Abstract
Acetyl-CoA synthase (ACS) catalyzes the synthesis of acetyl-CoA from CO, coenzyme A (CoA), and a methyl-group from the CH3-Co3+ site in the corrinoid iron-sulfur protein (CFeSP). These are the key steps in the Wood-Ljungdahl pathway of anaerobic CO and CO2 fixation. The active site of ACS is the A-cluster, which is an unusual nickel-iron-sulfur cluster. There is significant evidence for the catalytic intermediacy of a CO-bound paramagnetic Ni species, with an electronic configuration of [Fe4S4]2+-(Nip1+–CO)-(Nid2+), where Nip and Nid represent the Ni centers in the A-cluster that are proximal and distal to the [Fe4S4]2+ cluster. This well-characterized Nip1+–CO intermediate is often called NiFeC species. Photolysis of the Nip1+–CO state generates a novel Nip1+ species (Ared*) with a rhombic electron paramagnetic resonance spectrum (g-values of 2.56, 2.10, 2.01) and an extremely low (1 kJ/mol) barrier for recombination with CO. We suggest that the photolytically generated Ared* species is (or is similar to) the Nip1+ species that binds CO (to form the Nip1+–CO species) and the methyl group (to form Nip-CH3) in the ACS catalytic mechanism. The results provide support for a binding site (an “alcove”) for CO near Nip, indicated by X-ray crystallographic studies of the Xe-incubated enzyme. We propose that, during catalysis, a resting Nip2+ state predominates over the active Nip1+ species (Ared*) that is trapped by the coupling of a one-electron transfer step to the binding of CO, which pulls the equilibrium toward Nip1+-CO formation.
Keywords: CO dehydrogenase, acetyl-CoA synthase, photolysis, nickel, EPR spectroscopy, enzyme kinetics, iron-sulfur cluster
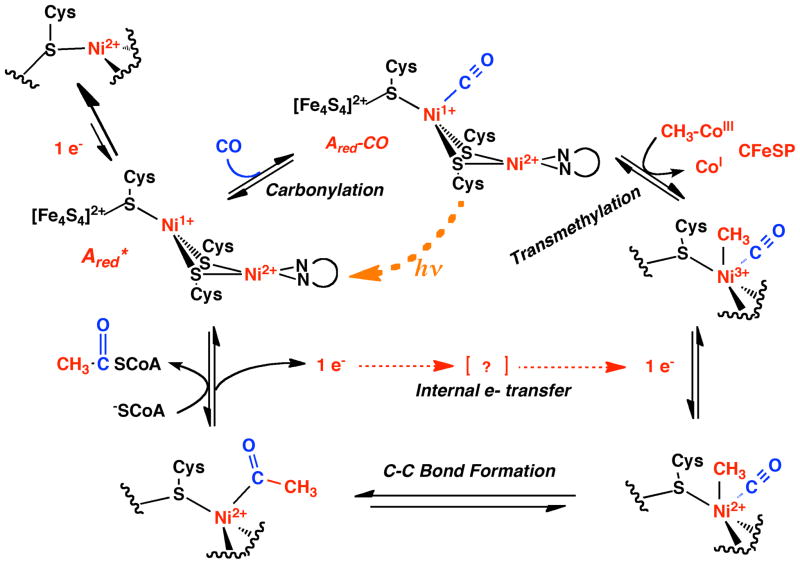
Carbon monoxide dehydrogenase/acetyl-CoA synthase (CODH/ACS) catalyzes the key steps in the Wood-Ljungdahl pathway of anaerobic CO2 fixation, which provides carbon and energy for a variety of anaerobic microbes (1–4). This bifunctional enzyme consists of two central CODH subunits, each of which is attached to an ACS subunit, forming an α2β2 complex (5, 6). In the CODH subunit, CO2 is reduced to CO, which then travels through a 70 Å tunnel to the A-cluster of ACS (7), Here, CO, a methyl-group from the CH3–Co3+ cofactor in the corrinoid iron-sulfur protein (CFeSP) and coenzyme A (CoA) (1) are converted to acetyl-CoA. Based on the results of X-ray crystallographic (5, 8) and biochemical (9, 10) studies, the active A-cluster is known to be composed of a [Fe4S4] cluster bridged through cysteine to the proximal Ni (Nip) of a dinuclear Ni center (Figure 1), an arrangement similar to the Fe-only hydrogenases in which a [Fe4S4] cluster and a binuclear Fe site are bridged by a Cys residue (11, 12). The substrate binding site is ambiguous; however, computational results (13) combined with biochemical and spectroscopic experiments (10, 14–16) and studies of model complexes (17, 18) suggest that Nip is the binding site. Therefore, the purpose of discussion, we will refer to the CO complex as involving Nip-CO, as shown in the mechanism in Figure 2. Nip changes ligation and oxidation states during catalysis, whereas the distal Ni (Nid), which is ligated by two deprotonated amides and two cysteine thiolates in a Cys-Gly-Cys motif, appears to remain square planar in the +2 oxidation state (17). Various details of the mechanism of acetyl-CoA synthesis are not yet established; for example, whether Nip forms paramagnetic or diamagnetic intermediates during the reaction is debated (1, 19–21). The paramagnetic mechanism, which is so-called because it includes a paramagnetic Ni(I)-CO species, is outlined in Figure 2. For simplicity, the ACS mechanism is described as an ordered reaction; however, recent isotope trapping experiments demonstrate that CO and the methyl group bind randomly to ACS, forming a ternary CH3-CO-ACS (or binary acetyl) intermediate that reacts with CoA to form acetyl-CoA (20).
Figure 1.
Structure of the A-Cluster of ACS and the gas-binding site. (A) The structure is based on that of PDB ID1OAO (6), (B) The structure is based on molecule P of PDB ID 2Z8Y (7), which was generated from a crystal structure obtained at high pressures of Xe. In this structure, Cu was present in the proximal site (Cup) and Ni was present in the distal site Nid. Some of the hydrophobic residues are shown that were proposed (7) to form a gas-binding site (the “alcove”) near the binuclear center (Nip and Nid).
Figure 2.
Paramagnetic mechanism of ACS. In this so-called “paramagnetic mechanism”, which is written simplistically as an ordered reaction (although it is a random order of CO or methyl addition), CO traps the thermodynamically unfavorable Ni1+ state (Ared*). However, this state can be trapped at low temperatures by photolysis of the CO ligand. The Ared-CO state is favored because of CO backbonding to a low valent Ni and because ACS has a CO binding site (“alcove”) near Nip. Transmethylation generates an unstable methyl-Ni3+ state that accepts an electron to generate the stable methyl-Ni2+, which undergoes carbonyl insertion to generate an acetyl-Ni2+. As the acetyl group undergoes thiolysis by the nucleophilic attack of CoA, two electrons bifurcate: one goes into the internal electron transfer pathway and the other is used to regenerate Ni1+. In the diamagnetic mechanism, so-called because none of the proposed intermediates are paramagnetic, the substrate(s) bind to a diamagnetic “Ni(0)” state to generate a diamagnetic methyl-Ni(II) or Ni(II)-CO species. The intramolecular electron transfer step would not be required in the “diamagnetic mechanism”. See the text for details.
Because binding of CO is not a redox reaction, the Nip center in Ared* (prior to binding substrates, CO or CH3) should have the same redox state as Nip1+-CO, namely Nip1+, as shown in Fig. 2. However, it is a conundrum that ACS lacks an electron paramagnetic resonance (EPR) signal before binding CO and becomes EPR-active afterwards, because this would indicate that CO binding converts the enzyme from a diamagnetic to a paramagnetic state. Ironically, the best-characterized intermediate in the ACS mechanism is the most controversial one; this is the paramagnetic CO-bound Nip species (Nip1+–CO) (called Ared-CO or the “NiFeC species”), which has been trapped, spectroscopically characterized and shown to be catalytically competent in acetyl-CoA synthesis (22). Based on density functional theory (DFT) computations that incorporate the results of EPR (15), Mössbauer (23, 24), electron nuclear double resonance (ENDOR) (25), infrared (26, 27) and X-ray crystallographic experiments (11, 12), the electronic configuration of Ared-CO has been described as [Fe4S4]2+-(Nip1+–CO)-(Nid2+) (13). The assignment of this as a catalytically competent intermediate is based on its formation and decay rates being faster than the overall rate of acetyl-CoA formation (22, 27, 28). Furthermore, the results of combined freeze quench EPR and stopped-flow fourier transform infrared (FTIR) experiments demonstrate that the NiFeC species is the sole metal-carbonyl species formed upon reaction of ACS with CO (27).
Besides Nip-CO, both methyl- (14, 22) and acetyl-ACS (5, 29) species have been trapped and these forms of the enzyme are EPR-silent and likely diamagnetic. The diamagnetic nature of methyl-ACS represents a challenge for the paramagnetic mechanism because rapid kinetic studies indicate that methyl transfer is an SN2 reaction in which the methyl group is transferred formally as a cation; thus, nucleophilic attack of Nip1+ or Nip1+-CO on a methyl cation should generate a paramagnetic methyl-Ni3+ (or acetyl-Ni3+) state. However, it is recognized that such a species would be highly oxidizing, possessing a Ni3+/2+ couple perhaps as high as +1.0 V (18, 19) and it has been proposed that a rapid coupled one-electron transfer could reduce the methyl-Ni3+ species to methyl-Ni2+ (1). Thus, the predominant observable species shown in Figure 2 that would accumulate to detectible levels during the catalytic mechanism would be Nip2+, Ni1+-CO, methyl-Ni2+ and acetyl-Ni2+, while the unstable Nip1+ and methyl-Ni3+ states would be present in low amounts and observable only by special methods, like those described in the present manuscript.
Characterization of the photolysis of M–CO complexes, e.g., of the Fe2+–CO state of heme in myoglobin or hemoglobin (30, 31), has yielded important insights into protein structure, function and dynamics. Detailed analysis of CO rebinding kinetics can give information on different ligand binding sites and mechanisms (32). Here, we show that photolysis of the catalytically competent Nip1+–CO species at low-temperatures generates a novel paramagnetic Nip1+ species with an extremely low barrier for recombination with CO. The photolysis and recombination have been characterized by EPR and IR measurements. The photolysis and spectroscopic studies described here provide significant novel insights into the mechanism of CO binding to ACS and into the electronic structure of the A-cluster.
MATERIALS AND METHODS
Protein Purification
The His-tagged a-subunit (ACS) of the Moorella thermoacetica CODH/ACS was prepared, purified and Ni-reconstituted under strictly anaerobic conditions below 1 ppm of O2 as described (20). For some ACS samples, Ni reconstitution was done for longer periods (2–3 days) and at higher temperature (45 ºC), which gave a higher NiFeC EPR spin quantity (60–75%).
FTIR Spectroscopy
The FTIR cell consisted of a 50 μm thick, air-tight, transparent sample compartment and an outside metal frame. The samples used for FTIR were prepared by incubating 100 μL of dithionite-reduced ACS (609 μM) with CO for 5 minutes, filling the FTIR cell in the anaerobic chamber using a blunt-ended syringe, removing the cell from the chamber, and freezing immediately in liquid nitrogen. Then, the FTIR cell was transferred into a liquid helium cooling cryostat to start the experiment. Identical samples were prepared in H2O and D2O. Similar results were obtained with the H2O- and D2O-prepared samples; however, having D2O as the solvent provided better access to the 1800–1600 cm−1 region of the spectrum, which includes the so-called ‘amide-I region’ where conformational changes in the polypeptide are observed.
EPR Spectroscopy
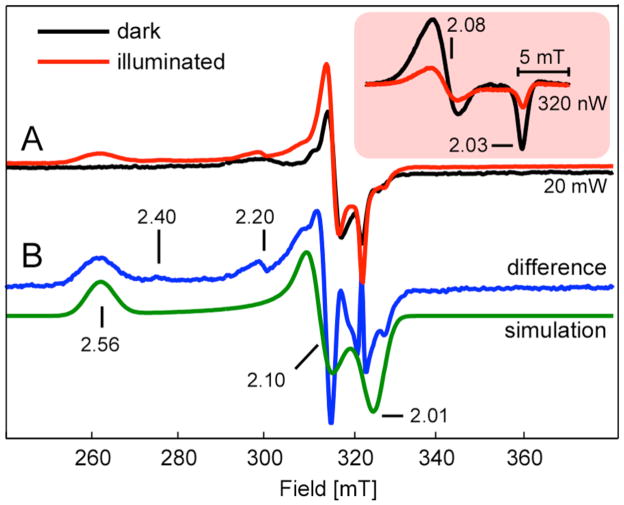
For the EPR-based detection of the photolysis reaction, the Ni1+–CO intermediate was formed by incubating 50–150 μM of ACS with 2 mM dithionite and then purging with 100% CO for 15 min in an EPR tube. The spin concentration of the resultant Ared–CO, measured by double integration relative to a Cu-perchlorate standard, was between 30–75% in different samples. Dithionite-reduced and methylated ACS samples were similarly prepared, but not treated with CO. Methylated ACS was prepared by incubating 65 μM of ACS with 2.5 equivalents of methylcobinamide and 4 mM Ti(III) citrate at 45 ºC for 2.5 hours, and then removing excess methylcobinamide by ultrafiltration. Acetylated ACS was prepared by purging the methylated ACS sample with 100% CO for 15 minutes in the EPR tube. X-band (9.4 GHz) continuous-wave EPR spectra were recorded under non-saturating, slow-passage conditions using a Bruker ECS106 spectrometer equipped with a TE102 cavity. Cryogenic temperatures were achieved and controlled using an Oxford Instruments ESR900 liquid helium cryostat in conjunction with an Oxford Instruments ITC503 temperature and gas flow controller. Spectrometer settings for the data presented in Figure 6 include: temperature = 4.7 K, excitation frequency = 9.480 GHz, microwave power = 20 mW, modulation amplitude = 0.5 mT, modulation frequency = 100 kHz, sweep rate = 4.1 mT/s. The microwave power had to be reduced to 320 nW to give the NiFeC signal shown in the Inset under non-saturating conditions.
Figure 6.
EPR spectra of Ared* and Ared-CO
(A) 20 mW X-band EPR spectra of Ared-CO in 50 % glycerol (v/v) (black) and Ared-CO after one hour of photolysis at 4.7 K (red line). (Inset) NiFeC signal measured using 320 nW of microwave power (B) Difference of the two spectra in panel A (blue line) and corresponding simulation (green line). See text for spectrometer settings and full simulation parameters.
Photolysis and recombination experiments
For the FTIR experiments, the sample photolysis was conducted in an Oxford cryostat with CaF2 external windows, ZnSe intermediate windows, and ZnS windows for the IR cell. Spectra were recorded at 4 cm−1 resolution with a Bruker V-70 FT-IR spectrometer using an MCT detector. For photolysis, a 100 W XENOPHOT® lamp (OSRAM HLX) source was located at the alternate source position of the Bruker spectrometer, and the sample was irradiated by turning the spectrometer mirrors to illuminate the sample with the alternative source. The estimated power on the sample was approximately 100 mW. The FTIR cell was irradiated at 4.7 K for different amounts of time as indicated in the Figure 3 legend. The recombination was observed after turning off the lamp, adjusting the temperature to one of the higher points indicated in Figure 4 and recording the kinetics at which the FTIR difference peak disappeared after the new temperature stabilized (within 90 sec).
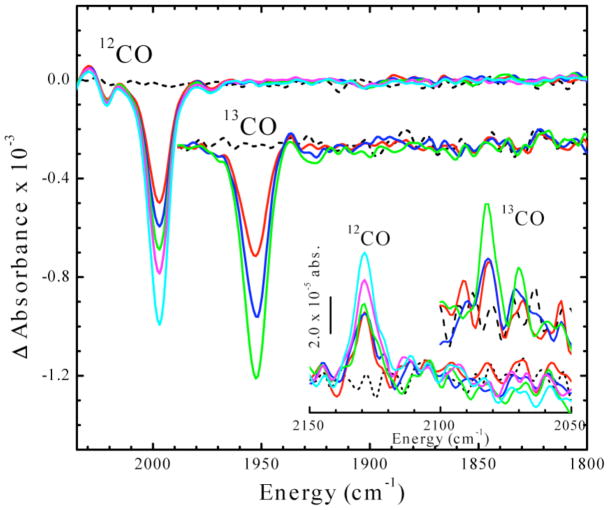
Figure 3.
Time dependence of IR absorbance changes upon photolysis of Ared-CO at 4.5 K. Inset: Development of features in the 2050–2150 cm−1 region, using 12CO and 13CO. See the Materials and Methods section for details of the FTIR experiment. Time points for 12CO data are: 0 (black), 3 (red), 6 (blue), 12 (green), 20 (magenta) and 40 (cyan) min. Time points for 13CO data are: 0 (black), 0.67 (red), 1.67 (blue) and 4 (green) min. The 13CO signal is enlarged 2.64-fold.
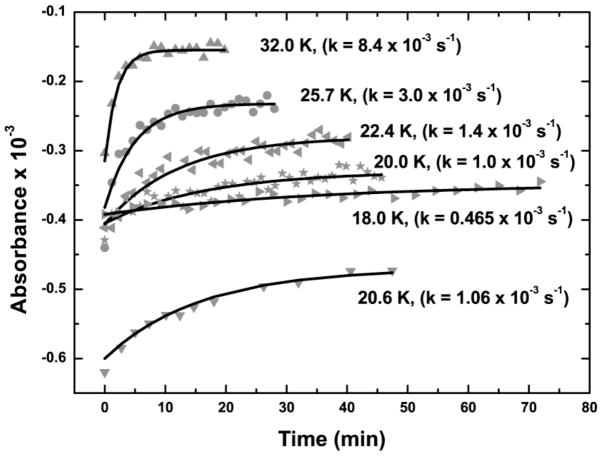
Figure 4.
Time courses of thermal recombination at various temperatures. The temperatures at which the recombinations were observed and the fitted exponential rate constants are indicated next to the curves.
For the EPR experiments, photolysis was performed by focusing the IR-filtered (10 cm of an aqueous 10 mM Cu(II)SO4 solution) light from a 300 W tungsten filament lamp into the light port of the EPR cavity. The sample was rotated four times in 90 ° intervals each followed by 15 min of continuous illumination to maximize photolysis. No further intensity changes in the NiFeC signal were observed after this one hour of illumination. The addition of 50 % glycerol (v/v) to samples of Ared–CO led to a significant increase in the efficacy of the photolysis. Without glycerol, we observed only a 20 % decrease in the NiFeC signal upon illumination. We attribute this change in yield to an increase in the optical quality of the frozen glass upon addition of glycerol. This has the effect of decreasing light scattering and allowing the incident light to penetrate more deeply into the 3 mm diameter EPR sample. For all reported data, a spectrum of the buffer collected under identical spectrometer conditions was subtracted. Data manipulations and spectral simulations were performed in MatLab using EasySpin 3.1 (33, 34). Parameters used for the EPR spectral simulation presented in Figure 6 included: S = 1/2, g = [2.558, 2.10, 2.01], g-strain = [0.06, 0.015, 0.015], line-width = 7 mT.
The recombination kinetics data obtained via FTIR spectroscopy were supported by analogous EPR experiments that monitored the recovery of the NiFeC signal—indicating successful recombination of CO and the transiently formed Ni1+ species—as a function of time once the tungsten lamp was extinguished. The sample was allowed to thermally equilibrate at 20.6 K for at least 15 minutes prior to initiating the experiment. This temperature was chosen for convenience and to allow for facile comparison of the kinetics measured via FTIR. The entire NiFeC signal was acquired in approximately 40 sec. Successive scans were collected individually and the intensity of the corresponding NiFeC signal was determined via double integration. Upon onset of the illumination period of eight minutes, the observed NiFeC signal diminishes significantly (10–12%, see Figure 8). Once the light is extinguished, we observed an immediate jump in the signal intensity followed by a much slower increase. It is likely that the first phase is due to sudden temperature changes in the sample caused by the cessation of illumination. Temperature stability returns rapidly, within one data point (i.e. 40 sec). This second phase is well-fit by a signal exponential function with a rate constant ≈ 0.0006–0.0012 sec−1, in agreement with the 20.8 K recombination rate constant of 0.001 sec−1 obtained from fitting the recovery of the ν(C≡O) band in the FTIR spectrum once the light is turned off (vide supra).
Figure 8.
Time course for recovery of NiFeC signal following illumination.
EPR spectral intensity of the NiFeC signal in Ared–CO with 50 % glycerol (v/v) as a function of time and white-light illumination (designated by the hash marks). Spectrometer settings: temperature 20.6 K, microwave frequency 9.4744 GHz, microwave power 6.41 μW, modulation frequency 100 kHz, modulation amplitude 0.5 mT, sweep rate 0.73 mT/sec.
RESULTS
FTIR studies of the photolysis of Ared-CO and recombination of CO with Ared*
Low-temperature (4.5 K) photolysis of Ared-CO generates a negative band at 1998 cm −1 in the difference FTIR spectrum (Figure 3). This is the first observation of any effect of photolysis on the FTIR (or EPR) spectra of Ared-CO. This feature shifts to lower frequency (1953 cm−1) when ACS is treated with 13CO. This band and a similar isotope shift were first observed in FTIR studies of CO binding to CODH/ACS and assigned as a terminal carbonyl bound to the Ni center in ACS2 (26). We attribute this loss of the ν(C≡O) stretching mode intensity to photo-induced breaking of the Ni–CO bond to generate CO and an Ared* species. Indeed, upon photolysis, a small positive band appears at 2129 cm−1 (2081 cm−1 when prepared with 13CO) that is associated with unbound CO. Upon raising the temperature, the negative CO bands disappear as the original Ni1+–CO species reappears (Figure 4); thus, photolysis must be conducted at low temperatures for Ared* to accumulate. Studies of the temperature dependence of the recombination rate reveal an exceptionally low activation energy (Ea) of 1 kJ/mol (Figure 5). For comparison, CO recombination to the Fe2+-heme in myoglobin has an Ea of 8.4 kJ/mol (35).
Figure 5.
Temperature dependence of the recombination rate of CO with Ared* to reform Ared-CO
Arrhenius plot based on the data shown in Fig. 4.
EPR studies of the photolysis of Ared-CO and recombination of CO with Ared*
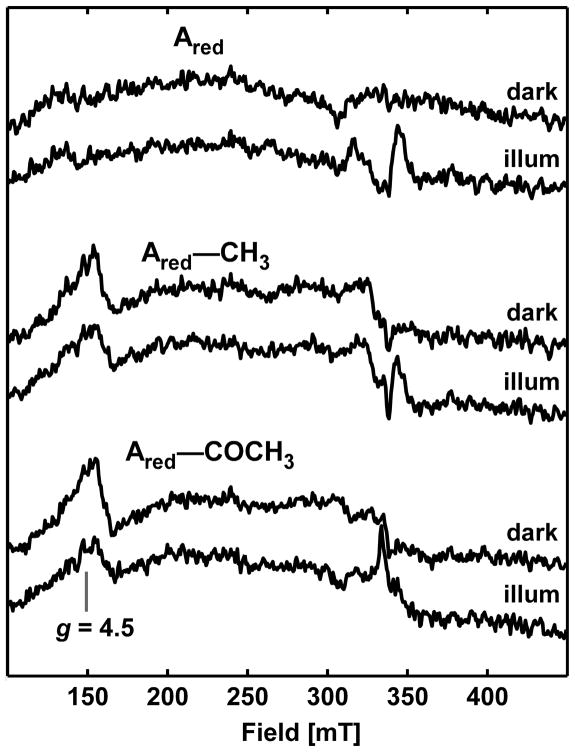
The photochemistry of Ared-CO was followed by EPR spectroscopy. The pre-photolysis spectrum of Ared-CO (black trace, inset Figure 6A) is identical to that previously observed for the NiFeC signal (15). After 1 h of continuous white-light illumination at 4.7 K, the NiFeC signal intensity diminishes by 66 % (red trace, inset Figure 6) as a new set of resonances at ca. 260, 310 and 325 mT appear (Figure 6A). After subtracting residual NiFeC contributions, the resultant Ared* signal is well simulated as an S = 1/2 spin system with g-values of 2.56, 2.10, 2.01 (Figure 6B). These signals are absent from the spectra of pre- or post-photolyzed ACS samples (Ared, Ared-CH3 or Ared-COCH3) lacking CO (Figure 7).
Figure 7.
X-band EPR spectra of reduced, methylated and acetylated ACS preparations. EPR spectra of Ared (top two spectra), Ared–CH3 (middle), and Ared–COCH3 (bottom) in 50 % glycerol (v/v) before (dark) and after (“illum”) one hour of white light illumination. In all cases, the spectrum of the buffer was subtracted. Spectrometer settings are identical to those used to acquire the data in Figure 6.
As in the FTIR experiment, photolysis of Ared-CO is only observed at low temperatures and, after photolysis, the NiFeC signal returns at a rate consistent with that measured by FTIR (Figure 8). Thus, the formation of the paramagnetic photoproduct (Ared*) observed by EPR spectroscopy appears to be correlated with loss of the CO ligand in Ared-CO, observed by IR.
Other minor features at g = 2.40, 2.20 are observed in the difference EPR spectrum. These minor signals may arise from a slightly altered Ared* state, which is consistent with the appearance, in some samples of CO-treated ACS, of an altered form of the NiFeC signal with g-values at 2.05, 2.028 (15). It is also possible that these low-intensity resonances represent a small amount of Nid reduction; however, this is unlikely because spectroscopic and electrochemical studies of the Ni2+/1+ couple in model complexes with an N2S2 thiolato- and carboxamido coordination environment reveal a very low midpoint potential (~ −1.26 V versus NHE) that would not be accessible in aqueous solution with biological reductants (18, 36). Thus, we suggest that the existence of these low intensity features in the EPR spectra for the Nip center indicate variations in electronic and geometric environments, as has been observed after low temperature photolysis of Mb-CO (37).
DISCUSSION
This manuscript describes photolysis studies of a catalytically competent metal-carbonyl species on ACS, the key enzyme in the Wood-Ljungdahl pathway of anaerobic CO2 and CO fixation. Low-temperature (4.5 K) photolysis of the Ared-CO species generates a negative band at 1998 cm−1 in the difference FTIR spectrum (Figure 3). This feature shifts by 45 cm−1 to lower frequency (1953 cm−1) when ACS is incubated with 13CO. This IR band has been previously observed in studies of CO binding to the M. thermoacetica CODH/ACS (26) and to the Carboxydothermus hydrogenoformans ACS (27) and was assigned to the stretching mode of a terminal carbonyl bound to the Ni center in ACS. The 1953 cm−1 IR band of Ared-13CO undergoes isotope substitution when the 13CO-incubated enzyme is reacted with natural abundance acetyl-CoA, indicating that the CO group of Ared-CO undergoes isotope exchange with the carbonyl group of acetyl-CoA (26). Furthermore, this IR band develops at catalytically relevant rates upon incubation of ACS with CO (27). These isotopic exchange experiments indicate that Ared-CO is catalytically relevant to the mechanism of acetyl-CoA synthesis (26, 27).
The IR band associated with Ared-CO forms at the same rate as an EPR signal with g-values at 2.074, 2.028 (27). This has been called the NiFeC signal because it undergoes hyperfine-induced broadening when the metal centers in ACS are isotopically substituted with 61Ni or 57Fe and when ACS is incubated with 13CO versus 12CO (15). Here we describe EPR spectroscopic studies of the photolysis of Ared-CO. When we had earlier irradiated samples of CO-incubated CODH/ACS at 77 K in EPR tubes that were transferred into the EPR cavity for analysis, we did not observe any effects of photolysis on the NiFeC EPR signal (unpublished results). We now recognize that, as seen in the IR experiment, in order to observe photolysis, the experiment must be performed at low temperatures; for example, after 1 h of continuous white-light illumination at 4.7 K, the EPR signal intensity of Ared-CO diminishes by 66 %.
These photolysis results have important implications on the structure of the CO binding site in ACS. We attribute the loss of the ν (C≡O) stretching mode intensity to photo-induced breaking of the Ni1+–CO bond to generate CO and a Ni1+ species (designated as Ared*), a concept that is supported by the g-values in the EPR experiments being similar to those of other Ni1+ complexes (see below) and by the appearance of a small positive IR band (at 2129 cm−1 when ACS is reacted with 12CO and 2081 cm−1 with 13CO) that is associated with unbound CO. The reason that such low temperatures are required to observe photolysis is that recombination of CO with Ared* to generate the original Ni1+–CO species has an activation energy (Ea) of only 1 kJ/mol. The minimal Ea for recombination of CO with Ared* suggests that the nascent CO molecule cannot diffuse far from the Nip site. For comparison, CO recombination to the Fe2+-heme in myoglobin has an Ea of 8.4 kJ/mol (35). FTIR (38, 39) and X-ray crystallographic (40) studies of these heme-based systems indicate that, after photolysis, CO exits the active site and recombination kinetics depend on CO migration inside the protein as well as on protein dynamics accompanying the movement of CO. As shown in Figure 1B, a hydrophobic pocket located by crystallographic studies of Xe-incubated CODH/ACS is only 3.9 Å from Nip and has been proposed to represent the entry point of CO to the A-cluster (7). Assuming a Nip-CO bond distance of 1.8 Å (41), CO would travel ~2 Å to reach this hydrophobic binding pocket after the bond is broken. We propose that photolysis of Ared-CO could relocate CO into this “alcove” from where it can rebind to Nip1+ with a minimal Ea. The low Ea for CO recombination also implies that the photolysis and rebinding of CO at liquid He temperatures is not accompanied by any major reorganization of the active site conformation.
The photolysis results also have important implications with respect to the electronic and electrochemical properties of the A-cluster of ACS. Coupled to photoinduced loss of the Ni1+-CO species is the formation of a novel S=1/2 species with g values of 2.558, 2.1 and 2.01, which are appropriate for assignment of the photolyzed state as a Ni(I) species (42–44). The unusually large g-anisotropy found for Ared* indicates that the unpaired electron is almost wholly localized on Nip with very little spin density on the adjacent metal centers. Complexes that exhibit similar EPR spectra include a three-coordinate bisphosphine diethylether Ni complex (g = 2.45, 2.11, 2.11) (42) and a tetrahedral tris-thioether Ni complex with bound CO (g = 2.64, 2.02, 1.95) (45). The Ni-L form of [NiFe] hydrogenase (g = 2.3, 2.12, 2.05), which is formed by photolyzing the H2-reduced enzyme at 40 K (46), has much less g-anisotropy because of the near square-pyramidal geometry of the Ni site and because exchange interactions with a neighboring Fe delocalize the electron density (47).
We do not observe any indication of the reduced [Fe4-S4] cluster (g-values at 2.06, 1.92, 1.80) (48) or of a Ti(III)citrate-reduced form of ACS that exhibits an axial EPR signal (g = 2.10, 2.03) (21). There also is no evidence for the reduction of the Nid site. As described above, reduction of Nid would be highly unlikely based on the very low midpoint potential of the Ni2+/1+ couple in model complexes with a similar coordination environment (18, 36). Because an electron from the photolytically generated Nip1+ species could potentially transfer to either of the metal centers to which it is bridged, the predominance of the Nip1+ state suggests that, even though the redox potential for the Ni2+/1+ couple of Nip must be extremely low, those for the [Fe4S4]2+/1+ cluster and for Nid2+/1+ must be even lower (or their reductions are kinetically disfavored).
Importantly, the photolysis experiments allow the observation of a novel EPR-active form of the A-cluster of ACS that is generated when CO is released; thus, this is the state that binds CO. As shown in Figure 2, we suggest that Ared* is a thermodynamically unfavorable Nip1+ state. The EPR signal of Ared* is not observed when ACS treated with reductants like Ti(III)-citrate or dithionite because, in the absence of CO, the Nip2+/1+ equilibrium strongly favors Ni(II) (Equation 1). In the presence of CO, the equilibrium shifts to favor the formation of Ni(I)-CO because CO tends to bind strongly to the low valent states of metal centers (the Km for CO in the CO/acetyl-CoA exchange is 10 μM (49)) and because there is a gas binding pocket near Nip (7). Thus, it appears that, even in the presence of low potential reductants with midpoint potentials below −500 mV, there is too little accumulation of the Nip1+ state to be observed; however, by kinetically coupling the reduction (Eq. 1) and CO binding (Eq 2) processes, a low valent Ni1+-CO complex becomes favorable. In a similar fashion, it was observed by cyclic voltammetry that the midpoint redox potentials of nickel(I) macrocyclic complexes shifted to a more positive value under 1 atm CO (50).
| Eq. 1 |
| Eq. 2 |
Figure 2 shows two one-electron transfer steps that occur during the catalytic cycle - one is used to reductively activate Ni2+ to Ni1+ in the one-electron coupled CO binding step and the other electron is used in the internal one-electron transfer associated with the methylation of ACS by the methylated CFeSP. These electrons ultimately come from CoA-dependent thiolysis of the acetyl-ACS intermediate to generate acetyl-CoA; thus, the overall reaction cycle does not involve net electron transfer. There is ample evidence for internal redox chemistry during acetyl-CoA synthesis; in fact, an unknown factor that was isolated based on its ability to stimulate the CO/acetyl-CoA exchange reaction turned out to be ferredoxin, but it could be replaced by other mediators (51). Although CoA is the ultimate electron donor, the immediate donor(s) of the reducing equivalents involved in conversion of Ni2+ to Ni1+-CO and of methyl-Ni3+ to methyl-Ni2+ during the transmethylation reaction is unknown. Because the [Fe4S4] cluster that is attached to Nip is a low potential center, we speculate that it could act as a conduit in the formation of Ared-CO. Although electron transfer to and from the [Fe4S4]2+/1+ cluster has been shown to be 200-fold slower than the rate of methyl group transfer (52), coupling the electron transfer step to carbonylation in a so-called “EC reaction” would significantly enhance the rate because the thermodynamic driving force would be greater. We speculate that Nid may be involved in the redox-coupled methyl transfer reaction. Like other cobalamin-dependent transmethylations (53), this is a nucleophilic SN2-type reaction (54, 55) that would initially generate a methyl-Ni3+ intermediate; however, unlike methyl-Co3+, methyl-Ni3+ is expected to be highly oxidizing and to undergo reduction to the methyl-Ni2+ state in the presence of even moderate reducing agents. The coordination environment of Nid resembles that of Ni-superoxide dismutase (Ni-SOD) (56, 57), which accesses both the Ni2+ and Ni3+ states during the catalytic cycle (56, 58). Furthermore, model studies have shown that Ni3+ is stabilized by the anionic amidate ligands found in Nid (59, 60). Regardless, the paramagnetic pathway and the internal electron transfer loop in Figure 2 describe a speculative, but testable, working hypothesis that would include roles for all three components of the A-Cluster.
In summary, we have demonstrated the photodissociation of CO from the Nip site in Ared-CO, which is a catalytically competent intermediate in acetyl-CoA synthesis (22, 27, 28). This is the first time a photolysis event has been observed on a catalytically competent metal-carbonyl intermediate on an enzyme, even though CO photodissociation was observed in Ni-based inorganic compounds (61) and in Mo- and Ni-dependent nitrogenase and hydrogenase, respectively, for which CO acts as an inhibitor rather than a substrate (62, 63). The other photoproduct (Ared*) is a Ni1+ species that is only long-lived at low temperatures (t1/2 = 24.8 min at 18 K) and has EPR properties similar to those of Ni1+ compounds with trigonal planar or tetrahedral geometries. The facile recombination of CO with Ared* can be attributed to the capture of photolyzed CO in a gas-binding pocket near Nip that has been observed in the crystal structure (7). The Nip2+/1+ couple must have a sufficiently low redox potential that the Nip1+ photoproduct trapped in this study at low temperatures does not accumulate during catalysis. We propose that, during the ACS catalytic mechanism, binding of CO traps this unstable Nip1+ species in a coupled one-electron transfer step, pulling the redox equilibrium toward formation of Ni1+–CO. Further spectroscopic and computational studies on Ared* are underway and are expected to yield essential insight into changes in the electronic and geometric structures of the A cluster that occur upon substrate binding.
Acknowledgments
We thank Elizabeth Pierce (Univ. of Michigan) for help in preparing some of the enzyme used in these experiments.
Abbreviations
- CODH
CO dehydrogenase
- ACS
acetyl-CoA synthase
- FTIR
Fourier transform infrared
- Nip
proximal nickel in the binuclear site of the A-cluster of ACS
- Nid
distal nickel in the binuclear site of the A-cluster of ACS
- DFT
density functional theory
- EPR
electron paramagnetic resonance
- Ared
the state of the A-cluster when ACS is treated with dithionite
- Ared*
the photolyzed state of Ared-CO
Footnotes
This research was supported by grants to S.W.R. (NIH, GM-39451), S.P.C. (NIH GM-65440, NSF CHE-0745353, and DOE OBER), and R.D.B. (NIH GM-48242).
In this citation, the M-CO band was assigned to CODH because it was not known until later (Kumar, M., Lu, W.-P., Liu, L., and Ragsdale, S. W. (1993) J. Am. Chem. Soc. 115, 11646-11647) that CO oxidation and acetyl-CoA synthesis occur at separate sites.
References
- 1.Ragsdale SW. Life with carbon monoxide. CRC Crit Rev Biochem Mol Biol. 2004;39:165–195. doi: 10.1080/10409230490496577. [DOI] [PubMed] [Google Scholar]
- 2.Ragsdale SW. Nickel and the Carbon Cycle. J Inorg Biochem. 2007;101:1657–1666. doi: 10.1016/j.jinorgbio.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ragsdale SW. Nickel-based enzyme systems. J Biol Chem. 2009;284:18571–18575. doi: 10.1074/jbc.R900020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller V, Imkamp F, Andreas Rauwolf Küsel K, Drake HL. Molecular and Cellular Biology of Acetogenic Bacteria. In: Nakano MM, Zuber P, editors. Strict and Facultative Anaerobes: Medical and Environmental Aspects. Horizon Bioscience; Wymondham, UK: 2004. pp. 251–281. [Google Scholar]
- 5.Doukov TI, Iverson TM, Seravalli J, Ragsdale SW, Drennan CL. A Ni-Fe-Cu center in a bifunctional carbon monoxide dehydrogenase/acetyl-CoA synthase. Science. 2002;298:567–572. doi: 10.1126/science.1075843. [DOI] [PubMed] [Google Scholar]
- 6.Darnault C, Volbeda A, Kim EJ, Legrand P, Vernede X, Lindahl PA, Fontecilla-Camps JC. Ni-Zn-[Fe(4)-S(4)] and Ni-Ni-[Fe(4)-S(4)] clusters in closed and open alpha subunits of acetyl-CoA synthase/carbon monoxide dehydrogenase. Nat Struct Biol. 2003;10:271–279. doi: 10.1038/nsb912. [DOI] [PubMed] [Google Scholar]
- 7.Doukov TI, Blasiak LC, Seravalli J, Ragsdale SW, Drennan CL. Xenon in and at the end of the tunnel of bifunctional carbon monoxide dehydrogenase/acetyl-CoA synthase. Biochemistry. 2008;47:3474–3483. doi: 10.1021/bi702386t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drennan CL, Doukov TI, Ragsdale SW. The metalloclusters of carbon monoxide dehydrogenase/acetyl-CoA synthase: a story in pictures. J Biol Inorg Chem. 2004;9:511–515. doi: 10.1007/s00775-004-0563-y. [DOI] [PubMed] [Google Scholar]
- 9.Seravalli J, Xiao YM, Gu WW, Cramer SP, Antholine WE, Krymov V, Gerfen GJ, Ragsdale SW. Evidence that NiNi acetyl-CoA synthase is active and that the CuNi enzyme is not. Biochemistry. 2004;43:3944–3955. doi: 10.1021/bi036194n. [DOI] [PubMed] [Google Scholar]
- 10.Seravalli J, Xiao Y, Gu W, Cramer SP, Antholine WE, Krymov V, Gerfen GJ, Ragsdale SW. Evidence That Ni-Ni Acetyl-CoA Synthase Is Active And That The Cu-Ni Enzyme Is Not. Biochem. 2004;43:3944–3955. doi: 10.1021/bi036194n. [DOI] [PubMed] [Google Scholar]
- 11.Peters JW, Lanzilotta WN, Lemon BJ, Seefeldt LC. X-ray crystal structure of the Fe-only hydrogenase (Cpl) from Clostridium pasteurianum to 1.8 angstrom resolution. Science. 1998;282:1853–1858. doi: 10.1126/science.282.5395.1853. [DOI] [PubMed] [Google Scholar]
- 12.Nicolet Y, Lemon BJ, Fontecilla-Camps JC, Peters JW. A novel FeS cluster in Fe-only hydrogenases. Trends Biochem Sci. 2000;25:138–143. doi: 10.1016/s0968-0004(99)01536-4. [DOI] [PubMed] [Google Scholar]
- 13.Schenker RP, Brunold TC. Computational studies on the A cluster of acetyl-coenzyme A synthase: geometric and electronic properties of the NiFeC species and mechanistic implications. J Am Chem Soc. 2003;125:13962–13963. doi: 10.1021/ja037893q. [DOI] [PubMed] [Google Scholar]
- 14.Barondeau DP, Lindahl PA. Methylation of carbon monoxide dehydrogenase from Clostridium thermoaceticum and mechanism of acetyl coenzyme A synthesis. J Am Chem Soc. 1997;119:3959–3970. [Google Scholar]
- 15.Ragsdale SW, Wood HG, Antholine WE. Evidence that an iron-nickel-carbon complex is formed by reaction of CO with the CO dehydrogenase from Clostridium thermoaceticum. Proc Natl Acad Sci USA. 1985;82:6811–6814. doi: 10.1073/pnas.82.20.6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bramlett MR, Tan X, Lindahl PA. Inactivation of Acetyl-CoA Synthase/Carbon Monoxide Dehydrogenase by Copper. J Am Chem Soc. 2003;125:9316–9317. doi: 10.1021/ja0352855. [DOI] [PubMed] [Google Scholar]
- 17.Harrop TC, Mascharak PK. Structural and spectroscopic models of the A-cluster of acetyl coenzyme a synthase/carbon monoxide dehydrogenase: Nature’s Monsanto acetic acid catalyst. Coord Chem Rev. 2005;249:3007–3024. [Google Scholar]
- 18.Mathrubootham V, Thomas J, Staples R, McCraken J, Shearer J, Hegg EL. Bisamidate and Mixed Amine/Amidate NiN(2)S(2) Complexes as Models for Nickel-Containing Acetyl Coenzyme A Synthase and Superoxide Dismutase: An Experimental and Computational Study. Inorg Chem. 2010;49:5393–5406. doi: 10.1021/ic9023053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hegg EL. Unraveling the structure and mechanism of acetyl-coenzyme A synthase. Acc Chem Res. 2004;37:775–783. doi: 10.1021/ar040002e. [DOI] [PubMed] [Google Scholar]
- 20.Seravalli J, Ragsdale SW. Pulse-chase studies of the synthesis of acetyl-CoA by carbon monoxide dehydrogenase/acetyl-CoA synthase - Evidence for a random mechanism of methyl and carbonyl addition. J Biol Chem. 2008;283:8384–8394. doi: 10.1074/jbc.M709470200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan X, Martinho M, Stubna A, Lindahl PA, Munck E. Mossbauer evidence for an exchange-coupled {[Fe4S4](1+) Ni-p(1+)} A-cluster in isolated alpha Subunits of acetyl-coenzyme a synthase/carbon monoxide dehydrogenase. J Am Chem Soc. 2008;130:6712–6713. doi: 10.1021/ja801981h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seravalli J, Kumar M, Ragsdale SW. Rapid kinetic studies of acetyl-CoA synthesis: Evidence supporting the catalytic intermediacy of a paramagnetic NiFeC species in the autotrophic Wood-Ljungdahl pathway. Biochemistry. 2002;41:1807–1819. doi: 10.1021/bi011687i. [DOI] [PubMed] [Google Scholar]
- 23.Lindahl PA, Ragsdale SW, Münck E. Mössbauer studies of CO dehydrogenase from Clostridium thermoaceticum. J Biol Chem. 1990;265:3880–3888. [PubMed] [Google Scholar]
- 24.Xia J, Hu Z, Popescu CV, Lindahl PA, Munck E. Mössbauer and EPR Study of the Ni-Activated a-Subunit of Carbon Monoxide Dehydrogenase from Clostridium thermoaceticum. J Am Chem Soc. 1997;119:8301–8312. [Google Scholar]
- 25.Fan C, Gorst CM, Ragsdale SW, Hoffman BM. Characterization of the Ni-Fe-C complex formed by reaction of carbon monoxide with the carbon monoxide dehydrogenase from Clostridium thermoaceticum by Q-band ENDOR. Biochem. 1991;30:431–435. doi: 10.1021/bi00216a018. [DOI] [PubMed] [Google Scholar]
- 26.Kumar M, Ragsdale SW. Characterization of the CO binding site of carbon monoxide dehydrogenase from Clostridium thermoaceticum by infrared spectroscopy. J Am Chem Soc. 1992;114:8713–8715. [Google Scholar]
- 27.George SJ, Seravalli J, Ragsdale SW. EPR and infrared spectroscopic evidence that a kinetically competent paramagnetic intermediate is formed when acetyl-coenzyme A synthase reacts with CO. J Am Chem Soc. 2005;127:13500–13501. doi: 10.1021/ja0528329. [DOI] [PubMed] [Google Scholar]
- 28.Gorst CM, Ragsdale SW. Characterization of a Ni-Fe-CO complex of CO dehydrogenase as a catalytically competent intermediate in the pathway of acetyl-CoA synthesis. J Biol Chem. 1991;266:20687–20693. [PubMed] [Google Scholar]
- 29.Gencic S, Grahame DA. Two Separate One-Electron Steps in the Reductive Activation of the A Cluster in Subunit beta of the ACDS Complex in Methanosarcina thermophila. Biochemistry. 2008;47:5544–5555. doi: 10.1021/bi7024035. [DOI] [PubMed] [Google Scholar]
- 30.Gibson QH. An apparatus for flash photolysis and its application to the reactions of myoglobin with gases. J Physiol. 1956;134:112–122. doi: 10.1113/jphysiol.1956.sp005627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartridge H, Roughton FJW. The velocity with which carbon monoxide displaces oxygen from combination with h ae moglobin - Part I. Proceedings of the Royal Society of London Series B-Containing Papers of a Biological Character. 1923;94:336–367. [Google Scholar]
- 32.Abbruzzetti S, Bruno S, Faggiano S, Ronda L, Grandi E, Mozzarelli A, Viappiani C. Characterization of ligand migration mechanisms inside hemoglobins from the analysis of geminate rebinding kinetics. Globins and Other Nitric Oxide-Reactive Proteins, Part B. 2008;437:329–345. doi: 10.1016/S0076-6879(07)37017-1. [DOI] [PubMed] [Google Scholar]
- 33.Stoll S, Britt RD. General and efficient simulation of pulse EPR spectra. Phys Chem Chem Phys. 2009;11:6614–6625. doi: 10.1039/b907277b. [DOI] [PubMed] [Google Scholar]
- 34.Stoll S, Schweiger A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. Journal of Magnetic Resonance. 2006;178:42–55. doi: 10.1016/j.jmr.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Austin RH, Beeson KW, Eisenstein L, Frauenfelder H, Gunsalus IC. Dynamics of ligand binding to myoglobin. Biochemistry. 1975;14:5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- 36.Hatlevik O, Blanksma MC, Mathrubootham V, Arif AM, Hegg EL. Modeling carbon monoxide dehydrogenase/acetyl-CoA synthase (CODH/ACS): a trinuclear nickel complex employing deprotonated amides and bridging thiolates. J Biol Inorg Chem. 2004;9:238–246. doi: 10.1007/s00775-003-0518-8. [DOI] [PubMed] [Google Scholar]
- 37.Roder H, Berendzen J, Bowne SF, Frauenfelder H, Sauke TB, Shyamsunder E, Weissman MB. Comparison of the magnetic properties of deoxy- and photodissociated myoglobin. Proc Natl Acad Sci U S A. 1984;81:2359–2363. doi: 10.1073/pnas.81.8.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Causgrove TP, Dyer RB. Protein Response to Photodissociation of Co from Carbonmonoxymyoglobin Probed by Time-Resolved Infrared-Spectroscopy of the Amide-I Band. Biochemistry. 1993;32:11985–11991. doi: 10.1021/bi00096a007. [DOI] [PubMed] [Google Scholar]
- 39.Plunkett SE, Chao JL, Tague TJ, Palmer RA. Time-Resolved Step-Scan Ft-Ir Spectroscopy of the Photodynamics of Carbonmonoxymyoglobin. Applied Spectroscopy. 1995;49:702–708. [Google Scholar]
- 40.Ostermann A, Waschipky R, Parak FG, Nienhaus GU. Ligand binding and conformational motions in myoglobin. Nature. 2000;404:205–208. doi: 10.1038/35004622. [DOI] [PubMed] [Google Scholar]
- 41.Furenlid LR, Renner MW, Szalda DJ, Fujiita E. J Am Chem Soc. 1991;113:883–892. [Google Scholar]
- 42.Saraev VV, Kraikivskii PB, Matveev DA, Zelinskii SN, Lammertsma K. EPR study of the oxidation reaction of nickel(0) phosphine complexes with Lewis and Bronsted acids. Inorg Chim Acta. 2006;359:2314–2320. [Google Scholar]
- 43.Lancaster JR. The Bioinorganic chemistry of nickel. VCH; New York, N.Y: 1988. [Google Scholar]
- 44.Albracht SPJ, Ankelfuchs D, Bocher R, Ellermann J, Moll J, Vanderzwaan JW, Thauer RK. 5 New Electron-Paramagnetic-Res Signals Assigned to Nickel in Methyl-Coenzyme M-Reductase from Methanobacterium-Thermoautotrophicum, Strain Marburg. Biochim Biophys Acta. 1988;955:86–102. [Google Scholar]
- 45.Craft JL, Mandimutsira BS, Flujita K, Riordan CG, Brunold TC. Spectroscopic and computational studies of a Ni+-CO model complex: Implications for the acetyl-CoA synthase catalytic mechanism. Inorg Chem. 2003;42:859–867. doi: 10.1021/ic020441e. [DOI] [PubMed] [Google Scholar]
- 46.Pandelia ME, Ogata H, Currell LJ, Flores M, Lubitz W. Inhibition of the [NiFe] hydrogenase from Desulfovibrio vulgaris Miyazaki F by carbon monoxide: An FTIR and EPR spectroscopic study. Biochim Biophys Acta. 1797:304–313. doi: 10.1016/j.bbabio.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Ogata H, Mizoguchi Y, Mizuno N, Miki K, Adachi S, Yasuoka N, Yagi T, Yamauchi O, Hirota S, Higuchi Y. Structural studies of the carbon monoxide complex of [NiFe]hydrogenase from Desulfovibrio vulgaris Miyazaki F: Suggestion for the initial activation site for dihydrogen. J Am Chem Soc. 2002;124:11628–11635. doi: 10.1021/ja012645k. [DOI] [PubMed] [Google Scholar]
- 48.Bramlett MR, Stubna A, Tan XS, Surovtsev IV, Munck E, Lindahl PA. Mossbauer and EPR study of recombinant acetyl-CoA synthase from Moorella thermoacetica. Biochemistry. 2006;45:8674–8685. doi: 10.1021/bi060003+. [DOI] [PubMed] [Google Scholar]
- 49.Raybuck SA, Bastian NR, Orne-Johnson WH, Walsh CT. Kinetic characterization of the carbon monoxide-acetyl-CoA (carbonyl group) exchange activity of the acetyl-CoA synthesizing CO dehydrogenase from Clostridium thermoaceticum. Biochem. 1988;27:7698–7702. doi: 10.1021/bi00420a019. [DOI] [PubMed] [Google Scholar]
- 50.Jung HJ, Bang H, Suh MP. Carbon Monoxide Binding to Ni(I) Macrocyclic Complexes Generated by Electrochemical Methods. Bull Korean Chem Soc. 2001;22:523–525. [Google Scholar]
- 51.Ragsdale SW, Wood HG. Acetate biosynthesis by acetogenic bacteria: evidence that carbon monoxide dehydrogenase is the condensing enzyme that catalyzes the final steps of the synthesis. J Biol Chem. 1985;260:3970–3977. [PubMed] [Google Scholar]
- 52.Tan X, Sewell C, Yang Q, Lindahl PA. Reduction and Methyl Transfer Kinetics of the alpha Subunit from Acetyl Coenzyme A Synthase. J Am Chem Soc. 2003;125:318–319. doi: 10.1021/ja028442t. [DOI] [PubMed] [Google Scholar]
- 53.Banerjee R, Ragsdale SW. The Many Faces of Vitamin B12: Catalysis by Cobalamin-dependent Enzymes. Ann Rev Biochem. 2003;72:209–247. doi: 10.1146/annurev.biochem.72.121801.161828. [DOI] [PubMed] [Google Scholar]
- 54.Menon S, Ragsdale SW. Role of the [4Fe-4S] cluster in reductive activation of the cobalt center of the corrinoid iron-sulfur protein from Clostridium thermoaceticum during acetyl-CoA synthesis. Biochemistry. 1998;37:5689–5698. doi: 10.1021/bi9727996. [DOI] [PubMed] [Google Scholar]
- 55.Menon S, Ragsdale SW. The role of an iron-sulfur cluster in an enzymatic methylation reaction: methylation of CO dehydrogenase/acetyl-CoA synthase by the methylated corrinoid iron-sulfur protein. J Biol Chem. 1999;274:11513–11518. doi: 10.1074/jbc.274.17.11513. [DOI] [PubMed] [Google Scholar]
- 56.Barondeau DP, Kassmann CJ, Bruns CK, Tainer JA, Getzoff ED. Nickel superoxide dismutase structure and mechanism. Biochemistry. 2004;43:8038–8047. doi: 10.1021/bi0496081. [DOI] [PubMed] [Google Scholar]
- 57.Wuerges J, Lee J, Yim Y, Kang S, Yim H, Djinovic-Carugo K. Crystal Structure Of Nickel-Containing Superoxide Dismutase. Proc Natl Acad Sci U S A. 2004;101:8569–8574. doi: 10.1073/pnas.0308514101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bryngelson PA, Maroney MJ. Nickel superoxide dismutase. In: Sigel A, Sigel H, Sigel RKO, editors. Nickel and its Surprising Impact in Nature. John Wiley and Sons; West Sussex, England: 2007. pp. 417–444. [Google Scholar]
- 59.Mullins CS, Grapperhaus CA, Kozlowski PM. Density functional theory investigations of NiN2S2 reactivity as a function of nitrogen donor type and N-H...S hydrogen bonding inspired by nickel-containing superoxide dismutase. J Biol Inorg Chem. 2006;11:617–625. doi: 10.1007/s00775-006-0109-6. [DOI] [PubMed] [Google Scholar]
- 60.Fiedler AT, Bryngelson PA, Maroney MJ, Brunold TC. Spectroscopic and computational studies of Ni superoxide dismutase: electronic structure contributions to enzymatic function. J Am Chem Soc. 2005;127:5449–5462. doi: 10.1021/ja042521i. [DOI] [PubMed] [Google Scholar]
- 61.Hennig H, Hofbauer K, Handke K, Stich R. Unusual reaction pathways in the photolysis of diazido(phosphane)nickel(II) complexes: Nitrenes as intermediates in the formation of nickel(0) complexes. Angewandte Chemie-International Edition in English. 1997;36:408–410. [Google Scholar]
- 62.Vanderzwaan JW, Albracht SPJ, Fontijn RD, Roelofs YBM. Electron-Paramagnetic-Res Evidence for Direct Interaction of Carbon-Monoxide with Nickel in Hydrogenase from Chromatium-Vinosum. Biochim Biophys Acta. 1986;872:208–215. [Google Scholar]
- 63.Maskos Z, Hales BJ. Photo-lability of CO bound to mo-nitrogenase from Azotobacter vinelandii. J Inorg Biochem. 2003;93:11–17. doi: 10.1016/s0162-0134(02)00480-4. [DOI] [PubMed] [Google Scholar]