Abstract
BACKGROUND
Emerging evidence has strongly implicated hereditary determinants for atrial fibrillation (AF). Loss-of-function mutations in KCNA5 encoding the ultra-rapid delayed rectifier potassium current (IKur) have been identified in AF families.
OBJECTIVE
Our study sought to present the clinical and biophysical phenotypes in a KCNQ5 mutation with deletion of 11 amino acids in the N-terminus of the protein, which was identified in patients with lone AF.
METHODS
Patients with AF, confirmed by ECG, were prospectively enrolled in the Vanderbilt AF Registry, which comprises clinical and genetic databases. A KCNQ5 mutation was generated by mutagenesis for the electrophysiology characterization.
RESULTS
We identified a novel 33-bp coding region deletion in 2 Caucasian probands. One proband was part of a kindred that included 4 other members with AF, and all were mutation carriers. The mutation results in deletion of 11 amino acids in the N-terminus of the protein, a proline-rich region as a binding site for Src homology 3 (SH3) domains associated with Src-family protein tyrosine kinase (TK) pathway. In transfected cells, the mutant caused ~60% decreased IKur vs wild-type (WT) (75±8 vs 180±15 pA/pF, P<0.01) and dominant-negative effect on WT current (105±10 pA/pF, P<0.01). Pretreatment with the Src inhibitor PP2 prevented v-Src TK from 90% suppressed WT current. By contrast, the mutant channel displayed no response to v-Src TK.
CONCLUSIONS
Our data implicate abnormal atrial repolarization control due to variable TK signaling as a mechanism in familial AF, and thereby suggest a role for modulation of this pathway in AF and its treatment.
Keywords: familial atrial fibrillation, KCNA5 channel, variants, genetics, tyrosine kinase
Introduction
Atrial fibrillation (AF) is a condition characterized by rapid, erratic electrical activation of the atrial myocardium, resulting in loss of effective contractility, an increased likelihood of clot formation and an increased risk of stroke. The rapid atrial contraction may be conducted to the ventricle exacerbating ventricular function and predisposing to heart failure. In addition, to causing substantial morbidity, AF is associated with increased mortality independent of coexisting risk factors.1 Prevalent in the aging population, AF is traditionally viewed as an acquired disorder occurring as a complication of cardiac and systemic diseases, such as hypertension, coronary artery disease, valvular heart disease, cardiomyopathies and thyroid disorders. However, recent recognition of familial aggregation has implicated a heritable basis for AF.2–7 A primary genetic defect is most likely in familial cases associated with early-onset idiopathic (“lone”) AF, a form of AF that is unassociated with structural heart disease.
A very frequent cellular accompaniment for induction of AF is shortening of the action potential duration (APD). Support for the concept that APD shortening plays a key role in the genesis of AF comes both from animal models8, 9 as well as reports of AF-associated gain-of-function mutations in long QT syndrome disease genes such as those encoding IKs (KCNQ1/KCNE2) 10, 11 or IK1 (KCNJ2) channels.12 Although such understanding provides a therapeutic rationale for prolonging the atrial refractory period in patients with AF, such an approach is not universally effective and can lead to proarrhythmia. Moreover, recent studies have identified loss-of-function mutations in KCNA5, the gene that encodes the ultrarapid delayed rectifier potassium current (IKur), in kindreds with AF.13, 14 The mutations were found to prolong the APD and trigger early after-depolarizations in human atrial myocytes, increasing susceptibility to stress-provoked triggered activity and AF.
IKur is an atrial-specific repolarizing current that has been proposed as a target for AF in humans.15 However, IKur is down-regulated in AF, and loss of function predisposes to AF.16 Here, we report a novel KCNA5 mutation in a kindred with familial AF. This indel disrupts a proline-rich motif involved in tyrosine kinase (TK) regulation of IKur,17 decreased unstimulated current, but renders the channel kinase-resistant. The data suggest a role of modulation of the TK signaling pathway in AF and its treatment.
Methods
Study subjects
Between November 2002 and October 2006, subjects with AF were prospectively enrolled in the Vanderbilt AF Registry, which comprises clinical and genetic databases.18 At enrollment a detailed medical and drug history was obtained in all patients. Patients were recruited from the Vanderbilt Cardiology and Arrhythmia Clinics, the emergency department, and in-patient services. Individuals age greater than 18 years with a diagnosis of AF, confirmed by electrocardiography (ECG), who presented because of symptoms or who were diagnosed during a routine physical examination were included in the AF Registry. Subjects were excluded if AF was diagnosed in the setting of recent cardiac surgery or were unable to give informed consent or report for follow-up. The study protocol was approved by the Institutional Review Board of Vanderbilt University and participants were enrolled following informed written consent.
Probands and their relatives were clinically classified by a consistently applied set of definitions. For the purposes of our study, AF was defined as replacement of sinus P waves by rapid oscillations or fibrillatory waves that varied in size, shape, and timing and were associated with an irregular ventricular response when atrioventricular conduction was intact. Documentation of AF on an ECG, rhythm strip, event recorder, or Holter monitor recording was necessary. Lone AF was defined as AF occurring in individuals at the age of ≤65 years without hypertension or overt structural heart disease as determined by clinical examination, ECG and echocardiography. An echocardiogram was obtained on all patients at time of enrollment into the Registry. The upper limits of normal for cardiac chamber dimension were based on age and body surface area.
Familial AF was defined as the presence of lone AF in one or more first degree relatives of the index case. Family history information was initially obtained from the medical record and was supplemented by a questionnaire detailing past medical history, family history, and clinical symptoms. For individuals with a positive family history, a more detailed pedigree was generated by history and review of medical records of relatives.
Mutation Screening
Whole blood was collected for genomic DNA extraction and analysis from all subjects. We resequenced high priority candidate ion channel genes (KCNQ1, KCNE1-5, KCNJ2, KCNA5, SCN5A [α- and β-subunits], L-type Ca2+) as well as non-ion channel candidates including GJA5, and NPPA (that encodes atrial natriuretic peptide [ANP]). Non-synonymous variants in sodium channel and potassium channels have been identified in ~10% of subjects. These cosegregate in extended kindreds (where they are available) and those studied to date show abnormal electrophysiology in vitro.
The coding and flanking intronic regions of KCNA5 were amplified by polymerase chain reaction (PCR) using primers designed to obtain fragments of appropriate size. PCR-amplified DNA fragments were analyzed using the RevealR Discovery System (based on temperature gradient capillary electrophoresis) to identify aberrant conformers, which were then directly sequenced.
DNA constructs and site-directed mutagenesis
Our sequence analysis identified a deletion of 11 amino acids (71-81del) at the N-terminus of KCNA5 (Kv1.5) in a familial AF kindred (Figure 1). This deletion construct was made by using site-directed mutagenesis. In brief, DNA encoding the Kv1.5 N-terminus and internal deletion were subcloned as NcoI fragments into pCI/CMV vector as appropriate. Sequence-confirmed polymerase chain reaction (PCR)-derived segments encoding N-terminal fragment of Kv1.5 (aa 1-82) was similarly subcloned into pCI/CMV vector. The deletion construct segment (Kv1.571-81del) was made by restriction digests or internal digestion followed by incubation with nuclease Bal31 for varying times. Digestions were stopped by addition of 20 mM EGTA, and the DNA was ligated and then recovered after transformation into Escherichia coli.
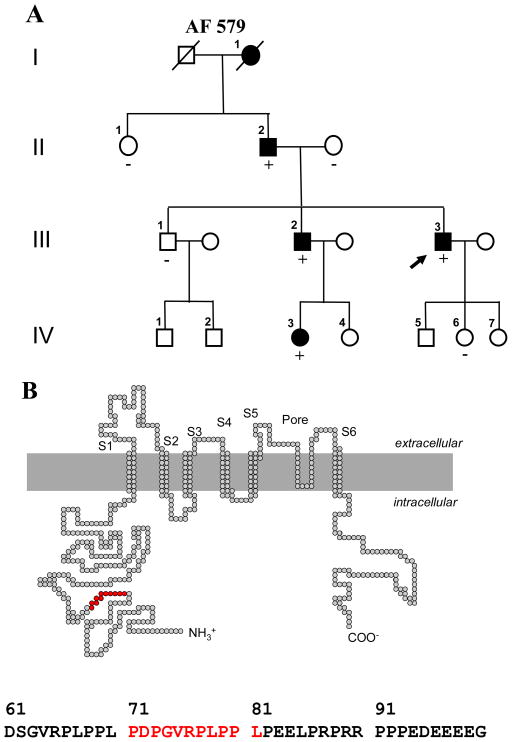
Figure 1.
A, family pedigree of Vanderbilt AF 579. Solid symbols denote AF, and open symbols individuals without a documented history of AF. Male subjects are shown as squares and female subjects as circles. The proband (arrow) and the presence (+) or absence (−) of the KCNA5 71-81del are indicated. B, location of the indel in the KCNA5 protein and a portion of the KCNA5 N-terminus amino acid showing the indel mutant sequence in red.
Tyrosine kinase (TK) isoform viral (v)-Src
V-Src plasmid was generously provided by Dr. Todd Holmes at New York University, NY. The Src TK isoform homology 3 (SH3) interacts with the proline-rich motif of human Kv1.5 channel to suppress IKur.17 The human Kv1.5 channel sequence contains one to two copies of the preferred Src SH3 domain binding motif RPLPXXP (Figure 1).17, 19 Collectively these findings demonstrate that there is an interaction between the Kv1.5 channel and Src in human heart tissue. In the present study, we identified a novel Kv1.5 mutation (Kv1.5 71-81del, a deletion of 11 amino acids at positions 71-81 in the N-terminus of the protein) in two AF patients and investigated biophysical properties of the deletion mutant and its interaction with viral (v)-Src.
FuGENE6-mediated channel expression and cell transfection
cDNAs for wild-type (WT) human KCNA5 (Kv1.5) and deletion mutant (Kv1.5 71-81del) were transiently transfected into Chinese hamster ovary (CHO) cells by using FuGENE6 methodology (Roche, USA). The cells lack endogenous outward currents and are thus suitable for K+ current studies. The cells were grown to confluence in F-12 nutrient mixture (HAM) medium (Invitrogen) supplemented with 10% horse serum at 37 oC. To generate Kv1.5 current heterologously, either WT Kv1.5 or the deletion plasmid (71-81del, 2 μg) was transiently transfected with and without v-Src (2 μg) into CHO cells by mixing with green fluorescence protein (GFP) and 12 μl of FuGENE6 (Roche) in 0.5 ml serum-free medium for 30 min, after which standard medium was restored for 48 hours in culture. Cells showing green fluorescence were chosen to identify successfully-transfected cells for the voltage-clamp electrophysiology analysis. For some acute experiments in which the Src inhibitor PP2 was used, v-Src (30 u/ml) was added into the intracellular pipette-filling solution for comparison. The cells were removed from the dish by brief trypsinization, and stored in standard medium for the experiments within the next 12 hours.
Electrophysiological study with whole-cell voltage clamping
The voltage-clamp studies were performed using the same methods as previously reported.20 To obtain current-voltage (I-V) relations for KCNQ5 current, cells were held at −80 mV. Activating currents were elicited with 500-ms depolarizing pulses from −60 to +60 mV in 10 mV steps, and tail currents were recorded upon return to −40 mV. Pulses were delivered every 15 sec. Current densities (in pA/pF) were obtained after normalization to cell surface area. The I-V relationships were determined by fitting data to the Boltzmann equation: I=1/{1+exp[(V1/2−Vt)/k]}, where I was for the current, V1/2 for the membrane potential at which 50% of the channels were activated, Vt for the testing potential, and k for the slope factor. The time constants for activation and deactivation were obtained by mono-exponentially fitting the currents to a Chebyshev equation with CLAMPFIT software. In addition, an action potential waveform was also used as the recording protocol to compare the currents. All electrophysiology experiments were conducted at 22–23°C.
Solutions and drugs
To record KCNA5-encoded current, the internal pipette filling solution contained (in mM): KCl 110, K4BAPTA 5, K2ATP 5, MgCl2 1 and HEPES 10. The solution was adjusted to pH 7.2 with KOH, yielding a final [K+]i of ~145 mM, as we have described previously.21, 22 The external solution was normal Tyrode’s, containing (in mM): NaCl 130, KCl 4, CaCl2 1.8, MgCl2 1, HEPES 10, and glucose 10, and was adjusted to pH 7.35 with NaOH. A potent inhibitor of the Src-family TK, PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo[3,4-d]pyrimidine), was purchased from Sigma-Aldrich Co. (St. Louis, MO).
Statistical analysis
Data are expressed as mean ± SEM. For comparisons among means of more than two groups, ANOVA was used, with post hoc pair-wise comparisons by Duncan’s test if significant differences among means were detected. If only two groups were being compared, Student’s t-test was used. A P-value <0.05 was considered statistically significant.
Results
Mutation screening
During the 5-year enrollment period, 610 patients with AF were approached and 580 (95%) subjects agreed to participate in the study. AF was diagnosed at a mean age of 50 ± 14 years. In the study cohort of 231 individuals with lone AF, screening for KCNA5 mutations in genomic DNA identified a novel 33-bp coding region deletion in 2 Caucasian probands with early onset lone AF. The variant results in deletion of 11 amino acids at positions 71-81 of the N-terminus (71-81del) (Figure 1B). The sequence change was not found in 200 patients with typical AF and 300 ethnically-matched population controls.
Family phenotype evaluation
The proband in Vanderbilt AF 579 (III-3) (Figure 1A) presented with symptoms of palpitations and racing heart beat as a teenager but was not diagnosed with AF until the age of 34. As he was experiencing less than one episode of AF a month, the proband was initially managed with flecainide and metoprolol with the ‘pill-in-the-pocket’ approach. However, over the last 4–5 years, he gradually began to experience more frequent symptomatic recurrences of paroxysmal AF and his symptoms are currently controlled with sotalol. A family history also showed that most of the affected family members developed lone AF at a relatively young age and most presented with symptomatic paroxysmal AF (Table 1). The probands’ and affected family members ECGs all showed typical coarse AF. Although the probands paternal grandmother’s (I-1) genotype is unknown, review of medical records does indicate that she presented with a stroke at the age of 52 years and was found to be in permanent AF. A second proband (Vanderbilt AF 142) presented with symptomatic paroxysmal lone AF at the age of 38. Although there does appear to be a family history of familial AF with two maternal aunts being diagnosed with early onset AF, no additional data is available for this kindred.
Table 1.
Clinical phenotype of affected individuals in Vanderbilt AF 579.
| Pedigree Number | Age at onset (yr) | Age at diagnosis (yr) | AF Type | Symptoms | Echo | LA size (mm) |
|---|---|---|---|---|---|---|
| I-1 | unknown | 52 | Permanent | Yes; stroke | LVH | 46 |
| II-2 | 48 | 54 | Persistent | Yes | Normal | 44 |
| III-2 | 48 | 56 | Paroxysmal | Yes | Normal | 42 |
| III-3 | 18 | 34 | Paroxysmal | Yes | Normal | 37 |
| IV-3 | 19 | 23 | Paroxysmal | Yes | Normal | 36 |
AF = atrial fibrillation; LA = left atrial.
KCNA5 deletion channels (71-81del) generate smaller current
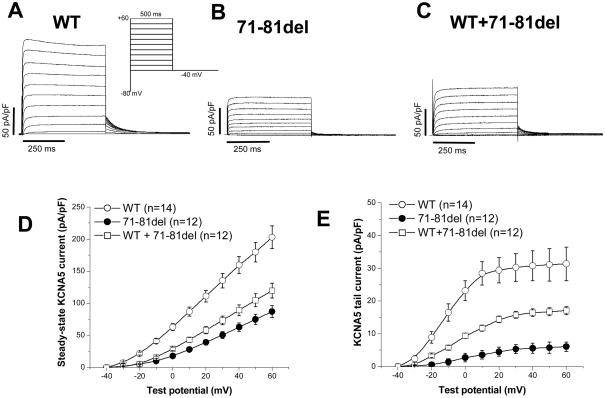
In transfected CHO cells, we found that the mutant (71-81del) channel resulted in ~60% decreased IKur compared to WT KCNA5 channels (Figure 2A/B). Co-expression of the 71-81del and WT channels at 1:1 ratio resulted in >50% less current than expected from WT experiments, i.e. a modest dominant negative effect (Figure 2C). The current-voltage relations in three groups of cells are summarized in Figure 2D for steady-state current and 2E for tail current. In addition, the deletion channel 71-81del caused a markedly positive shift of half-activation voltage (V1/2) by ~10 mV (P<0.01, Table 2). In addition, the deactivation phase of the KCNA5 current was markedly slowed in the mutant channel (P<0.01). However, the activation course of the KCNA5 current was not altered by the deletion channels.
Figure 2.
The 71-81del in the N-terminus of KCNA5 expressed a reduced current. Panels A, B and C show wild-type (WT), the 71-81del and the WT+71-81 del currents respectively with a dominant negative effect. Panels D and E summarize both steady-state and tail currents in the three groups.
Table 2.
The effects of the 71-81del on KCNA5 channel properties.
| N | Activating current (pA/pF, at +40 mV) | Tail current (pA/pF, at −40 mV) | V1/2 (mV) | τdeactivation (ms, at −40 mV) | |
|---|---|---|---|---|---|
| WT | 14 | 159.6±13.3 | 30.8±4.6 | −12.1±1.1 | 38.2±4.8 |
| 71-81del | 12 | 62.7±6.8a | 5.6±1.5a | 3.4±0.8a | 82.1±5.9a |
| 71-81del+WT | 12 | 89.2±8.4a,b | 16.4±1.2a,c | −2.1±0.6a,b | 57.3±7.1a,b |
Values are shown as mean ± SEM;
P<0.01 vs WT-1;
P<0.05 vs 71-81 del;
P<0.01 vs 71-81 del. The current was represented with 1-sec pulsing to +40 mV from a holding potential of −80 mV and the tail current was recorded upon return to −40 mV. The mid-points (V1/2) of the channel activation were obtained by fitting all tail data at various potentials.
The mutant-generated current was resistant to inhibition by TK isoform v-Src
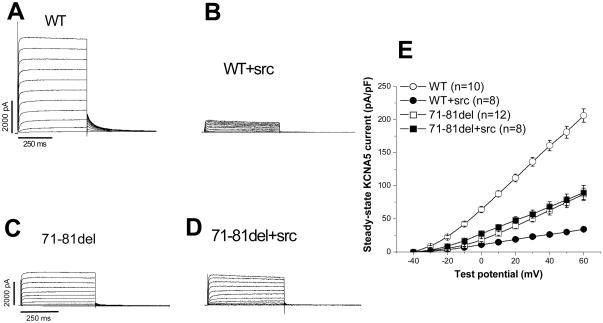
We next examined the effects of co-expressing v-Src with WT KCNA5 on IKur. As in previous studies,17 this resulted in striking suppression of WT KCNA5 current (Figure 3A/B): at +50 mV, the current amplitude was 29.8±2.8 pA/pF compared to 180.9±9.1 pA/pF in WT channel without v-Src coexpression (P<0.001, n=8–10 each). However, the 71-81del expressed current was resistant to v-Src suppression (Figure 3C/D): at +50 mV, the 71-81del current remained unchanged with and without v-Src (78.6±9.1 pA/pF vs. 74.9±8.1 pA/pF, p>0.05, n=8–12 each). These results are summarized in Figure 3E.
Figure 3.
A tyrosine kinase (TK) isoform v-Src coexpressed with KCNA5 suppressed WT current but had no effect on the mutant 71-81del expressed current. A, WT current; B, coexpression of v-Src with WT KCNA5 channel reduced the current; C, 71-81del expressed reduced the current; D, the 71-81del fail to respond to v-Src; E, current-voltage relationships in the four groups.
The Src-TK inhibitor PP2 prevented KCNA5 current suppression by v-Src
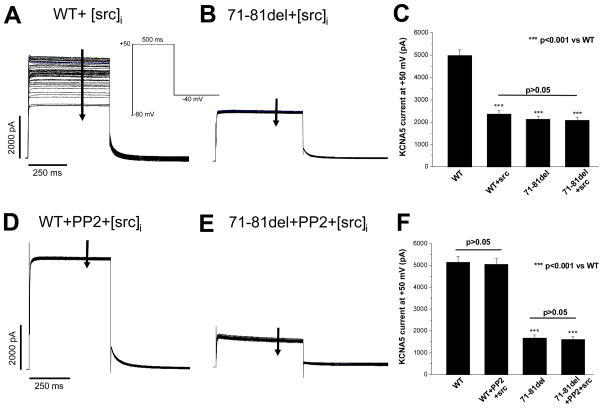
We then tested the hypothesis that a selective Src-TK inhibitor would negate the inhibitory effect of Src on WT KCNA5 channel, but have no effect on the mutant channel. To accomplish these experiments, we first demonstrated that acute exposure to v-Src in the pipette resulted in suppression of WT but not mutant channels. Figure 4A shows that adding v-Src (30 u/ml) to the intracellular pipette-filling solution rapidly reduced WT KCNA5 current during repetitive pulsing; however, this effect was not seen with the 71-81del mutant (Figure 4B). A summary of these data is presented in Figure 4C (n=6 each). These acute effects on KCNA5 channels by v-Src further support our results with chronic co-expression of v-Src with WT KCNA5 channels (Figure 3A/B).
Figure 4.
Suppression of KCNA5 current by Src is prevented by the selective Src inhibitor PP2 (10 μM). A, intracellular addition of v-Src (30 u/ml) progressively suppressed WT KCNA5 current elicited by repetitive pulses to +50 mV from −80 mV with the protocol shown. B, intracellular Src application did not affect the 71-81del expressed current. C, effects of intracellular Src application on WT and the 71-81del currents. D, pretreatment with PP2 (10 μM) for 30 minutes prevented the intracellular Src-induced suppression of WT current. E, intracellular Src addition did not alter the 71-81del expressed current. F, effects of intracellular Src application on WT and 71-81del currents in the presence PP2 pretreatment. The number of cells in each group was 6. Statistical differences among these groups are shown.
We then repeated this experiment after bath pretreatment for 30 minutes with the Src inhibitor PP2 at a concentration of 10 μM.23 PP2 alone had no effect on the KCNA5 current: at +50 mV, the current amplitudes at the end of a pulse were 5047±262 pA (without PP2) vs. 5093±259 pA (with PP2, P>0.05, n=6 each). With PP2 pretreatment however, the marked reduction in IKur after break-in to cells expressing WT channels seen in Figure 4A was absent (Figure 4D). Cells expressing the mutant channels displayed no effect of v-Src in the pipette (Figure 4B) and there was also no change with PP2 pretreatment (Figure 4E). The effects of intracellular v-Src application on WT and 71-81del currents in the presence PP2 pretreatment are summarized in Figure 4F (n=6 each).
Discussion
AF is the most common cardiac arrhythmia affecting ~2% of the US population and results in substantial morbidity and mortality. Evidence for the heritability of AF susceptibility has come from several sources including analysis of AF kindreds who exhibit the arrhythmia as a primary electrical disease, the analysis of AF presenting in the setting of another familial disease, and the analysis of genetic backgrounds that may predispose to AF.7 In this study, we have identified a novel KCNA5 mutation in a kindred with early-onset lone familial AF. This indel disrupts a proline-rich motif involved in TK regulation of IKur, reduces WT current but renders the channel kinase-resistant. Thereby, these changes might cause action potential shortening for the development of AF.
Modular binding domains such as SH3 are important mediators of specific protein-protein interactions between signaling proteins. SH3 domains bind to proline-rich regions in partner proteins.17 Several species with KCNA5 channel, including human, dog, and rabbit,24 contain one to two copies of the preferred Src SH3 domain binding motif RPLPXXP.19 In particular, KCNA5 channel contains two repeats of the sequence RPLPPLP between amino acid residues 65 and 82 of the channel protein.24 The novel indel identified in our AF cohort results in loss of one of the proline-rich binding motifs, thereby in turn preventing suppression of KCNA5 current by tyrosine phosphorylation of v-Src.
The reduction in IKur amplitude would be expected to prolong atrial action potentials as with the previously described KCNA5 AF-associated mutation.13 However, the results with TK stimulation raise a second possible mechanism. TK stimulation is a response to a variety of exogenous perturbations, such as atrial stretch.25, 26 Therefore, it may be that the physiologic response of IKur to such perturbations is marked current suppression (e.g. Figure 3B), and that the mutant fails to demonstrate this response. In this case, a perturbation that ordinarily resulted in atrial AP prolongation (due to reduced IKur) would be absent in the mutant, and AF due to AP shortening might be the result. Indeed, one possibility that our data suggest is that atrial cells are under a degree of tonic TK stimulation; in this case, the functional consequences of the 71-81del mutation would reflect increased current. One way to better define the mechanism of AF in this kindred is to perform the in vitro studies in HL-1 cells. However, the presence of 27, 28 prominent cardiac-like potassium channel currents similar to IKr and ITo in HL-1 cells would make interpretation of such studies challenging even if we were able to introduce exogenous KCNA5 constructs into these cells. Clearly further studies in myocytes and whole hearts will be required to sort out the role of TK modulation of IKur in response to exogenous stressors.
Most experimental models of AF, as well as clinical cases of AF, are associated with atrial APD shortening29 with premature atrial depolarization as the most common (> 90%) initiating event in the onset of paroxysmal AF.30 It is therefore no surprise that APD abbreviation by gain-of-function mutations in genes encoding IKs and IK1, increase the propensity for AF.10, 12, 31 In contrast, we have identified a novel KCNA5 indel that could function either as a loss- or gain-of-function mutation and results in TK-resistant IKur channels that generate larger current than WT with shorter atrial AP durations. Support for such mutations creating the substrate for AF comes from recent reports associating KCNA5 loss-of-function mutations with familial AF13, 14 and recent demonstration that IKur block likely abbreviates AP duration and effective refractory period in a canine AF model.32 Although shortening of the atrial AP duration would tend to argue against it, the possibility that AF in this kindred might represent atrial torsades de pointes should be considered as mutant KCNQ1 channels might prolong atrial repolarization and produce atrial torsades de pointes.33, 34
The KCNA5 mutation was absent in 200 patients with typical AF and 300 controls, in line with the reported limited variability in the coding region of this gene and ruling out this indel as a common polymorphism in the population at large. The association with AF susceptibility was underscored in vitro. First, the indel reduced IKur with a dominant negative effect. Second, the indel disrupted TK modulation by v-Src implicating a tonic level of activation of TK and suppression of IKur. Third, the electrophysiological substrate for AF in carriers of the indel mutation was recapitulated at the cellular level when the specific Src inhibitor PP2 prevented the intracellular Src-induced suppression of WT current underscoring thereby the requirement of operational KCNA5 channels for adequate repolarization reserve.
Studies of kindreds with AF suggest a genetic basis for the condition, and mutations in several cardiac potassium channel genes have been linked to familial AF.10, 12 Although specific mutations in the KCNQ1 gene have been observed in families with AF,10, 35 the role of such mutations in AF remains unclear as often such isolated or “private” mutations are in residues of unknown function, effects on channel conductance are variable and in most cases it may be difficult to discriminate rare polymorphisms of no functional significance and true mutations. However, the low prevalence of KCNQ1 mutations in large AF cohorts, including ours, suggests that mutations in this gene are not a major cause of AF.36, 37 In our AF kindred, there were 5 family members with AF, the KCNA5 indel cosegregated with AF and functional data provide strong support for the 71-81 indel being functional and disease-associated.
Several limitations of the present study warrant consideration. First, the AF kindred is small and thereby segregation analysis was limited. Family data for the second proband with the indel is not available. Second, no novel mutations were identified in the ethnically-defined controls. However, comprehensive resequencing of the population-based controls was not performed and this may condition interpretation of the results of this study. Although it is possible that additional novel KCNA5 mutations may have been identified, the association of the indel with AF is supported primarily by segregation analysis and the fact that the indel occurred in a highly conserved region of the KCNA5 protein and was associated with a functional phenotype.
In summary, we report a novel KCNA5 variant in a kindred with early-onset lone familial AF. This indel disrupts a proline-rich motif involved in TK modulation of IKur, reduces WT current but renders the channel kinase-resistant. These data implicate abnormal atrial potential control due to abnormal TK signaling as a mechanism in this familial form of AF, and thereby suggest a role for modulation of this pathway in AF and its treatment.
Acknowledgments
This work was supported in part by NIH awards (HL65962, HL49989 and HL92217) and the American Heart Association award 0940116N. Data from this study have been deposited at the Pharmacogenetics Knowledge Base (www.pharmGKB.org).
ABBREVIATIONS
- KCNA5 (Kv1.5)
the gene encoding cardiac ultra-rapidly activating delayed rectifier potassium current (IKur)
- AF
atrial fibrillation
- CHO
Chinese hamster ovary cells
- TK
tyrosine kinase
- v-Src
viral isoform of tyrosine kinase
Footnotes
Disclosures
None
References
- 1.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 2.Darbar D, Herron KJ, Ballew JD, Jahangir A, Gersh BJ, Shen WK, Hammill SC, Packer DL, Olson TM. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol. 2003;41:2185–2192. doi: 10.1016/s0735-1097(03)00465-0. [DOI] [PubMed] [Google Scholar]
- 3.Ellinor PT, Yoerger DM, Ruskin JN, Macrae CA. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005:1–6. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 4.Arnar DO, Thorvaldsson S, Manolio TA, Thorgeirsson G, Kristjansson K, Hakonarson H, Stefansson K. Familial aggregation of atrial fibrillation in Iceland. Eur Heart J. 2006;27:708–712. doi: 10.1093/eurheartj/ehi727. [DOI] [PubMed] [Google Scholar]
- 5.Darbar D, Kannankeril PJ, Donahue BS, Kucera G, Stubblefield T, Haines JL, George AL, Jr, Roden DM. Cardiac sodium channel (SCN5A) variants associated with atrial fibrillation. Circulation. 2008;117:1927–1935. doi: 10.1161/CIRCULATIONAHA.107.757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darbar D, Hardy A, Haines JL, Roden DM. Prolonged signal-averaged P-wave duration as an intermediate phenotype for familial atrial fibrillation. J Am Coll Cardiol. 2008;51:1083–1089. doi: 10.1016/j.jacc.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darbar D. Genetics of atrial fibrillation: Rare mutations, common polymorphisms, and clinical relevance. Heart Rhythm. 2008;5:483–486. doi: 10.1016/j.hrthm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92(7):1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 9.Van Wagoner DR. Electrophysiological remodeling in human atrial fibrillation. Pacing Clin Electrophysiol. 2003;26(7 Pt 2):1572–1575. doi: 10.1046/j.1460-9592.2003.t01-1-00234.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen YH, Xu SJ, Bendahhou S, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 11.Makiyama T, Akao M, Shizuta S, et al. A novel SCN5A gain-of-function mutation M1875T associated with familial atrial fibrillation. J Am Coll Cardiol. 2008;52(16):1326–1334. doi: 10.1016/j.jacc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Xia M, Jin Q, Bendahhou S, et al. A Kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem Biophys Res Commun. 2005;332:1012–1019. doi: 10.1016/j.bbrc.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 13.Olson TM, Alekseev AE, Liu XK, et al. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Li J, Lin X, et al. Novel KCNA5 loss-of-function mutations responsible for atrial fibrillation. J Hum Genet. 2009;3:3. doi: 10.1038/jhg.2009.26. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlich JR, Biliczki P, Hohnloser SH, Nattel S. Atrial-selective approaches for the treatment of atrial fibrillation. J Am Coll Cardiol. 2008;51:787–792. doi: 10.1016/j.jacc.2007.08.067. [DOI] [PubMed] [Google Scholar]
- 16.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 17.Holmes TC, Fadool DA, Ren R, Levitan IB. Association of Src tyrosine kinase with a human potassium channel mediated by SH3 domain. Science. 1996;274:2089–2091. doi: 10.1126/science.274.5295.2089. [DOI] [PubMed] [Google Scholar]
- 18.Darbar D, Motsinger AA, Ritchie MD, Gainer JV, Roden DM. Polymorphism modulates symptomatic response to antiarrhythmic drug therapy in patients with lone atrial fibrillation. Heart Rhythm. 2007;4:743–749. doi: 10.1016/j.hrthm.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rickles RJ, Botfield MC, Weng Z, Taylor JA, Green OM, Brugge JS, Zoller MJ. Identification of Src, Fyn, Lyn, PI3K and Abl SH3 domain ligands using phage display libraries. Embo J. 1994;13:5598–5604. doi: 10.1002/j.1460-2075.1994.tb06897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu K, Yang T, Viswanathan PC, Roden DM. New mechanism contributing to drug-induced arrhythmia: rescue of a misprocessed LQT3 mutant. Circulation. 2005;112:3239–3246. doi: 10.1161/CIRCULATIONAHA.105.564008. [DOI] [PubMed] [Google Scholar]
- 21.Yang T, Kanki H, Roden DM. Phosphorylation of the IKs channel complex inhibits drug block: novel mechanism underlying variable antiarrhythmic drug actions. Circulation. 2003;108:132–134. doi: 10.1161/01.CIR.0000082708.86266.B8. [DOI] [PubMed] [Google Scholar]
- 22.Kanki H, Kupershmidt S, Yang T, Wells S, Roden DM. A structural requirement for processing the cardiac K+ channel KCNQ1. J Biol Chem. 2004;279:33976–33983. doi: 10.1074/jbc.M404539200. [DOI] [PubMed] [Google Scholar]
- 23.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 24.Tamkun MM, Knoth KM, Walbridge JA, Kroemer H, Roden DM, Glover DM. Molecular cloning and characterization of two voltage-gated K+ channel cDNAs from human ventricle. Faseb J. 1991;5:331–337. doi: 10.1096/fasebj.5.3.2001794. [DOI] [PubMed] [Google Scholar]
- 25.Taskinen P, Toth M, Vuolteenaho O, Magga J, Ruskoaho H. Inhibition of atrial wall stretch-induced cardiac hormone secretion by lavendustin A, a potent tyrosine kinase inhibitor. Endocrinology. 1999;140:4198–4207. doi: 10.1210/endo.140.9.6967. [DOI] [PubMed] [Google Scholar]
- 26.Ueyama T, Kawashima S, Sakoda T, Rikitake Y, Ishida T, Kawai M, Yamashita T, Ishido S, Hotta H, Yokoyama M. Requirement of activation of the extracellular signal-regulated kinase cascade in myocardial cell hypertrophy. J Mol Cell Cardiol. 2000;32:947–960. doi: 10.1006/jmcc.2000.1135. [DOI] [PubMed] [Google Scholar]
- 27.Claycomb WC, Lanson NA, Jr, Stallworth BS, et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, Shen W, Rottman JN, Wikswo JP, Murray KT. Rapid stimulation causes electrical remodeling in cultured atrial myocytes. J Mol Cell Cardiol. 2005;38:299–308. doi: 10.1016/j.yjmcc.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415(6868):219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 30.Vincenti A, Brambilla R, Fumagalli MG, Merola R, Pedretti S. Onset mechanism of paroxysmal atrial fibrillation detected by ambulatory Holter monitoring. Europace. 2006;8:204–210. doi: 10.1093/europace/euj043. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Xia M, Jin Q, et al. Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am J Hum Genet. 2004;75:899–905. doi: 10.1086/425342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burashnikov A, Antzelevitch C. Can inhibition of IKur promote atrial fibrillation? Heart Rhythm. 2008;5:1304–1309. doi: 10.1016/j.hrthm.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satoh T, Zipes DP. Cesium-induced atrial tachycardia degenerating into atrial fibrillation in dogs: atrial torsades de pointes? J Cardiovasc Electrophysiol. 1998;9:970–975. doi: 10.1111/j.1540-8167.1998.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 34.Kirchhof P, Eckardt L, Franz MR, Monnig G, Loh P, Wedekind H, Schulze-Bahr E, Breithardt G, Haverkamp W. Prolonged atrial action potential durations and polymorphic atrial tachyarrhythmias in patients with long QT syndrome. J Cardiovasc Electrophysiol. 2003;14:1027–1033. doi: 10.1046/j.1540-8167.2003.03165.x. [DOI] [PubMed] [Google Scholar]
- 35.Otway R, Vandenberg JI, Guo G, et al. Stretch-sensitive KCNQ1 mutation A link between genetic and environmental factors in the pathogenesis of atrial fibrillation? J Am Coll Cardiol. 2007;49:578–586. doi: 10.1016/j.jacc.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 36.Ellinor PT, Moore RK, Patton KK, Ruskin JN, Pollak MR, Macrae CA. Mutations in the long QT gene, KCNQ1, are an uncommon cause of atrial fibrillation. Heart. 2004;90:1487–1488. doi: 10.1136/hrt.2003.027227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellinor PT, Petrov-Kondratov VI, Zakharova E, Nam EG, Macrae CA. Potassium Channel Gene Mutations Rarely Cause Atrial Fibrillation. BMC Med Genet. 2006;7:70. doi: 10.1186/1471-2350-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]