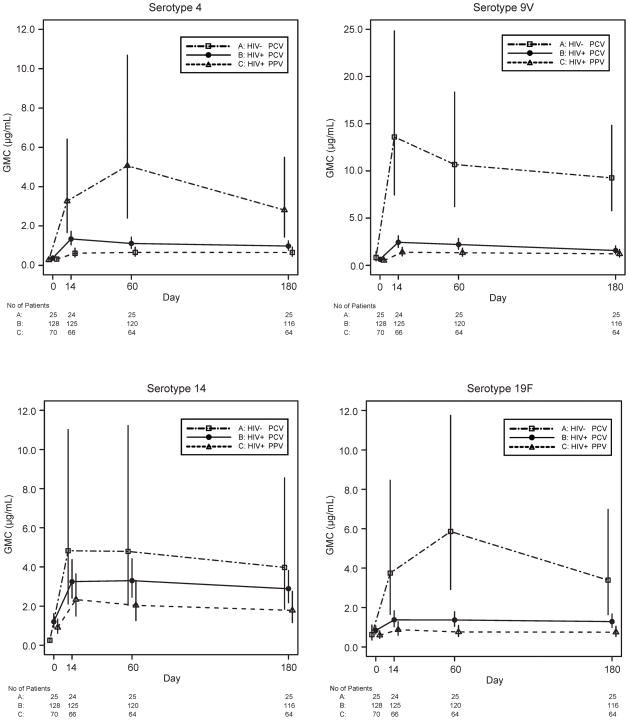
Among HIV-infected subjects, both PPV and PCV elicited significant increases in capsule-specific IgG GMC from baseline to days 14, 60, and 180 for each serotype (Figure 1). The only post-immunization values that were not significantly greater than baseline were to serotype 19F in the PPV arm at days 60 and 180.
Figure 1.
Geometric mean concentrations (GMC) in μg/mL with 95% confidence intervals for each of the four serotypes and each arm at baseline and days 14, 60, and 180 post-vaccination.
Among HIV-infected subjects, a greater proportion of those receiving PCV reached the primary endpoint (two-fold rises in IgG for at least two of the four serotypes on day 60 with levels ≥1000 ng/mL) than those receiving PPV (57% vs. 36%, respectively; OR 2.6, 95% CI 1.4–5.0, p=0.004) (Table 2). At day 60, PCV compared with PPV elicited greater response frequencies for serotypes 4 and 19F and were also more likely to achieve a positive response for at least three of the four serotypes (31% vs. 14%, respectively; OR 2.6, 95% CI 1.1–6.0, p=0.02). Results were similar when omitting the criterion of achieving a level of ≥1000 ng/ml. However, the differences between the HIV-infected PCV and PPV arm for positive antibody response seen at days 14 and 60 were not sustained through day 180 (Table 2).
Table 2.
Antibody Responses for the HIV-Infected PCV and PPV Arms
| At Least 2 of 4 Serotypes | At least 3 of 4 Serotypes | Serotype 4 | Serotype 9V | Serotype 14 | Serotype 19F | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) With a Positive Immune Response1 | ||||||||||||
| Day | PCV | PPV | PCV | PPV | PCV | PPV | PCV PPV | PCV PPV | PCV PPV | |||
| 14 | 67 (54) | 23 (35) | 37 (30) | 11 (17) | 54 (43) | 17 (26) | 68 (54) | 22 (33) | 57 (46) | 27 (41) | 37 (30) | 12 (18) |
| 60 | 68 (57) | 23 (36) | 37 (31) | 9 (14) | 48 (40) | 15 (23) | 63 (53) | 25 (39) | 61 (51) | 28 (44) | 42 (35) | 12 (19) |
| 180 | 47 (41) | 20 (31) | 26 (22) | 11 (17) | 37 (32) | 16 (25) | 49 (42) | 24 (38) | 47 (41) | 22 (34) | 38 (33) | 13 (20) |
| Adjusted Odds Ratios for a Positive Immune Response (PCV to PPV) With 95% Confidence Intervals and P-values | ||||||||||||
| Day | OR (95% CI) p-value | OR (95% CI) p-value | OR (95% CI) p-value | OR (95% CI) p-value | OR (95% CI) p-value | OR (95% CI) p-value | ||||||
| 14 | 2.4 (1.2, 4.6) 0.009 | 2.2 (1.0, 4.9) 0.05 | 2.4 (1.2, 4.7) 0.01 | 2.5 (1.3, 4.8) 0.007 | 1.3 (0.7, 2.5) 0.39 | 1.9 (0.9, 4.1) 0.09 | ||||||
| 60 | 2.6 (1.4, 5.0) 0.004 | 2.6 (1.1, 6.0) 0.02 | 2.3 (1.1, 4.7) 0.02 | 1.7 (0.9, 3.1) 0.12 | 1.5 (0.8, 3.0) 0.19 | 3.5 (1.5, 7.9) 0.004 | ||||||
| 180 | 1.4 (0.7, 2.7) 0.34 | 1.3 (0.6, 2.9) 0.55 | 1.4 (0.7, 2.8) 0.36 | 1.1 (0.6, 2.1) 0.80 | 1.3 (0.7, 2.6) 0.39 | 1.8 (0.9, 3.8) 0.12 | ||||||
| Adjusted Differences (PCV-PPV) for Change from Baseline in IgG Concentration (log10 ng/mL) With 95% Confidence Intervals and P-values | ||||||||||||
| Day | Difference (CI) p-value | Difference (CI) p-value | Difference (CI) p-value | Difference (CI) p-value | ||||||||
| 14 | 0.31 (0.15, 0.48) <0.001 | 0.19 (0.03, 0.35) 0.02 | 0.07 (−0.11, 0.25) 0.43 | 0.10 (−0.06, 0.26) 0.23 | ||||||||
| 60 | 0.21 (0.04, 0.38) 0.02 | 0.17 (0.01, 0.32) 0.04 | 0.14 (−0.05, 0.34) 0.14 | 0.19 (0.03, 0.34) 0.02 | ||||||||
| 180 | 0.14 (−0.01, 0.29) 0.07 | 0.05 (−0.11, 0.21) 0.55 | 0.11 (−0.07, 0.28) 0.23 | 0.13 (−0.01, 0.28) 0.08 | ||||||||
Primary endpoint: positive immune response to at least 2 of 4 serotypes at day 60
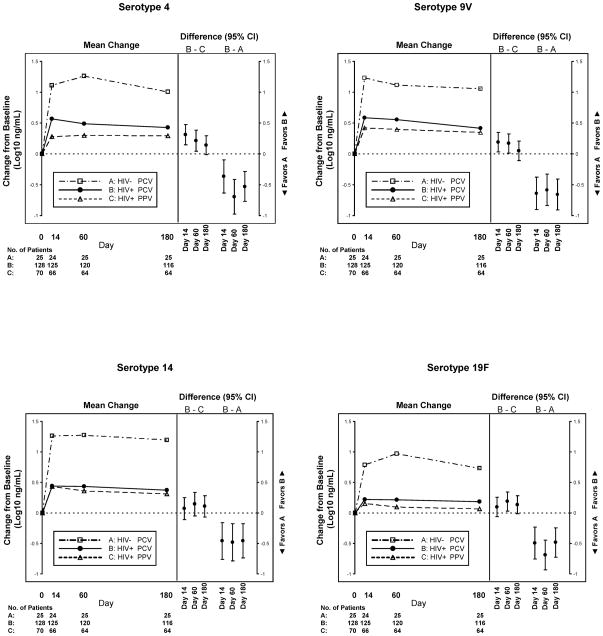
Similar to the categorical analyses, changes from baseline IgG concentrations were greater in the PCV arm compared with the PPV arm for three of four serotypes at day 60, but differences were no longer present on day 180 (Figure 2 and Table 2). There were no significant differences in change in IgG concentrations at day 60 for Caucasian compared with African American adults for the PCV and PPV arms separately or when combined. For the combined HIV-infected arms, the mean (SD) changes in IgG concentration (log10 ng/mL) at day 60 for the PCV and PPV arms, respectively, were 0.41 (0.62) vs. 0.35 (0.64), p=0.51 for serotype 4; 0.55 (0.55) vs. 0.38 (0.57), p=0.11 for serotype 9V; 0.43 (0.68) vs. 0.32 (0.71), p=0.46 for serotype 14; and 0.20 (0.53) vs. 0.11 (0.56), p=0.73 for serotype 19F.
Figure 2.
Results from models estimating the change in IgG concentration (log10 ng/mL) from baseline to days 14, 60 and 180. For each plot, the panel on the left shows the mean changes for Group A (HIV-uninfected PCV arm), Group B (HIV-infected PCV arm) and Group C (HIV-infected PPV arm); while the panel on the right shows the differences (with 95% confidence intervals) between Groups A and B and between Groups B and C for the change from baseline to each post-vaccination visit. All models were adjusted for age, ethnicity, prior pneumonia, and baseline serotype concentration; models comparing Groups B and C were also adjusted for CD4 cell count, HIV RNA level, and use of HAART at baseline.
The differences between the HIV-infected PCV and PPV arms in positive antibody response frequencies for the primary endpoint did not vary according to age (≤40, >40 years), ethnicity (Caucasian, other), prior history of pneumonia, baseline CD4+ T cell count (≤350, 351–500, 501–750, >750 cells/mm3), time between revaccination and prior PPV (3–5, 5–8 years), or HAART use at baseline.
Changes in CD4+ T Cell Counts and HIV RNA Levels after Revaccination
We observed no statistically significant decreases in CD4+ T cell counts or increases in plasma HIV RNA levels from baseline to any time point in either vaccine arm (data not shown). In addition, changes in CD4+ T cell counts and plasma HIV RNA levels after revaccination were similar in both vaccine arms. The pooled median (IQR) change in CD4+ T cell count from baseline in both arms was 20 cells/mm3 (−37, 94) at day 14; 18 cells/mm3 (−52, 88) at day 60; and 22 cells/mm3 (−45, 102) at day 180. Moreover, an increase of at least 0.3 in log10 HIV RNA at day 14 was noted in only 5% vs. 9%, (p=0.38) with PCV and PPV, respectively.
Factors Associated with Vaccine Responses among HIV-Infected Persons
Exploratory analyses of factors associated with a positive antibody response at day 60 to at least two of four serotypes and for each serotype separately were performed for each HIV vaccine arm and pooled together. Ethnicity, history of pneumonia, time since last PPV, and HIV factors (CDC stage, CD4+ T cell count, HIV RNA level, and HAART use) were not significantly associated with vaccine responses for either vaccine arm or when pooled. Younger age (≤40 vs. >40 years) was associated with improved antibody responses to serotypes 4 (unadjusted OR 2.0, 95% CI 1.1–3.7, p=0.03) and 14 (unadjusted OR 2.0, 95% CI 1.1–3.6, p=0.02) in the pooled vaccine groups, with a trend for a positive antibody response to at least two of four serotypes (unadjusted OR 1.7, 95% CI 0.9–3.1, p=0.08). Time since HIV diagnosis (≤10 years vs. > 10 years) was also associated with improved antibody responses to serotype 4 in the pooled group (unadjusted OR 2.3, 95% CI 1.2–4.3, p=0.009)
Comparisons of Capsule-Specific IgG Responses to PCV among HIV-Infected and HIV-Uninfected Participants
Compared with the HIV-uninfected subjects, HIV-infected persons receiving PCV generated lower capsule-specific IgG responses to all serotypes (Figure 1). HIV-infected subjects also exhibited a lower odds of generating a positive antibody response to at least two of four serotypes at day 60 (57% vs. 88%, OR 0.2, 95% CI 0.1–0.7, p=0.01) (Table 3) and for three of the four serotypes (4, 9V, 19F). These differences in response rate persisted through day 180 for two of the four serotypes individually (4, 9V). At each visit, and for all serotypes, the changes in IgG concentration from baseline were also significantly higher among HIV-uninfected compared to HIV-infected subjects who received PCV (Figure 2 and Table 3).
Table 3.
Antibody Responses for the HIV-Infected PCV and HIV-Uninfected PCV Arms
| At Least 2 of 4 Serotypes | At least 3 of 4 Serotypes | Serotype 4 | Serotype 9V | Serotype 14 | Serotype 19F | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) With a Positive Immune Response1 | ||||||||||||
| Day | HIV+ | HIV− | HIV+ | HIV− | HIV+ HIV− | HIV+ HIV− | HIV+ HIV− | HIV+ HIV− | ||||
| 14 | 67 (54) | 22 (92) | 37 (30) | 16 (67) | 54 (43) | 17 (71) | 68 (54) | 22 (92) | 57 (46) | 18 (75) | 37 (30) | 14 (58) |
| 60 | 68 (57) | 22 (88) | 37 (31) | 16 (64) | 48 (40) | 18 (72) | 63 (53) | 21 (84) | 61 (51) | 18 (72) | 42 (35) | 20 (80) |
| 180 | 47 (41) | 21 (84) | 26 (22) | 16 (64) | 37 (32) | 18 (72) | 49 (42) | 22 (88) | 47 (41) | 18 (72) | 38 (33) | 12 (48) |
| Adjusted Odds Ratios for a Positive Immune Response (HIV-Infected to HIV-Uninfected) With 95% Confidence Intervals and P-values | ||||||||||||
| Day | OR (95% CI) p-value | OR (95% CI) p-value | OR (95% CI) p-value | OR (95% CI) p-value | OR (95% CI) p-value | OR (95% CI) p-value | ||||||
| 14 | 0.1 (0.0, 0.7) 0.01 | 0.3 (0.1, 0.7) 0.01 | 0.4 (0.2, 1.2) 0.09 | 0.1 (0.0, 0.60) 0.01 | 0.5 (0.2, 1.5) 0.20 | 0.3 (0.1, 0.8) 0.02 | ||||||
| 60 | 0.2 (0.1, 0.7) 0.01 | 0.3 (0.1, 0.8) 0.01 | 0.3 (0.1, 0.7) 0.008 | 0.2 (0.1, 0.7) 0.01 | 0.8 (0.3, 2.3) 0.67 | 0.1 (0.0, 0.4) <0.001 | ||||||
| 180 | 0.1 (0.0, 0.4) <0.001 | 0.2 (0.1, 0.4) <0.001 | 0.2 (0.1, 0.4) <0.001 | 0.1 (0.0, 0.4) <0.001 | 0.4 (0.1, 1.1) 0.08 | 0.5 (0.2, 1.3) 0.17 | ||||||
| Adjusted Differences (HIV-Infected PCV–HIV-Uninfected PCV) for Change from Baseline in IgG Concentration (log10 ng/mL) With 95% Confidence Intervals and P-values | ||||||||||||
| Day | Difference (CI) p-value | Difference (CI) p-value | Difference (CI) p-value | Difference (CI) p-value | ||||||||
| 14 | −0.37 (−0.64, −0.10) 0.008 | −0.64 (−0.90, −0.38) <0.001 | −0.46 (−0.76, −0.16) 0.003 | −0.50 (−0.76, −0.23) <0.001 | ||||||||
| 60 | −0.69 (−0.97, −0.41) <0.001 | −0.58 (−0.83, −0.33) <0.001 | −0.48 (−0.79, −0.17) 0.002 | −0.69 (−0.93, −0.45) <0.001 | ||||||||
| 180 | −0.53 (−0.77, −0.29) <0.001 | −0.66 (−0.90, −0.42) <0.001 | −0.46 (−0.74, −0.18) 0.002 | −0.48 (−0.73, −0.24) <0.001 | ||||||||
Primary endpoint: positive immune response to at least 2 of 4 serotypes at day 60
Adverse Events (AE)
Both vaccines were generally well tolerated among HIV-infected participants. During the first seven days after revaccination with PCV and PPV, 39% and 32% experienced at least one AE, respectively (p=0.29; Table 4). The frequency and pattern of adverse events were similar between the HIV-infected vaccine arms. Most AEs were mild and most commonly were local tenderness and myalgias. Only one HIV-infected participant (who received PCV) developed a serious AE that was possibly related to the vaccine: encephalitis developing 41 days after revaccination presumed due to herpes simplex virus and improved with acyclovir.
Table 4.
Adverse Events (AE) Temporally (Within 7 Days) Related to Pneumococcal Vaccination
| HIV-infected PCV N=131 | HIV-infected PPV N=73 | P-value1 | HIV-uninfected PCV N=25 | P-value2 | |
|---|---|---|---|---|---|
| Any AE | 51 (38.9%) | 23 (31.5%) | 0.29 | 24 (96.0%) | <0.001 |
| Number of AE | 0.55 | <0.001 | |||
| None | 80 (61.1%) | 50 (68.5%) | 1 (4.0%) | ||
| One | 36 (27.5%) | 17 (23.3%) | 13 (52.0%) | ||
| Two or more | 15 (11.5%) | 6 (8.2%) | 11 (44.0%) | ||
| Total Number of AE | 72 | 38 | 38 | ||
| Severity | 0.17 | 0.29 | |||
| Mild - Grade 1 | 68 (94.4%) | 33 (86.8%) | 33 (86.8%) | ||
| Moderate - Grade 2 | 4 (5.6%) | 5 (13.2%) | 5 (13.2%) | ||
| Severe/Life-threatening – Grades 3 or 4 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Specific AE | |||||
| Local Tenderness | 36 (27.5%) | 14 (19.2%) | 23 (92.0%) | ||
| Malaise | 5 (3.8%) | 3 (4.1%) | 7 (28.0%) | ||
| Myalgia | 11 (8.4%) | 7 (9.6%) | 5 (20.0%) | ||
| Headache | 3 (2.3%) | 4 (5.5%) | 1 (4.0%) | ||
| Erythema | 1 (0.8%) | 3 (4.1%) | 3 (12.0%) | ||
| Local Swelling | 0 (0.0%) | 0 (0.0%) | 3 (12.0%) | ||
| Local Site Reaction | 7 (5.3%) | 1 (1.4%) | 3 (12.0%) | ||
| Injection Site - Other | 3 (2.3%) | 1 (1.4%) | 0 (0.0%) | ||
| Pyrexia | 1 (0.8%) | 0 (0.0%) | 4 (16.0%) | ||
| Diarrhea | 2 (1.5%) | 0 (0.0%) | 0 (0.0%) | ||
| Dizziness | 0 (0.0%) | 3 (4.1%) | 0 (0.0%) | ||
| Other related | 1 (0.8%) | 1 (1.4%) | 3 (12.0%) |
P-value is comparing the HIV-infected PCV and PPV arms
P-value is comparing the HIV-infected and HIV-uninfected PCV arms
Compared with HIV-infected persons who received PCV, HIV-uninfected subjects were more likely to experience at least one AE (39% vs. 96%; p<0.001) (Table 4). Again, the majority of AE among HIV-uninfected subjects were mild (87%) and most often involved local tenderness, malaise, and myalgia; none was severe/life-threatening. All vaccine-related AE in the HIV-uninfected group occurred within seven days.
Discussion
We evaluated whether revaccination with PCV would provide a more robust response than PPV among HIV-infected adults. PCV was initially more immunogenic than PPV, a result consistent with most [18, 20] but not all, studies [24]. However, this difference waned by day 180, suggesting that revaccination using this novel vaccine may provide little added clinical advantage compared with PPV.
A recent study in Malawi that evaluated a two-dose series of PCV among HIV-infected adults after recovery from invasive pneumococcal infection found that PCVwas effective at preventing pneumococcal disease due to vaccine serotypes [25]. However, that study examined PCV as primary vaccination rather than its use in revaccination, as performed in our study.
We also found that despite the ability of HIV-infected persons to generate statistically significant increases in antibody levels after pneumococcal vaccination, their concentrations and frequencies of responses to PCV were significantly lower than those in HIV-uninfected subjects receiving the same vaccine. These differences were present even though this HIV cohort had relatively high CD4+ T cell counts (median 533 cells/mm3), high antiretroviral coverage, and few concurrent comorbidities.
Antibody responses to both pneumococcal vaccines likely depend, in part, on CD4+ T cell function [18, 20, 21, 24, 26, 27]. However, persistent CD4+ T cell defects, which may not be measured by the absolute current CD4+ T cell count nor completely recoverable with HAART, may be present. The etiology of these persistent CD4+ T cell defects is likely due, at least in part, to immune activation of CD4+ T cells, B cells and dendritic cells. Although our cohort had well-controlled viremia (77% had undetectable HIV RNA levels), immune activation may persist, albeit less prominently, despite viral suppression [28]. Independent of HIV infection, responses to pneumococcal revaccination in older adults also appears to elicit a very limited magnitude of response [29–31]. Although the exact nature of these underlying immune deficits is unknown, immunosenescence may also contribute to poorer responses among subjects receiving both primary and revaccination.
In our ethnically diverse cohort, we did not observe differential antibody responses by ethnicity. Earlier reports demonstrated an increased incidence of invasive pneumococcal disease in African Americans compared to Caucasians [32]. Furthermore, among HIV-infected adults some studies have shown that black Americans and Africans, compared with Caucasians, are not protected from pneumococcal infections after receipt of PPV [33, 34]. However, we found no evidence for differences in immunogenicity with revaccination in African American compared with Caucasian HIV-infected adults with open access to care and little illicit drug use.
Overall, both PPV and PCV were well tolerated, consistent with other studies [20, 22, 35]. Rates of adverse reactions were similar, and as previously described [20, 22], changes in HIV RNA and CD4+ T cell numbers were few and comparable in the vaccine groups. However, the HIV-uninfected cohort receiving PCV experienced a significantly higher rate of vaccine reactions, which may have been related with more robust immune responses.
Our study has potential limitations. First, enrollment was halted prior to full recruitment, so it is possible that important findings may have been missed due to truncated enrollment. Second, although we used reported criteria [19], there is no defined correlate of protective pneumococcal immunity in adults. However, the pattern for each of several analyses of antibody responses yielded similar patterns. Third, prior pneumococcal vaccination may result in blunted responses to subsequent revaccination [30]; however, most of this effect was noted in the first year after initial vaccination [29], whereas the median time to revaccination in our cohort was five years. Finally, although we limited our study to four serotypes shared between PPV and PCV, it seems doubtful that large differences were missed by focusing on the serotypes chosen. PCV contains seven pneumococcal serotypes; newer generations of conjugate vaccines (i.e., 10- and 13-valent) are now available. Our study was designed to determine whether revaccination with a pneumococcal conjugate vaccine could generate superior antibody responses compared with those to a polysaccharide vaccine in HIV-infected adults, effects that should not be influenced by the valence of either PPV or PCV.
Strengths of this work include its precedence as the first study to determine whether PCV is a promising revaccination strategy after distant primary vaccination with PPV. In addition, this study is one of the largest in HIV-infected adults to evaluate pneumococcal vaccine responses. Finally, we characterized vaccine responses in a relatively healthy, well-defined HIV cohort with open access to care.
In summary, although revaccination with PCV was more immunogenic than PPV among HIV-infected adults at day 60, all such differences appeared to wane by day 180. Further, despite high median CD4+ T cell counts and use of HAART by the majority of our cohort, HIV-infected persons produced antibody concentrations that were significantly lower than did the HIV-uninfected group, although the former had been previously vaccinated whereas the latter had not. Our data suggest that the use of PCV may not provide substantial additional protective benefit over PPV in defense against S. pneumoniae infections among HIV-infected adults. These data suggest that more effective revaccination strategies, including vaccines that elicit more robust and sustained antibody responses, are needed to more consistently prevent pneumococcal disease in HIV-infected adults.
Acknowledgments
Support for this work (IDCRP RV150) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed through the Uniformed Services University of the Health Sciences. This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072. Additional support was obtained from the Veterans Affairs Research Service.
The IDCRP HIV Working Group is comprised of: Susan Banks, Irma Barahona, CDR Mary Bavaro MD, Carolyn Brandt RN, LCDR Helen Chun MD, Cathy Decker MD, Conner Eggleston, LTC Tomas Ferguson MD, COL Susan Fraser MD, COL Cliff Hawkes MD, Arthur Johnson MD, Erica Johnson MD, Alan Lifson MD, Grace Macalino PhD, CDR Jason Maguire MD, Scott Merritt, Christie Morse, LTC Robert O’Connell MD, MAJ Jason Okulicz MD, Sheila Peel PhD, Michael Polis MD, John Powers MD, Roseanne Ressner MD, CAPT (ret) Sybil Tasker MD, COL (ret) Edmund Tramont MD, CAPT (ret) Mark Wallace MD, CDR Timothy Whitman MD, COL Glenn Wortmann MD, CDR Michael Zapor MD.
Footnotes
Conflict of Interest: None
The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, the DoD or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
This work is original and has not been published elsewhere. Part of these data was presented at the 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, CA, February 2010.
References
- 1.Janoff EN, Breiman RF, Daley CL, Hopewell PC. Pneumococcal disease during HIV infection. Epidemiologic, clinical, and immunologic perspectives. Ann Intern Med. 1992;117:314–24. doi: 10.7326/0003-4819-117-4-314. [DOI] [PubMed] [Google Scholar]
- 2.Barry PM, Zetola N, Keruly JC, Moore RD, Gebo KA, Lucas GM. Invasive pneumococcal disease in a cohort of HIV-infected adults: incidence and risk factors, 1990–2003. AIDS. 2006;20:437–44. doi: 10.1097/01.aids.0000206507.54901.84. [DOI] [PubMed] [Google Scholar]
- 3.Frankel RE, Virata M, Hardalo C, Altice FL, Friedland G. Invasive pneumococcal disease: clinical features, serotypes, and antimicrobial resistance patterns in cases involving patients with and without human immunodeficiency virus infection. Clin Infect Dis. 1996;23:577–84. doi: 10.1093/clinids/23.3.577. [DOI] [PubMed] [Google Scholar]
- 4.Hibbs JR, Douglas JM, Jr, Judson FN, McGill WL, Rietmeijer CA, Janoff EN. Prevalence of human immunodeficiency virus infection, mortality rate, and serogroup distribution among patients with pneumococcal bacteremia at Denver General Hospital, 1984–1994. Clin Infect Dis. 1997;25:195–9. doi: 10.1086/514538. [DOI] [PubMed] [Google Scholar]
- 5.McEllistrem MC, Mendelsohn AB, Pass MA, et al. Recurrent invasive pneumococcal disease in individuals with human immunodeficiency virus infection. J Infect Dis. 2002;185:1364–8. doi: 10.1086/339882. [DOI] [PubMed] [Google Scholar]
- 6.Jordano Q, Falcó V, Almirante B, et al. Invasive pneumococcal disease in patients infected with HIV: still a threat in the era of highly active antiretroviral therapy. Clin Infect Dis. 2004;38:1623–8. doi: 10.1086/420933. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H Centers for Disease Control and Prevention (CDC); National Institutes of Health; HIV Medicine Association of the Infectious Diseases Society of America. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(RR-4):1–207. [PubMed] [Google Scholar]
- 8.Hausdorff W, Bryant J, Paradiso P, Siber G. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis. 2000;30:100–21. doi: 10.1086/313608. [DOI] [PubMed] [Google Scholar]
- 9.Flannery B, Schrag S, Bennett NM, et al. Active Bacterial Core Surveillance/Emerging Infections Program Network. Impact of childhood vaccination on racial disparities in invasive Streptococcus pneumoniae infections. JAMA. 2004;291:2197–203. doi: 10.1001/jama.291.18.2197. [DOI] [PubMed] [Google Scholar]
- 10.Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006;295:1668–74. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]
- 11.Whitney CG, Farley MM, Hadler J, et al. Active Bacterial Core Surveillance of the Emerging Infections Program Network. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 12.Lexau CA, Lynfield R, Danila R, et al. Active Bacterial Core Surveillance Team. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043–51. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 13.Meisel R, Kuypers L, Dirksen U, et al. Pneumococcal conjugate vaccine provides early protective antibody responses in children after related and unrelated allogeneic hematopoietic stem cell transplantation. Blood. 2007;109:2322–6. doi: 10.1182/blood-2006-06-032284. [DOI] [PubMed] [Google Scholar]
- 14.Cordonnier C, Labopin M, Chesnel V, et al. Randomized study of early versus late immunization with pneumococcal conjugate vaccine after allogeneic stem cell transplantation. Clin Infect Dis. 2009;48:1392–401. doi: 10.1086/598324. [DOI] [PubMed] [Google Scholar]
- 15.Molrine DC, Antin JH, Guinan EC, et al. Donor immunization with pneumococcal conjugate vaccine and early protective antibody responses following allogeneic hematopoietic cell transplantation. Blood. 2003;101:831–6. doi: 10.1182/blood-2002-03-0832. [DOI] [PubMed] [Google Scholar]
- 16.Kumar D, Chen MH, Welsh B, et al. A randomized, double-blind trial of pneumococcal vaccination in adult allogeneic stem cell transplant donors and recipients. Clin Infect Dis. 2007;45:1576–82. doi: 10.1086/523583. [DOI] [PubMed] [Google Scholar]
- 17.Madhi SA, Klugman KP, Kuwanda L, Cutland C, Käyhty H, Adrian P. Quantitative and qualitative anamnestic immune responses to pneumococcal conjugate vaccine in HIV-infected and HIV-uninfected children 5 years after vaccination. J Infect Dis. 2009;199:1168–76. doi: 10.1086/597388. [DOI] [PubMed] [Google Scholar]
- 18.King JC, Jr, Vink PE, Farley JJ, et al. Comparison of the safety and immunogenicity of a pneumococcal conjugate with a licensed polysaccharide vaccine in human immunodeficiency virus and non-human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1996;15:192–6. doi: 10.1097/00006454-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Lesprit P, Pédrono G, Molina JM, et al. Immunological efficacy of a prime-boost pneumococcal vaccination in HIV-infected adults. AIDS. 2007;21:2425–34. doi: 10.1097/QAD.0b013e3282887e91. [DOI] [PubMed] [Google Scholar]
- 20.Feikin DR, Elie CM, Goetz MB, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine. 2001;20:545–53. doi: 10.1016/s0264-410x(01)00347-4. [DOI] [PubMed] [Google Scholar]
- 21.Kroon FP, van Dissel JT, Ravensbergen E, Nibbering PH, van Furth R. Enhanced antibody response to pneumococcal polysaccharide vaccine after prior immunization with conjugate pneumococcal vaccine in HIV-infected adults. Vaccine. 2001;19:886–94. doi: 10.1016/s0264-410x(00)00232-2. [DOI] [PubMed] [Google Scholar]
- 22.Tasker SA, Wallace MR, Rubins JB, Paxton WB, O’Brien J, Janoff EN. Revaccination with 23-valent pneumococcal vaccine for patients infected with human immunodeficiency virus type 1: clinical, immunologic, and virologic responses. Clin Infect Dis. 2002;34:813–21. doi: 10.1086/339044. [DOI] [PubMed] [Google Scholar]
- 23.Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (Version 1.0 - December 2004) [Accessed on June 1, 2005]; Available at http://rcc.tech-res.com/safetyandpharmacovigilance/
- 24.Ahmed F, Steinhoff MC, Rodriguez-Barradas MC, Hamilton RG, Musher DM, Nelson KE. Effect of human immunodeficiency virus type 1 infection on the antibody response to a glycoprotein conjugate pneumococcal vaccine: results from a randomized trial. J Infect Dis. 1996;173:83–90. doi: 10.1093/infdis/173.1.83. [DOI] [PubMed] [Google Scholar]
- 25.French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med. 2010;362:812–22. doi: 10.1056/NEJMoa0903029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miiro G, Kayhty H, Watera C, et al. Conjugate pneumococcal vaccine in HIV-infected Ugandans and the effect of past receipt of polysaccharide vaccine. J Infect Dis. 2005;192:1801–5. doi: 10.1086/497144. [DOI] [PubMed] [Google Scholar]
- 27.Colino J, Chattopadhyay G, Sen G, et al. Parameters underlying distinct T cell-dependent polysaccharide-specific IgG responses to an intact gram-positive bacterium versus a soluble conjugate vaccine. J Immunol. 2009;183:1551–9. doi: 10.4049/jimmunol.0900238. [DOI] [PubMed] [Google Scholar]
- 28.Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med. 2009;60:471–84. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musher DM, Rueda AM, Nahm MH, Graviss EA, Rodriguez-Barradas MC. Initial and subsequent response to pneumococcal polysaccharide and protein-conjugate vaccines administered sequentially to adults who have recovered from pneumococcal pneumonia. J Infect Dis. 2008;198:1019–27. doi: 10.1086/591629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson LA, Janoff EN. Pneumococcal vaccination of elderly adults: new paradigms for protection. Clin Infect Dis. 2008;47:1328–38. doi: 10.1086/592691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldblatt D, Southern J, Andrews N, et al. The immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50–80 years. Clin Infect Dis. 2009;49:1318–25. doi: 10.1086/606046. [DOI] [PubMed] [Google Scholar]
- 32.Bennett NM, Buffington J, LaForce FM. Pneumococcal bacteremia in Monroe County, New York. Am J Public Health. 1992;82:1513–6. doi: 10.2105/ajph.82.11.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breiman RF, Keller DW, Phelan MA, et al. Evaluation of effectiveness of the 23-valent pneumococcal capsular polysaccharide vaccine for HIV-infected patients. Arch Intern Med. 2000;160:2633–8. doi: 10.1001/archinte.160.17.2633. [DOI] [PubMed] [Google Scholar]
- 34.French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355:2106–11. doi: 10.1016/s0140-6736(00)02377-1. [DOI] [PubMed] [Google Scholar]
- 35.Amendola A, Tanzi E, Zappa A, et al. Safety and immunogenicity of 23-valent pneumococcal polysaccharide vaccine in HIV-1 infected former drug users. Vaccine. 2002;20:3720–4. doi: 10.1016/s0264-410x(02)00357-2. [DOI] [PubMed] [Google Scholar]