Abstract
Background
Women with fecal incontinence (FI) and rectal urgency have increased rectal stiffness and sensation.
Aims
To evaluate the effects of clonidine, an (α2-adrenergic agonist, in FI.
Methods
In this open-label uncontrolled study, bowel symptoms and anorectal functions (anal pressures, rectal compliance, and sensation) were assessed before and during treatment with transdermal clonidine (0.2 mg daily, 4 weeks) in 12 women with urge-predominant FI.
Results
Clonidine reduced the frequency (17.8 ± 3.1 before vs 8.8 ± 3.9 after, p = 0.03), and number of days with FI (11.8 ± 1.6 before vs 6.1 ± 1.8 after, p = 0.02), FI symptom severity score (max = 13, 8.3 ± 0.7 vs 5.6 ± 0.9, p<0.01), and allowed patients to defer defecation for a longer duration (p = 0.03). While overall effects on anorectal functions were not significant, the treatment-associated reduction in FI episodes was associated with increased rectal compliance (r = −0.58, p < 0.05) and reduced rectal sensation. (r = −0.73, p = 0.007 versus desire to defecate pressure threshold).
Conclusions
Clonidine improves symptoms in women with FI; this improvement is associated with increased rectal compliance and reduced rectal sensitivity. A controlled study is necessary to confirm these observations.
Keywords: clonidine, fecal incontinence, rectal compliance, rectal capacity, urgency
Fecal incontinence (FI) is a relatively common problem, particularly among women and the elderly, which can significantly impair daily functioning and also contribute to institutionalization.(1) Current management relies upon conservative measures to modify diet and bowel habits and biofeedback therapy for patients who do not respond to other conservative measures. (2) Although amitryptiline and loperamide improved diarrhea and rectal urgency in uncontrolled studies, (3-5) the use of these agents is often limited by constipation. The role of anal sphincteroplasty is unclear since continence is preserved in ≤ 40% of patients at 3-5 years after surgery. (6) Sacral nerve stimulation can also improve FI.
While most attention has focused on anal sphincter injury and weakness, there is increasing evidence for rectal sensorimotor dysfunctions in FI. For example, we observed reduced rectal capacity in 27% and reduced rectal compliance in 23% of women with FI. (7, 8) Reduced rectal capacity was more common in women with (i.e, 36%) than without (i.e, 8%) urgency. These observations have been confirmed by other groups. (9, 10)
Clonidine is an α2-adrenergic agonist that inhibits gastrointestinal motor activity by presynaptically inhibiting acetylcholine release from nerves in the myenteric plexus and at the neuromuscular junction. (11) Adrenergic α2 receptors are also widely distributed on nociceptive pathways in the spinal cord, brain stem, and forebrain. (12) In humans, clonidine (i) reduced colonic tone but not the colonic response to a meal, increased colonic compliance, and reduced colonic perception in healthy subjects, (13) (ii) reduced rectal compliance and rectal sensation in healthy subjects, (14) and (iii) improved symptoms in diarrhea-predominant IBS. (15) Therefore, this study evaluated the effects of clonidine on symptoms and anorectal sensorimotor functions in FI. Our hypotheses were that clonidine will improve symptoms and anorectal sensorimotor dysfunctions in women with urge-predominant FI.
MATERIAL & METHODS
Study Design
After obtaining written informed consent, bowel symptoms and anorectal sensorimotor functions were assessed before and after clonidine treatment in 12 women (age 58 ± 3 years, [Mean ± SEM] BMI 32.4 ± 1.8 kg/m2) with urge-predominant FI in this study which was approved by the Institutional Review Board at Mayo Clinic. This study was initiated before publicly available registries were developed. All patients had a clinical assessment to exclude significant organic disease. The study comprised a 4-week baseline period without medication followed by a 4-week treatment phase. Bowel habits were recorded in daily diaries for 4 weeks at baseline and then for 4 weeks during treatment with clonidine. Anorectal functions were evaluated before and during treatment with clonidine.
Participants
To be included in the study, patients had to complain of fecal incontinence for 6 months or longer. Only subjects with urge or combined FI, as defined below, and 4 or more incontinent episodes during the 4-week baseline period were permitted to participate in the 4-week treatment phase. Exclusion criteria included diabetes mellitus with neuropathy, congenital anorectal malformations, previous rectal surgery (such as rectopexy or rectal resection); chronic inflammatory bowel disease; pelvic radiation; active anal abscesses and fistulae; neurologic diseases such as clinically significant peripheral neuropathy or spinal cord injury. Except for the following conditions (i.e., hypothyroidism with euthyroid status on pharmacological supplementation, degenerative joint disease, urinary incontinence, clinically stable bronchial asthma, hypertension, hyperlipidemia, or anxiety/depression), patients with chronic medical conditions were also excluded from the study.
Medication
A clonidine patch (Catapres #2 patch, Boehringer Ingelheim, Ridgefield, Ct), which is designed to deliver 0.2 mg clonidine daily, was applied to a fresh skin site at weekly intervals during the 4-week treatment phase. The transdermal preparation provides stable plasma concentrations over 7 days and has a lower incidence of systemic side effects (i.e., drowsiness and hypotension) than oral clonidine. (16, 17) Participants were not permitted to use other medications which affect gastrointestinal motility or sensation (e.g., anticholinergic agents). However, use of loperamide as a rescue medication for intolerable diarrhea was permitted and recorded.
Symptom Assessments
At baseline, patients completed a validated questionnaire pertaining to bowel symptoms, abdominal discomfort, as well as severity and circumstances surrounding FI. (18) (19) These responses were used to grade the severity of FI by a validated scoring system (i.e., Fecal Incontinence and Continence Assessment [FICA]) incorporating the type, frequency, amount, and characteristics (i.e., urge, passive, or combined) of FI. (Table 1) (18) FI was characterized as urge, passive, combined (i.e., urge and passive) or neither, based on patient responses to the questionnaire. Those patients who reported they were “often“ or “usually“ incontinent because they had “great urgency and could not reach the toilet on time“ were considered to have urge incontinence. Those patients who reported they were “often“ or “usually“ “unaware when the leakage was actually happening“ were considered to have “passive“ incontinence.
Table 1.
Effects of Clonidine on Symptoms
| Before clonidine | During clonidine | P value | |
|---|---|---|---|
| Bowel habits | |||
| Stool frequency/day | 2.5 ± 0.7 | 2.2 ± 0.6 | 0.05 |
| Stool consistency (Bristol stool form score) |
4.3 ± 1.2 | 4.3 ± 1.3 | ns |
| Duration for which defecation could be deferred (minutes) |
1.6 ± 0.5 | 3.0 ± 0.9 | 0.02<p≤0.05 |
| Rectal urgency (% of bowel movements preceded by urgency) |
57 ± 16 | 45 ± 13 | 0.06 |
| Proportion of complete bowel movements |
76 ± 9 % | 81 ± 8% | 0.07 |
| Fecal incontinence | |||
| Number of days with FI | 11.8 ± 1.6 | 6.1 ± 1.8 | 0.02 |
| Number of FI episodes | 17.8 ± 3.1 | 8.8 ± 3.9 | 0.03 |
| Proportion of incontinent bowel movements |
28 ± 8% | 16 ± 5% | 0.005 |
| Volume of FI | |||
| Staining only (%) | 50 ± 10 | 65 ± 10 | 0.02 |
| Moderate FI (%) | 28 ± 10 | 14 ± 10 | ns |
| Full bowel movement (%) | 22 ± 10 | 20 ± 10 | ns |
| Symptom severity score (max = 13) | 8.3 ± 0.7 | 5.6 ± 0.9 | <0.01 |
| VAS Symptom Relief Score | 1.9 ± 0.4 | 5.2 ± 0.7 | < 0.001 |
| Quality of life scale – lifestyle | 2.6 ± 0.3 | 2.7 ± 0.3 | ns |
| Quality of life scale – coping/behavior | 1.7 ± 0.2 | 2.0 ± 0.2 | ns |
| Quality of life scale – embarrassment | 1.9 ± 0.3 | 2.3 ± 0.3 | ns |
| Use of loperamide | |||
| Mean ± SEM | |||
Participants recorded the details of every bowel movement in a diary for 4 weeks before and 4 weeks during clonidine treatment. For every continent and incontinent bowel movement, subjects recorded presence of urgency prior to defecation, the duration for which defecation could be deferred, stool form quantified by the Bristol scale, (20) and satisfaction after defecation (e.g., sense of incomplete evacuation).
In addition to daily bowel diaries, patients completed a weekly questionnaire which evaluated the severity of FI and its impact on quality of life. (21) Satisfaction with treatment was also assessed by a 10 cm VAS scale anchored by the terms “not satisfied at all“ and “completely satisfied“. Quality of life was evaluated by a validated instrument.
Assessment of Anorectal Sensorimotor Functions
After 2 sodium phosphate enemas (Fleets #, C.B. Fleet, Lynchburg, VA), anorectal testing (i.e., anal manometry and assessment of rectal compliance and sensation by a barostat) was conducted in the left lateral position. Average anal resting and squeeze pressures were measured using the station pull-through technique and summarized as described previously. (22) Rectal compliance and sensation were recorded using previously validated techniques by an “infinitely“ compliant 7-cm long balloon with a maximum volume of 500 ml (Hefty Baggies, Mobil Chemical Co., Pittsford, NY) linked to an electronic rigid piston barostat (Mayo Clinic, Rochester, MN). (7) An initial or conditioning distention followed by a rectal staircase distention (from 0 to 44 mm Hg or to maximum tolerated pressure, whichever came first, in 4-mm Hg steps at 1 minute intervals) was performed. Rectal pressure-volume relationships were analyzed by a power exponential function and summarized by the pressure corresponding to half maximum volume Prhalf) and rectal capacity (i.e., maximum volume). (7, 23) Rectal compliance and sensory thresholds for first sensation, desire to defecate, and urgency were recorded during the staircase distention; the threshold was the first sensation of each symptom. In addition, subjects also rated the intensity of perception for the desire to defecate and discomfort on two separate 100 mm long visual analog scales (VAS) during balloon distentions 8, 16 and 24 mm Hg greater than the operating pressure applied in random order; (13, 24) each distention was maintained for 1 minute, with an inter-stimulus interval of 1 minute, during which the balloon was deflated to operating pressure.
Statistical Analysis
For each subject, the bowel diary data (e.g., stool frequency and form, proportion of bowel movements associated with rectal urgency, and straining to begin and to end defecation) were first averaged per day and then separately for baseline and post-clonidine diaries. Anorectal functions were summarized as anal resting and squeeze pressures, rectal compliance and capacity, rectal sensory thresholds for first sensation, desire to defecate, and urgency, and visual analog scores for rectal perception during phasic distentions 8, 16, and 24 mmHg above operating pressure.
Bowel symptoms and anorectal sensorimotor functions before versus after therapy were compared using parametric (paired t-test) or nonparametric (signed rank test) methods, as warranted. Data were analyzed per intent-to-treat analysis. The pressure level values for sensory thresholds that were not perceived by a given subject were considered as “censored“ using an approach described previously. [Andrews, 2007 # 1744] For example, when the first sensation threshold was not perceived, the pressure level for this threshold sensation was recorded as the pressure value for the desire to defecate, or urgency thresholds, whichever came earlier, but labeled as “censored“ (in contrast to pressure levels in which the subject indicated the first sensation threshold was perceived, which implied the “event“ occurred). Similarly, if the desire to defecate sensation was not perceived by a subject, then this sensation threshold pressure level was “censored“ using the urgency threshold pressure or the highest pressure during the pressure-volume curve, whichever came first. If the threshold for urgency was not perceived, then this pressure value was “censored“ at the highest pressure during the pressure-volume curve. Then the sensory thresholds for first sensation, desire to defecate and urgency between the first and second studies were compared using separate proportional hazard regression models for each threshold type using a strata option to account for the matching (before vs. during therapy pairs). These models estimated the "risk" (probability) for reporting a specific sensation threshold over increasing pressure steps analogous to a survival analysis of the risk for an event (e.g. death) over time. The associations between changes (before vs. after clonidine) in anorectal functions with changes in clinical features were assessed using the Spearman correlation coefficient.
RESULTS
Clinical Features
Of 19 enrolled patients, 7 withdrew during the screening phase, and 12 completed the study. Of these 7 patients, symptoms improved in 4 patients prior to starting bowel diaries, 1 patient had an abnormal electrocardiogram while logistical constraints precluded participation in 2 patients. The 12 patients who completed the study had FI for 4 ± 1 years (Mean ± SEM); 5 had symptoms of urge- and 7 had combined (i.e., passive and urge) FI. All 12 patients had FI at least once a week; 4 reported daily symptoms. Ten patients were incontinent for liquid and formed stools while 2 patients were only incontinent for liquid stools. The typical amount of leakage was small (i.e., staining only, n = 4), moderate (i.e., more than staining but less than a full bowel movement, n = 6), or large (i.e., a full bowel movement, n = 2). Thus, the FICA incontinence symptom severity score suggested moderate (7 patients) or severe FI (5 patients).
Six women each had known potential obstetric risk factors for FI (i.e., more than 4 vaginal deliveries, 3rd or 4th degree perineal tear, or a forceps-assisted delivery) and a hysterectomy. Seven women had a cholecystectomy and 1 had a history of an anorectal abscess. Seven patients had anorectal imaging with endoanal ultrasound or MRI. Imaging revealed normal internal and external anal sphincters (3 patients), or only internal sphincter abnormalities (i.e., atrophy or scar, 2 patients), only external sphincter abnormalities (1 patient) or internal and external anal sphincter abnormalities (1 patient).
Effects of Clonidine on Bowel Habits and Fecal Incontinence
Clonidine tended to reduce (p= 0.05) stool frequency but did not significantly affect stool consistency. (Table 1) The proportion of bowel movements preceded by rectal urgency was also lower (p = 0.06) and patients could defer defecation for a longer duration (0.02<p ≤0.05) after than before clonidine. The sense of satisfaction after defecation also improved but differences were not statistically significant.
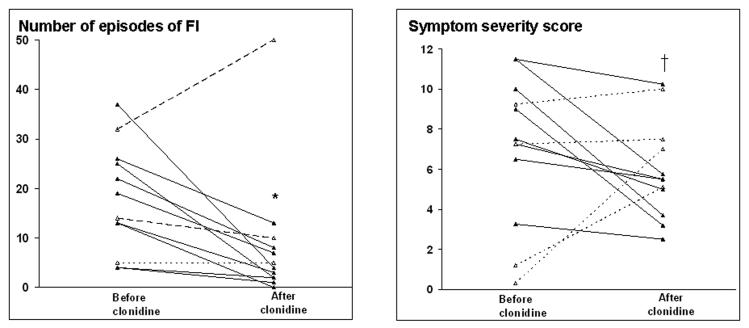
Clonidine also improved fecal continence, summarized as the number of episodes of FI (p = 0.03), the number of days with FI (p = 0.02), and the proportion of incontinent bowel movements (p= 0.005). (Table 1, Figure 1) During treatment, 9 of 12 patients reported a 50% reduction in the number of episodes of FI and 8 of these patients also reported a 50% reduction in the number of incontinent days. The Bristol stool form score was higher (p < 0.01), reflecting less formed stools, for incontinent (5.0 ± 0.3) than continent (4.1 ± 0.3) bowel movements. The volume of FI was also lower during than before clonidine, i.e., more episodes were associated with staining only (50 ± 10% before versus 65 ± 10% during clonidine, p = 0.02). The FI symptom severity score declined (p < 0.02) from 8.2 ± 0.7 before to 5.6 ± 0.9 during therapy and the VAS symptom relief score improved (p < 0.001) from 1.9 ± 0.4 during the first 4 weeks to 5.2 ± 0.7 during the last 4 weeks. The proportion of days on which patients used loperamide was not significantly different (p = 0.28) before (13 ± 10%) versus after (8 ± 10%) clonidine.
Figure 1. Effect of clonidine on frequency of FI (left panel) and FI symptom severity score (right panel).
Solid and dotted lines reflect subjects in whom fecal continence did and did not improve respectively with clonidine. * p = 0.03, † p < 0.01
Effects on Anorectal Functions
At baseline, 2 patients had reduced anal resting and 12 had reduced anal squeeze pressures. Clonidine did not significantly affect anal resting or squeeze pressures, rectal capacity, or compliance. (Table 2) However, the maximum tolerated pressure during the compliance curve was higher (p = 0.027) after (42 ± 1 mmHg) than before (37 ± 2 mmHg). Similarly, perception of rectal distention, expressed either as sensory thresholds or VAS scores, were not significantly different after versus before clonidine.
Table 2.
Effects of Clonidine on Anorectal Sensorimotor Functions
| Before clonidine | During clonidine | |
|---|---|---|
| Anal resting pressure | 41 ± 3 | 41 ± 2 |
| Anal squeeze pressure | 71 ± 7 | 74 ± 6 |
|
Maximum tolerated pressure during compliance curve (mmHg) |
37 ± 2 | 42 ± 1† |
| Rectal capacity (mL) | 245 ± 64 | 256 ± 51 |
| Rectal compliance (Prhalf) | 16.7 ± 1.4 | 18.4 ± 2.5 |
| Rectal sensation | ||
| Threshold for desire to defecate | ||
| Pressure | 16.7 ± 2.0 | 16.7 ± 2.3 |
| Volume (mL) | 111 ± 17 | 113 ± 23 |
| Threshold for urgency | ||
| Pressure | 23.4 ± 2.2 | 24.4 ± 2.9 |
| Volume (mL) | 169 ± 16.5 | 167 ± 23.1 |
| VAS Score (urgency, 8 mm*) | 30 ± 7.5 | 30 ± 8.4 |
| VAS Score (urgency, 16 mm*) | 46 ± 7.3 | 44 ± 8.4 |
| VAS Score (urgency, 24 mm*) | 62 ± 5.9 | 66 ± 7.9 |
Values are Mean ± SEM in mmHg except where stated
Distending pressure = Operating pressure + specified pressure
p = 0.027 vs pre clonidine
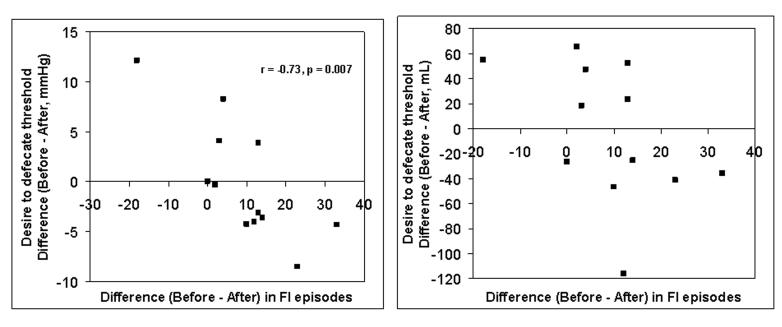
However, 7 of 12 patients tolerated a higher maximum distending pressure, and had a higher maximum rectal capacity after than before clonidine; maximum distending pressure and capacity were either unchanged (4 patients) or lower (1 patient) in the remaining patients. (Figure 2) Differences (before – after treatment) in rectal capacity were significantly correlated with corresponding differences in volume (r = 0.58, p < 0.05) but not pressure thresholds (r = 0.47, p = 0.12). Also, the observed differences (before treatment vs. after clonidine) in symptoms and anorectal functions were associated. Thus, differences in the number of FI episodes (after – before clonidine) were correlated with changes in the Prhalf for rectal compliance (r = −0.58, p < 0.05), maximum tolerated pressure during rectal pressure-volume relationships (r = −0.57, p = 0.05), rectal capacity (r = −0.51, p = 0.09), and pressure (r = −0.73, p = 0.007) but not volume (r = −0.45, p = 0.14) thresholds for the desire to defecate. (Figure 3) Because symptom improvement was significantly correlated with an increase in Prhalf and the pressure threshold for desire to defecate, treatment with clonidine likely improved in FI through increased rectal compliance and reduced rectal sensitivity.
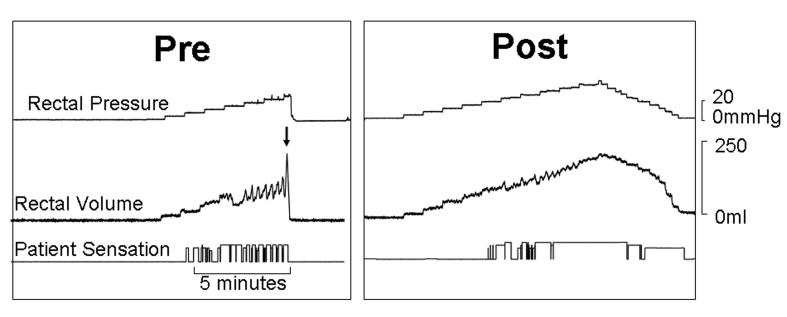
Figure 2. Comparison of rectal pressure-volume relationships and perception before (left panel) and during (right panel) clonidine therapy in a single subject.
Before clonidine, rectal distention induced pronounced balloon volume fluctuations reflecting rectal contractions, culminating in balloon expulsion at 24 mmHg (arrow). After clonidine, rectal contractility was less pronounced and balloon distention was tolerated up to 40 mmHg.
Figure 3. Comparison of clonidine's effects on fecal incontinence versus pressure (left panel) and volume (right panel) thresholds for the desire to defecate during rectal distention.
Observe that a more pronounced reduction (before – after) in the number of incontinence episodes was associated with a negative difference (i.e., before – after therapy) in pressure thresholds, suggesting that thresholds were higher, indicating reduced rectal sensation after therapy.
Adverse Effects
Six patients reported no side effects. Five patients reported a skin reaction at the patch site, 3 experienced fatigue, and 2 each had a dry mouth and orthostatic symptoms. These side effects were mild, with the exception of one patient in whom the dose of clonidine was reduced to 0.1 mg daily in the 3rd week because of fatigue, orthostatic symptoms, and a low blood pressure.
Sample Size for Future Clinical Trials
Based on the variation during the baseline period in the current study, a parallel group study with 20 subjects randomized to placebo or clonidine would provide approximately 80% power to detect a difference (before – after) of 5 days in the number of incontinent days between treatmentgroups (e.g., 12 days [placebo] versus 7 days [clonidine]) and a difference of 10 FI episodes over 4 weeks (e.g., 18 [placebo] versus 8 [clonidine]).
DISCUSSION
There is very limited, mostly uncontrolled, evidence to support the approaches currently used to manage FI. (6, 25, 26) Clonidine was reasonably well-tolerated and significantly improved bowel symptoms in this uncontrolled open-label study of women with moderately severe FI. Eight of 12 patients (66%) reported at least a 50% reduction in the number of incontinent episodes and a majority of patients were more satisfied after than before treatment. However, these findings need to be confirmed by a controlled study with longer follow-up.
Conceivably, clonidine may improve fecal continence by modulating bowel disturbances and/or anorectal sensorimotor dysfunctions. Clonidine (0.1 mg orally bid) improved bowel symptoms but did not affect gastrointestinal or colonic transit in IBS. (15) In that study also, clonidine did not affect stool frequency, and while effects on stool consistency were statistically significant, they were relatively modest. These observations suggest it is unlikely that clonidine improves fecal continence primarily by altering colonic motor functions but do not exclude this explanation since it is conceivable that scintigraphy, which summarizes colonic transit over 24 hours, may not identify subtle motor effects (e.g., an inhibitory effect of clonidine on colonic high-amplitude propagated contractions). While overall effects on anal pressures, rectal capacity, compliance, and sensation were not significant, the maximum tolerated pressure during rectal distention was higher in 7 patients after compared to before clonidine. Conceivably, the finding that clonidine's effects on rectal compliance were less pronounced than previously observed in healthy subjects (27) may suggest that fibrosis rather than increased tone contributes to rectal stiffness in women with FI. A controlled study is required to clarify whether the change in maximum tolerated pressure during rectal distention reflects drug effect or habituation of visceral perception to repeated distention, which is conceivable but unlikely. (28) Indeed, assessments of colonic and rectal pressure-volume relationships and sensation on the same and different days are reasonably reproducible among healthy subjects. (22, 29, 30) In 1 study of 15 patients with irritable bowel syndrome, thresholds for discomfort during rectal distention were similar at baseline and 4 months and increased only at 8 and 12 months thereafter, suggestive of habituation and/or fluctuations in the natural history of the disease. (28) Of note, in this study, pre- and post-treatment assessments were performed at 1 month intervals. Reduced rectal capacity and rectal hypersensitivity have been associated with the symptom of urgency in FI. (7-9) However, this is the first study to document an association between improved symptoms and rectal sensorimotor functions, which substantiates the concept that rectal sensorimotor dysfunctions contribute to the symptoms of FI. Though rectal capacity was correlated with the volume sensory threshold for the desire to defecate, the correlation coefficient (r = 0.6), was modest suggesting that clonidine may have independent effects on rectal sensation; indeed, adrenergic α2 receptors are also widely distributed on nociceptive pathways in the spinal cord, brain stem, and forebrain. (12)
The number of incontinent episodes and days are the most widely used indices to evaluate the response to therapy. However, these indices do not incorporate the type of incontinence (i.e., solid or liquid stool). Moreover, these indices and some scales for rating the severity of FI (31, 32) do not assess the symptom of urgency or the amount of leakage, which also affect the severity of FI. Using the FICA symptom scoring system, which does evaluate urgency and the amount of leakage, we observed that clonidine also reduced symptom severity, demonstrating that this scale is responsive to therapy. (18, 19) However, quality of life did not improve, perhaps because this was a relatively short study. This dose of clonidine was generally well tolerated and the dose was reduced in 1 patient only. While drowsiness and dry mouth are more common after oral than transdermal clonidine, (17) 5 of 12 patients (40%) developed allergic skin reactions to transdermal clonidine patches in this study. Typically, these skin reactions cannot be mitigated by switching patches to different sites. The prevalence of allergic contact sensitivity to transdermal clonidine varies from 0 to 50%, increases over time, and is more frequent in Caucasian women than others. (33)
In summary, this uncontrolled study with a small number of subjects suggests that clonidine may significantly improves symptoms in women with urge-predominant fecal continence. While overall effects on anorectal sensorimotor functions were not significant, improved symptoms were associated with an improvement in anorectal functions. These findings need to be confirmed in a larger controlled study of clonidine for FI. (22)
Acknowledgments
This study was funded in part by USPHS NIH Grant R01 DKDK78924 and Grant 1 UL1 RR024150* from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
STATEMENT OF INTERESTS
The authors have no conflict of interest.
REFERENCES
- 1.Bharucha AE, Zinsmeister AR, Locke GR, Seide B, McKeon K, Schleck CD, et al. Prevalence and burden of fecal incontinence: A population based study in women. Gastroenterology. 2005;129:42–49. doi: 10.1053/j.gastro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Heymen S, Scarlett Y, Jones K, Ringel Y, Drossman DA, Whitehead W. Randomized controlled trial shows biofeedback to be superior to alternative treatments for fecal incontinence. Diseases of the Colon & Rectum. 2009;52:1730–1737. doi: 10.1007/DCR.0b013e3181b55455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Read M, Read NW, Barber DC, Duthie HL. Effects of loperamide on anal sphincter function in patients complaining of chronic diarrhea with fecal incontinence and urgency. Digestive Diseases and Sciences. 1982;27(9):807–14. doi: 10.1007/BF01391374. [DOI] [PubMed] [Google Scholar]
- 4.Sun WM, Read NW, Verlinden M. Effects of loperamide oxide on gastrointestinal transit time and anorectal function in patients with chronic diarrhoea and faecal incontinence. Scandinavian Journal of Gastroenterology. 1997;32(1):34–8. doi: 10.3109/00365529709025060. [DOI] [PubMed] [Google Scholar]
- 5.Santoro GA, Eitan BZ, Pryde A, Bartolo DC. Open study of low-dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 2000;43:1676–81. doi: 10.1007/BF02236848. [DOI] [PubMed] [Google Scholar]
- 6.Cheung O, Wald A. Review article: the management of pelvic floor disorders. Aliment Pharmacol Ther. 2004;19(5):481–95. doi: 10.1111/j.1365-2036.2004.01886.x. [DOI] [PubMed] [Google Scholar]
- 7.Bharucha AE, Fletcher JG, Harper CM, Hough D, Daube JR, Stevens C, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic fecal incontinence. Gut. 2005;54:546–55. doi: 10.1136/gut.2004.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews C, Bharucha AE, Camilleri M, Low PA, Seide B, Burton D, et al. Rectal sensorimotor dysfunction in women with fecal incontinence. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2007;292(1):G282–9. doi: 10.1152/ajpgi.00176.2006. [DOI] [PubMed] [Google Scholar]
- 9.Siproudhis L, El Abkari M, El Alaoui M, Juguet F, Bretagne JF. Low rectal volumes in patients suffering from fecal incontinence: what does it mean? Alimentary Pharmacology & Therapeutics. 2005;22(10):989–96. doi: 10.1111/j.1365-2036.2005.02675.x. [DOI] [PubMed] [Google Scholar]
- 10.Deutekom M, Dobben AC, Terra MP, Engel AF, Stoker J, Bossuyt PM, et al. Clinical presentation of fecal incontinence and anorectal function: what is the relationship? American Journal of Gastroenterology. 2007;102(2):351–61. doi: 10.1111/j.1572-0241.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- 11.Tack JF, Wood JD. Actions of noradrenaline on myenteric neurons in the guinea pig gastric antrum. Journal of the Autonomic Nervous System. 1992;41(1-2):67–77. doi: 10.1016/0165-1838(92)90128-4. [DOI] [PubMed] [Google Scholar]
- 12.Unnerstall JR, Kopajtic TA, Kuhar MJ. Distribution of alpha 2 agonist binding sites in the rat and human central nervous system: analysis of some functional, anatomic correlates of the pharmacologic effects of clonidine and related adrenergic agents. Brain Research. 1984;319(1):69–101. doi: 10.1016/0165-0173(84)90030-4. [DOI] [PubMed] [Google Scholar]
- 13.Bharucha AE, Camilleri M, Zinsmeister AR, Hanson RB. Adrenergic modulation of human colonic motor and sensory function. American Journal of Physiology. 1997;273(5 Pt 1):G997–1006. doi: 10.1152/ajpgi.1997.273.5.G997. [DOI] [PubMed] [Google Scholar]
- 14.Malcolm A, Camilleri M, Kost L, Burton D, Fett S, Zinsmeister A. Towards Identifying Optimal Doses For Alpha-2 adrenergic Modulation of Colonic and Rectal Motor and Sensory Function. Alimentary Pharmacology and Therapeutics. 2000;14:783–93. doi: 10.1046/j.1365-2036.2000.00757.x. [DOI] [PubMed] [Google Scholar]
- 15.Camilleri M, Kim D-Y, McKinzie S, Kim H-J, Thomforde G, Burton D, et al. A randomized, controlled exploratory study of clonidine in diarrhea-predominant irritable bowel syndrome. Clinical Gastroenterology & Hepatology. 2003;1(2):111–121. doi: 10.1053/cgh.2003.50019. [DOI] [PubMed] [Google Scholar]
- 16.MacGregor TR, Matzek KM, Keirns JJ, van Wayjen RG, van den Ende A, van Tol RG. Pharmacokinetics of transdermally delivered clonidine. Clinical Pharmacology & Therapeutics. 1985;38(3):278–84. doi: 10.1038/clpt.1985.171. [DOI] [PubMed] [Google Scholar]
- 17.Fujimura A, Ebihara A, Ohashi K, Shiga T, Kumagai Y, Nakashima H, et al. Comparison of the pharmacokinetics, pharmacodynamics, and safety of oral (Catapres) and transdermal (M-5041T) clonidine in healthy subjects. Journal of Clinical Pharmacology. 1994;34(3):260–5. doi: 10.1002/j.1552-4604.1994.tb03996.x. [DOI] [PubMed] [Google Scholar]
- 18.Bharucha AE, Locke GR, Seide B, Zinsmeister AR. A New Questionnaire for Constipation and Fecal Incontinence. Alimentary Pharmacology & Therapeutics. 2004;20:355–364. doi: 10.1111/j.1365-2036.2004.02028.x. [DOI] [PubMed] [Google Scholar]
- 19.Bharucha AE, Zinsmeister AR, Locke GR, Schleck C, McKeon K, Melton LJ. Symptoms and quality of life in community women with fecal incontinence. Clinical Gastroenterology & Hepatology. 2006;4(8):1004–9. doi: 10.1016/j.cgh.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Heaton KW, O'Donnell LJ. An office guide to whole-gut transit time. Patients' recollection of their stool form. Journal of Clinical Gastroenterology. 1994;19(1):28–30. doi: 10.1097/00004836-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, et al. Fecal incontinence quality of life scale: quality of life instrument for patients with fecal incontinence. Diseases of the Colon and Rectum. 2000;43(1):9–16. doi: 10.1007/BF02237236. discussion 16-7. [DOI] [PubMed] [Google Scholar]
- 22.Bharucha AE, Seide B, Fox JC, Zinsmeister AR. Day-to-day Reproducibility of Anorectal Sensorimotor Assessments in Healthy Subjects. Neurogastroenterology & Motility. 2004;16:241–50. doi: 10.1111/j.1365-2982.2004.00499.x. [DOI] [PubMed] [Google Scholar]
- 23.Law NM, Bharucha AE, Undale AS, Zinsmeister AR. Cholinergic stimulation enhances colonic motor activity, transit, and sensation in humans. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2001;281(5):G1228–37. doi: 10.1152/ajpgi.2001.281.5.G1228. [DOI] [PubMed] [Google Scholar]
- 24.Ford MJ, Camilleri M, Wiste JA, Hanson RB. Differences in colonic tone and phasic response to a meal in the transverse and sigmoid human colon. Gut. 1995;37(2):264–9. doi: 10.1136/gut.37.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bharucha A. Fecal Incontinence. Gastroenterology. 2003;124(6):1672–1685. doi: 10.1016/s0016-5085(03)00329-9. [DOI] [PubMed] [Google Scholar]
- 26.Scarlett Y. Medical management of fecal incontinence. Gastroenterology. 2004;126(1 Suppl 1):S55–63. doi: 10.1053/j.gastro.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Viramontes BE, Malcolm A, Camilleri M, Szarka LA, McKinzie S, Burton DD, et al. Effects of an alpha(2)-adrenergic agonist on gastrointestinal transit, colonic motility, and sensation in humans. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2001;281(6):G1468–76. doi: 10.1152/ajpgi.2001.281.6.G1468. [DOI] [PubMed] [Google Scholar]
- 28.Naliboff BD, Berman S, Suyenobu B, Labus JS, Chang L, Stains J, et al. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131(2):352–65. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Bharucha AE, Hubmayr RD, Ferber IJ, Zinsmeister AR. Viscoelastic properties of the human colon. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2001;281(2):G459–66. doi: 10.1152/ajpgi.2001.281.2.G459. [DOI] [PubMed] [Google Scholar]
- 30.Hammer HF, Phillips SF, Camilleri M, Hanson RB. Rectal tone, distensibility, and perception: reproducibility and response to different distensions. American Journal of Physiology. 1998;274(3 Pt 1):G584–90. doi: 10.1152/ajpgi.1998.274.3.G584. [DOI] [PubMed] [Google Scholar]
- 31.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Diseases of the Colon and Rectum. 1993;36(1):77–97. doi: 10.1007/BF02050307. [DOI] [PubMed] [Google Scholar]
- 32.Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, et al. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Diseases of the Colon and Rectum. 1999;42(12):1525–32. doi: 10.1007/BF02236199. [DOI] [PubMed] [Google Scholar]
- 33.Murphy M, Carmichael AJ. Transdermal drug delivery systems and skin sensitivity reactions. Incidence and management. American Journal of Clinical Dermatology. 2000;1(6):361–8. doi: 10.2165/00128071-200001060-00004. [DOI] [PubMed] [Google Scholar]