Abstract
Introduction and Hypothesis
This study seeks to quantify differences in anterior vaginal wall prolapse during sequential Valsalva attempts on dynamic magnetic resonance imaging (MRI).
Methods
Subjects were taken from an ongoing case-control study evaluating anterior vaginal wall prolapse. Women with a prolapse whose leading edge extended ≥ 1cm beyond the hymenal ring were included (n=40). All subjects performed 3 maximal Valsalvas efforts while mid-sagittal dynamic MRI scans were obtained. Bladder descent between the first, second and third maximal Valsalva efforts were compared.
Results
Forty percent of women had a greater than 2cm increase in prolapse size from their first to third Valsalva attempt. 95% of women extended their prolapse further with a third Valsalva.
Conclusions
As is true during clinical examination, several attempts may be required to have maximal anterior compartment prolapse present during dynamic MRI of the pelvic floor.
Keywords: Magnetic Resonance Imaging, Pelvic Organ Prolapse, Valsalva
INTRODUCTION
Dynamic magnetic resonance imaging (MRI) allows visualization of both soft tissue and bony movement during maneuvers such as Valsalva. This has become an important tool in pelvic floor research because it allows the observation and quantification of changes in the relative positions of pelvic floor structures and has provided insight into possible disease mechanisms involved in pelvic organ prolapse [1,2]. However, just as with clinical assessment of prolapse using the pelvic organ support quantification (POP-Q) exam, the clinician or the researcher must be careful that the patient demonstrates the maximal extent of her prolapse during dynamic MRI.
Our group has extensive experience in pelvic floor research. Over the last 10 years we have performed over 1400 research MRI scans under strict protocols, including nearly 1200 dynamic MRI scans with women who also had exams with POP-Q measurements. We have employed various methods of coaching subjects on how to achieve their maximal prolapse size during dynamic MRI before reaching our current paradigm. During this process, we attempted to become proficient at coaching subjects to reproduce the maximal prolapse in the MRI scanner. The purpose of this project was to quantify the effect of multiple Valsalva attempts in producing maximal prolapse during MRI scans.
MATERIALS AND METHODS
This was a secondary analysis from an on-going IRB approved case-control study (1999-0395) evaluating anterior vaginal wall prolapse. Women were excluded from the parent study if they had previous surgery for prolapse, incontinence, genital anomalies, or were pregnant within the past year. Subjects for this analysis were enrolled between February 2007 and April 2008 and had a prolapse whose leading edge extended ≥ 1cm beyond the hymenal ring as determined using the Pelvic Organ Prolapse Quantification (POP-Q) [3] system on clinical exam (n=40). Subjects underwent pelvic examination and urodynamic testing by urogynecologists followed by magnetic resonance scans of the pelvis.
Subjects were instructed on how to perform a Valsalva maneuver at several time points prior to the dynamic MRI: 1) upon presentation for their clinical examination, subjects were shown a Power Point presentation on how to perform Valsalva maneuvers during the MRI sequences; 2) during the clinic exam, subjects were coached and practiced how to perform a Valsalva and 3) prior to the MRI sequence, subjects were again instructed in regards to the straining maneuvers to be performed during the examination. Finally, a study team member who had knowledge of the clinical POP-Q measurements results monitored each MRI scan to assure that the same extent of prolapse was achieved during imaging as was present on clinical exam.
MRI for these study subjects was performed on a 3 Tesla system (Philips Medical Systems, Best, The Netherlands) using a 6-channel cardiac phased array coil with the subject in the supine position with legs together and in a semiflexed position. For dynamic imaging, an image of the pelvis in the mid-sagittal plane was obtained approximately every 1.4 seconds for 30 seconds using a single-shot turbo spin-echo (SSTSE) sequence (TR: approx 1300ms, TE: 105ms, slice thickness: 6mm, field of view 34cm, matrix: 256 × 90, and 1 NSA (number of signals averaged)). A set of 20 successive images was acquired in 30 seconds during rest and graded Valsalva effort.
The operator instructed the subject to strain minimally for 5 seconds, moderately for 5 seconds, and maximally for 5 seconds. She was then instructed to breathe normally and relax for another 5 to 7 seconds before ending the acquisition. Usually 3 images were acquired at rest during suspended inspiration, 12 during the graded Valsalva effort and 5 during post-Valsalva relaxation and normal breathing. All subjects performed at least 3 maximal Valsalvas efforts during mid-sagittal dynamic MRI scans. The images were placed in a cine loop using RadPix (Version 3.15, Weadock Software, LLC, Ann Arbor, MI) so they could be viewed as a movie clip to evaluate the sequence. The maximal Valsalva image for each effort was selected for analysis by the primary author (JT) and reviewed by the second author (YH).
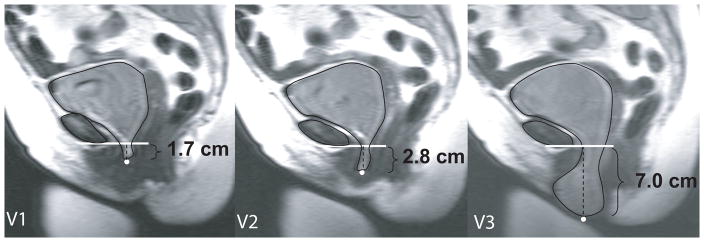
The most dependent bladder point was marked in each maximal Valsalva image. A line perpendicular to the body axis was placed at the inferior edge of the pubic bone. The most dependent point of the anterior vaginal wall was marked. This reference line was chosen for this research project rather than the sacrococcygeal inverior pubic point (SCIPP) line as it eliminated differences in measurements with more ventral or dorsal placement of the bladder point. The vertical distance between the horizontal reference line and the bladder point was then measured (Figure 1). Bladder descent between the first, second and third maximal Valsalva efforts was compared.
Figure 1. Increase in cystocele size with subsequent Valsalva efforts.
Demonstration that one (V1), or even two (V2), Valsalva attempts does not fully capture the extent of the prolapse. Data for this individual is seen in Figure 2 with white squares at reference points (red squares on color image).
RESULTS
A total of 40 women were enrolled. The mean age of participants was 59.7 ± 11.7 years. Eighty percent of the subjects were Caucasian. Mean prolapse size (POP-Q point Ba) was +3.2 ± 2.4 cm (range +1 to +12). Median vaginal parity was 2 (range 1–8) and eight women had a prior hysterectomy.
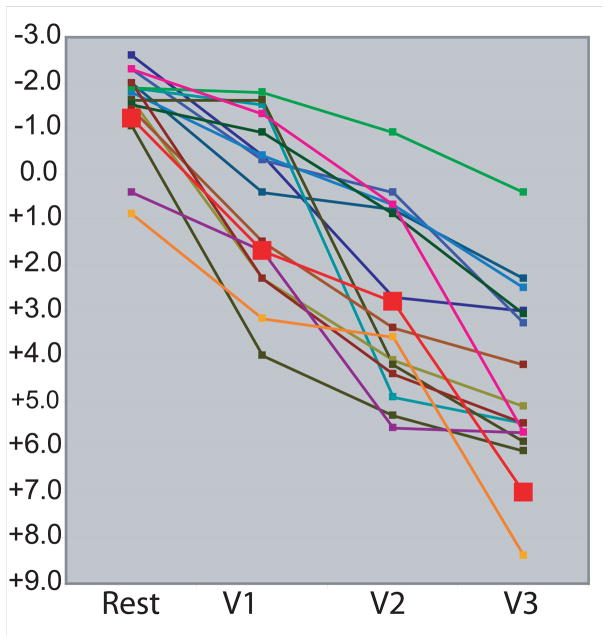
Anterior vaginal wall prolapse changed significantly (p<.001) with number of subsequent Valsalva efforts (Table 1). More women overall (95% vs 87.5%) extended their prolapse further with a third Valsalva attempt (2 cm) versus a second (1.1 cm). Of the women who extended their prolapse overall, 17.5% increased their prolapse more than 2 cm with a second attempt; this number increases to 40% when a third attempt was made. Figure 2 depicts, individually, the change that occurred when a third attempt was made in 40% of women. On dynamic MRI, this increase in cystocele size can be seen with subsequent Valsalva maneuvers in the exemplar of Figure 1. Those with smaller prolapse (+1 to +3) were less likely to have more than a 2 cm difference than those with a prolapse of +4 or greater (18% vs. 67%) (Table 2).
Table 1.
Change in Cyctocele Size with Subsequent Valsalva attempts (n=40)
| V = Maximum Valsalva | V1 to V2 | V1 to V3 |
|---|---|---|
| Mean increase in size overall | 1.1cm ±1.5 | 2.0cm ± 2.1 |
| Range of change | −.4cm–6.4cm | −.1cm–7.5cm |
| Overall Women with size increase | 87.5% (35) | 95% (38) |
| ≥ 1cm | 37.5% (15) | 52.5% (21) |
| Mean increase in size | 2.4cm | 3.4cm |
| ≥ 2cm | 17.5% (7) | 40% (16) |
| Mean increase in size | 3.6cm | 4.0cm |
Figure 2. Individual progression of prolapse in women with change of at least 2 cm.
The progression of prolapse from rest through each maximum Valsalva attempt (V1, V2, V3) for change ≥ 2cm. Prolapse progression from Figure 1 represented by bold black line with white boxes (red boxes on color image). “0” represents the level of the inferior edge of the pubic bone which is the solid line in Figure 1.
Table 2.
Most dramatic change associated with prolapse size (≥ 2 cm change by prolapse size)
| BA | Change ≥ 2 cm | Total | Percent |
|---|---|---|---|
| 1 | 2 | 14 | 12% |
| 2 | 1 | 5 | 20% |
| 3 | 1 | 3 | 33% |
| 4 | 5 | 8 | 63% |
| 5 | 5 | 6 | 83% |
| 6 | 1 | 2 | 50% |
| 9 | 1 | 1 | 100% |
| 12 | 0 | 1 | 0% |
| Total | 16 | 40 | 40% |
DISCUSSION
In this study of women with anterior vaginal wall prolapse that is at least 1 cm below the hymenal ring, we found that 40% of women have at least a 2 cm increase in prolapse size on dynamic MRI when the Valsalva is repeated 3 times. As is true during clinical pelvic examination, it often takes multiple efforts to have a prolapse present at its maximal extent during dynamic MRI studies. These differences occurred in women who had been instructed on how to perform a proper Valsalva and who had demonstrated their ability to do so during pelvic examination, indicating that this is likely not a result of women not knowing how to effectively Valsalva. In order to minimize the discrepancy between clinical exam and MRI one attempt is often not sufficient to attain maximum prolapse size. Not surprisingly, this effect is most marked in large prolapses (Table 2). With smaller prolapse (Bp 1cm below the hymen), for example, the difference between subsequent Valsalva maneuvers may only be 1 cm if the first attempt produces a cystocele that would be at the hymen on POP-Q exam. On the other hand, a woman with a cystocele that is 5 cm below the hymen may have a much larger difference.
Obtaining dynamic MR images which show the maximal development of prolapse during Valsalva has important research applications. MRI studies have been used to examine various mechanisms of prolapse [1,2,4,5] as well as to develop biomechanical models of prolapse [6]. Capitalizing on the power of MRI to study prolapse depends on images being made that accurately represent a clinical situation. For example, Cortes [7] found that 37% of women were “overdiagnosed” with prolapse clinically and 29% were under-diagnosed when compared to MRI. The authors suggested that these differences may have been the result of either patient mobility restrictions or the result of organ competition. It is unclear, however, how many Valsalva attempts were made by the patients in that study. If multiple Valsalvas had been performed, this discrepancy may have been minimized. In our experience, knowledge of the maximal prolapse size reached during clinical examination by the individuals conducting the MRI study is also critical in determining if an adequate MRI study has been accomplished. Even though the reference points used for clinical POP-Q (hymenal ring) and MRI (pubic axis line) differ, having this knowledge allows an experienced examiner to determine if maximal prolapse is achieved in the MRI scanner. A coordinated plan of coaching techniques and assessment of whether the prolapse is fully developed has substantially improved our ability to capture full prolapse size during dynamic MRI scans and thus, assuring us that an adequate study is achieved.
There are several different elements involved in consistent reproducibility of pelvic floor measurements. One is the acquisition of similar images during repeated examinations and the second is inter-rater reliability. The present study addresses the first of these issues. Earlier work addresses inter-rater and intra-rater reliability in the same images and is important in applying the technique on a wider research and clinical basis [8]. When different examiners evaluate the same images it is possible to obtain high inter-rater reliability with certain landmarks. For example, consistent measures of levator plate angle (r = 0.90), levator hiatus (r = 0.97), and perineal body location (r = 0.93) can be obtained [4]. Other measures may be more challenging; for example, the intraclass correlation coefficients (ICC) for soft tissue parameters such as hiatus length have been documented to range from 0.24 to 0.78 [9]. There are many factors that influence the development of prolapse during examination such as patient inhibitions about passing gas or leaking urine and the degree of filling of bladder and bowel. Since women experience symptoms throughout the day, arbitrarily choosing one state of visceral filling may not have advantages over evaluating the prolapse in it’s natural, unadulterated state.
A variety of factors such as differences in imaging and measurement techniques may account for the discrepancy of these findings to those of our group. Clear and consistent landmark and reference point definitions (e.g. whether to use the inner or outer side of the periosteum as a marker) is important to achieve consistency. However, despite this, the fact that highly trained examiners may have difficulty in achieving reproducible findings suggests that more work needs to be done to improve the reliability of dynamic MRI before it can be more widely applied. At present, there are several different reference lines in use. In a recent review, Broekhuis has observed that there is no consensus regarding which line to use [11]. Further investigations into the variability that using different reference lines is needed. By placing the reference line at the pubic symphysis, if the pelvic moves upward or downward in the scanner, this is taken into account although rotational movement is not.” In addition, research concerning the effects of intra abdominal pressure and bladder volume on prolapse development are needed. Also investigating how MRI findings relate to findings on ultrasound should deepen our knowledge about the results obtained from these complimentary morphologic techniques.
Obtaining dynamic MRI exams at the maximal extent of prolapse is also fundamentally important and is the contribution of this study. Clinicians intuitively understand that prolapse size differs depending on strength, duration and number of efforts, as well as on the filling of bladder and rectum and time of day. Most MRI studies are extremely varied with respect to the instructions given to patients and how many attempts were made to achieve maximum prolapse size [10–12]. This study and further work will facilitate a standardizing protocol that best produces a maximum prolapse.
Acknowledgments
This research was supported by a grant from the National Institute of Health Grants R01 HD 38665 and ORWH P50 HD44406.
Footnotes
There are no conflicts of interest to disclose.
References
- 1.Summers A, Winkel LA, Hussain HK, DeLancey JO. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006 May;194(5):1438–43. doi: 10.1016/j.ajog.2006.01.057. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu Y, Chen L, Summer A, Ashton-Miller JA, DeLancey JOL. Anterior Vaginal Wall Length and Degree of Anterior Compartment Prolapse Seen on Dynamic MRI. Int Urogynecol J. 2007 Jan;19(1):137–142. doi: 10.1007/s00192-007-0405-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JO, Klarskov P, Shull BL, Smith AR. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996 Jul;175(1):10–7. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 4.Hsu Y, Summers A, Hussain HK, Guire KE, DeLancey JO. Levator plate angle in women with pelvic organ prolapse compared to women with normal support using dynamic MR imaging. Am J Obstet Gynecol. 2006 May;194(5):1427–33. doi: 10.1016/j.ajog.2006.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu Y, Lewicky-Gaupp C, DeLancey JO. Posterior compartment anatomy as seen in magnetic resonance imaging and 3-dimensional reconstruction from asymptomatic nulliparas. Am J Obstet Gynecol. 2008 Jun;198(6):651. doi: 10.1016/j.ajog.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Hsu Y, Ashton-Miller JA, DeLancey JO. Measurement of the pubic portion of the levator ani muscle in women with unilateral defects in 3-D models from MR images. Int J Gynaecol Obstet. 2006 Mar;92(3):234–41. doi: 10.1016/j.ijgo.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortes E, Reid WM, Singh K, Berger L. Clinical examination and dynamic magnetic resonance imaging in vaginal vault prolapse. Obstet Gynecol. 2004 Jan;103(1):41–6. doi: 10.1097/01.AOG.0000102704.29607.FC. [DOI] [PubMed] [Google Scholar]
- 8.Morgan DM, Umek W, Stein T, Hsu Y, Guire K, Delancey JO. Interrater reliability of assessing levator ani muscle defects with magnetic resonance images. Int Urogynecol J Pelvic Floor Dysfunct. 2007 Jul;18(7):773–8. doi: 10.1007/s00192-006-0224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lockhart ME, Fielding JR, Richter HE, Brubaker L, Salomon CG, Ye W, Hakim CM, Wai CY, Stolpen AH, Weber AM. Reproducibility of dynamic MR imaging pelvic measurements: a multi-institutional study. Radiology. 2008 Nov;249(2):534–40. doi: 10.1148/radiol.2492072009. Epub 2008 Sep 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodfield CA, Hampton BS, Sung V, Brody JM. Magnetic resonance imaging of pelvic organ prolapse: comparing pubococcygeal and midpubic lines with clinical staging. 2009 Jun;20(6):695–701. doi: 10.1007/s00192-009-0865-2. Epub 2009 Mar 25. [DOI] [PubMed] [Google Scholar]
- 11.Broekhuis SR, Fütterer JJ, Barentsz JO, Vierhout ME, Kluivers KB. A systematic review of clinical studies on dynamic magnetic resonance imaging of pelvic organ prolapse: the use of reference lines and anatomical landmarks. Int Urogynecol J Pelvic Floor Dysfunct. 2009 Jun;20(6):721–9. doi: 10.1007/s00192-009-0848-3. Epub 2009 Mar 7. [DOI] [PubMed] [Google Scholar]
- 12.Handa VL, Lockhart ME, Kenton KS, Bradley CS, Fielding JR, Cundiff GW, Salomon CG, Hakim C, Ye W, Richter HE. Magnetic resonance assessment of pelvic anatomy and pelvic floor disorders after childbirth. Int Urogynecol J Pelvic Floor Dysfunct. 2009 Feb;20(2):133–9. doi: 10.1007/s00192-008-0736-2. Epub 2008 Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]