Abstract
Our understanding of primary biliary cirrhosis (PBC) has been significantly enhanced by the rigorous dissection of the multilineage T and B cell response against the immunodominant mitochondrial autoantigen, the E2 component of the pyruvate dehydrogenase complex (PDC-E2). PDC-E2 is a ubiquitous protein present in mitochondria of nucleated cells. However, the damage in PBC is confined to small biliary epithelial cells (BEC). We have previously demonstrated that BEC translocate immunologically intact PDC-E2 to apoptotic bodies, creating an apotope. To define the significance of this observation, we studied the ability of biliary or control epithelial apotopes to induce cytokine secretion from mature monocyte derived macrophages (MDMΦ) from either patients with PBC or controls in the presence or absence of anti-mitochondrial antibodies (AMA). We demonstrate that there is intense inflammatory cytokine production in the presence of the unique triad of BEC apotopes, macrophages from patients with PBC and AMA. The cytokine secretion is inhibited by anti-CD16 and not due to differences in apotope uptake. Moreover, MDMΦ from PBC patients cultured with BEC apoptotic bodies in the presence of AMA markedly increased TNF-related apoptosis-inducing ligand expression.
Conclusion
These results provide a mechanism for the biliary specificity of PBC, the recurrence of disease following liver transplantation and the success of ursodiol in treatment. They further emphasize a critical role of the innate immune system in the perpetuation of this autoimmune disease.
Keywords: autoimmunity, biliary epithelial cell, macrophages, cytokines, Fc receptor
There have been significant advances in our understanding of primary biliary cirrhosis (PBC) (1), including dissection of the autoreactive CD4 and CD8 responses (2–5) and the molecular characteristics of anti-mitochondrial antibodies (AMA) (6–7). Results of these studies suggest that PBC ensues from a multi-lineage loss of tolerance to the E2 component of the pyruvate dehydrogenase complex (PDC-E2), the immunodominant autoantigen of PBC (8–9). While the mechanisms involved in the loss of tolerance remain unknown, the role of genetic susceptibility (10) and environmental factors that modify the auto-antigen motif and contribute to the breakdown of tolerance have gained attention (11–12). However, a major unanswered question regarding the pathogenesis of PBC is the specific targeting of the small biliary duct epithelial cell. All nucleated cells have mitochondria, yet only small biliary epithelial cells (BEC) and to a lesser extent salivary gland cells are the targets of the autoimmune attack in PBC.
Apoptotic cells are normally efficiently cleared after engulfment by ‘professional’ phagocytes followed by an anti-inflammatory response (13–14). When such uptake is impaired, cell lysis can release intracellular components that are a potential source of autoantigenic stimulation and autoimmunity onset (15). We have recently demonstrated that, in contrast to other epithelial cells, small BEC translocate immunologically intact PDC-E2 to apoptotic bodies formed during apoptosis (16). We and others have called the epitope expressed on apoptotic cells an apotope (17–18) and we submit that the biliary apotope has important biological and clinical significance in PBC. To investigate the unique target cell specificity of PBC, we have studied the ability of apoptotic bodies from either small BECs or control epithelial cells to induce cytokine secretion from macrophages of either patients with PBC, or control subjects in the presence or absence of AMA.
We report that the unique triad consisting of macrophages from patients with PBC, BEC apotopes, and AMA leads to a burst of inflammatory cytokines. This finding implicates that AMA contributes to bile duct damage and explains the tissue specificity of PBC. Moreover, our results offer an explanation for the recurrence of PBC after liver transplantation (19), the relative failure of immunosuppressive drugs to modify what is considered a model autoimmune disease (20), as well as the efficacy of ursodiol in PBC, a drug that has anti-apoptotic properties (21).
MATERIALS AND METHODS
Subjects
Fresh heparinized peripheral blood samples were obtained from patients diagnosed with PBC (n=25), unaffected controls (n=20), subjects with primary sclerosing cholangitis (PSC) (n=6), and subjects with systemic lupus erythematosus (SLE) (n=3) (1, 22–23). All patients with PBC were women and had detectable AMA. Mean age was 56 years old (range 43–66 years) and 70% of were taking ursodiol. Patients with PBC had histological stage I (n=7), stage II (n=15) or stage III (n=3) and were excluded if they had stage IV histological disease. Subjects were also excluded if they had malignancies or were using immunosuppressive drugs. Patients and controls were matched for age and sex and separate unrelated donors were used for each independent experiment. The IRB of the University of California at Davis approved the study protocol and all subjects provided written informed consent.
Generation of monocyte-derived macrophages (MDMΦ)
Human mononuclear cells were isolated under endotoxin-free conditions from buffy coats of centrifuged peripheral blood followed by anti-CD14 microbead assisted magnetic cell sorting (Miltenyi Biotec). The purity of the monocytic population was >95% as assessed by flow cytometry. Aliquots of monocytes (0.5 × 106/ml) were then re-suspended in RPMI culture medium containing 10% heat inactivated fetal bovine serum, 90 U/mL penicillin and 90 µg/mL streptomycin supplemented with 100 ng/mL GM-CSF and cultured for at least 5 days in a humidified 5% CO2 incubator. Viability was confirmed by trypan blue dye exclusion. Only preparations in which the percentage of CD14+ cells was > 95% and the viability > 90% were utilized.
Cell culture
Human intra-hepatic biliary epithelial cells (HIBEC) from 2 healthy donors were purchased from ScienCell. Data from both sets of healthy donors were similar and are combined herein. Primary cultures of human keratinocytes, a gift from Dr. Rivkah Isseroff (University of California, Davis), were used as controls based on our previous data (16). Previous data on apoptosis also utilized bronchial epithelial cells, HeLa and CaCo-2 cells. Both the human intrahepatic biliary epithelial cells and the keratinocytes were obtained from normal human tissue and cryopreserved immediately after isolation. All experiments were performed using cells between passage 2 and 8. HIBEC were cultured in sterile Epithelial Cell Medium (ScienCell) supplemented with 2% fetal bovine serum, epithelial cell growth supplement (ScienCell) and 1% penicillin. Keratinocytes were cultured in Epi-Life Medium (Gibco-Invitrogen) supplemented with bovine pituitary extract, bovine insulin hydrocortisone, bovine transferrin and human epidermal growth factor. Cells were cultured at 37 °C in a humidified 5% CO2 incubator.
Apoptosis induction and isolation of apoptotic bodies
Apoptosis was induced with bile salts as described (16). After induction of apoptosis, supernatants were collected and centrifuged twice (500 g, 5 min) to remove intact cells. The supernatant fluid was then passed through a 1.2 µm non-pyrogenic, hydrophilic syringe filter. After centrifugation at 100,000 g for 45 min, the pellet containing apoptotic bodies was re-suspended in RPMI media. The number of apoptotic bodies was determined and the suspension immediately used for co-culture with MDMΦ.
Antibody reagents and AMA purification
Human IgG was purified from sera of PBC patients with high levels of AMA using a Protein G column (Pierce). Similarly, serum IgG from unaffected controls was purified. IgA-AMA from a patient diagnosed with PBC and POEMS syndrome was purified using Jacalin-agarose beads (Pierce); serum IgA from normals was used as control. All experiments were conducted under sterile conditions and solutions filtered through a 0.22 µm filter.
Culture assay
Prior to co-culture, all cells were washed in RPMI. Aliquots of 105 MDMΦ were suspended in 200 µl of RPMI 1640 media with 10% fetal bovine serum and plated in a 96 well culture plate with apoptotic bodies at a ratio of 10 apoptotic bodies per cell. At the same time, AMA or control antibodies were added to the culture (100 µg/ml). Controls for each experiment included apoptotic bodies prepared from keratinocytes, control (non-AMA) antibodies and appropriate isotype-matched immunoglobulin. For a positive control, lipopolysaccharide was included in the culture medium at a final concentration of 10 µg/ml. MDMΦ cultured alone were used as a negative control. Other controls included cultures of MDMΦ with apoptotic bodies alone and MDMΦ with antibody alone. Anti-CD16 antibody (clone 3G8) and anti-CD16/32 were purchased from BD Pharmingen and Miltenyi Biotec, respectively, and used to block the fragment crystallizable receptor (FcR) (10 µg/ml). Treatment of MCDMΦ with anti-CD16 was initiated 30 min before adding apoptotic bodies. For full M1 polarization, MDMΦ from healthy controls were stimulated with 100 U/ml IFN-γ (Boehringer Ingelheim) and 1 ng/ml LPS (Cambrex) during the last 24 h of culture. All cultures were performed in RPMI 1640 for 24 h at 37 °C. Cell culture supernatants were collected, centrifuged to remove cell debris, harvested and stored at −80°C until use. All samples were run in duplicate.
Cytokine detection
Culture supernatants were analyzed by the Luminex multiplex platform using the Human Cytokine Antibody Bead Kit (Millipore). The assay kit allowed for the detection of IL-2, IL-4, IL-6, IL-10, IL-12p40, IFN-γ, TNF-α, GM-CSF, and MIP-1β. Briefly, analyte specific antibody conjugated beads were incubated with 50 µL of biotinylated detection antibody solution and 50 µL of either standard, or 25 µL of sample in 25 µL of assay diluent for 3 h at room temperature, in the dark, on an orbital shaker (500–600 rpm). After the wells were washed and aspirated by vacuum manifold aspiration, 100 µL of strepavidin-conjugated R-phycoerythrin (PE) was added to each well and incubated for 30 min at room temperature, in the dark, on an orbital shaker (500–600 rpm). PE fluorescence was measured using the Luminex100 LabMAP system. Cytokine concentrations from mean fluorescence values obtained were calculated from standard curves of each cytokine tested, using Bio-Plex Manager™ software. M-CSF was measured in sera using an ELISA kit (Antigenix America) (24).
Evaluation of cell phenotypes
Phenotypic analysis of peripheral blood mononuclear cells (PBMC) and MDMΦ was assessed on a FACScan flow cytometer upgraded by Cytec Development, using fluorochrome-conjugated monoclonal antibodies against cell surface markers CD3 (BD Biosciences), CD1a (Biolegend), CD14 (Caltag), CD16 (Biolegend), CCR2 (RD Systems), CX3CR1 (Biolegend), CD80 (eBioscience), CD83 (Caltag), and HLA-DR (Pharmigen). The appropriate fluorochrome-conjugated isotype control antibodies were used as negative controls. Acquired data were analyzed using the CELLQUEST Pro (BD Immunocytometry Systems) and FlowJo (Tree Star, Inc.) software packages.
Immunofluorescence and confocal microscopy
Uptake of apoptotic bodies by MDMΦ was evaluated by immunofluorescence microscopy. The apoptotic bodies utilized in these assays were isolated as follows. HIBECs or control keratinocytes were first stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) (3 µM) (Molecular Probes) for 20 min at 37°C. CFSE labeled cells were then induced to undergo apoptosis with bile salts. Following 3 h of incubation, apoptotic bodies were isolated from cellular supernatants as above. MDMΦ were co-incubated for 24 h with CFSE-labeled apoptotic bodies from HIBEC or the keratinocyte cultures in the presence of human AMA-IgG or control- IgG (100 µg/ml). When anti-CD16 was used, it was added to the culture system 30 min prior to AMA. Cells were then fixed in 3.7% formaldehyde (5 min, room temperature) and stained with PE-conjugated anti-human CD14 antibody diluted 1:200 overnight at 4°C. These cells were incubated with 4',6-diamidino-2-phenylindole (DAPI) (Invitrogen) to stain the nucleus. Controls consisting of incubating cells with secondary antibody alone did not demonstrate any detectable staining under the same conditions. For the phagocytosis experiments, MDMΦ were cultured in glass-bottom dishes and apoptotic bodies added to the culture medium at a constant ratio. Immunofluorescence-labeled samples were examined using a LSM5 Pascal confocal laser scanning microscope (Zeiss) with a 100X oil-immersion objective.
Phagocytosis was evaluated by randomly counting 100 MDMΦ per well at 100X using an oil immersion objective. The percentage of phagocytosis was calculated by counting the number of macrophages that had ingested at least one apoptotic body. The phagocytic index was expressed as the percentage of phagocytosis multiplied by the mean number of phagocytosed bodies per macrophage and was evaluated at 0, 4 and 24 h of incubation.
TNF-related apoptosis-inducing ligand (TRAIL) and Fas ligand (FasL) expression
Total RNA was extracted from MDMΦ using the QIAGEN RNeasy Mini Kit (Qiagen). One microgram of total RNA was reverse-transcribed and quantified by real-time PCR on an ABI Prism 7900HT Sequence Detection System. Amplification was performed for 40 cycles in a total volume of 12 µl and products detected using SYBR Green. The relative expression level of each target gene was determined by normalizing its mRNA level to the internal control glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primers used were: FasL forward 5’-AAAGTGGCCCATTTAACAGGC-3’, reverse 5’-AAAGCAGGACAATTCCATAGGTG-3’; TRAIL forward5’-ATGGCTATGATGGAGGTCCAG-3’, reverse 5’-TTGTCCTGCATCTGCTTCAGC-3’; GAPDH forward 5’-CCATGGAGAAGGCTGGGG-3’, reverse 5’-CAAAGTTGTCATGGATGACC-3’.
Statistical analysis
Statistical differences between groups were determined using a two tailed Mann-Whitney non-parametric test with 95% confidence interval (CI). All results were expressed as mean ± standard error (SEM). The percentages of phagocytosis among different cell populations were compared using a Fisher's Exact Test. We used the Prism statistical package (GraphPad Software Inc.) to carry out the analyses.
RESULTS
Macrophages from patients with PBC cultured with apoptotic bodies from HIBEC secrete pro-inflammatory cytokines only in the presence of AMA
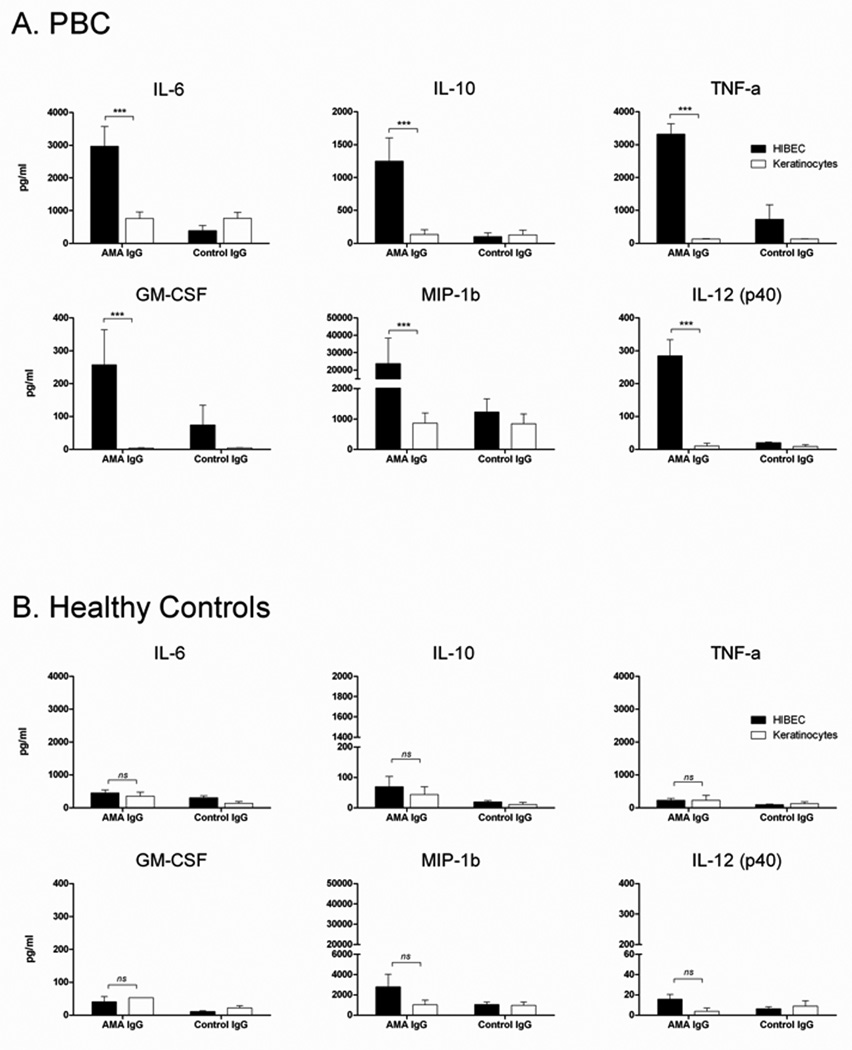
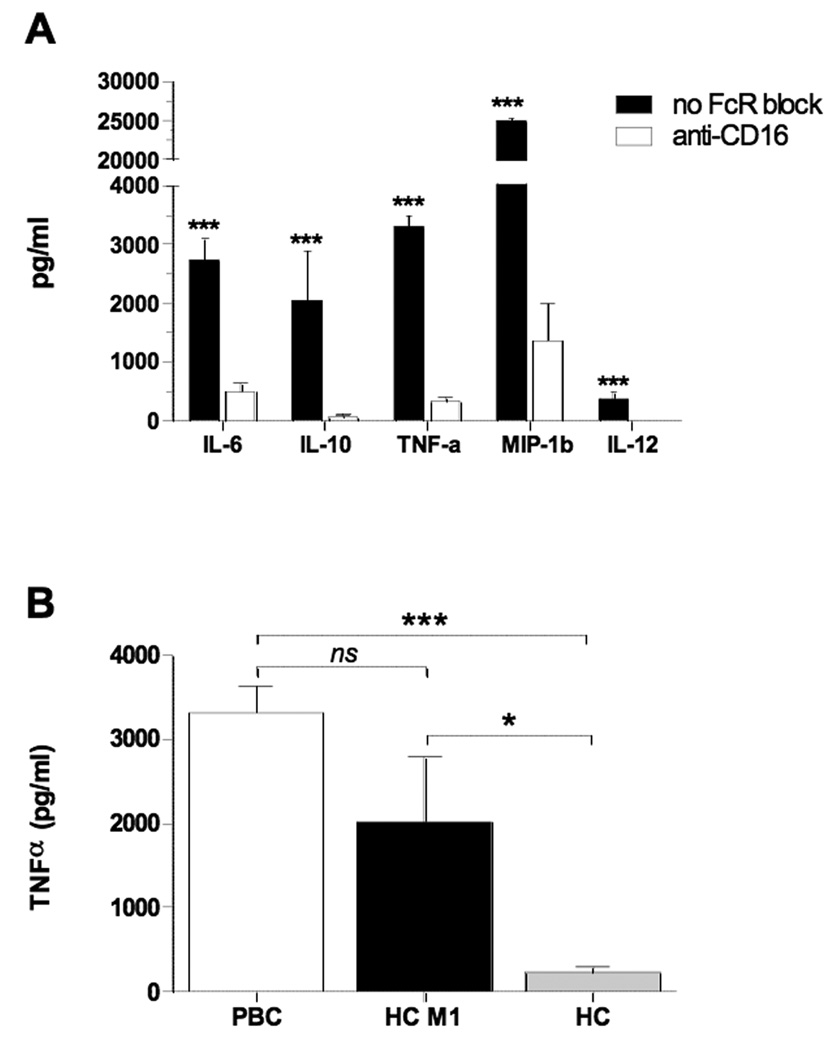
To determine factors that stimulate cytokine secretion in PBC, we incubated MDMΦ from patients with PBC together with AMA and apoptotic bodies from HIBEC; this led to increased secretion of pro-inflammatory cytokines. In the presence of AMA, there was a 10-fold higher level of secreted TNF-α and 2-fold higher levels of secreted IL-6, Il-10, MIP-1b and IL12p40 compared to cytokine secretion in the presence of control IgG (p<0.001) (Figure 1A). Although GM-CSF was added to the monocyte cultures to stimulate macrophage differentiation, its concentration in the culture supernatant was significantly higher in patients with PBC than controls (p<0.001), suggesting supplemental production of GM-CSF. The patient’s histological stage did not affect the cytokine levels or profiles secreted from the cultured cells. The level of cytokine release from MDMΦ of unaffected controls was independent of the presence of antibody (either AMA or control) or the source of the apoptotic bodies (HIBEC or control) (Figure 1B). Other controls included cultures of MDMΦ of patients with PBC (n=25) incubated only with apoptotic bodies from either HIBEC or control epithelial cells, which showed no significant level of cytokine production. This was also true when apoptotic bodies were incubated with MDMΦ from 20 unaffected donors (data not shown).
Figure 1. Macrophages from PBC cultured with apoptotic bodies from HIBEC secrete pro-inflammatory cytokines in the presence of AMA.
MDMΦ from patients with PBC (A) or unaffected controls (B) were cultured with apoptotic bodies and antibody (AMA IgG or control IgG). The triad of MDMΦ from patients with PBC (A), but not from controls (B), apoptotic bodies from HIBEC cells, and AMA leads to significantly increased secretion of TNF-α, IL-6, IL-10, MIP-1b, and IL12p40 compared to IgG control. Each experiment was performed in duplicate. Level of significance is denoted as ***p<0.001, based on a two tailed Mann-Whitney test with 95% CI.
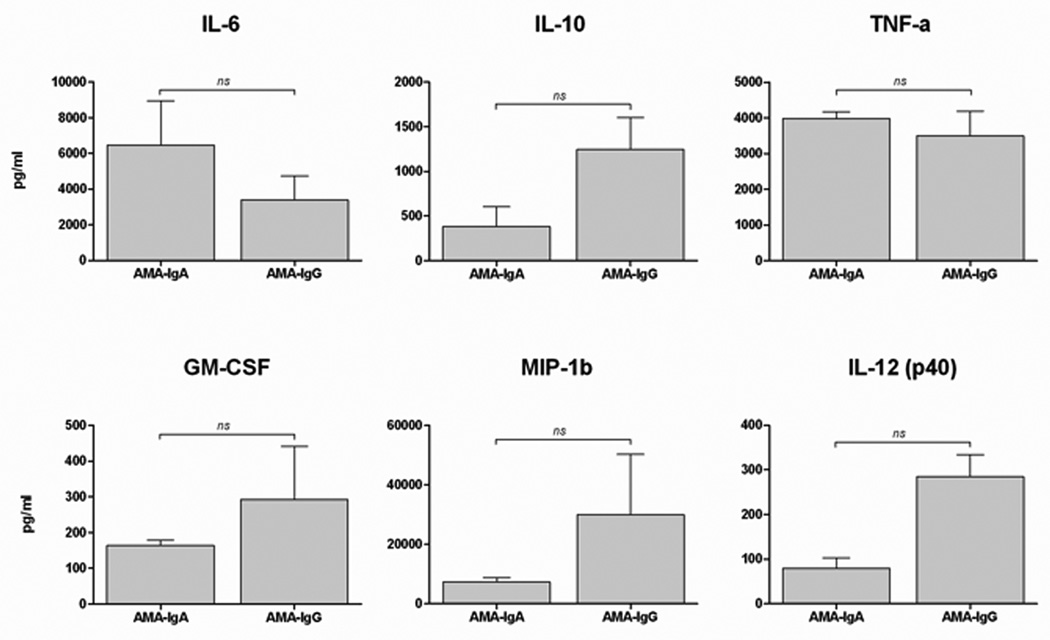
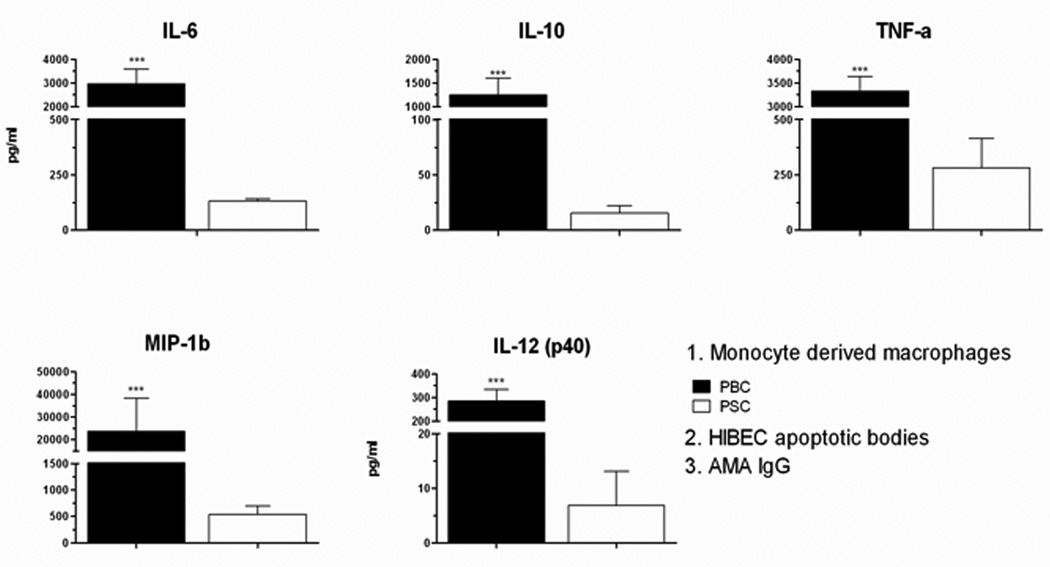
Both IgA and IgG AMA isotypes equally stimulated secretion of cytokines from MDMΦ of patients with PBC in the presence of HIBEC apoptotic bodies (Figure 2). We also evaluated the inflammatory response of MDMΦ from patients with PSC (n=6) and SLE (n=3) under the same conditions in the presence of AMA and HIBEC apoptotic bodies. The MDMΦ cells from patients with PBC demonstrated significantly higher levels of cytokine production than those from PSC (Figure 3) or SLE (data not shown). Hence, AMA-stimulated cytokine secretion was specific for PBC in presence of HIBEC apoptotic bodies.
Figure 2. Both IgA and IgG isotypes stimulate MDMΦ from patients with PBC.
MDMΦ from 10 patients with PBC and 10 controls were cultured with HIBEC apoptotic bodies in the presence of two different isotypes of AMA (IgG and IgA). Supernatants were collected after 24 h of incubation and cytokine concentration determined. While cytokine levels were markedly increased (see Figure 1A), there was no significant difference (ns) in levels of TNF-α, IL-6, Il-10, MIP-1b, IL12p40 and GM-CSF between AMA-IgG and IgA.
Figure 3. MDMΦ from PBC secrete significantly higher levels of cytokines compared to PSC patients.
Cytokine secretion in supernatants collected at 24 hours from MDMΦ isolated from patients with PBC (n=25) or PSC (n=6) cultured with HIBEC apoptotic bodies in the presence of AMA. Levels of TNF-α, IL-6, Il-10, MIP-1b, and IL12p40 were significantly higher in cultures with MDMΦ from PBC compared to PSC. Statistical difference was analyzed by a two tailed Mann-Whitney test (95% CI), *** p < 0.001. All samples were run in duplicate.
Phenotype of monocytes and MDMΦ from patients with PBC and controls
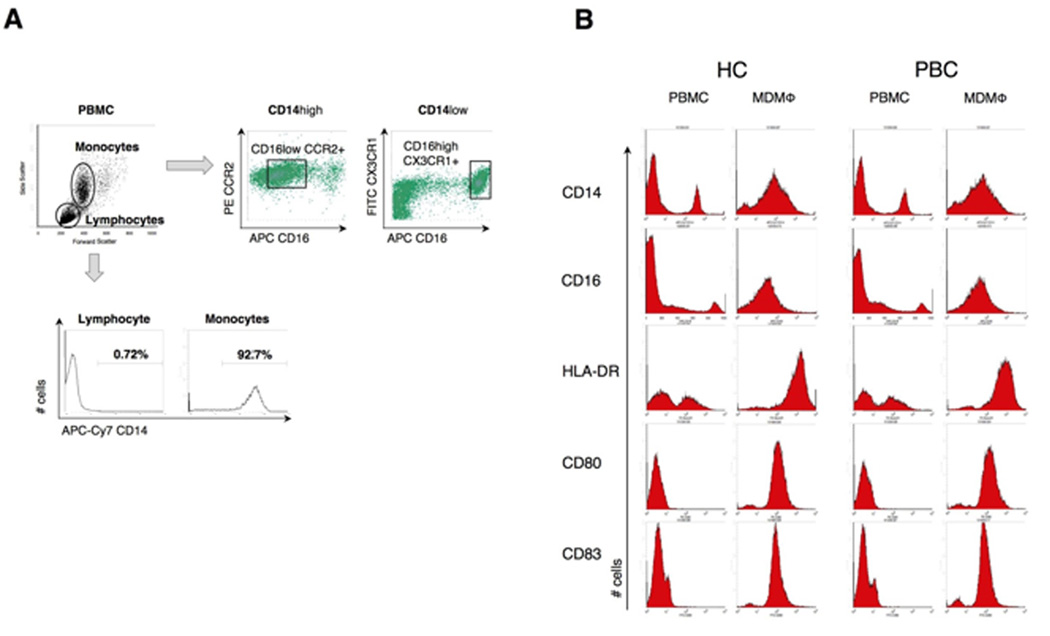
To address the question of whether cytokine secretion was a consequence of a different state of MDMΦ activation in patients with different diseases, we determined the phenotypes of monocytes from PBMC and MDMΦ. Freshly isolated PBMC and MDMΦ were stained with fluorochrome-conjugated antibodies to CD14, CD16, CCR2, CX3CR1, HLA-DR, CD80 and CD83 and phenotypes assessed by flow cytometry (Figure 4). Monocytes from freshly isolated PBMC were gated on the basis of CD14 expression into CD14 high and CD14 low sub-populations (Figure 4A) (25–26). These gated subsets were then analyzed for the expression of CD16 and chemokine receptors and two classical subpopulations of monocytes were identified: classical CD14highCD16- CCR2+ (87%) and a minor subset of CD14lowCD16+CX3CR1+ (13%). This monocyte phenotype from PBC patients was essentially the same as the monocyte phenotype of controls subjects. CD14+ monocytes were then stimulated with GM-CSF for 5 days and cultured in vitro with apoptotic bodies. Stimulated MDMΦ expressed higher levels of CD83, CD80 and HLA-DR compared to pre-stimulated monocytes (Fig. 4B and Table 1) (25, 27). The same profile of increased CD 80, CD83 and HLA-DR was also noted in MDMΦ from control donors (Table 1), as well as from patients with PSC and SLE (data not shown). To assess whether M-CSF determines different state of MDMΦ activation, serum levels of M-CSF from patients with PBC and controls were analyzed; however, no differences were revealed (data not shown).
Figure 4. Phenotype of circulating monocytes and MDMΦ as determined by FACS analysis of monocyte subsets and phenotype of monocytes and MDMΦ in patients with PBC and controls.
Cells were stained with fluorochrome-conjugated antibodies to CD14, CD16, CCR2, CX3CR1, HLA-DR, CD80 and CD83. (A) Monocytes and lymphocytes from PBMCs were gated on the basis of forward and side scatter profiles and two monocyte subpopulations were identified: classical CD14highCD16- CCR2+ (87%) and a minor subset of CD14lowCD16+CX3CR1+ (13%). (B) The enriched population of CD14+ monocytes were stimulated to develop MDMΦ and cultured with apoptotic bodies. No differences in the phenotypes of PBMC and MDMΦ were observed between PBC and HC.
Table 1.
Phenotype of monocytes from PBMC and MDMΦ from patients with PBC and healthy controls (HC).
| PBMC | MDMΦ | |||||
|---|---|---|---|---|---|---|
| PBC | HC | p | PBC | HC | p | |
| Tot CD14 (106/ml) | 1.1 ± 0.18 | 1.34 ± 0.13 | ns | 0.68 ± 0.13 | 0.66 ± 0.12 | ns |
|
CD14+ CD16low CCR2+ (106/ml) |
0.32 ± 0.09 | 0.37 ± 0.13 | ns | NA | ||
|
CD14low CD16+CX3CR1+ (106/ml) |
0.06 ± 0.02 | 0.08 ± 0.02 | ns | NA | ||
| CD14+ CD83+ (MFI) | 17.07 ± 1.56 | 13.89 ± 0.87 | ns | 114.01 ± 6.53 | 121.59 ± 4.32 | ns |
| CD14+ CD80+ (MFI) | 6.07 ± 0.18 | 5.87 ± 0.18 | ns | 137.45 ± 11.43 | 138.84 ± 8.50 | ns |
| CD14+ HLA DR+ (MFI) | 218.99 ± 28.81 | 242.31 ± 16.88 | ns | 776.62 ± 88.02 | 964.46 ± 88.08 | ns |
FcγR mediates cytokine production
To determine the role of Fcγ receptor in cytokine production, we evaluated responses of MDMΦ pre-incubated with or without anti-CD16 monoclonal antibody to block FcγRIII, followed by the addition of the apoptotic bodies and IgG AMA or control sera. MDMΦ from patients with PBC had markedly reduced cytokine production in the presence of anti-CD16 (p<0.001) (Figure 5A). After blocking FcγR, the cytokine levels were similar to those secreted by MDMΦ from patients with PBC incubated with HIBEC apoptotic bodies in the presence of control IgG.
Figure 5. Stimulation of PBC macrophages in the presence of AMA and HIBEC apoptotic bodies is mediated by the Fc Receptor and is a consequence of M1 polarization.
(A) Comparison of cytokine secretion from MDMΦ from patients with PBC (n=8) cultured with HIBEC apoptotic bodies in the presence of AMA with (white bars) or without (black bars) pre-treatment with CD16 monoclonal antibody (clone 3G8). Cytokine levels were markedly decreased when FcγR was blocked (***p< 0.001). (B) Comparison of TNFα secretion from MDMΦ from patients with PBC (n = 8) and healthy controls (n = 8) after M1 polarization (HC M1), and untreated (HC) cultured with HIBEC apoptotic bodies in the presence of AMA. (***p< 0.001, *p<0.05).
M1 polarized MDMΦ from healthy controls show strong reactivity against AMA and apoptotic bodies from biliary cells
We hypothesized that the pro-inflammatory cytokine response of MDMΦ from PBC patients but not controls might have resulted from M1 polarization of macrophages in PBC patients. When MDMΦ from healthy controls were polarized toward an M1 phenotype with IFNγ and LPS and co-cultured with AMA and HIBEC apoptotic bodies, they secreted significantly greater amounts of TNFα compared to untreated MDMΦ from the same subjects, but not compared to MDMΦ from PBC patients (Figure 5B).
Engulfment of apoptotic bodies by monocyte-derived phagocytes is reduced in patients with PBC
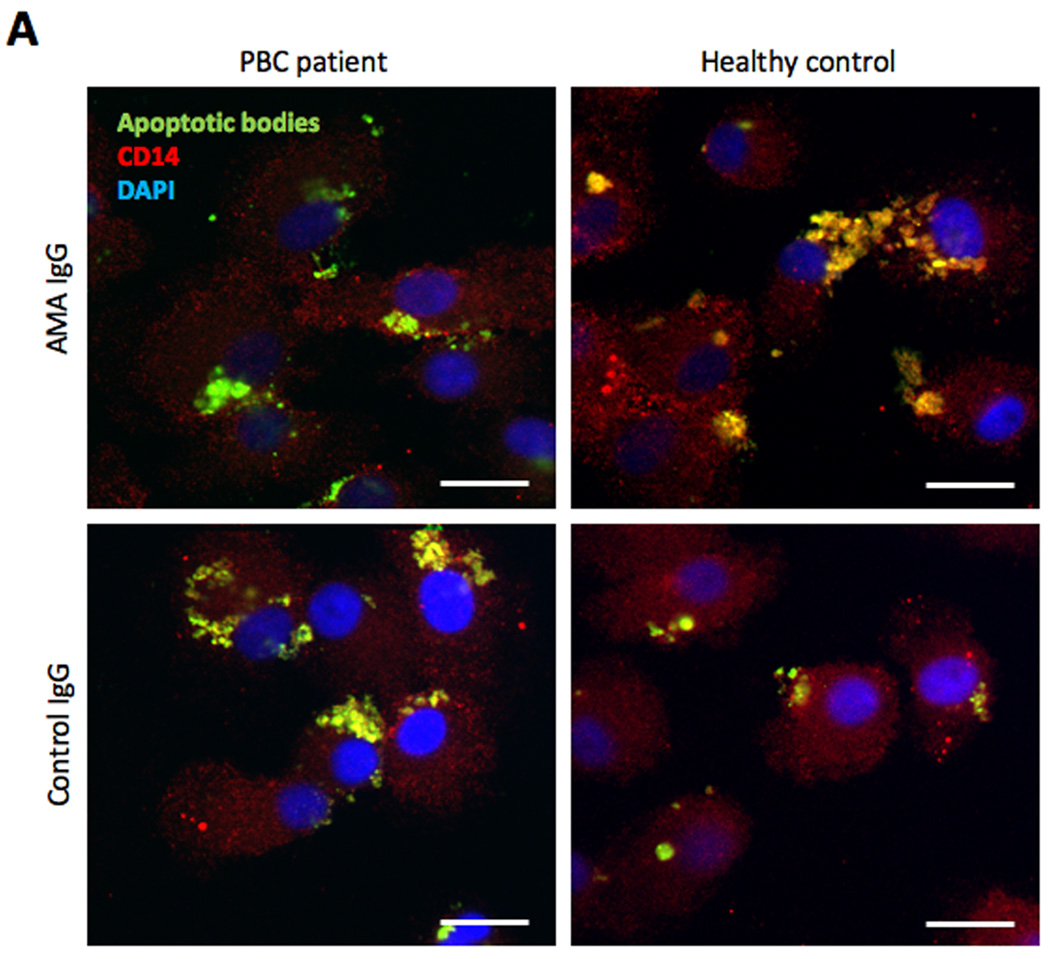
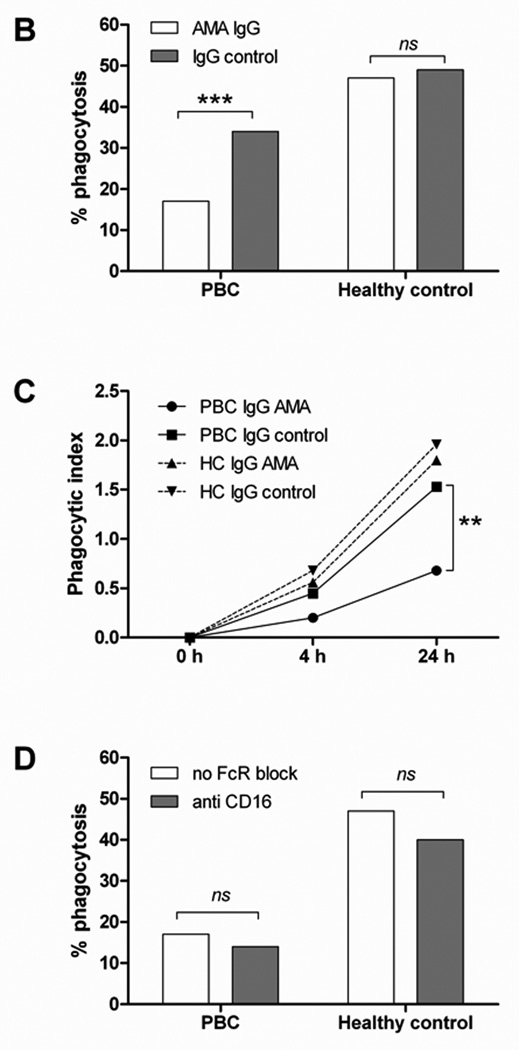
To assess whether MDMΦ cytokine production was associated with their ability to phagocytose apoptotic bodies, MDMΦ from patients with PBC and controls were co-incubated for 24 h with CFSE-labeled apoptotic bodies from HIBEC in the presence of human AMA-IgG or control-IgG and percentage of phagocytosis and phagocytic index were determined. MDMΦ from PBC and all control groups phagocytose apoptotic bodies during the 24 h incubation period (Figure 6A). However, MDMΦ from PBC demonstrated a reduced uptake (17% vs 47%, p<0.001) of apoptotic bodies (Figure 6B) associated with a decrease of the phagocytic index at 4 and 24 h (Figure 6C). Blocking the FcγR with anti-CD16 did not modify the ability of MDMΦ from both patients with PBC or controls to take up apoptotic bodies (Figure 6D), suggesting that cytokine production and phagocytosis follow independent pathways.
Figure 6. Uptake of apoptotic bodies.
(A) Confocal imaging of MDMΦ from a representative patient with PBC and an unaffected control co-incubated for 24 h with CFSE-labeled apoptotic bodies from HIBEC in the presence of human AMA-IgG or control- IgG. After culture the cells were fixed and stained with PE-conjugated anti-human CD14 (red), DAPI was used to stain the nucleus (blue), CFSE stained apoptotic bodies are shown in green. In all four conditions, apoptotic bodies (green) are located inside the cells, which indicate that MDMΦ actively engulfed apoptotic bodies after 24 h (arrows). Scale bar 20 µm. (B) Comparison of phagocytic efficacy, expressed as percentage of phagocytosis, of MDMΦ from PBC and controls; MDMΦ from PBC had a reduced uptake of HIBEC apoptotic bodies in the presence of AMA (17% vs 47%, ***p<0.001), this difference was not noted when IgG control was used (34% vs 49%, p = ns). Percentage of phagocytosis was calculated by counting the number of macrophages that had ingested at least one HIBEC apoptotic body. (Fisher's Exact Test). (C) The phagocytic index (PI) was expressed as percentage of phagocytosis multiplied by the mean number of phagocytosed bodies per macrophage (ABMΦ): PI = (% phagocytosis x mean ABMΦ/100) and was evaluated at 0, 4 and 24 h of incubation. ** p< 0.01 (D) The ability of MDMΦ from PBC and controls to uptake HIBEC apoptotic bodies, expressed as percentage of phagocytosis, was studied in presence of AMA, with or without FcγR block. No significant difference (ns) in the percentage of phagocytosis was observed when FcγR was blocked in both patients and controls. Statistical difference was determined by Fisher's Exact Test.
TRAIL expression is increased in MDMΦ from PBC patients cultured with apoptotic bodies from HIBEC in the presence of AMA
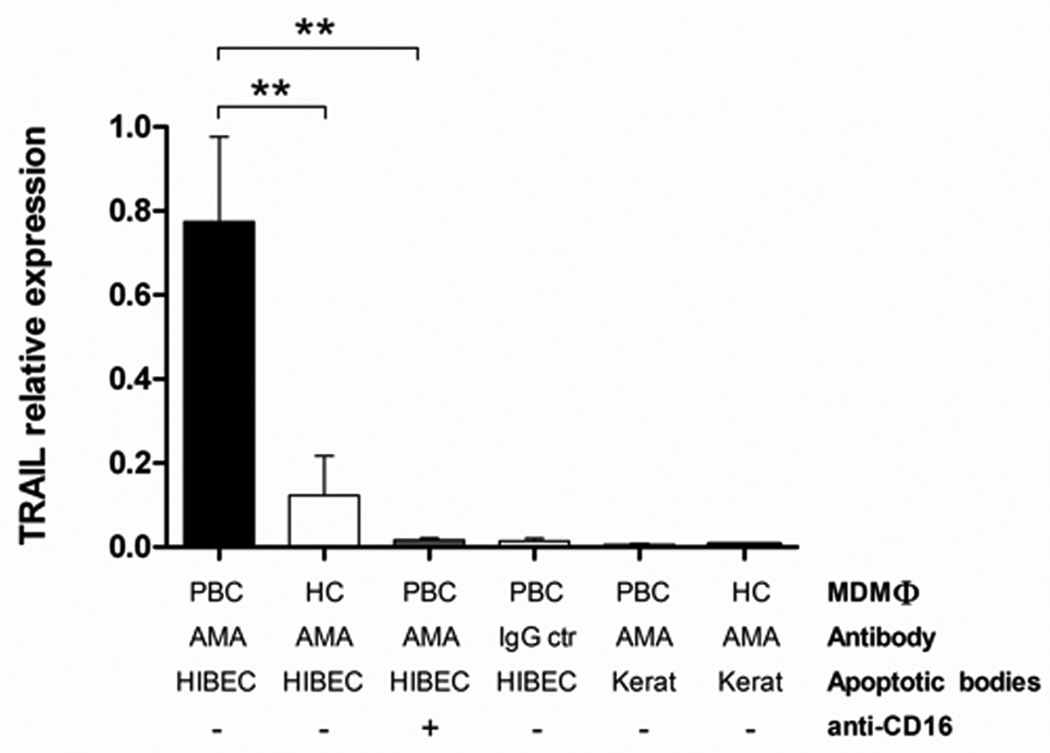
TRAIL and FasL are pro-apoptotic ligands (28) and TRAIL can induce BEC cell damage (29). Given the high levels of TNF-α secreted by MDMΦ from PBC patients when co-cultured with HIBEC apoptotic bodies in the presence of AMA, we quantified mRNAs encoding TRAIL and FasL in MDMΦ following incubation with apoptotic bodies and antibodies. TRAIL mRNA expression was markedly increased in MDMΦ from PBC patients when co-cultured with HIBEC apoptotic bodies in the presence of AMA (Figure 7). In contrast, no significant differences in the expression of FasL were noted (data not showed). These results suggest that TRAIL but not FasL plays a role in the pro-inflammatory response of MDMΦ in PBC.
Figure 7. TRAIL expression is increased in MDMΦ from PBC patients cultured with apoptotic bodies from HIBEC in the presence of AMA.
The relative expression levels of TRAIL in MDMΦ are compared with regards to MDMΦ, AMA, cell type of apoptotic bodies, and use of anti-CD16. Note that TRAIL expression is increased at least 4 fold (**p < 0.01) in PBC MDMΦ, AMA and apoptotic bodies from HIBEC and significantly reduced (**p < 0.01) when the cells are pretreated with anti-CD16.
DISCUSSION
Our present results and those of our previous studies provide insights into why the autoimmune damage in PBC is primarily confined to cells of the biliary epithelial tract. We previously reported that PDC-E2, the immunodominant autoantigen of PBC, remains immunologically intact in BECs following apoptosis and localizes within apoptotic bodies where it is accessible to AMA (16, 30). This phenomenon was specific to BECs and did not occur in other epithelial cell types tested. We now demonstrate that the triad of MDMΦ from patients with PBC, AMA and apoptotic bodies from BECs can stimulate a burst of pro-inflammatory cytokines. We hypothesize that within the intrahepatic biliary environment, the normal turnover of BECs becomes the source of the PDC-E2 apotope. PDC-E2 contained in the apoptotic bodies would be recognized by circulating AMA, and the apotope-AMA complex would then stimulate macrophages from subjects with a susceptible genetic background. This eventually leads to a localized burst of pro-inflammatory cytokines, and the ensuing inflammation would be associated with increased apoptosis of surrounding cells, including BECs, and perpetuation of local inflammation with chronic damage of the biliary tract. These data complete previous work from our group demonstrating that immune complexes including autoantibodies against PDC-E2 can be efficiently internalized, processed, and presented by antigen presenting cells (APCs) to induce specific cytotoxic T lymphocytes in PBC patients (31). We emphasize that the BECs used in our experiments were from normal donors. Hence our results imply that the BECs in PBC are “innocent victims” based on its apotope, and thus there is no specific phenotype of BECs in patients with PBC.
Our results can explain several phenomena that occur in PBC. First, they explain why only BECs are targeted in PBC. They further suggest that we should examine salivary gland epithelium to see whether apoptotic bodies derived from these cells, which are also affected in the disease, are enriched for PBC-specific autoantigens. Moreover, PBC frequently recurs in patients following liver transplantation (19), in a situation where absence of MHC matching would preclude presentation of PDC-E2 to elements of the adaptive immune system and compromise the ability of CD8+ T-cells to attack BECs (5). Indeed, apoptosis of BECs in the newly transplanted liver completes the triad required for inflammatory cytokine release and provides a milieu in which disease can recur. Furthermore, since steroids mainly suppress T cell-mediated immunity (32), our results explain the unsatisfactory response of PBC to classic immunosuppressive agents (20) and why ursodiol, an agent that suppresses apoptosis (21), is effective in slowing disease progression in PBC (21). Our results also have broader implications for other organ-specific autoimmune diseases in which the target autoantigen is widely distributed (33–36), such as in neonatal cardiomyopathy of anti-Ro positive mothers (37–38).
Macrophages are essential effector cells that undergo various forms of activation in response to cytokines and microbial signals. In particular, IFN-γ and IL-4 activate distinct functional programs, inducing M1 (or classic) and M2 (or alternative) activation, which mirror the Th1/Th2 dichotomy (39–40). It is known that the innate immune system of patients with PBC demonstrates a higher reactivity than controls. Indeed, peripheral monocytes from patients with PBC secrete high levels of cytokines (41). Moreover, the frequency and absolute number of blood and liver natural killer cells in patients with PBC are increased, as well as their cytotoxic activity and perforin expression (42). However, very little is known about the state of macrophage activation in autoimmunity. Our data emphasize an essential role of the innate immune system in PBC, and suggest that MDMΦ in patients with PBC are polarized, most likely to M1, as a consequence of the specific microenvironment (43–44). This would explain the strong reactivity of MDMΦ from patients when they interact with AMA and apoptotic bodies of HIBEC. A minority of patients with PBC, less than 5% when tested by Luminex (45), does not have detectable serum AMA. According to our model, these patients should either have biliary disease mediated by a different mechanism or antibodies that recognize still unidentified apotopes. In this regard, so-called “AMA negative” patients with PBC are more likely to produce antinuclear antibodies than those with detectable AMA (1). Future studies should test for the possible role of known nuclear autoantigens in the inflammatory cytokine response in PBC.
Recent data from a genome wide association study (46) and animal models (47) have identified the IL-12 signaling pathway as a central player in the etiopathogenesis of PBC. In our study, MDMΦ from patients with PBC in the presence of HIBEC apoptotic bodies and AMA secrete high levels of IL-12p40. Hence, our results provide additional support for a central role of IL-12 signaling in PBC pathogenesis. Our data further demonstrate that activation of MDMΦ from patients with PBC is mediated by FcγRIII. Activating FcγRs may facilitate antigen presentation and dendritic-cell maturation (48), while in the late phase of the immune response, the inhibitory FcγRIIb may down-regulate B-cell activation upon cross-linking with activating receptors (49). Our current results suggest that the immune complex of AMA and BEC apotope specifically engages the FcγRIII and stimulates cytokine production. FcγRIII mediated activation of APCs is known to be pro-inflammatory (50); however, it is usually associated with stimulation of phagocytosis (51). Similar to neonatal cardiomyopathy (38), we demonstrate a reduced uptake of HIBEC apoptotic bodies by MDMΦ in the presence of AMA that is not CD16 mediated. This could decrease clearance of antigen and contribute to the perpetuation of the innate immune response. However, our data also suggest that FcγRIII is not the only mechanism as AMA-IgA is also able to stimulate macrophages from patients with PBC in the presence of HIBEC apoptotic bodies.
Macrophage activation may lead to direct damage of BECs, perhaps due to high levels of TNF-α or TRAIL, which belongs to the TNF family of cytokines and signals through receptors that contain cytoplasmic death domains (28). TRAIL can induce BEC cell damage (29) and bile acids can increase TRAIL effects through the up regulation of its receptor and the cellular responses to TRAIL have been related to the inflammatory status of the liver, indicating a link between cholestasis and TRAIL signaling (52). Our results thus highlight the essential role of the innate immune system in PBC and demonstrate a role of AMA in BEC damage. Clearly, there are functional differences in the monocytic population from PBC that respond to apoptotic blebs of bile duct cells only in the presence of AMA. Future studies must now define the specific responsive monocytic population using primary monocytes and pDCs as well as GM-CSF or GM-CSF/IL-4 stimulated macrophages and mDC. Identification of this population will be important towards defining functional signaling pathways using differential phosphorylation of specific signal proteins in cells stimulated with blebs/AMA. These studies, including the potential use of next-generation deep sequencing will determine not only all genes in the activated signaling pathways, but also collectively define why PBC monocytes react to induce a pro-inflammatory environment at the site of tissue damage.
Acknowledgments
Grant Support: financial support provided by National Institutes of Health, DK39588 (MEG) and DK41876 (GJG).
Abbreviations
- PBC
primary biliary cirrhosis
- AMA
anti-mitochondrial antibodies
- PDC-E2
E2 component of the pyruvate dehydrogenase complex
- BEC
biliary epithelial cells
- MDMΦ
monocyte derived macrophages
- PSC
primary sclerosing cholangitis
- SLE
systemic lupus erythematosus
- HIBEC
human intrahepatic biliary epithelial cells
- FcR
fragment crystallizable receptor
- PE
R-phycoerythrin
- PBMC
peripheral blood mononuclear cells
- MFI
median fluorescence intensity
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- DAPI
4',6-diamidino-2-phenylindole
- TRAIL
TNF-related apoptosis-inducing ligand
- FasL
Fas ligand
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- CI
confidence interval
- SEM
standard error of the mean
- APCs
antigen presenting cells
- pDC
plasmacytoid dendritic cells
- mDC
myeloid dendritic cells
Footnotes
Conflict of interest: the authors declare that no conflict of interest exists
Contributor Information
Ana Lleo, Email: alleo@ucdavis.edu.
Christopher L. Bowlus, Email: clbowlus@ucdavis.edu.
Guo-Xiang Yang, Email: gxyang@ucdavis.edu.
Pietro Invernizzi, Email: pietro.invernizzi@humanitas.it.
Mauro Podda, Email: mauro.podda@unimi.it.
Judy Van de Water, Email: javandewater@ucdavis.edu.
Aftab A. Ansari, Email: pathaaa@emory.edu.
Ross L. Coppel, Email: ross.coppel@med.monash.edu.au.
Howard J. Worman, Email: hjw14@columbia.edu.
Gregory J. Gores, Email: gores.gregory@mayo.edu.
M. Eric Gershwin, Email: megershwin@ucdavis.edu.
REFERENCES
- 1.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 2.Kawano A, Shimoda S, Kamihira T, Ishikawa F, Niiro H, Soejima Y, Taketomi A, et al. Peripheral tolerance and the qualitative characteristics of autoreactive T cell clones in primary biliary cirrhosis. J Immunol. 2007;179:3315–3324. doi: 10.4049/jimmunol.179.5.3315. [DOI] [PubMed] [Google Scholar]
- 3.Shimoda S, Nakamura M, Ishibashi H, Hayashida K, Niho Y. HLA DRB4 0101-restricted immunodominant T cell autoepitope of pyruvate dehydrogenase complex in primary biliary cirrhosis: evidence of molecular mimicry in human autoimmune diseases. J Exp Med. 1995;181:1835–1845. doi: 10.1084/jem.181.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimoda S, Miyakawa H, Nakamura M, Ishibashi H, Kikuchi K, Kita H, Niiro H, et al. CD4 T-cell autoreactivity to the mitochondrial autoantigen PDC-E2 in AMA-negative primary biliary cirrhosis. J Autoimmun. 2008;31:110–115. doi: 10.1016/j.jaut.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Yang GX, Lian ZX, Chuang YH, Moritoki Y, Lan RY, Wakabayashi K, Ansari AA, et al. Adoptive transfer of CD8(+) T cells from transforming growth factor beta receptor type II (dominant negative form) induces autoimmune cholangitis in mice. Hepatology. 2008;47:1974–1982. doi: 10.1002/hep.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Migliaccio C, Nishio A, Van de Water J, Ansari AA, Leung PS, Nakanuma Y, Coppel RL, et al. Monoclonal antibodies to mitochondrial E2 components define autoepitopes in primary biliary cirrhosis. J Immunol. 1998;161:5157–5163. [PubMed] [Google Scholar]
- 7.Mattalia A, Quaranta S, Leung PS, Bauducci M, Van de Water J, Calvo PL, Danielle F, et al. Characterization of antimitochondrial antibodies in health adults. Hepatology. 1998;27:656–661. doi: 10.1002/hep.510270303. [DOI] [PubMed] [Google Scholar]
- 8.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525–3531. [PubMed] [Google Scholar]
- 9.Van de Water J, Gershwin ME, Leung P, Ansari A, Coppel RL. The autoepitope of the 74-kD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. J Exp Med. 1988;167:1791–1799. doi: 10.1084/jem.167.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selmi C, Mayo MJ, Bach N, Ishibashi H, Invernizzi P, Gish RG, Gordon SC, et al. Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology. 2004;127:485–492. doi: 10.1053/j.gastro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, Lindor KD, et al. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194–1202. doi: 10.1002/hep.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakabayashi K, Lian ZX, Leung PS, Moritoki Y, Tsuneyama K, Kurth MJ, Lam KS, et al. Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology. 2008;48:531–540. doi: 10.1002/hep.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 14.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 15.Nagata SHR, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, Gores GJ, et al. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49:871–879. doi: 10.1002/hep.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed JH, Jackson MW, Gordon TP. A B cell apotope of Ro 60 in systemic lupus erythematosus. Arthritis Rheum. 2008;58:1125–1129. doi: 10.1002/art.23377. [DOI] [PubMed] [Google Scholar]
- 18.Reed JH, Jackson MW, Gordon TP. B cell apotopes of the 60-kDa Ro/SSA and La/SSB autoantigens. J Autoimmun. 2008;31:263–267. doi: 10.1016/j.jaut.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Van de Water J, Gerson LB, Ferrell LD, Lake JR, Coppel RL, Batts KP, Wiesner RH, et al. Immunohistochemical evidence of disease recurrence after liver transplantation for primary biliary cirrhosis. Hepatology. 1996;24:1079–1084. doi: 10.1002/hep.510240517. [DOI] [PubMed] [Google Scholar]
- 20.Silveira MG, Lindor KD. Treatment of primary biliary cirrhosis: therapy with choleretic and immunosuppressive agents. Clin Liver Dis. 2008;12:425–443. doi: 10.1016/j.cld.2008.02.008. x–xi. [DOI] [PubMed] [Google Scholar]
- 21.Chamulitrat W, Burhenne J, Rehlen T, Pathil A, Stremmel W. Bile salt-phospholipid conjugate ursodeoxycholyl lysophosphatidylethanolamide as a hepatoprotective agent. Hepatology. 2009;50:143–154. doi: 10.1002/hep.22955. [DOI] [PubMed] [Google Scholar]
- 22.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 23.Chapman RW, Arborgh BA, Rhodes JM, Summerfield JA, Dick R, Scheuer PJ, Sherlock S. Primary sclerosing cholangitis: a review of its clinical features, cholangiography, and hepatic histology. Gut. 1980;21:870–877. doi: 10.1136/gut.21.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menke J, Rabacal WA, Byrne KT, Iwata Y, Schwartz MM, Stanley ER, Schwarting A, et al. Circulating CSF-1 promotes monocyte and macrophage phenotypes that enhance lupus nephritis. J Am Soc Nephrol. 2009;20:2581–2592. doi: 10.1681/ASN.2009050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ancuta P, Liu KY, Misra V, Wacleche VS, Gosselin A, Zhou X, Gabuzda D. Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16− monocyte subsets. BMC Genomics. 2009;10:403. doi: 10.1186/1471-2164-10-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 27.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 28.Schutze S, Tchikov V, Schneider-Brachert W. Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat Rev Mol Cell Biol. 2008;9:655–662. doi: 10.1038/nrm2430. [DOI] [PubMed] [Google Scholar]
- 29.Takeda K, Kojima Y, Ikejima K, Harada K, Yamashina S, Okumura K, Aoyama T, et al. Death receptor 5 mediated-apoptosis contributes to cholestatic liver disease. Proc Natl Acad Sci U S A. 2008;105:10895–10900. doi: 10.1073/pnas.0802702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odin JA, Huebert RC, Casciola-Rosen L, LaRusso NF, Rosen A. Bcl-2-dependent oxidation of pyruvate dehydrogenase-E2, a primary biliary cirrhosis autoantigen, during apoptosis. J Clin Invest. 2001;108:223–232. doi: 10.1172/JCI10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kita H, Lian ZX, Van de Water J, He XS, Matsumura S, Kaplan M, Luketic V, et al. Identification of HLA-A2-restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J Exp Med. 2002;195:113–123. doi: 10.1084/jem.20010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leung DY, Bloom JW. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2003;111:3–22. doi: 10.1067/mai.2003.97. quiz 23. [DOI] [PubMed] [Google Scholar]
- 33.Clancy RM, Buyon JP, Ikeda K, Nozawa K, Argyle DA, Friedman DM, Chan EK. Maternal antibody responses to the 52-kd SSA/RO p200 peptide and the development of fetal conduction defects. Arthritis Rheum. 2005;52:3079–3086. doi: 10.1002/art.21289. [DOI] [PubMed] [Google Scholar]
- 34.Miranda-Carus ME, Askanase AD, Clancy RM, Di Donato F, Chou TM, Libera MR, Chan EK, et al. Anti-SSA/Ro and anti-SSB/La autoantibodies bind the surface of apoptotic fetal cardiocytes and promote secretion of TNF-alpha by macrophages. J Immunol. 2000;165:5345–5351. doi: 10.4049/jimmunol.165.9.5345. [DOI] [PubMed] [Google Scholar]
- 35.Karnabi E, Qu Y, Wadgaonkar R, Mancarella S, Yue Y, Chahine M, Clancy RM, et al. Congenital heart block: Identification of autoantibody binding site on the extracellular loop (domain I, S5-S6) of alpha(1D) L-type Ca channel. J Autoimmun. 2009 doi: 10.1016/j.jaut.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neufing PJ, Clancy RM, Jackson MW, Tran HB, Buyon JP, Gordon TP. Exposure and binding of selected immunodominant La/SSB epitopes on human apoptotic cells. Arthritis Rheum. 2005;52:3934–3942. doi: 10.1002/art.21486. [DOI] [PubMed] [Google Scholar]
- 37.Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–182. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 38.Clancy RM, Neufing PJ, Zheng P, O'Mahony M, Nimmerjahn F, Gordon TP, Buyon JP. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116:2413–2422. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantovani A, Locati M. Orchestration of macrophage polarization. Blood. 2009;114:3135–3136. doi: 10.1182/blood-2009-07-231795. [DOI] [PubMed] [Google Scholar]
- 41.Mao TK, Lian ZX, Selmi C, Ichiki Y, Ashwood P, Ansari AA, Coppel RL, et al. Altered monocyte responses to defined TLR ligands in patients with primary biliary cirrhosis. Hepatology. 2005;42:802–808. doi: 10.1002/hep.20859. [DOI] [PubMed] [Google Scholar]
- 42.Chuang YH, Lian ZX, Tsuneyama K, Chiang BL, Ansari AA, Coppel RL, Gershwin ME. Increased killing activity and decreased cytokine production in NK cells in patients with primary biliary cirrhosis. J Autoimmun. 2006;26:232–240. doi: 10.1016/j.jaut.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun. 2008;31:252–256. doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagano T, Yamamoto K, Matsumoto S, Okamoto R, Tagashira M, Ibuki N, Matsumura S, et al. Cytokine profile in the liver of primary biliary cirrhosis. J Clin Immunol. 1999;19:422–427. doi: 10.1023/a:1020511002025. [DOI] [PubMed] [Google Scholar]
- 45.Oertelt S, Rieger R, Selmi C, Invernizzi P, Ansari AA, Coppel RL, Podda M, et al. A sensitive bead assay for antimitochondrial antibodies: Chipping away at AMA-negative primary biliary cirrhosis. Hepatology. 2007;45:659–665. doi: 10.1002/hep.21583. [DOI] [PubMed] [Google Scholar]
- 46.Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Gu X, Walker EJ, et al. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360:2544–2555. doi: 10.1056/NEJMoa0810440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida K, Yang GX, Zhang W, Tsuda M, Tsuneyama K, Moritoki Y, Ansari AA, et al. Deletion of interleukin-12p40 suppresses autoimmune cholangitis in dominant negative transforming growth factor beta receptor type II mice. Hepatology. 2009;50:1494–1500. doi: 10.1002/hep.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest. 2005;115:2914–2923. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamir I, Stolpa JC, Helgason CD, Nakamura K, Bruhns P, Daeron M, Cambier JC. The RasGAP-binding protein p62dok is a mediator of inhibitory FcgammaRIIB signals in B cells. Immunity. 2000;12:347–358. doi: 10.1016/s1074-7613(00)80187-9. [DOI] [PubMed] [Google Scholar]
- 50.Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol. 2002;2:580–592. doi: 10.1038/nri856. [DOI] [PubMed] [Google Scholar]
- 51.Frisoni L, McPhie L, Colonna L, Sriram U, Monestier M, Gallucci S, Caricchio R. Nuclear autoantigen translocation and autoantibody opsonization lead to increased dendritic cell phagocytosis and presentation of nuclear antigens: a novel pathogenic pathway for autoimmunity? J Immunol. 2005;175:2692–2701. doi: 10.4049/jimmunol.175.4.2692. [DOI] [PubMed] [Google Scholar]
- 52.Higuchi H, Grambihler A, Canbay A, Bronk SF, Gores GJ. Bile acids up-regulate death receptor 5/TRAIL-receptor 2 expression via a c-Jun N-terminal kinase-dependent pathway involving Sp1. J Biol Chem. 2004;279:51–60. doi: 10.1074/jbc.M309476200. [DOI] [PubMed] [Google Scholar]