Abstract
Because fibromyalgia (FM) patients frequently report activity-dependent deep tissue pains, impulse input from painful body regions may be relevant for their musculoskeletal complaints. In addition, peripheral impulse input may induce and maintain thermal and mechanical hyperalgesia of FM patients. If so, activity and rest may alternately enhance and diminish intensity of FM pain. However, the effects of exercise on pain are ambiguous in studies of FM. Whereas exercise-only studies demonstrated increased pain and hyperalgesia during and after physical activity, some exercise studies that included rest periods resulted in decreased FM pain and increased function. To further clarify these effects, we examined the effects of alternating exercise with rest on clinical pain and thermal/mechanical hyperalgesia of 34 FM patients and 36 age-matched healthy controls (NC). Using an ergometer, all subjects performed arm exercise to exhaustion twice alternating with 15 min rest periods. Although strenuous muscle activity was reported as painful by most FM subjects, overall clinical pain consistently decreased during the rest periods. Additionally, FM subjects’ pain sensitivity to mechanical pressure decreased after each exercise and rest session.
Conclusion
Alternating strenuous exercise with brief rest periods not only decreased overall clinical pain of FM subjects but also their mechanical hyperalgesia. No prolonged worsening of overall FM pain and hyperalgesia occurred despite vigorous muscle activity. Our findings contribute further evidence that FM pain and hyperalgesia are at least partially maintained by muscle impulse input and that some types of exercises may be beneficial for FM.
Perspective
FM is a pain-amplification syndrome that depends at least in part on peripheral tissue impulse input. Whereas muscle activity increased overall pain, short rest periods produced analgesic effects.
Keywords: Analgesia, Fibromyalgia, Chronic pain, Nociception, Facilitation, Inhibition, Exercise
Introduction
Many individuals with FM adopt sedentary lifestyles 11 resulting in cardio-pulmonary fitness well below average levels 5,7,10. While underlying pain, fatigue, and depression likely contribute to such sedentary lifestyles and low levels of fitness, several exercise studies indicate that individuals with FM are able to perform not only low to moderate intensity aerobic muscle activities 15,25, flexibility and muscle-strengthening exercises 20,22, but also high level cardio-pulmonary activities 18,50. However, while exercise is recognized as an important part of FM management, not all of the clinically relevant and practically important aspects of exercise related symptom improvement have been identified. Exercise activates opioid and noradrenergic stress response systems in proportion to the magnitude and duration of exercise 1,42. These endogenous systems also respond to nociceptive input and reduce or limit the intensity of evoked pain sensations and related affective states 36. Accordingly, appropriate levels of exercise should also reduce pain sensitivity, and two reports support this hypothesis 21,23.
An important factor with respect to exercise-related pain sensitivity is the source of nociceptive input. For example, morphine more powerfully attenuates C-fiber mediated pain sensations than pain evoked by A-delta fiber input 12,39. Because exercise activates endogenous opioid 26 and other analgesic mechanisms 45, the present study predominantly evaluated effects of strenuous exercise on pain in FM and NC subjects that is predominantly mediated by C-fiber input.
Pain modulation depends on the amount and duration of exercise-induced stress and the status of pain modulatory systems activated by exercise. Whereas acute stressors have been shown to attenuate nociceptive reflexes 2,13,24,35 and may inhibit pain, chronic stress appears to increase pain sensitivity in some behavioral assays 41,52. Similarly, acute activation of endogenous opioid systems is clearly antinociceptive, but this system may become less effective under conditions of chronic pain 27,43. Therefore, activation of such systems by exercise could have different effects on nociceptive processing in NC and FM patients.
Although some aerobic exercise programs seem to attenuate clinical pain in FM patients 29,31,53, these reports stand in contrast to complaints by FM patients of chronic muscle pain that appears to worsen with physical exercise 32,33. The latter reports are consistent with recent observations that nociceptive input from skeletal muscle can contribute to the hyperalgesia of FM patients 47.
Many studies of FM patients have demonstrated the presence of mechanical and heat hyperalgesia 46,48,49. The present study directly compares the effects of strenuous exercise and rest not only on mechanical and heat hyperalgesia but also on clinical pain of FM patients. Using a classic double reversal design, we hypothesized that FM endogenous pain inhibitory systems will be effectively activated by repeated periods of strenuous exercise and this effect would become most evident during subsequent short rest periods. Such attenuation of clinical pain and hyperalgesia would not only provide evidence for effective pain inhibitory systems in FM patients but also address potential concern that exercise could result in prolonged enhancement of clinical pain.
Methods
Study Participants
36 normal control (NC) participants and 34 FM subjects were recruited from the local community and FM support groups. Informed consent was obtained from all subjects and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The University of Florida Institutional Review Board approved the procedures and protocol for this study. Prior to testing, all subjects underwent a clinical examination and were excluded from the study if they had abnormal findings unrelated to FM. Use of analgesics, including non-steroidal anti-inflammatory drugs (NSAID) and acetaminophen, was not allowed during the study. All subjects were asked to discontinue analgesics for the duration of five drug half-lives before testing, except narcotics which had to be stopped at least two weeks prior to study entry. Low dose muscle relaxants and/or amitriptyline (≤ 10 mg/day) were permissible during the study for treatment of FM-related insomnia.
Experimental Design
A parallel group classic reversal design (A-B-A-B) was used to evaluate the effects of 2 rest and 2 arm exercise periods on clinical pain as well as primary and secondary hyperalgesia of NC and FM subjects 3,4,19. The study protocol started with a 15 min rest period in a darkened, quiet room of ambient temperature followed by arm exercise to volitional exhaustion, intolerable pain, or 15 min duration. This sequence was once repeated in the same order (double reversal design). During the rest periods the subjects reclined on a comfortable lounge chair with foot elevation. The exercise testing was conducted in an adjacent room using a calibrated arm ergometer (Monark, Sweden).
Rest Period
During each of two 15 min rest periods the participants were asked every 5 minutes to rate their overall clinical pain, anxiety and fatigue using a mechanical visual analogue (VAS) scale 38.
Arm Exercise Testing
For exercise testing a friction braked arm ergometer was used. This ergometer was comprised of a Monark exercise bicycle with handle bars instead of foot pedals. The ergometer was stably mounted on a table with the handle bars at shoulder height. As with all Monark ergometers flywheel resistance could be adjusted with a friction brake. For this experiment all participants exercised with the friction brake setting of 1kp (= 60 watts). They were instructed to rotate the flywheel consistently at 60 revolutions per minute (rpm). To achieve this goal they were asked to synchronize each rotation of the flywheel with the pulse of a metronome set at 1 Hertz (Hz). Thus, with every pulse of the metronome one complete revolution of the flywheel had to be achieved by each participant. During each exercise period the participants were asked to rate the intensity of their clinical pain, anxiety, and fatigue once per minute using a mechanical VAS scale. In addition, they were instructed to rate their perceived exertion once per minute using the Borg scale (see below).
Borg Ratings of Perceived Exertion (RPE)
The Borg scale ranges from 6 to 20, where 6 means “no exertion at all” and 20 “maximal exertion” 6. Participants were asked to select the number from the Borg scale that best described their level of exertion. All participants were asked to rate their level of exertion during arm exercises. They were instructed that their rating of perceived exertion should reflect how heavy and strenuous the exercise felt, including all sensations and feelings of physical stress, effort, and fatigue. No single factor such as arm or shoulder pain or shortness of breath should dominate their exertion rating, instead they were asked to focus on their total feeling of exertion.
Ratings of Pain
Ratings of Experimental Pain
A 15 cm mechanical visual analogue scale (0–10) was used for ratings of experimental pain during mechanical and heat stimulation 38. The scale was anchored on the left with “no pain at all” and on the right with “the most intense pain imaginable”.
Ratings of Somatic Pain
The same mechanical VAS (0–10) was also used for ratings of somatic pain of all study participants before and after the experimental protocol. Although the NC subjects were required to be pain free at enrollment their somatic pain ratings were obtained before and after the testing session to capture possible new onset pains like back pain, headaches, etc.
Thermal probe
A Peltier thermode with a contact surface of 3 × 3 cm (9 cm2) (TSA-2001, Medoc Advanced Medical Systems, Ramat Yishai, Israel) was used for the heat stimuli. For heat pain testing the preheated probe was brought into firm contact with the skin of the volar forearm.
Heat Stimuli
Experimental pain was elicited by 10 sec heat pulses to the volar surface of the forearm. Three 46 deg C 10 sec heat pulses were applied to three different areas of the forearm separated by 10 cm, in counterbalanced order. At the end of each 10 sec heat stimulus the participants were immediately asked to rate the intensity of their experimental pain sensations using the VAS.
Pressure Pain Threshold Testing
Using a calibrated electronic algometer (Somedic AB, Horby, Sweden) pressure pain thresholds (PPT) were obtained at the trapezius muscle (TrapM) tender point (TP) of each shoulder, the web space between 1st and 2nd fingers (adductor pollicis muscle) of both hands, and midpoint of the tibialis anterior muscle of the leg. The rubber tip of the algometer was 1 cm in diameter. After the algometer was placed on the examined site pressure was gradually increased by 50 kPa/sec until pain threshold was reached. The subjects were instructed to push a hand-held button when the sensation changed from pressure to pain at the examination site. PPT testing was stopped at that moment and the results were electronically recorded. Three PPTs were obtained at each body site after each rest and exercise period.
Anxiety and Fatigue Ratings
The subjects rated their anxiety and fatigue several times during each rest period using a 15 cm mechanical VAS. The scale was limited on the right with “no anxiety at all” or no fatigue at all” and on the left with “most intense anxiety imaginable” or “most intense fatigue imaginable”.
Tender Point Testing
Nine paired TPs as defined by the ACR Criteria 54 and two control points (at the center of the right forearm and the right thumbnail) were assessed by a trained investigator using a Wagner Dolorimeter (Force Measurement, Greenwich, CT). The rubber tip of the Dolorimeter was 1 cm in diameter. The Dolorimeter was placed on the examination site, and pressure was gradually increased by 1kg/sec. The subjects were instructed to report when the sensation at the examination site changed from pressure to pain. Pressure testing was stopped at that moment and the result recorded as positive (1) if maximal pressure was ≤ 4 kg. If no pain was elicited at ≥ 4 kg the test result was recorded as negative (0).
Statistical Analysis
Statistical analyses were conducted using SPSS 16.0 software (SPSS, Inc., Chicago, IL). All group results were averaged (SD). A series of mixed model ANOVAs for repeated measures was utilized to test experimental pain ratings for differences within and between groups (Alpha level = .05). A priori hypotheses were evaluated by simple contrasts (two-tailed).
Results
Study participants
We recruited 36 middle-aged healthy pain-free female subjects [mean age (SD): 44.7 (11.0) years] and 34 female FM subjects [44.6 (12.2) years] using advertisements posted throughout the University of Florida, Gainesville. All FM subjects fulfilled the 1990 ACR Criteria for FM 54. NC and FM subjects were found to have 5.4 and 16.9 TP, respectively (p < .001). All participants were right handed including 61 Caucasian Non-Hispanics, 7 African-American and 2 Hispanic subjects.
Ratings of Somatic Pain in NC and FM Subjects
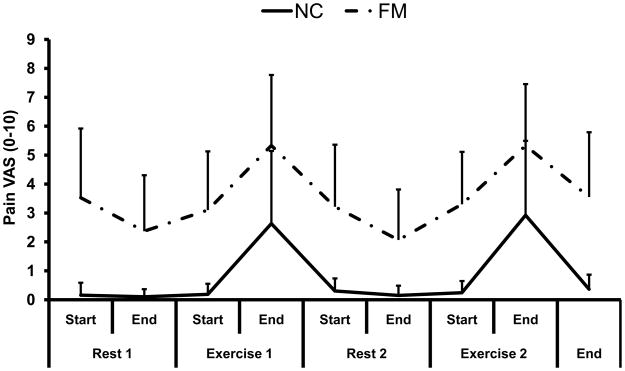
At the beginning of the study the healthy subjects reported only minimal somatic pain [.3 (.6) VAS units], whereas FM subjects’ average overall somatic pain was 4.0 (2.3) VAS units (p < .001). Using a double reversal design, overall pain of NC and FM subjects increased during arm exercises and decreased with rest as shown in Figure 1 (for more details, see below).
Figure 1.
Demonstration of a classic double reversal design as a useful tool for assessing the effects of arm exercise alternating with rest on clinical pain of 36 NC and 34 FM subjects. All subjects consistently reported increasing pain with exercise and attenuation of pain with rest.
Exercise Duration and Ratings of Perceived Exhaustion (RPE)
Exercise Duration
NC subjects were able to exercise for 8.4 (2.4) min and 7.8 (2.4) min during exercise period 1 and 2, respectively. FM subjects, however, exercised for only 5.8 (2.7) min and 5.1 (2.6) min, respectively. A repeated measure ANOVA with exercise period (2) as within factor and diagnostic group (2) as between factor showed a significant main effect for exercise period (F(62) = 12.5; p = .001) and diagnostic group (F(1,62) = 18.9; p < .001). The interaction effect of exercise period × diagnostic group was not significant (F(1,62) = .03; p > .05). Thus, NC subject were able to exercise slightly longer than FM. Although the duration of the second exercise period was slightly shorter for both groups, this difference was not statistically significant (p > .05).
RPE
NC and FM subjects were asked to perform arm exercises to exhaustion or until intolerable pain occurred. All subjects stopped because of exhaustion and not of pain, including all FM participants. Because there was no significant difference in RPE between the two exercise periods within each diagnostic group (all p > .05), we combined the RPE of each group. RPE of NC and FM subjects was 15.7 (3.2) and 16.6 (2.0) Borg units, respectively, and this difference was not statistically significant (p > .05).
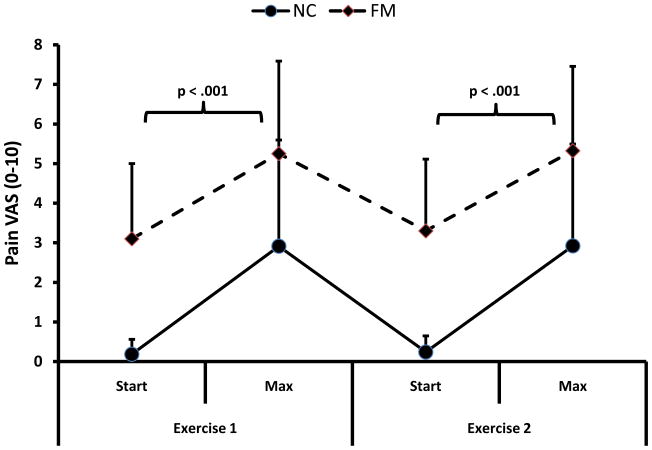
Effects of Arm Exercise on Pain
This analysis compares the pain ratings during 2 exercise periods in FM and NC subjects. During the 1st period of arm exercises the pain ratings of NC and FM subjects increased from .6 (.7) and 3.1 (2.2) VAS units to 2.8 (2.7) and 5.3 (2.3) VAS units, respectively. Pain ratings rapidly declined over 5 min to .3 (.4) and 3.4 (2.0) for NC and FM subject. During the 2nd period the pain ratings of NC and FM subjects increased from .7 (1.2) and 3.3 (1.8) VAS units to 2.9 (2.7) and 5.2 (2.2) VAS units (see Fig. 2). Within approximately 5 min they returned to baseline. A repeated measures ANOVA with time (2) and exercise period (2) as within and diagnostic group (2) as between subjects’ factor showed significant main effects for time (F(1,62) = 75.8 = p < .001) and diagnostic group (F(1,66) = 47.6; p < .001), indicating that overall pain significantly increased in NC and FM subjects during exercise. However, there was no significant interaction effect between time and diagnostic group noted (F(1,62) = .3; p > .05) demonstrating that the increase of exercise induced pain was similar for both diagnostic groups. The significant main effect of diagnostic group indicated that pain ratings of FM subjects were higher than NC. There was, however, no significant difference in pain ratings between exercise periods (F(1,62) = .5; p > .05) demonstrating that strenuous exercise alternating with rest was well tolerated. A correlational analysis was done to explore the impact of exercise duration on pain. The lack of significant associations between pain and exercise duration (p > .05), however, argues against this hypothesis and supports subjects’ reports that their exercise duration was limited by exhaustion and by pain.
Figure 2.
Average (SD) pain ratings of NC and FM subjects during 2 periods of arm exercise. Pain ratings of FM subjects were significantly higher than those of NC before and after each exercise period (p < .001). Arm exercises significantly increased pain ratings of NC and FM subjects (p < .001). However, the overall pain increase from arm exercise was not different between NC and FM groups (p > .05).
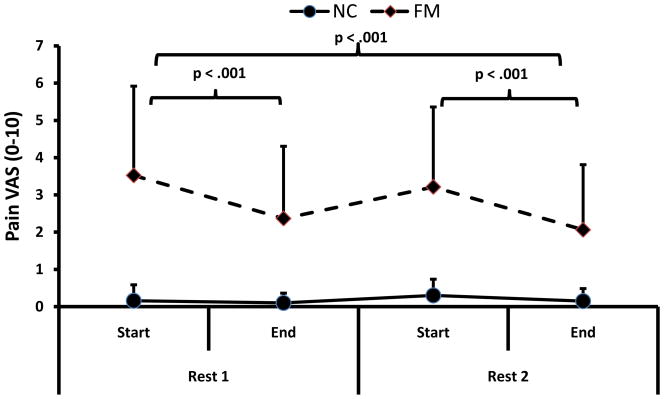
Effects of Rest on Pain
This analysis examines the effects of 2 rest periods on clinical pain in FM and NC subjects. During the 1st rest period (15 min) the pain ratings of NC and FM subjects changed from .2 (.4) and 3.5 (2.4) VAS units to .1 (.3) and 2.4 (2.4) VAS units, respectively (see Fig 3). Subsequently, rest alternated with arm exercises to exhaustion and then all subjects rested again for 15 min. During the 2nd rest period the pain ratings of NC and FM subjects changed from .3 (.4) and 3.2 (2.1) VAS units to .2 (.3) and 2.1 (1.7) VAS units, respectively. A repeated measures ANOVA with exercise periods (2) and time (2) as within and diagnostic group (2) as between subjects’ factor showed significant main effects for time (F(1,68) = 60.4; p < .001) and diagnostic group (F(1,68) = 70.2; p < .001). There was also a statistically significant interaction effect of time × diagnostic group noted (F(1,68) = 41.8; p < .001). These results showed that pain ratings of FM subjects were higher than those of NCs and that pain decreased faster for FM than NC subjects (However, the lesser effects of rest on pain of NC may be explained by floor effects). In addition, the magnitude of pain reductions was similar during each rest period in NC and FM subjects (F(1,68) = .7; p > .05). Thus, each rest period resulted in significant pain reductions of FM patients and this effect was neither significantly diminished nor enhanced by exercise.
Figure 3.
Average (SD) pain ratings of NC and FM subjects during 2 rest periods. Pain ratings of NC were minimal before the rest periods and significantly decreased during the rest periods (p < .001). FM subjects’ overall pain ratings were significantly higher than NCs’ before, during, and after the rest periods (p < .001). 15 min of rest significantly attenuated clinical pain ratings of FM subjects (p < .001) and this effect become stronger over time (p < .001). These beneficial effects of effects of rest were not attenuated by exercise.
Effects of Exercise and Rest on Mechanical and Thermal Hyperalgesia
Effects of Exercise on PPT
Average PPTs obtained at the TrapM of NC and FM subjects before and after exercise period 1 & 2 are shown in Table 1. A mixed model ANOVA with time (4) as between groups and diagnostic group (2) as within groups factors showed a main effect for diagnostic group (F(1,69) = 36.6; p < .001) and for time (F(3,207) = 5.1; p = .002). Simple contrasts showed a significant increase of PPT from the beginning of exercise period 1 to the end of exercise period 2 for FM and NC subjects (F(1,69) = 7.2; p = .009). These findings demonstrate significantly greater mechanical hyperalgesia for FM subjects than NC at any time during the exercise periods. In addition, FM subjects became significantly less hyperalgesic and NC subjects became hypoalgesic.
Table 1.
Pressure Pain Thresholds (PPT) of NC and FM subjects Before and After Arm Exercise 1 and 2
| PPT-Before Exercise 1(kPa) | SD | PPT-After Exercise 1(kPa) | SD | PPT-Before Exercise 2(kPa) | SD | PPT-After Exercise 2(kPa) | SD | |
|---|---|---|---|---|---|---|---|---|
| NC (n=35) | 381.4 | 161.8 | 397.8 | 180.2 | 366.3 | 160.6 | 397.3* | 177.2 |
| FM (n=36) | 154.0 | 70.7 | 187.0 | 106.7 | 198.3 | 115.6 | 207.8* | 130.2 |
p = .01
Effects on Heat Hyperalgesia
Average heat pain ratings obtained at the forearms of NC and FM subjects before and after exercise periods 1 & 2 are shown in Table 2. A mixed model ANOVA with time (4) as between groups and diagnostic group (2) as within groups factors showed a main effect for diagnostic group (F(1,55) = 20.8; p < .001), indicating that FM subjects’ heat pain ratings were greater than NC’s at any time during the exercise periods. There was no significant main effect detected for time (F(3,165) = 1.4; p > .05).
Table 2.
Heat-Pain Ratings (VAS) of NC and FM subjects Before and After Arm Exercise 1 and 2
| Heat Pain Ratings-Before Exercise 1(VAS) | SD | Heat Pain Ratings -After Exercise 1(VAS) | SD | Heat Pain Ratings -Before Exercise 2(VAS) | SD | Heat Pain Ratings -After Exercise 2(VAS) | SD | |
|---|---|---|---|---|---|---|---|---|
| NC (n=28) | 2.3 | 1.6 | 2.3* | 1.6 | 2.8 | 2.0 | 2.4* | 1.9 |
| FM (n=29) | 5.0 | 2.2 | 4.6* | 2.3 | 4.6 | 2.3 | 4.5* | 2.0 |
All subjects received 10 sec heat pulses (46 deg C) to the forearms in counterbalanced fashion
simple contrasts of Before Exercise and After Exercise heat pain ratings were non-significant (p > .05)
Ratings of Anxiety and Fatigue
Ratings of anxiety and fatigue were low for NC and moderate for FM subjects before each rest period (see Table 3 & 4). A series of mixed model ANOVA was used to analyze the effects of time and number of rest periods on anxiety and fatigue in NC and FM subjects.
Table 3.
Anxiety Ratings of NC and FM Subjects Before and After Rest 1 and 2
| Anxiety Ratings-Before Rest 1(VAS) | SD | Anxiety Ratings -After Rest 1(VAS) | SD | Anxiety Ratings -Before Rest 2(VAS) | SD | Anxiety Ratings -After Rest 2(VAS) | SD | |
|---|---|---|---|---|---|---|---|---|
| NC (n=29) | 0.3 | 0.9 | 0.2 | 0.7 | 0.2 | 0.7 | 0.2 | 0.8 |
| FM (n=36) | 1.9 | 2.2 | 1.5* | 2.0 | 1.5 | 1.3 | 1.2* | 1.7 |
significant main effect of time (p = .02).
Table 4.
Fatigue Ratings of NC and FM Subjects Before and After Rest 1 and 2
| Fatigue Ratings-Before Rest 1(VAS) | SD | Fatigue Ratings -After Rest 1(VAS) | SD | Fatigue Ratings -Before Rest 2(VAS) | SD | Fatigue Ratings -After Rest 2(VAS) | SD | |
|---|---|---|---|---|---|---|---|---|
| NC (n=29) | 1.2 | 1.8 | 0.9** | 1.5 | 2.8 | 2.0 | 2.4** | 1.9 |
| FM (n=36) | 4.4 | 2.5 | 3.3** | 2.4 | 4.2 | 1.3 | 3.5** | 2.5 |
significant main effect of time point (p < .001). The lack of significant time point x rest period interaction indicates that fatigue reductions were similar during each rest period.
Anxiety
The mixed model ANOVA of anxiety ratings (see Table 3) demonstrated significant main effects for individual time points (F(3,189) = 3.4; p = .02), rest period (F(1,63) = 5.8; p = .02), and group membership (F(1,63 = 22.6; p < .001), indicating that anxiety ratings significantly decreased over time in FM and NC subjects. The interaction effect of time points x group membership, however, was not significant (F(3,189) = 2.1, p > .06), showing that the reductions of anxiety during rest were similar over time for FM subjects and NC. The significant main effect for diagnostic group, however, demonstrated that anxiety ratings of FM subjects were higher than NC. The significant main effect for rest periods indicated that anxiety ratings were higher during the 1st compared to the 2nd rest-period. Although this effect was greater for FM subjects than NC (F(1,63) = 4.6; p = .04), this is most likely due to floor effects of NC ratings.
Fatigue
The mixed model ANOVA of fatigue ratings (see Table 4) showed significant main effects for time points (F(3,189) = 14.2; p < .001) and group membership (F(1,63 = 35.4; p < .001). These findings indicated that fatigue ratings were higher in FM than NC subjects and significantly decreased over time. The interaction effect of time points x group membership was also significant (F(1,63) = 6.7, p = .01), showing that reductions of fatigue during rest were greater for FM subjects than NC. The lack of significant main effects of rest period on fatigue ratings (F(1,63) = .3; p > .05) demonstrated that fatigue reductions during rest were similar during each rest period.
Discussion
Using a classic double reversal design 19 this study is the first to demonstrate that exercise consistently increases and rest reduces FM pain and fatigue. Moreover, some effects appear to be cumulative because large reductions in mechanical hyperalgesia occurred at the end of two brief exercise bouts alternating with 15 min rest periods. These sequential effects provide strong support for a role of peripheral impulse input in induction and maintenance of FM pain 47. In addition, these results encourage a therapeutic strategy of using rest as well as exercise and also provide evidence for effective exercise-induced anti-hyperalgesic mechanisms in FM. Although FM patients reported a short-lasting increase in overall pain intensity during arm exercises, their clinical pain always rapidly declined during the rest periods to below baseline levels allaying potential concerns that exercise might produce prolonged exacerbation of FM pain 28. Although the mean duration of each exercise period in this study was quite short (approx. 5 min) for FM and NC subjects alike, most likely due to low physical fitness of the participants, high physical exertion ratings (RPE) indicate excellent efforts by all participants. Thus short periods of exercise alternating with rest seem to be an effective adjunctive therapy for FM symptoms.
Exercise and Pain
Over the last 20 years exercise has become an integral part of the nonpharmacological treatment of FM patients after patients randomized to 20 weeks of high-intensity exercise had greater improvements in fitness, tender point pain thresholds, and patient/physician global assessment ratings than patients randomized to flexibility training 30. The acceptance of high-intensity exercise by chronic pain patients, however, is limited because of its association with more activity-related pain than low intensity exercise 51 and due to high attrition rates (up to 67%) 34.
Four meta-analyses have examined the benefits of exercise for individuals with FM 8,9,17,40. Whereas most of these reviews examined the combined effects of exercise and other non-pharmacological interventions, only one meta-analysis focused exclusively on the effects of exercise 9. This meta-analysis limited its study to exercise interventions that met or exceeded established criteria for improving aerobic conditioning or strengthening. There was moderate quality evidence that short-term aerobic-only exercise provides medium effect-size improvements of global well-being and physical function. Self reported pain decreased in about half of the included high-quality studies but this effect was not significant. There was, however, limited evidence that strength-only exercise has a positive effect on pain, global well-being, physical function, tender points, and depression. Although exercise did not decrease self-reported pain in many exercise studies, it is important to note that by the end of short-term exercise programs, pain had also not increased.
Peripheral Impulse Input Dynamically Induces and Maintains Clinical FM Pain and Hyperalgesia
FM is characterized by widespread hyperalgesia to mechanical, thermal, chemical, and electrical stimuli 14,44. Despite convincing evidence for central sensitization of nociceptive pain pathways in FM, increasing support exists for the role of peripheral tissue impulse input in the initiation and maintenance of this chronic pain syndrome. A previous study tested the effects of trapezius muscle (TrapM) tender point injections with 1% lidocaine on local pain thresholds as well as on remote heat hyperalgesia at the forearm of FM patients 47. Both local TrapM hyperalgesia and remote heat hyperalgesia (forearm) were significantly reduced after muscle injections, emphasizing the important role of peripheral impulse input in maintaining central sensitization in FM. The present results add to this interpretation by showing that short periods of arm exercise temporarily increase clinical pain, emphasizing the important role of peripheral impulse input for FM hyperalgesia and pain. In this respect, the interaction between peripheral impulse input and central sensitization may be similar to other persistent pain conditions like irritable bowel syndrome 37 and complex regional pain syndrome 16.
Conclusions
Short bouts of strenuous arm exercise were rated as painful by FM patients while brief rest periods consistently decreased their overall clinical pain, fatigue, and mechanical hyperalgesia. The reversibility of these findings strongly argues against unspecific effects on pain and hyperalgesia, including time. Therefore, this type of exercise regimen appears to be beneficial for FM symptoms. Importantly, no prolonged worsening of overall FM pain, fatigue, and hyperalgesia occurred despite vigorous muscle activity. Increase in pain during exercise and decreased pain during rest contribute further evidence that FM pain is at least partially maintained by muscle impulse input.
Acknowledgments
This work was supported by NIH grants NS041670 and AR053541. The expert technical assistance of Amber M. Schwier is greatly appreciated.
Footnotes
None of the authors has a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angelopoulos TJ, Denys BG, Weikart C, Dasilva SG, Michael TJ, Robertson RJ. Endogenous opioids may modulate catecholamine secretion during high intensity exercise. Eur J Appl Physiol. 1995;70:195–199. doi: 10.1007/BF00238563. [DOI] [PubMed] [Google Scholar]
- 2.Baker AK, Hoffmann VL, Meert TF. Interactions of NMDA antagonists and an alpha 2 agonist with mu, delta and kappa opioids in an acute nociception assay. Acta Anaesthesiol Belg. 2002;53:203–212. [PubMed] [Google Scholar]
- 3.Barlow DH, Blanchard EB, Hayes SC, Epstein LH. Single-case designs and clinical biofeedback experimentation. Biofeedback Self Regul. 1977;2:221–239. doi: 10.1007/BF00998648. [DOI] [PubMed] [Google Scholar]
- 4.Barlow DH, Hersen M. Single-case experimental designs. Uses in applied clinical research. Arch Gen Psychiatry. 1973;29:319–325. doi: 10.1001/archpsyc.1973.04200030017003. [DOI] [PubMed] [Google Scholar]
- 5.Bennett RM, Clark SR, Goldberg L, Nelson D, Bonafede RP, Porter J, Specht D. Aerobic fitness in patients with fibrositis. A controlled study of respiratory gas exchange and 133xenon clearance from exercising muscle. Arthritis Rheum. 1989;32:454–460. doi: 10.1002/anr.1780320415. [DOI] [PubMed] [Google Scholar]
- 6.Borg G. Human Kinetics. Champaign, Ill.: 1998. Borg’s perceived exertion and pain scales. [Google Scholar]
- 7.Burckhardt CS, Clark SR, Padrick KP. Use of the modified Balke treadmill protocol for determining the aerobic capacity of women with fibromyalgia. Arthritis Care Res. 1989;2:165–167. doi: 10.1002/anr.1790020412. [DOI] [PubMed] [Google Scholar]
- 8.Busch A, Barber KAR, Overend TJ, Peloso PMJ, Schachter CL. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev. 2007;4 doi: 10.1002/14651858.CD003786.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busch AJ, Schachter CL, Overend TJ, Peloso PMJ, Barber KAR. Exercise for treating fibromyalgia: A systematic review. J Rheumatol. 2008;35:1130–1144. [PubMed] [Google Scholar]
- 10.Clark SR. Prescribing exercise for fibromyalgia patients. Arthritis Care Res. 1994;7:221–225. doi: 10.1002/art.1790070410. [DOI] [PubMed] [Google Scholar]
- 11.Clark SR, Burckhardt CS, O’Reilly CA, Bennett RM. Fitness characteristics and perceived exertion in women with fibromyalgia. J Musculoske Pain. 1993;1:191–197. [Google Scholar]
- 12.Cooper BY, Vierck CJ, Yeomans DC. Selective reduction of second pain sensations by systemic morphine in humans. Pain. 1986;24:93–116. doi: 10.1016/0304-3959(86)90030-8. [DOI] [PubMed] [Google Scholar]
- 13.De Kock M, Meert TF. Alpha 2-adrenoceptor agonists and stress-induced analgesia in rats: influence of stressors and methods of analysis. Pharmacol Biochem Behav. 1997;58:109–117. doi: 10.1016/s0091-3057(96)00462-5. [DOI] [PubMed] [Google Scholar]
- 14.Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48:1420–1429. doi: 10.1002/art.10893. [DOI] [PubMed] [Google Scholar]
- 15.Gowans SE, deHueck A, Voss S, Silaj A, Abbey SE, Reynolds WJ. Effect of a randomized, controlled trial of exercise on mood and physical function in individuals with fibromyalgia. Arthritis Care Res. 2001;45:519–529. doi: 10.1002/1529-0131(200112)45:6<519::aid-art377>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175–194. doi: 10.1016/0304-3959(92)90259-E. [DOI] [PubMed] [Google Scholar]
- 17.Hadhazy V. Mind and body therapy for fibromyalgia. Cochrane Database of Systematic Reviews. 2003;1:2003. doi: 10.1002/14651858.CD001980.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hakkinen A, Hakkinen K, Hannonen P, Alen M. Strength training induced adaptations in neuromuscular function of premenopausal women with fibromyalgia: comparison with healthy women. Ann Rheum Dis. 2001;60:21–26. doi: 10.1136/ard.60.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes SC. Single case experimental design and empirical clinical practice. J Consult Clin Psychol. 1981;49:193–211. doi: 10.1037//0022-006x.49.2.193. [DOI] [PubMed] [Google Scholar]
- 20.Isomeri R, Mikkelsson M, Latikka P, Kammonen K. Effects of amitrityline and cardiovascular fitness training on pain in patients with primary fibromyalgia. J Musculoskelet Pain. 1993;2:253–260. [Google Scholar]
- 21.Janal MN, Colt EW, Clark WC, Glusman M. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: effects of naloxone. Pain. 1984;19:13–25. doi: 10.1016/0304-3959(84)90061-7. [DOI] [PubMed] [Google Scholar]
- 22.Jones KD, Clark SR. Individualizing the exercise prescription for persons with fibromyalgia. Rheum Dis Clin North Am. 2002;28:419. doi: 10.1016/s0889-857x(01)00010-2. [DOI] [PubMed] [Google Scholar]
- 23.Kemppainen P, Pertovaara A, Huopaniemi T, Johansson G, Karonen SL. Modification of dental pain and cutaneous thermal sensitivity by physical exercise in man. Brain Res. 1985;360:33–40. doi: 10.1016/0006-8993(85)91217-x. [DOI] [PubMed] [Google Scholar]
- 24.King CD, Devine DP, Vierck CJ, Rodgers J, Yezierski RP. Differential effects of stress on escape and reflex responses to nociceptive thermal stimuli in the rat. Brain Res. 2003;987:214–222. doi: 10.1016/s0006-8993(03)03339-0. [DOI] [PubMed] [Google Scholar]
- 25.King SJ, Wessel J, Bhambhani Y, Sholter D, Maksymowych W. The effects of exercise and education, individually or combined, in women with fibromyalgia. J Rheumatol. 2002;29:2620–2627. [PubMed] [Google Scholar]
- 26.Koltyn KF. Analgesia following exercise: a review. Sports Med. 2000;29:85–98. doi: 10.2165/00007256-200029020-00002. [DOI] [PubMed] [Google Scholar]
- 27.Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 28.Maquet D, Croisier JL, Renard C, Crielaard JM. Muscle performance in patients with fibromyalgia. Joint Bone Spine. 2002;69:293–299. doi: 10.1016/s1297-319x(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 29.Martin L, Nutting A, MacIntosh BR, Edworthy SM, Butterwick D, Cook J. An exercise program in the treatment of fibromyalgia. J Rheumatol. 1996;23:1050–1053. [PubMed] [Google Scholar]
- 30.McCain GA, Bell DA, Mai FM, Halliday PD. A controlled study of the effects of a supervised cardiovascular fitness training program on the manifestations of primary fibromyalgia. Arthritis Rheum. 1988;31:1135–1141. doi: 10.1002/art.1780310908. [DOI] [PubMed] [Google Scholar]
- 31.Meiworm L, Jakob E, Walker UA, Peter HH, Keul J. Patients with fibromyalgia benefit from aerobic endurance exercise. Clin Rheumatol. 2000;19:253–257. doi: 10.1007/s100670070040. [DOI] [PubMed] [Google Scholar]
- 32.Mengshoel AM, Vollestad NK, Forre O. Pain and fatigue induced by exercise in fibromyalgia patients and sedentary healthy subjects. Clin Exp Rheumatol. 1995;13:477–482. [PubMed] [Google Scholar]
- 33.Miles MP, Clarkson PM. Exercise-induced muscle pain, soreness, and cramps. J Sports Med Phys Fitness. 1994;34:203–216. [PubMed] [Google Scholar]
- 34.Norregaard J, Lykkegaard JJ, Mehlsen J, DanneskioldSamsoe B. Exercise training in treatment of fibromyalgia. J Musculoskelet Pain. 1997;5:71–79. [Google Scholar]
- 35.Owens PC, Smith R. Opioid peptides in blood and cerebrospinal fluid during acute stress. Baillieres Clin Endocrinol Metab. 1987;1:415–437. doi: 10.1016/s0950-351x(87)80070-8. [DOI] [PubMed] [Google Scholar]
- 36.Peng YB, Lin Q, Willis WD. Involvement of alpha-2 adrenoceptors in the periaqueductal gray-induced inhibition of dorsal horn cell activity in rats. J Pharmacol Exp Ther. 1996;278:125–135. [PubMed] [Google Scholar]
- 37.Price DD, Craggs JG, Zhou QQ, Verne GN, Perlstein WM, Robinson ME. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: Evidence from human psychophysics, animal models, and neuroimaging. Neuroimage. 2009;47:995–1001. doi: 10.1016/j.neuroimage.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price DD, Harkins SW. Psychophysical approaches to pain measurement and assessment. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. New York: Guilford Press; 1992. pp. 114–134. [Google Scholar]
- 39.Price DD, Vander-Gruen A, Miller J, Rafii A, Price C. A psychophysical analysis of morphine analgesia. Pain. 1985;22:261–269. doi: 10.1016/0304-3959(85)90026-0. [DOI] [PubMed] [Google Scholar]
- 40.Rossy LA, Buckelew SP, Dorr N, Hagglund KJ, Thayer JF, McIntosh MJ, Hewett JE, Johnson JC. A meta-analysis of fibromyalgia treatment interventions. Ann Behav Med. 1999;21:180–191. doi: 10.1007/BF02908299. [DOI] [PubMed] [Google Scholar]
- 41.Satoh M, Kuraishi Y, Kawamura M. Effects of intrathecal antibodies to substance P, calcitonin gene-related peptide and galanin on repeated cold stress-induced hyperalgesia: comparison with carrageenan-induced hyperalgesia. Pain. 1992;49:273–278. doi: 10.1016/0304-3959(92)90151-Z. [DOI] [PubMed] [Google Scholar]
- 42.Schwarz L, Kindermann W. Changes in beta-endorphin levels in response to aerobic and anaerobic exercise. Sports Med. 1992;13:25–36. doi: 10.2165/00007256-199213010-00003. [DOI] [PubMed] [Google Scholar]
- 43.Sigurdsson A, Maixner W. Effects of experimental and clinical noxious counterirritants on pain perception. Pain. 1994;57:265–275. doi: 10.1016/0304-3959(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 44.Sorensen J, Graven-Nielsen T, Henriksson KG, Bengtsson M, Arendt-Nielsen L. Hyperexcitability in fibromyalgia. J Rheumatol. 1998;25:152–155. [PubMed] [Google Scholar]
- 45.Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. Exercise activates the endocannabinoid system. Neuroreport. 2003;14:2209–2211. doi: 10.1097/00001756-200312020-00015. [DOI] [PubMed] [Google Scholar]
- 46.Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain. 2008;12:1078–1089. doi: 10.1016/j.ejpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staud R, Nagel S, Robinson ME, Price DD. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo controlled trial. Pain. 2009;145:96–104. doi: 10.1016/j.pain.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007;8:893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Staud R, Spaeth M. Psychophysical and neurochemical abnormalities of pain processing in fibromyalgia. CNS Spectr. 2008;13:12–17. doi: 10.1017/s109285290002678x. [DOI] [PubMed] [Google Scholar]
- 50.Valkeinen H, Hakkinen A, Hannonen P, Hakkinen K, Alen M. Acute heavy-resistance exercise-induced pain and neuromuscular fatigue in elderly women with fibromyalgia and in healthy controls - Effects of strength training. Arthritis Rheum. 2006;54:1334–1339. doi: 10.1002/art.21751. [DOI] [PubMed] [Google Scholar]
- 51.van Santen M, Bolwijn P, Landewe R, Verstappen F, Bakker C, Hidding A, van der Heijde D, Houben H, van der Linden S. High or low intensity aerobic fitness training in fibromyalgia: Does it matter? J Rheumatol. 2002;29:582–587. [PubMed] [Google Scholar]
- 52.Vidal C, Jacob JJ. Stress hyperalgesia in rats: an experimental animal model of anxiogenic hyperalgesia in human. Life Sci. 1982;31:1241–1244. doi: 10.1016/0024-3205(82)90352-6. [DOI] [PubMed] [Google Scholar]
- 53.Wigers SH, Stiles TC, Vogel PA. Effects of aerobic exercise versus stress management treatment in fibromyalgia. A 4.5 year prospective study. Scand J Rheumatol. 1996;25:77–86. doi: 10.3109/03009749609069212. [DOI] [PubMed] [Google Scholar]
- 54.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]