Abstract
Background
Otitis media is an extremely common pediatric infection, and is mostly caused by bacteria that are carried within the nasopharyngeal microbiota. It is clear that most otitis media cases involve simultaneous infection with multiple agents.
Methods
Chinchillas were infected with nontypeable Haemophilus influenzae, Streptococcus pneumoniae, or a combination of both organisms, and the course of disease was compared. In vitro experiments were also performed to address how coinfection impacts biofilm formation.
Results
The incidence of systemic disease was reduced in coinfected animals as compared to those infected with pneumococcus alone. Pneumococci were present within surface-attached biofilms in coinfected animals, and a greater proportion of translucent colony type was observed in the coinfected animals. As this colony type has been associated with pneumococcal biofilms, the impact of coinfection on pneumococcal biofilm formation was investigated. The results clearly show enhanced biofilm formation in vitro by pneumococci in the presence of H. influenzae.
Conclusions
Based on these data, we conclude that coinfection with H. influenzae facilitates pneumococcal biofilm formation and persistence on the middle-ear mucosal surface. This enhanced biofilm persistence correlates with delayed emergence of opaque colony variants within the bacterial population, and a resulting decrease in systemic infection.
Keywords: Polymicrobial infection, Streptococcus pneumoniae, Haemophilus influenzae, otitis media, biofilm
INTRODUCTION
Otitis media (OM) is an extremely common pediatric infection, affecting the majority of all children [1]. OM arises as a consequence of impaired drainage of the middle-ear chamber, which facilitates colonization by bacterial opportunists residing in the nasopharynx that include nontypeable Haemophilus influenzae and Streptococcus pneumoniae (pneumococcus) [2]. OM often presents as a chronic or recurrent infection in which bacteria persist within biofilm communities [3]. Biofilms have been observed on middle ear mucosal tissues from patients undergoing tympanostomy [4], and it is now clear that biofilms promote bacterial persistence during chronic/recurrent OM infections [5].
Recent epidemiologic studies have shown that the majority of cases of OM and other opportunistic airway infections involve simultaneous infection with multiple pathogens [6]. Prior work using murine models indicates that H. influenzae components can prime enhanced innate responses that contain pneumococcal infection [7-9]. In this study, we used the chinchilla animal infection model for OM to test the impact of coinfection with H. influenzae and pneumococcus on bacterial clearance/persistence and OM disease. The results clearly show that coinfection with H. influenzae promotes pneumococcal biofilm formation and persistence in localized infections.
MATERIALS AND METHODS
Bacterial strains and growth conditions
Streptococcus pneumoniae TIGR4 is a well-studied clinical isolate for which a complete genomic sequence is available [10], and which we have recently shown to form biofilms during experimental otitis media infections [11]. Pneumococci were grown on Todd-Hewitt yeast extract (THY) agar (Difco) with 4 μg/mL of gentamicin (Sigma) added, brain heart infusion (BHI) agar (Difco) with 5% sheep's blood and gentamicin or trypticase soy agar (BD) with 315 U/mL of catalase (Worthington) added, as indicated in the text. Nontypeable Haemophilus influenzae 86-028NP is an otitis media isolate that has been fully sequenced [12] and which likewise is known to cause otitis media featuring biofilms in the chinchilla infection model [5, 13-17]. Bacteria were grown on BHI agar supplemented with hemin (ICN Biochemicals) and nicotinamide adenine dinucleotide (NAD, Sigma) and 3 μg/mL of vancomycin (Sigma).
Chinchilla infections
Healthy adult chinchillas (Chinchilla lanigera) were purchased from Rauscher's chinchilla ranch (LaRue, OH) and allowed to acclimate to the vivarium for 1 week prior to infection. All animals were examined by otoscopy prior to infection, and none had any clinical signs of middle-ear infection or other overt disease. The chinchilla infection protocols were performed essentially as described previously [5, 11, 13, 14]. S. pneumoniae TIGR4 and/or nontypeable H. influenzae 86-028NP were diluted using pyrogen-free phosphate-buffered saline (PBS), and the bacterial density was confirmed by plate-count. Chinchillas (3-5 animals/group/time point) were anesthetized with isofluorane and inoculated via transbullar injection with 0.1 mL of bacterial suspension containing either S. pneumoniae, H. influenzae or both bacterial species, as indicated in the text. Infectious doses ranged from 102-105 colony-forming units, as indicated in the individual experiments. Groups of animals were euthanized at 3, 7, 14 or 21 days post-infection. Animals exhibiting overt symptoms of systemic disease were euthanized. Blood was collected at euthanasia and plated to determine the presence of bacteremia. After euthanasia, the superior bullae were opened to expose the middle ear cavity as previously described [14], and presence of visible biofilm was assessed. If present, middle ear effusion fluids were collected. The middle ear cavity was then lavaged with 1 mL of sterile PBS. Effusion and lavage fluids were serially diluted and assessed by plate-count. For animals that received both bacteria, fluid was plated on two separate plates, one selective for H. influenzae (supplemented BHI + vancomycin) and one selective for S. pneumoniae (THY + gentamicin or TSB + catalase). Middle ear bullae were aseptically removed and homogenized using a Power Gen 1000 homogenizer (Fisher Scientific); the bullar homogenates were plated to assess tissue-associated bacterial load. Representative bullae were fixed in 4% paraformaldehyde for microscopy studies. All of the chinchilla infection protocols were approved by the Wake Forest University Health Sciences Institutional Animal Care and Use Committee.
Microscopy
Biofilms were excised from the middle ear chamber, rinsed with PBS, then placed in Cryomolds (Sakura Finetek USA, Torrance, CA) with OCT compound (Sakura Finetek USA, Torrance, CA) and frozen at -70° C. Serial 5 μm sections were cut with Accu-Edge Low Profile Blade (Feather Safety Razor, Japan) at -20° C, and placed on adhesive slides and stored at –70° C. For immunofluorescent staining, slides were brought to room temperature, washed with PBS, blocked with 1% bovine serum albumin (Sigma) and then stained with monoclonal antibody specific for pneumococcal type 4 capsule (kindly provided by Dr. Moon Nahm, UAB Bacterial Pathogen Reference Laboratory) and rabbit anti-H. influenzae antiserum [13] as primary antibodies. Donkey anti-rabbit IgG AlexaFluor 488 (Invitrogen, Eugene, OR) or goat anti-rabbit IgG Texas Red (Invitrogen, Eugene, OR) were used as secondary antibodies. Stained slides were mounted with Prolong Gold anti-fade reagent (Invitrogen, Eugene, Oregon). Samples were visualized using a Zeiss LSM510 confocal laser-scanning microscope.
Static Biofilms
Assessment of biofilm formation in static cultures was performed essentially as described previously [11, 18]. H. influenzae 86-028NP (~107 CFU) was plated in each well of a 24-well plate in a total volume of 1.5 mL of supplemented BHI or Morse's minimal media [19]. After 24 h, supernatants were removed and replaced with 1.5 mL of supplemented Morse's minimal media + 10% horse serum. S. pneumoniae TIGR4 (~107 CFU) was added to the coinfection and S. pneumoniae wells. After 48 h, the supernatant was removed and the surface-attached bacteria (biofilm) were collected by scraping, and resuspended in 0.2 ml of sterile PBS; this suspension was then diluted and plated for bacterial counts.
RESULTS
Competitive infections
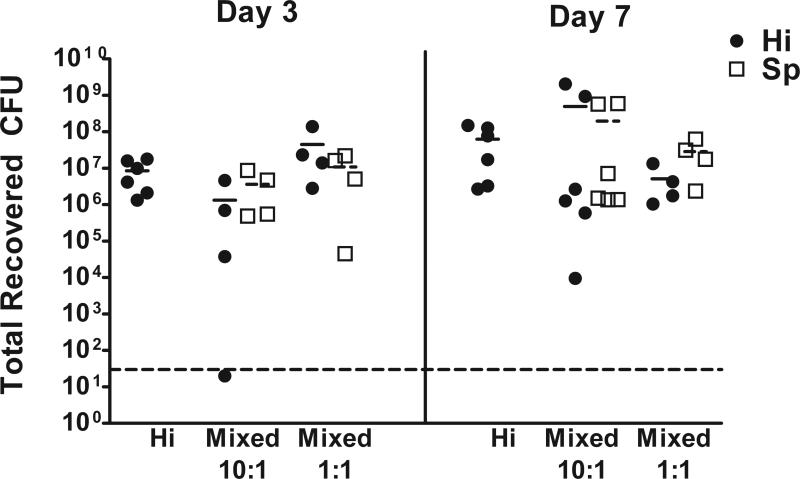
The impact of H. influenzae/pneumococcal coinfection on bacterial persistence and otitis media disease was assessed using infectious doses previously established for monospecies infections with each organism [11, 13]. Groups of chinchillas were inoculated with either ~103 CFU of H. influenzae, ~150 CFU of S. pneumoniae TIGR4, or like infectious doses of both species. On day 3, equivalent bacterial counts for both bacterial species were obtained from both middle ear fluids (Figure 1A) and bullar homogenates (Figure 1B). However, by day 7 post-infection, no pneumococci were recovered from the coinfected animals (Figure 1). Notably, while a significant percentage of animals infected with pneumococci developed systemic disease, no systemic disease was apparent in the coinfected animals (Supplemental Table 1). To determine if polymicrobial biofilms were formed and if there was a difference in biofilm formation in animals infected with H. influenzae, pneumococcus, or both, the numbers of ears containing biofilm were enumerated and biofilms were removed and analyzed by confocal laser scanning microscopy (CLSM). There was no difference in the number of ears with biofilms in coinfection versus single infection animals, although S. pneumoniae alone animals tended to form small biofilms, while coinfected animals formed large biofilms that filled the majority of the middle ear space (data not shown). Based on these data, we conclude that in the conditions of this experiment, H. influenzae and pneumococcus can establish a competitive relationship during otitis media infection. Moreover, coinfection appeared to moderate the progression of pneumococcal disease to fatal systemic infection (Supplemental Table 1).
Figure 1. Bacterial competition during experimental OM coinfections.
Total recovered CFU from the middle ears of infected animals on days 3 and 7 post-infection. All animals received inocula of ~103 CFU of H. influenzae (Hi) and/or ~150 CFU of S. pneumoniae (Sp) as indicated. Each data point represents one ear. The dashed line indicates the limit of detection, the short solid and dashed lines represent the mean CFU for H. influenzae and S. pneumoniae for each group, respectively.
Stable coinfections
While the above results clearly showed that H. influenzae could out-compete pneumococci in some infection conditions, it should be noted that the infectious doses used may have afforded a competitive advantage to H. influenzae simply due to greater numbers of these bacteria. Therefore, groups of animals were infected with either 1:1 or 10:1 ratios of H. influenzae to pneumococcus. No animals were given S. pneumoniae alone because at these doses, the majority of animals would be expected to rapidly develop systemic disease (preliminary results). Notably, at 3 and 7 days post-infection, H. influenzae and S. pneumoniae were present in equivalent numbers in the middle ears of mixed infection animals from both the 1:1 ratio and 10:1 ratio groups (Figure 2). At these inoculating doses, there was a marked difference in the prevalence of visible biofilm in the infection groups; 89% of the ears of mixed infection animals (16/18) contained biofilm, while biofilms were observed in 50% (6/12) of animals infected with either species alone. In addition, biofilms from coinfected animals were much larger and generally filled the entire middle ear chamber (data not shown). Confocal images of biofilms from mixed infection animals were very similar to those seen in the previous experiment, where both bacterial species are present in close proximity to one another.
Figure 2. Persistent polymicrobial infection during exerimental OM coinfections.
Symbols represent total recovered CFU of each species recovered from the middle ears of animals. All animals received ~104 CFU of H. influenzae (Hi), coinfected animals also received ~103 CFU (10:1 ratio) or ~104 CFU of S. pneumoniae (Sp). Each point represents one ear. The dashed line indicates the limit of detection, the short solid and dashed lines represent the mean CFU for H. influenzae and S. pneumoniae for each group, respectively.
With the results of these infection studies in hand, we asked how long a stable coinfection was maintained. Groups of chinchillas were infected with H. influenzae alone or a 10:1 ratio of H. influenzae/S. pneumoniae, and euthanized at 7, 14 and 21 days post-infection. As in the preceding experiment, equivalent numbers of H. influenzae and S. pneumoniae were recovered from the middle ears of single and mixed infection animals 7 days post-infection (Figure 3). On day 14, S. pneumoniae was not recovered from the middle ears of every animal except one, where it was found in both ears. Interestingly, the only animal with S. pneumoniae in its ears had the lowest levels of H. influenzae (light and dark blue circles and squares respectively, Figure 3). In contrast to day 14, on day 21, the majority of coinfected animals had higher numbers of S. pneumoniae than H. influenzae. The most likely explanation for this is that, although S. pneumoniae was undetectable in the effusion, it was able to persist within biofilm communities, or at a second site within the upper airway, at day 14 and re-established an infection when the conditions are more favorable.
Figure 3. Duration of stable polymicrobial infection.
Data points represent total recovered CFU from the middle ears of infected animals 7, 14 and 21 days post-infection. Coinfected animals received ~104 CFU of H. influenzae (Hi) and ~104 CFU of S. pneumoniae (Sp), whereas another group of animals received ~104 CFU of H. influenzae. Each point represents one ear. Dark and light colored symbols represent the right and left ears of one animal with respect to both bacterial species. The dashed line indicates the limit of detection, the short solid and dashed lines represent the mean CFU for H. influenzae and S. pneumoniae for each group, respectively.
There were equivalent numbers of ears with biofilm recovered from single and coinfected animals on days 7 and 14; however, on day 21, 4/6 mixed infection ears contained biofilm, whereas no biofilms were observed in animals infected with a single species (data not shown). Figure 4 shows the amount of biofilm recovered within the middle-ear chamber of animals 14 and 21 days post-infection (Figure 4A-C). Cryosections of biofilms excised from the middle-ear chamber were stained with mouse monoclonal anti-serotype 4 capsular antibody to detect S. pneumoniae or a polyclonal rabbit anti-Haemophilus antibody, and analyzed by confocal laser scanning microscopy (CLSM). CLSM images from coinfected animals showed the presence of both bacterial species on 7, 14 and 21 days post-infection (Figure 4D-F and Supplemental Figure).
Figure 4. Imaging of polymicrobial biofilms.
A-C. Gross images of sectioned bullae removed from mock (A) and coinfected animals 14 (B) and 21 (C) days post infection. D-F. Confocal laser scanning microscopy images of biofilms removed from mixed infection animals 7, 14, and 21 days post infection. The green is staining for H. influenzae and the red is staining the capsule of S. pneumoniae. Yellow indicates colocalization of the two bacterial species.
Temporal variation of inoculation has no affect on bacterial persistence
We next wanted to determine if a preexisting infection with one of the bacterial species would prevent colonization with the other bacterial species or alter the type of coinfection. To address this question, two sets of experiments were performed. In the first experiment, 3 groups of chinchillas were infected. The first received H. influenzae on day 0 and PBS on day 7 (Hi → PBS), the second received H. influenzae on day 0 and of S. pneumoniae on day 7 (Hi → Sp) and the last received PBS on day 0 and S. pneumoniae on day 7 (Sp → PBS). All animals were euthanized 14 days after the initial infection. One animal (represented with dark and light green symbols) had no recovery of S. pneumoniae and had high levels of H. influenzae, similar to results seen in Figure 1 above (Figure 5A). At the other end of the spectrum, one animal (represented with light and dark orange symbols) had no H. influenzae in either ear, but had high numbers of S. pneumoniae. The other three coinfected animals had equivalent numbers of both bacterial species in each ear. Although the outcome varied with each animal, the experiment clearly shows that a preexisting H. influenzae infection does not prevent S. pneumoniae from establishing an infection. However, no systemic disease was observed in animals with preexisting H. influenzae infection (Supplementary Table 1).
Figure 5. Temporal variation of coinfections.

Counts were obtained from middle-ear fluids or bullar homogenates 14 days following initial infection. A. Animals received either H. influenzae or PBS on day 0 and S. pneumoniae or PBS on day 7. B. Animals received either CFU of S. pneumoniae or PBS on day 0 and H. influenzae or PBS on day 7. Each point represents one ear. Dark and light colored symbols represent the right and left ears of one animal with respect to both bacterial species. The dashed line indicates the limit of detection, the short solid and dashed lines represent the mean CFU for H. influenzae and S. pneumoniae for each group, respectively.
All ears from coinfected animals contained biofilm (10/10), as compared to 4/8 ears from S. pneumoniae infected animals and 6/8 ears from H. influenzae infected animals. As seen in previous experiments, biofilms from coinfected animals consistently appeared larger than those from animals infected with either species alone. In the second set of experiments, we wanted to determine if a preexisting S. pneumoniae infection could prevent H. influenzae from establishing an infection. The same experimental groups were used, except the timing of inoculation of H. influenzae and S. pneumoniae was reversed. Groups of animals were given either S. pneumoniae or PBS on day 0. The majority of animals infected with S. pneumoniae developed systemic disease and were thus euthanized before day 7 (Supplemental Table 1). The two surviving animals that received S. pneumoniae and the four animals given PBS on day 0 were inoculated with H. influenzae on day 7. All animals were euthanized on day 14. The two coinfected animals established stable coinfections containing equivalent numbers of H. influenzae and S. pneumoniae in their middle ears (Figure 5B), showing that a preexisting S. pneumoniae infection does not prevent H. influenzae colonization. All four ears from coinfected animals contained visble biofilms, and immunofluorescence showed the presence of both bacterial species (data not shown).
The presence of H. influenzae increases pneumococcal biofilm formation in vitro
One observation made during the in vivo studies was that S. pneumoniae formed biofilms with a greater frequency and of a larger size in the presence of H. influenzae than alone. Thus, the impact of H. influenzae on pneumococcal biofilm formation was assessed using an in vitro static biofilm assay. The results show significantly greater numbers of S. pneumoniae in surface-attached communities containing H. influenzae than in those containing pneumococcus alone (Figure 6A). Of note, these results were more dramatic when performed in chemically-defined minimal media (Figure 6B).
Figure 6. H. influenzae promotes pneumococcal biofilm formation in vitro.
Total recovered S. pneumoniae CFU from 48 h static biofilms in supplemented BHI (A) or minimal media (B). The black bars represents the S. pneumoniae recovered from co-infection with H. influenzae and the white bars represents the S. pneumoniae recovered from S. pneumoniae alone static biofilms. Bars represent the mean +/- the standard error of the mean from 4 independent wells from one representitive experiment. Statistical significance was assessed using a Mann Whitney nonparametric t-test. **P < 0.005.
Coinfection increases the translucent colony type within pneumococcal populations in vivo
Pneumococci undergo phase-variation between two distinct colony phenotypes; opaque colonies produce greater amounts of capsular polysaccharide and are associated with systemic infections, whereas translucent colonies have less capsular polysaccharide and greater choline-containing cell wall teichoic acid on their surfaces. Notably, the translucent populations predominate during the early stages of infection and within the tissue-associated populations that presumably include biofilms. Thus, we hypothesized that H. influenzae may influence pneumococcal colony phenotype. If this were the case, we would expect to see a higher percentage of translucent colonies in S. pneumoniae removed from mixed infection animals than in animals that received S. pneumoniae alone. To address this hypothesis, animals were infected with H. influenzae alone, S. pneumoniae, or both organisms. The colony type within the pneumococcal inocula was determined to be ~ 40% translucent. Animals were euthanized 7 days post-infection and colony counts and opacity from both the effusion (planktonic bacteria) and homogenized bullae (surface associated bacteria) were assessed. All infection groups established stable middle ear infections with equivalent numbers of both bacterial species in both the effusion and homogenized bullae (Figures 7A and 7B). In the effusion, the percentage of translucent colonies fell to ~20% for both S. pneumoniae alone and S. pneumoniae plus H. influenzae animals (Figure 7C). However, in the bullae, the percentage of translucent colonies increased significantly to ~50% in mixed infection animals and dropped to ~20% in S. pneumoniae alone animals (Figure 7C, p = 0.015).
Figure 7. Coinfection with H. influenzae promotes persistence of pneumococcal translucent colony variants.
Total recovered CFU from A. the middle ears and B. homogenized bullae of mixed and single infection animals 7 days post-infection. Animals were given 104 CFU of H. influenzae alone (Hi) alone or ~104 CFU of H. influenzae and ~104 CFU of S. pneumoniae (Sp). S. pneumoniae alone animals only received 100 CFU. Each point represents one ear. The dashed line indicates the limit of detection, the short solid and dashed lines represent the mean CFU for H. influenzae and S. pneumoniae for each group, respectively. C. The percentage of translucent S. pneumoniae colonies from each infection group from the effusion and bullae. Bars are the mean +/- SEM from 6 ears. Statistical significance was assessed using a Mann Whitney nonparametric t-test. *P = 0.015.
DISCUSSION
While it is clear that the majority of OM infections involve multiple species, most of our current knowledge regarding the bacterial pathogenesis of OM has been derived from infections using pure cultures of single organisms. Since H. influenzae and pneumococcus collectively account for the majority of OM infections [2], it is of particular importance to understand how these species influence one another. Prior work provides clear evidence for modulation of pneumococcal disease by H. influenzae, most notably by priming enhanced host innate responses to clear the pneumococcal infection [7-9]. The results of our work clearly establish that preceding or concurrent infection with H. influenzae moderates the progression and severity of pneumococcal disease in the chinchilla. Additional studies with phagocytes in vitro showed no difference in pneumococcal killing in accordance with coinfection (data not shown).
Our data also clearly show that the presence of H. influenzae significantly increases the percentage of translucent pneumococcal colonies, as compared to the populations recovered from animals that received pneumococcus alone. Translucent pneumococcal variants have a decreased propensity toward systemic infections [20], and instead are more adherent to abiotic and host cell surfaces [21]. Work from a number of groups has established that biofilm communities contain predominantly translucent colony variants [22-26]. Notably, the H. influenzae biofilm matrix consists of sialylated lipooligosaccharides [15, 18, 27]. Recent work has clearly established that pneumococcal neuraminidases promote biofilm formation [28], a result that is recapitulated in the presence of free sialic acid [29]. The observed enhancement of pneumococcal biofilm formation by H. influenzae was unaffected in neuraminidase mutant strains or H. influenzae siaB mutants lacking sialylated matrix [18], which would seem inconsistent with a specific role for sialic acid in this phenotype. It may be that H. influenzae plays a more generic role, such as enhanced retention of pneumococci on a surface with an established biofilm matrix scaffold. Based on our results, we conclude that enhancement of pneumococcal persistence within the biofilm phase on mucosal surfaces can delay or even ablate the progression to systemic disease. It is important to note that in contrast to the systemic infections observed in most animal model systems, the majority of pneumococcal infections in patients are localized mucosal infections. It is clear from many studies that particular host immunodeficiencies (most notably, complement deficiencies) can predispose to systemic infection with pneumococci and other bacteria [30, 31]. In light of the results of this study, it may be important to consider the possibility that systemic pneumococcal infection results not only from host susceptibility but also from infection and colonization by a bacterial population in which pneumococci predominate. It is equally important to consider the possibility that elimination of one or more components of the nasopharyngeal microbiota by vaccination or therapy may have unforeseen sequelae, not only in terms of opening host niches for colonization but also, potentially, by changing the course of infection by opportunists within this population.
Supplementary Material
Supplemental Table
Supplemental Table 1
Supplemental Figure
Three-dimensional imaging of polymicrobial biofilms recovered from infected chinchillas. Biofilms were excised from the middle-ears of infected chinchillas at varying times (7 d, 14 d, 21 d) post-infection, fixed and cryosectioned as detailed in Methods section . Images of biofilms were constructed from stacked Z-series images collected by confocal laser scanning microscopy.
A. In vivo biofilm 7 d post-infection
B. In vivo biofilm 14 d post-infection
C. In vivo biofilm 21 d post-infection
ACKNOWLEDGEMENTS
The authors gratefully acknowledge expert technical assistance of Gayle Foster, David Ornelles, and Ken Grant. Drs. Karen Haas and Sean Reid provided helpful feedback on this work and comments on the manuscript. Monoclonal antibody recognizing pneumococcal type 4 polysaccharide was generously provided by Dr. Moon Nahm via the UAB Bacterial Respiratory Pathogen Reference Laboratory.
Financial support:
National Institutes of Health, National Institute of Deafness and Other Communication Disorders (grant DC10051 to W.E.S.) and National Institutes of Health, National Institute of Allergy and Infectious Diseases (training grant T32AI07401 to K.E.D.W.).
Footnotes
Potential conflicts of interest: Authors report no relevant conflicts of interest.
REFERENCES
- 1.Paradise JL, Rockette HE, Colborn DK, Bernard BS, Smith CG, Kurs-Lasky M, Janosky JE. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997;99:318–333. doi: 10.1542/peds.99.3.318. [DOI] [PubMed] [Google Scholar]
- 2.Pichichero ME, Casey JR. Evolving microbiology and molecular epidemiology of acute otitis media in the pneumococcal conjugate vaccine era. Pediatr Infect Dis J. 2007;26:S12–16. doi: 10.1097/INF.0b013e318154b25d. [DOI] [PubMed] [Google Scholar]
- 3.Bakaletz LO. Bacterial biofilms in otitis media: evidence and relevance. Pediatr Infect Dis J. 2007;26:S17–19. doi: 10.1097/INF.0b013e318154b273. [DOI] [PubMed] [Google Scholar]
- 4.Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle ear mucosa of children with chronic otitis media. JAMA. 2006;296:202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong W, Juneau R, Pang B, Swords WE. Survival of bacterial biofilms within neutrophil extracellular traps promotes nontypeable Haemophilus influenzae persistence in the chinchilla model for otitis media. J Innate Immun. 2009;1:215–224. doi: 10.1159/000205937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis. 2008;14:1584–1591. doi: 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratner AJ, Aguilar JL, Shchepetov M, Lysenko ES, Weiser JN. Nod1 mediates cytoplasmic sensing of combinations of extracellular bacteria. Cell Microbiol. 2007;9:1343–1351. doi: 10.1111/j.1462-5822.2006.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lysenko ES, Ratner AJ, Nelson AL, Weiser JN. The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces. PLoS Pathog. 2005;1:e1. doi: 10.1371/journal.ppat.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lysenko ES, Clarke TB, Shchepetov M, et al. Nod1 signaling overcomes resistance of S. pneumoniae to opsonophagocytic killing. PLoS Pathog. 2007;3:e118. doi: 10.1371/journal.ppat.0030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tettelin H, Nelson KE, Paulsen IT, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 11.Reid SD, Hong W, Dew KE, et al. Streptococcus pneumoniae forms surface-attached communities in the middle ear of experimentally infected chinchillas. J Infect Dis. 2009;199:786–794. doi: 10.1086/597042. [DOI] [PubMed] [Google Scholar]
- 12.Harrison A, Dyer DW, Gillaspy A, et al. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J Bacteriol. 2005;187:4627–4636. doi: 10.1128/JB.187.13.4627-4636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong W, Mason K, Jurcisek JA, Novotny LA, Bakaletz LO, Swords WE. Phosphorylcholine decreases early inflammation and promotes the establishment of stable biofilm communities of nontypeable Haemophilus influenzae strain 86-028NP in a chinchilla model of otitis media. Infect Immun. 2007;75:958–965. doi: 10.1128/IAI.01691-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong W, Pang B, West-Barnette S, Swords WE. Phosphorylcholine expression by nontypeable Haemophilus influenzae correlates with maturation of biofilm communities in vitro and in vivo. J Bacteriol. 2007;189:8300–8307. doi: 10.1128/JB.00532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jurcisek JA, Greiner L, Watanabe H, Zaleski A, Apicella MA, Bakaletz LO. Role of sialic acid and complex carbohydrate biosynthesis in biofilm formation by nontypeable Haemophilus influenzae in the chinchilla middle ear. Infect Immun. 2005;73:3210–3218. doi: 10.1128/IAI.73.6.3210-3218.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurcisek JA, Bookwalter J, Baker B, et al. The PilA protein of nontypeable Haemophilus influenzae plays a role in biofilm formation, adherence to epithelial cells and colonization of the mammalian upper respiratory tract. Mol Microbiol. 2007;65:1288–1299. doi: 10.1111/j.1365-2958.2007.05864.x. [DOI] [PubMed] [Google Scholar]
- 17.Jurcisek JA, Bakaletz LO. Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J Bacteriol. 2007;189:3868–3875. doi: 10.1128/JB.01935-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swords WE, Moore ML, Godzicki L, Bukofzer G, Mitten MJ, VonCannon J. Sialylation of lipooligosaccharides promotes biofilm formation by nontypeable Haemophilus influenzae. Infect Immun. 2004;72:106–113. doi: 10.1128/IAI.72.1.106-113.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morse SA. Purine metabolism in Neisseria gonorrhoeae: The requirement for hypoxanthine. Can J Microbiol. 1980;26:13–20. doi: 10.1139/m80-003. [DOI] [PubMed] [Google Scholar]
- 20.Weiser JN, Austrian R, Sreenivasan PK, Masure HR. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun. 1994;62:2582–2589. doi: 10.1128/iai.62.6.2582-2589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cundell DR, Weiser JN, Shen J, Young A, Tuomanen EI. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect Immun. 1995;63:757–761. doi: 10.1128/iai.63.3.757-761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allegrucci M, Sauer K. Characterization of colony morphology variants isolated from Streptococcus pneumoniae biofilms. J Bacteriol. 2007;189:2030–2038. doi: 10.1128/JB.01369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allegrucci M, Sauer K. Formation of Streptococcus pneumoniae non-phase-variable colony variants is due to increased mutation frequency present under biofilm growth conditions. J Bacteriol. 2008;190:6330–6339. doi: 10.1128/JB.00707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allegrucci M, Hu FZ, Shen K, et al. Phenotypic characterization of Streptococcus pneumoniae biofilm development. J Bacteriol. 2006;188:2325–2335. doi: 10.1128/JB.188.7.2325-2335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McEllistrem MC, Ransford JV, Khan SA. Characterization of in vitro biofilm-associated pneumococcal phase variants of a clinically relevant serotype 3 clone. J Clin Microbiol. 2007;45:97–101. doi: 10.1128/JCM.01658-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oggioni MR, Trappetti C, Kadioglu A, et al. Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Mol Microbiol. 2006;61:1196–1210. doi: 10.1111/j.1365-2958.2006.05310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greiner L, Watanabe H, Phillips NJ, et al. Nontypeable Haemophilus influenzae strain 2019 produces a biofilm containing N-acetylneuraminic acid that may mimic sialylated O-linked glycans. Infect Immun. 2004;72:4249–4260. doi: 10.1128/IAI.72.7.4249-4260.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker D, Soong G, Planet P, Brower J, Ratner AJ, Prince A. The NanA neuraminidase of Streptococcus pneumoniae is involved in biofilm formation. Infect Immun. 2009;77:3722–3730. doi: 10.1128/IAI.00228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trappetti C, Kadioglu A, Carter M, et al. Sialic acid: a preventable signal for pneumococcal biofilm formation, colonization, and invasion of the host. J Infect Dis. 2009;199:1497–1505. doi: 10.1086/598483. [DOI] [PubMed] [Google Scholar]
- 30.Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–395. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman SL, Vogler LB, Feigin RD, Johnston RB., Jr Recurrent septicemia associated with congenital deficiency of C2 and partial deficiency of factor B and the alternative complement pathway. N Engl J Med. 1978;299:290–292. doi: 10.1056/NEJM197808102990606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table
Supplemental Table 1
Supplemental Figure
Three-dimensional imaging of polymicrobial biofilms recovered from infected chinchillas. Biofilms were excised from the middle-ears of infected chinchillas at varying times (7 d, 14 d, 21 d) post-infection, fixed and cryosectioned as detailed in Methods section . Images of biofilms were constructed from stacked Z-series images collected by confocal laser scanning microscopy.
A. In vivo biofilm 7 d post-infection
B. In vivo biofilm 14 d post-infection
C. In vivo biofilm 21 d post-infection