Abstract
Cell transformation by the v-rel oncogene is mediated by the aberrant expression of genes that are normally tightly regulated by other Rel/NF-κB family members. Although a number of genes inappropriately activated or suppressed by v-Rel have been identified, their contributions to the v-Rel transformation process have been poorly characterized. Here, we examine the role of individual AP-1 proteins in v-Rel-mediated transformation. v-Rel transformed cells exhibit elevated RNA and protein expression of c-Fos, c-Jun, and ATF2 and sustained repression of Fra-2. c-Fos and c-Jun are essential in both the initiation and maintenance of v-Rel-mediated transformation while Fra-2 is dispensable. By employing a c-Jun dimerization mutant, we further identified Fos:Jun heterodimers as major contributors to the v-Rel transformation process. The inability of c-Rel to induce the expression of c-Fos and c-Jun contributes to its weaker oncogenic potential relative to v-Rel. Our studies also demonstrate that v-Rel may induce AP-1 members by directly upregulating gene expression (c-fos and ATF2) and by activating pathways that stimulate AP-1 activity. While elevated expression of ATF2 is also required for v-Rel-mediated transformation, its ectopic overexpression is inhibitory. Investigating the mode of ATF2 regulation revealed a positive feedback mechanism whereby ATF2 induces p38 MAPK phosphorylation to further induce its own activity. In addition, these studies identified Ha-Ras as an effector of v-Rel mediated transformation and reveal a novel role for ATF2 in the inhibition of the Ras-Raf-MEK-ERK signaling pathway. Overall, these studies reveal distinct and complex roles of AP-1 proteins in Rel/NF-κB oncogenesis.
Keywords: v-Rel, Rel/NF-κB, transformation, oncogenesis, AP-1, Ras, MAPK
Introduction
The Rel/NF-κB family of transcription factors plays important roles in immune and inflammatory responses, proliferation, and apoptosis (Aggarwal, 2004; Bonizzi and Karin, 2004). Vertebrate members of this evolutionary conserved family include NF-κB1 (p50), NF-κB2 (p52), RelA (p65), RelB, and c-Rel. In unstimulated cells, Rel/NF-κB proteins are retained in the cytoplasm through association with IκB proteins. Activation of IκB kinases (IKKs) results in the degradation of IκB proteins, allowing the nuclear translocation of Rel/NF-κB complexes where they regulate the expression of a wide range of target genes through binding to specific decameric DNA sites (κB sites) (Chen and Ghosh, 1999).
Elevated expression of Rel/NF-κB proteins and/or disruption in IKK/IκB regulation has been linked to several types of cancer (Courtois and Gilmore, 2006; Rayet and Gelinas, 1999). The earliest evidence of a role for Rel/NF-κB proteins in oncogenic cell transformation resulted from studies with v-rel. The v-rel oncogene is derived from the transduction of c-rel into an avian retrovirus (Stephens et al., 1983). While c-Rel is only weakly oncogenic, v-Rel is the most efficient transforming member of the Rel/NF-κB family. It induces avian and mammalian lymphoid cell tumors and, in vitro, can efficiently transform cells of hematopoietic origin and chicken embryonic fibroblasts (Liss and Bose, 2008). v-Rel derives its strong oncogenicity from alterations in DNA binding specificity and transactivation potential as well as its ability to escape inhibition by IκB proteins (Gilmore, 1999). Transformation by v-Rel is mediated by the aberrant expression of genes that are normally tightly regulated by Rel/NF-κB proteins.
Our previous studies demonstrated that v-Rel selectively alters the expression of several AP-1 family members and that general inhibition of AP-1 activity dramatically reduced the ability of v-Rel to transform both fibroblasts and lymphoid cells (Kralova et al., 1998). AP-1 proteins are a family of transcription factors that regulate cell proliferation, differentiation, and apoptosis in response to external stimuli (Hess et al., 2004). The AP-1 family is composed of Jun, Fos, and ATF proteins, all of which contain a common basic-region leucine zipper important for dimerization and DNA binding. Jun family members (c-Jun, JunB, JunD) have the ability to form stable homodimers as well as heterodimers with Fos and ATF family members. Similarly, members of the ATF family (ATF2, ATF3, and ATF4) can homodimerize and selectively heterodimerize with Jun proteins. Members of the Fos family (c-Fos, FosB, Fra-1, Fra-2), however, can only form heterodimers. Jun homodimers and Jun-Fos dimers preferentially bind to the TPA-responsive element (TRE; 5'-TGA(C/G)TCA-3') whereas Jun-ATF dimers and ATF homodimers display a strong affinity for DNA sites containing cAMP-responsive elements (CRE; 5'-TGACGTCA-3') (Angel et al., 1988; Angel et al., 1987; Hai and Curran, 1991). In addition to changes in gene expression, AP-1 activity is regulated by post-translational modifications, including phosphorylation by mitogen-activated protein kinases (MAPKs) (Karin et al., 1997). Phosphorylation of pre-existing and newly synthesized AP-1 components by the JNK, ERK, or p38 MAPK cascades activates and stabilizes the dimeric AP-1 complexes, thereby enhancing their transactivation potential.
In this report, the role of individual AP-1 proteins in the v-Rel transformation process was evaluated. Our results demonstrate that c-Fos and c-Jun are key players in v-Rel-mediated transformation, and the inability of c-Rel to induce their expression contributes to its weaker oncogenic potential. While elevated expression of ATF2 appears to be required for v-Rel-mediated transformation, its ectopic overexpression is inhibitory. Characterization of the negative effect of ATF2 on transformation by v-Rel revealed a novel role for ATF2 in the regulation of the Ras-Raf-MEK-ERK signaling pathway.
Results
Altered expression of AP-1 family members in v-Rel transformed cells
For a detailed analysis of the role of individual AP-1 proteins in v-Rel-mediated transformation, we assessed the ability of c-Rel and v-Rel to alter the expression of c-fos, c-jun, fra-2, and ATF2 at both the RNA and protein levels. c-Jun, c-Fos and ATF2 expression was upregulated in v-Rel-transformed chicken embryonic fibroblasts (CEFs) and lymphoid cells (DT40 and DT95) and, to a moderate extent, in cells transformed by c-Rel (Supplemental Figure 1a and b). Fra-2 expression remained unchanged in cells ectopically expressing c-Rel; however, it was downregulated at both the RNA and protein level in v-Rel transformed CEFs and DT40 cells.
v-Rel directly activates the human c-jun promoter, and the presence of similar κB sites in the promoter of the avian homolog suggests that it is a direct target of v-Rel (Fujii et al., 1997). Employing a combination of luciferase reporter assays, electrophoretic mobility shift assays (EMSAs), and chromatin immunoprecipitation experiments, we demonstrated that the induction of c-fos by v-Rel is also likely due to direct promoter activation (Supplemental Figure 2a–d). ATF2 is also a candidate v-Rel target gene as there are two κB sites in its proximal promoter that can be bound by v-Rel-containing complexes (Supplemental Figure 2e). In addition to elevating the expression of AP-1 proteins, v-Rel efficiently induced the activity of luciferase reporter constructs specific for both Fos:Jun and ATF:Jun dimers (Supplemental Figure 1c), indicating that, in contrast to c-Rel, the expression of v-Rel is sufficient to activate the expression and activity of these distinct AP-1 pathways.
AP-1 proteins exhibit altered DNA binding profiles in v-Rel transformed cells
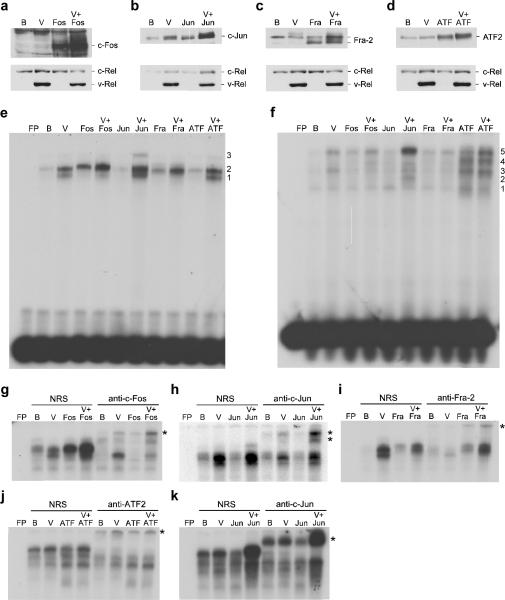
To directly assess contributions of individual AP-1 family members in v-Rel-mediated transformation, bicistronic retroviral vectors were constructed to express c-fos, c-jun, fra-2, and ATF2 alone or in combination with v-rel. DT40 cells infected with viruses expressing v-Rel alone induced the expression of c-Fos, c-Jun, and ATF2 while suppressing the expression of Fra-2 (Figure 1a–d). In addition, the co-expression of v-Rel with c-fos, c-jun, fra-2, and ATF2 resulted in higher levels of these AP-1 gene products than observed in cells expressing control viruses or viruses expressing v-Rel alone.
Figure 1.
Expression and DNA binding activity of AP-1 family members expressed from bicistronic retroviruses. DT40 cells were infected with control BIS retroviruses (B), BIS viruses expressing v-Rel (V), c-Fos, c-Jun, Fra-2, and ATF2 alone, or viruses that co-expressed v-Rel with each of the AP-1 family members. Whole cell lysates (25 μg) from infected cells were analyzed by Western blot to evaluate the expression of c-Fos, c-Jun, Fra-2, ATF2, and Rel proteins (panels a–d). Proteins detected by the antisera are indicated on the right of each panel. Nuclear extracts from infected cells were used in EMSAs with [32P]-labeled probes containing AP-1 DNA binding sites specific for Fos:Jun (panel e) or ATF2:Jun heterodimers (panel f). Free probe (FP) and the viruses expressed in the cells that extracts were derived from are indicated above each panel. Numbers to the right of each panel indicate the location of complexes described in the text. Supershift analyses were performed with the extracts described above employing normal rabbit serum (NRS) or antisera specific to c-Fos, c-Jun, Fra-2, and ATF2. Probes containing AP-1 DNA binding sites specific for Fos:Jun (panel g–i) or ATF2:Jun heterodimers (panel j–k) were employed for these studies. The asterisk to the right of each panel indicates the location of the supershifted band(s).
To determine whether these elevated AP-1 protein levels resulted in a corresponding change in AP-1 DNA binding activity, EMSAs were performed with nuclear extracts from DT40 cells infected with these viruses. The overexpression of v-Rel alone resulted in a strong induction of two AP-1 complexes that bound to a site specific for Fos:Jun dimers (Figure 1e, bands 1 and 2). Supershift analysis indicated that these complexes were composed of c-Fos, c-Jun, and Fra-2 (Figure 1g–i). Overexpression of c-Fos or Fra-2 alone resulted in enhanced DNA binding activity over that of control BIS-infected cells. Furthermore, nuclear extracts from cells that co-expressed v-Rel with c-Fos or Fra-2 exhibited increased binding activity of the slower migrating complex (band 2) and reduced binding of the faster migrating complex (band 1). Overexpression of c-Jun alone did not alter AP-1 DNA binding activity; however, coexpression of v-Rel with c-Jun resulted in an increase in both DNA binding complexes observed in cells expressing v-Rel alone. An additional slower migrating form was also observed in these cells (band 3). Supershift analysis demonstrated the altered DNA binding profiles observed in extracts from cells ectopically expressing AP-1 proteins were due to the presence of the overexpressed protein (Figure 1g–i). Overexpression of ATF2 alone or with v-Rel did not alter AP-1 DNA binding activity on this site.
In addition to altering DNA binding activity on a Fos:Jun specific binding site, v-Rel induced DNA binding on a site specific for ATF2:Jun dimers (Figure 1f, bands 3 and 5). Supershift analysis revealed the presence of c-Jun and ATF2 in these complexes (Figure 1j and k). Overexpression of ATF2 alone or with v-Rel resulted in an increase in DNA binding activity. In addition, the co-expression of c-Jun with v-Rel strongly induced the slowest migrating complex (band 5). Supershift analysis demonstrated the altered DNA binding profile observed above was due to the presence of overexpressed c-Jun or ATF2 (Figure 1j and k). As expected, the overexpression of c-Fos or Fra-2 did not alter binding on this site. Taken together, these results demonstrate that induction of c-Fos, c-Jun, and ATF2 by v-Rel correlates with their enhanced DNA binding activity and that the bicistronic retroviral vectors can effectively elevate the levels and DNA binding activity of c-Fos, c-Jun, Fra-2, and ATF2 to a greater extent than cells expressing v-Rel alone.
Elevated expression of c-Fos and c-Jun enhances transformation of Rel proteins
To determine whether alterations in the activity of c-Fos, c-Jun, Fra-2, and ATF2 can alter the transformation potential of v-Rel, primary splenic lymphocytes were infected with the bicistronic viruses described above. Overexpression of individual AP-1 proteins from these viruses failed to transform cells (Table 1). However, consistent with their induction by v-Rel, the co-expression of c-Fos or c-Jun with v-Rel resulted in a three- to four-fold enhancement in colony formation relative to viruses expressing v-Rel alone. Overexpression of Fra-2 resulted in a modest increase in colony formation, suggesting that the downregulation of Fra-2 by v-Rel is not an important event during transformation and therefore, the involvement of Fra-2 in v-Rel-mediated transformation was not investigated further. Unexpectedly, viruses co-expressing ATF2 and v-Rel were less effective at transforming splenic lymphocytes than viruses expressing v-Rel alone.
Table 1.
AP-1 family members alter the transformation potential of v-Rel in splenic lymphocytes
| Relative Colony Formation 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Retrovirus | Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | Exp. 5 | Exp. 6 | Avg ± Std. Dev. | p-value |
| BIS-V | 100 | 100 | 100 | 100 | 100 | 100 | 100 ± 0 | |
| BIS-V+c-fos | 414 | 382 | 199 | 448 | 589 | - | 406 ± 140 | 0.008 |
| BIS-V+c-jun | 311 | 332 | 172 | 297 | 364 | - | 295 ± 73 | 0.004 |
| BIS-V + fra-2 | 156 | 130 | 104 | 707 | 238 | - | 267 ± 251 | 0.211 |
| BIS-V + ATF2 | 43 | 44 | 37 | 12 | 77 | 43 | 43 ± 21 | 0.001 |
Splenic lymphocytes were infected with indicated retroviruses, plated in soft agar, and colonies were scored microscopically after 10 days. The number of colonies (ranging from 53 to 731) from BIS-V infections were normalized to 100 for each experiment. No colonies were observed with control BIS viruses or BIS viruses expressing AP-1 family members alone.
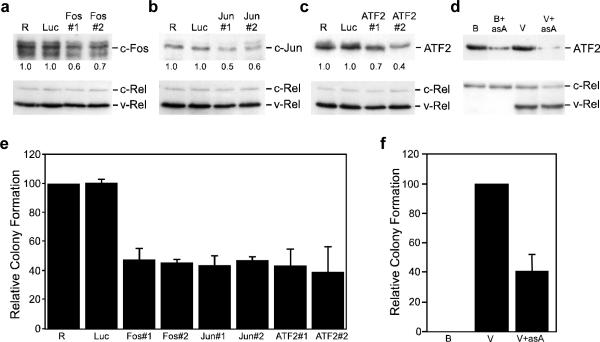
To gain additional insight into the contribution of elevated levels of c-Fos, c-Jun, and ATF2 to v-Rel-mediated transformation, retroviral vectors were constructed to express shRNAs that targeted c-fos, c-jun, and ATF2. The ability of a specific shRNA to effectively reduce the levels of its targeted endogenous AP-1 family member was evaluated in 160/2 cells, a v-Rel-transformed cell line. Cells were infected with empty viruses (RCAS), viruses encoding shRNAs specific for each AP-1 family member, or an shRNA specific for luciferase (luc) as a negative control. The expression of c-Fos, c-Jun, and ATF2 was reduced by 30–60% in cells expressing the specific shRNAs relative to cells infected with empty virus or virus encoding an shRNA specific for luciferase (Figure 2a–c).
Figure 2.
Reduced levels of AP-1 family members inhibit transformation by v-Rel. (a–c) Empty RCAS retroviruses (R) or RCAS viruses expressing an shRNA specific to luciferase (Luc), c-fos (a), c-jun (b), or ATF2 (c) were used to infect 160/2 cells. Whole cell lysates (25–50 μg) from infected cells were harvested ten days after infection and analyzed by Western blot for the expression of c-Fos, c-Jun, ATF2, and Rel proteins. Proteins detected by Western blot are indicated to the right of each panel. The signal corresponding to each AP-1 protein was normalized to that observed in control RCAS infections and values are shown below the top panel. (d) Control BIS retroviruses (B) and BIS viruses expressing v-Rel (V) or antisense ATF2 alone (B+asA), or in combination (V+asA) were used to infect DT40 cells. Whole cell lysates (25 μg) from infected cells were analyzed by Western blot for the expression of ATF2 and Rel proteins. (e) The RCAS-infected 160/2 cells described above were also plated in soft agar and scored for colony formation after eight days. The number of colonies from each experiment was normalized to control RCAS-infected cells. The average and standard deviation of three independent experiments is shown. (f) The BIS viruses described above were used to infect splenic lymphocytes from three-week old chickens. Infected cells were plated in soft agar three days later and colonies scored after ten days. The average and standard deviation of four independent experiments is shown.
The shRNA-encoding retroviruses were then evaluated for their ability to alter the transformed phenotype of 160/2 cells. Viruses encoding shRNAs specific for c-Fos, c-Jun, and ATF2 reduced the ability of these cells to form colonies in soft agar by approximately 60% relative to cells infected with empty control virus (Figure 2e). In contrast, cells infected with viruses encoding an shRNA specific for luciferase had no effect on colony formation. These results demonstrate a direct correlation between the levels of c-Fos, c-Jun, and ATF2 with the ability of v-Rel transformed cells to form colonies in soft agar.
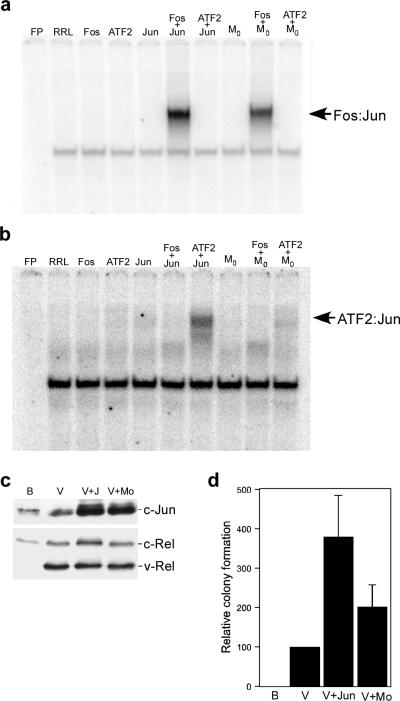
Alterations in the expression of c-Fos and c-Jun affecting the transformed phenotype suggested a role for these proteins in v-Rel-mediated transformation (Table 1 and Figure 2). To directly evaluate the potential contribution of Fos:Jun heterodimers to transformation by v-Rel, we employed the c-Jun dimerization mutant M0, which preferentially dimerizes with c-Fos, but not ATF2 (Figure 3a and b) (van Dam and Castellazzi, 2001). The use of bicistronic retroviruses demonstrated that coexpression of M0 and v-Rel enhanced the ability of v-Rel to transform primary splenic lymphocytes about half as efficiently as c-Jun (Figure 3c and d). These results suggest that Fos:Jun dimers are major contributors to v-Rel-mediated transformation as a result of the overexpression of c-Jun.
Figure 3.
Transformation of splenic lymphocytes by viruses co-expressing v-Rel and a c-Jun dimerization mutant. (a and b) DNA binding of the c-Jun dimerization mutant Mo. c-Fos, ATF2, c-Jun, and Mo were in vitro synthesized alone or in combination. Co-translated c-Fos and ATF2 were present in approximately two-fold excess relative to the c-Jun protein synthesized in the reaction. Unprogrammed rabbit reticulocyte lysate (RRL) and in vitro translated proteins were used in EMSAs with [32P]-labeled DNA binding sites specific for Fos:Jun (panel a) and ATF2:Jun (panel b) dimers. (c) Expression of c-Jun proteins in DT40 cells. DT40 cells were infected with control BIS retroviruses (B), BIS viruses expressing v-Rel (V), or viruses that co-expressed v-Rel with c-Jun or Mo. Whole cell lysates (25 μg) from infected cells were analyzed by Western blot to evaluate the expression of Jun and Rel proteins. Proteins detected by the antisera are indicated on the right of each panel. (d) Transformation of cells co-expressing v-Rel and Jun proteins. The viruses described above were used to infect splenic lymphocytes from three-week old chickens. Infected cells were plated in soft agar three days later and colonies scored after ten days. The average and standard deviation of three independent experiments is shown.
The observation that overexpression of ATF2 reduced the transformation potential of v-Rel in splenic lymphocytes (Table 1) is an apparent contradiction to the results of the shRNA experiments where reduced ATF2 expression inhibited colony formation of 160/2 cells (Figure 2). To determine if this was due to differences between the use of primary cells and transformed cell lines, we constructed bicistronic retroviruses expressing ATF2 in the antisense orientation alone or with v-Rel. Viruses expressing antisense ATF2 effectively reduced expression of endogenous ATF2 in DT40 cells as well as the levels of ATF2 induced by v-Rel (Figure 2d). Furthermore, splenic lymphocytes infected with viruses co-expressing antisense ATF2 with v-Rel exhibited a 60% reduction in colony formation relative to cells infected with viruses expressing v-Rel alone (Figure 2f). The diminished transformation potential of v-Rel observed when endogenous levels of ATF2 were reduced is consistent with the upregulation of this AP-1 family member by v-Rel being important for transformation.
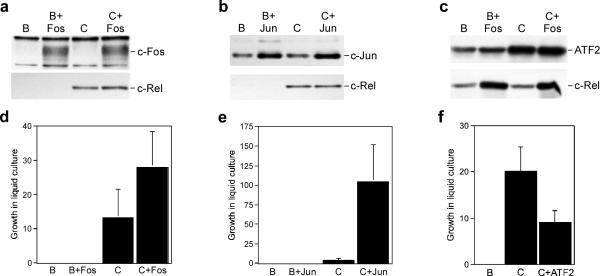
To evaluate whether the weak transformation potential of c-Rel results from its inability to activate AP-1, bicistronic retroviruses co-expressing c-Rel with c-Fos, c-Jun, or ATF2 were constructed (Figure 4a–c). Although splenic lymphocytes infected with these viruses did not form colonies in soft agar, overexpression of c-Fos or c-Jun enhanced the ability of c-Rel to transform splenic lymphocytes in liquid transformation assays (Figure 4d and e). In contrast, overexpression of ATF2 resulted in a reduction in the ability of c-Rel to transform cells (Figure 4f). These results suggest that the reduced oncogenic potential of c-Rel relative to v-Rel is due, at least in part, to its inability to elevate expression of c-Jun and c-Fos.
Figure 4.
Differential effect of enhanced AP-1 expression on the transformation potential of c-Rel. (a–c) Whole cell lysates were prepared from CEFs expressing control BIS retroviruses (B), BIS viruses expressing c-Rel (C), c-Fos, c-Jun, and ATF2 alone, or viruses that co-expressed c-Rel with each of the AP-1 family members. The expression of c-Fos, c-Jun, ATF2, and c-Rel was analyzed by Western blot. Proteins detected by the antisera are indicated on the right of each panel. (d–f) The viruses described above were used to infect splenic lymphocytes from three-week old chickens. Three days later, infected cells were serially diluted in 96-well plates and growth was scored microscopically after ten days. The average and standard deviation of three independent experiments is shown.
Overexpression of ATF2 inhibits v-Rel-induced activation of Ha-Ras
The experiments presented above indicated an ambiguous role of ATF2 in v-Rel-mediated transformation. v-Rel induces the expression of ATF2 and when this expression is reduced, it results in an attenuated oncogenic phenotype. However, further elevation of ATF2 levels inhibits transformation by Rel proteins. Since the transcriptional activity of ATF2 is largely regulated by the phosphorylation of Thr69 and Thr71, we evaluated whether the phosphorylation status of these sites may contribute to the inhibition of transformation by v-Rel. Levels of total and phosphorylated ATF2 were measured by Western blot analysis using lysates from CEFs and DT40 cells expressing control BIS viruses, BIS expressing v-Rel or ATF2 alone, or co-expressing v-Rel and ATF2 (Figure 5a). In both cell types, the expression of v-Rel resulted in slightly elevated levels of phosphorylated ATF2 relative to control cells. Cells that overexpressed ATF2 alone or coexpressed ATF2 and v-Rel exhibited higher levels of phosphorylated ATF2 than cells expressing v-Rel alone. These results suggest that the expression of ATF2 is able to activate the pathway(s) that lead to its own phosphorylation.
Figure 5.
Expression of ATF2 alters the activity of MAPKs. (a) Whole cell lysates were prepared from CEFs and DT40 cells expressing control BIS virus (B) and BIS expressing ATF2 (A) and v-Rel alone (V) or in combination (V+A). The expression of ATF2 phosphorylated on Thr69 and Thr71 (p-ATF2), total ATF2, c-Rel, and v-Rel was determined by Western Blot. (b) Whole cell lysates from CEFs and DT40 cells expressing control BIS virus or BIS expressing ATF2 were analyzed by Western blot for the levels of total and active, phosphorylated forms of ERK, JNK, and p38 MAPKs. Expression of ATF2 was also determined. (c) DT40 cells infected with BIS (B) or BIS expressing ATF2 (B+ATF2) were treated for one hour with 10 μM of inhibitors to p38 (SB202190), JNK (SP600125), or MEK1/2 (U0126) kinases, their respective negative controls (SB202474, JNK Inhibitor II Negative Control, U0124), or DMSO alone. Whole cell lysates from treated cells were analyzed by Western blot to determine the levels of phosphorylated and total ATF2. The phosphorylated and total levels of known downstream targets of the p38 (MAPKAP2), JNK (c-Jun), and MEK1/2 (ERK) kinases were analyzed to verify efficacy of the inhibitors.
Since the JNK, p38, and ERK MAPK pathways have all been implicated in the phosphorylation of ATF2 under various conditions, we investigated whether the activity of one or more of these pathways was altered by the expression of ATF2 (Morton et al., 2004). Whole cell lysates from CEFs and DT40 cells expressing control BIS virus or BIS viruses expressing ATF2 were analyzed by Western blot to determine the activation state of various members of the MAPK pathways (Figure 5b). In CEFs and DT40 cells overexpressing ATF2, an increase in the levels of active, phosphorylated p38 was observed. A slight activation of JNK was observed in DT40 cells overexpressing ATF2, but this activity was not altered in CEFs. The levels of active ERK were reduced in both CEFs and DT40 cells overexpressing ATF2.
Chemical inhibitors specific to each MAPK pathway were employed to further determine which pathway was responsible for phosphorylation of ATF2. DT40 cells infected with control BIS viruses or BIS viruses expressing ATF2 were treated with inhibitors specific to the p38, JNK, or ERK pathways and whole cell lysates were prepared. Western blot analysis demonstrated that inhibition of p38, but not JNK or ERK, reduced the levels of phosphorylated ATF2 in cells overexpressing ATF2 (Figure 5c). The experiments described above suggest that overexpression of ATF2 results in the activation of p38, which leads to enhanced phosphorylation of ATF2.
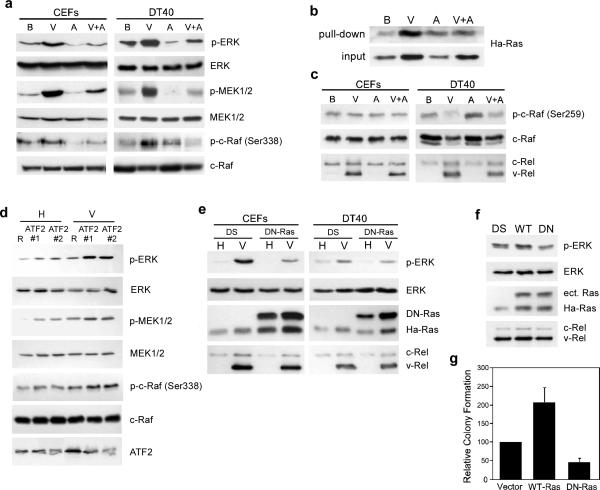
Our observation that the levels of phosphorylated, active ERK are decreased upon overexpression of ATF2 was especially interesting since ERK is one of the downstream effectors of Ha-Ras (Robbins et al., 1992). Therefore, we investigated whether v-Rel activates the ERK pathway, and if the overexpression of ATF2 disrupts this process. The levels of total and active forms of the upstream components of the ERK pathway were determined by Western blot analysis using whole cell lysates from CEFs and DT40 cells infected with control BIS viruses or BIS viruses expressing v-Rel or ATF2, alone or in combination. The expression of v-Rel in fibroblasts and lymphoid cells resulted in high levels of phosphorylated c-Raf (Ser338), MEK1/2, and ERK (components of the Ras pathway) (Figure 6a). No increase in the total levels of these proteins was observed. Consistent with the ability of ATF2 to reduce levels of active, phosphorylated ERK, the co-expression of ATF2 with v-Rel inhibited v-Rel-mediated activation of c-Raf, MEK1/2, and ERK in fibroblasts and lymphoid cells.
Figure 6.
ATF2 suppresses Ha-Ras activation in v-Rel-expressing cells. (a) Activity of the Raf-MEK-ERK pathway in cells expressing v-Rel. Whole cell lysates were prepared from CEFs and DT40 cells expressing control BIS virus (B) and BIS expressing ATF2 (A) and v-Rel alone (V) or in combination (V+A). The expression of phosphorylated ERK (p-ERK), total ERK, phosphorylated MEK1/2 (p-MEK1/2), total MEK1/2, phosphorylated c-Raf (p-c-Raf Ser338), and total c-Raf was determined by Western Blot analysis. The expression of v-Rel and c-Rel in these lysates was demonstrated in Figure 5a. (b) Regulation of Ha-Ras activity by v-Rel and ATF2. Levels of active Ha-Ras in cell lysates from DT40 cells expressing viruses described above were determined using the Active Ras Pull-Down and Detection Kit (Thermo Scientific). The levels of active (top panel) and total (bottom panel) Ha-Ras in these lysates are shown. (c) Regulation of an inhibitory phosphorylation site on c-Raf by v-Rel and ATF2. Western blot analysis was employed to determine the levels of total and phosphorylated c-Raf (Ser259), c-Rel, and v-Rel in whole cell lysates prepared from the CEFs and DT40 cells described above. (d) Reduction in ATF2 levels increases the activation of the Ras signaling pathway. DT40 cells infected with helper virus CSV (H) or viruses expressing v-Rel (V) were superinfected with control RCAS virus (R) or those expressing two different shRNAs against ATF2 (ATF2#1 and ATF2#2). Whole cell lysates were prepared 10 days post-superinfection and analyzed by Western blot for the levels of total and phosphorylated ERK, MEK1/2, and c-Raf as well as ATF2. (e) Dominant negative HA-Ras inhibits ERK activation in v-Rel-expressing cells. CEFs and DT40 cells infected with helper virus CSV (H) or viruses expressing v-Rel (V) were superinfected with control DS virus or viruses expressing a dominant negative Ha-Ras mutant (DN-Ras). Whole cell lysates were prepared and analyzed by Western blot for the levels of total and phosphorylated ERK, DN-Ras, endogenous HA-Ras, c-Rel, and v-Rel. (f) Ha-Ras regulates ERK activation in a v-Rel transformed cell line. 160/2 cells were infected with control DS virus or viruses expressing wild-type Ha-Ras (WT) or DNRas (DN). Whole cell lysates were prepared and analyzed by Western blot for the levels of total and phosphorylated ERK, ectopic Ras (ect. Ras), endogenous Ha-Ras, c-Rel, and v-Rel. (g) Alterations in Ha-Ras activity affect colony formation of v-Rel transformed cells. 160/2 cells described above were plated in soft agar and scored for colony formation after seven days.
Phosphorylation of c-Raf at Ser338 is dependent on the activation of Ras (Chaudhary et al., 2000; Sun et al., 2000). To directly measure the activity of Ha-Ras, pull-down experiments were performed. Cell lysates from DT40 cells infected with viruses described above were incubated with GST-fusion proteins containing the Ras binding domain of c-Raf and bound proteins were resolved by SDS-PAGE. Since only active, GTP-bound Ras can bind to the substrate, the level of bound Ha-Ras is reflective of its activation state. Western blot analysis demonstrated that in cells expressing v-Rel, there are higher levels of active, GTP-bound Ras than in control cells (Figure 6b). Furthermore, the co-expression of v-Rel with ATF2 reduced the levels of activated Ha-Ras to levels similar to those observed in control cells. In addition to evaluating the levels of active, GTP-bound Ras, we determined the levels of total Ha-Ras protein in these cells regardless of its activation state. Interestingly, v-Rel also increased the levels of total Ha-Ras in these cells, while the overexpresson of ATF2 had no effect.
Although ATF2 inhibited v-Rel-induced activation of the Ras-Raf-MEK-ERK pathway, the overexpression of ATF2 alone in DT40 cells resulted in the slight activation of Ha-Ras (Figure 6b). This activation of Ha-Ras corresponded to the elevated levels of c-Raf phosphorylated on Ser338 in DT40 cells (Figure 6a). Despite the activation of Ha-Ras and c-Raf in DT40 cells overexpressing ATF2, reduced levels of active, phosphorylated MEK1/2 and ERK were observed. To understand this apparent contradiction, we evaluated the state of additional phosphorylation sites on c-Raf (Figure 6c). This analysis revealed that the overexpression of ATF2 also induces the phosphorylation of c-Raf at Ser259 in DT40 cells. This enhanced phosphorylation of c-Raf was not observed in fibroblast cultures. The phosphorylation of c-Raf at Ser259 has been shown to participate in the binding of 14-3-3 to inhibit c-Raf activity (Chong et al., 2001).
In complementary experiments, the effect of reducing endogenous levels of ATF2 on the activation of the Ras pathway was analyzed (Figure 6d). v-Rel-expressing and control DT40 cells were infected with control viruses or viruses expressing shRNAs against ATF2. Reduced ATF2 levels correlated with increased levels of phoshorylated ERK, MEK1/2, and c-Raf (Ser338) in both control DT40 cells and those expressing v-Rel. This effect, however, was more dramatic in cells expressing v-Rel.
To directly test the role of Ras in transformation of cells by v-Rel, retroviruses expressing a dominant negative mutant of Ha-Ras (DN-Ras) were employed. The expression of DN-Ras blocked the induction of phosphorylated, active ERK by v-Rel in both CEF cultures and DT40 cells, demonstrating the importance of the Ras pathway for v-Rel-mediated ERK activation (Figure 6e). To define the contribution of Ha-Ras to transformation by v-Rel, the v-Rel transformed cell line 160/2 was infected with control virus or viruses expressing wild-type Ha-Ras or DN-Ras. The levels of active, phosphorylated ERK were evaluated in lysates from these cells (Figure 6f). Reflective of the role Ha-Ras plays in ERK activation, cells infected with viruses expressing wild-type Ha-Ras expressed higher levels of phosphorylated ERK, whereas cells expressing DN-Ras exhibited reduced levels of active ERK. In addition to their effect on the activity of the ERK pathway, the expression of DN-Ras resulted in a 50% decrease in colony formation, while cells overexpressing Ha-Ras formed colonies twice as efficiently as control cells (Figure 6g). Overall, these results reveal Ha-Ras as an effector of v-Rel transformation and demonstrate an inhibitory function of ATF2 in the Ras signaling pathway.
Discussion
In this study, we report specific AP-1 family members as targets of v-Rel and demonstrate their ability to mediate v-Rel transformation. Coexpression of c-Fos or c-Jun with v-Rel strongly promoted the transforming ability of v-Rel in primary splenic lymphocytes whereas inhibiting c-Fos and c-Jun expression decreased colony formation in a v-Rel transformed cell line (Table 1 and Figure 2). Overall, our studies demonstrate that c-Jun and c-Fos are important players in both the initiation and maintenance of v-Rel transformation. Unlike v-Rel, c-Rel failed to significantly alter the expression of c-Fos and c-Jun relative to control cells. However, the overexpression of c-Jun and c-Fos strongly enhanced the ability of c-Rel to transform primary splenic lymphocytes in liquid transformation assays, demonstrating that the differential expression of AP-1 proteins by v-Rel and c-Rel contributes to the enhanced oncogenicity of v-Rel relative to c-Rel (Figure 4).
These results might also be extended to understanding transformation of human lymphoid cells by Rel/NF-κB. Gene amplifications of c-Rel and constitutive, nuclear Rel/NF-κB activity are among the hallmarks of certain human lymphoid malignancies, such as Hodgkin's lymphoma, diffuse large B-cell lymphoma, and anaplastic large-cell lymphomas (Chin et al., 2009; Kalaitzidis et al., 2002; Mathas et al., 2005). Interestingly, overexpression of AP-1 proteins has been linked to some of these human cancers caused by deregulated Rel/NF-κB signaling (Mathas et al., 2002; Rassidakis et al., 2005; Shaulian, 2010). By supporting proliferation, inhibiting apoptosis, and synergizing with Rel/NF-κB to activate gene expression for a pro-tumorigenic response, AP-1 proteins are suggested to play an important role in lymphomagenesis. However, the mechanism by which these lymphomas give rise to aberrant AP-1 expression is not clear. Mammalian Rel/NF-κB factors are involved in positively modulating AP-1 expression. For instance, decreased expression of c-fos was observed in IKK1−/− and IKK2−/− MEFs, or when the DNA binding activity of RelA/NF-κB1 heterodimers was inhibited in pancreatic cells (Fujioka et al., 2004). Our studies with v-Rel suggest that deregulated Rel/NF-κB activity can induce AP-1 activation via direct upregulation of gene expression and activation of pathways that stimulate AP-1 activity (Supplemental Figure 1 and 2).
The contribution of ATF2 to oncogenesis is poorly defined. Overexpression of ATF2 alone does not transform cells; however, several lines of evidence suggest a role for ATF2 in promoting oncogenesis. ATF2 has been found to enhance proliferation in both human and mouse cancer cell lines and is implicated in prostate and breast cancers (Lewis et al., 2005; Ricote et al., 2006; Vlahopoulos et al., 2008; Zoumpourlis et al., 2000). Conversely, other studies have revealed that mice with reduced ATF2 expression are predisposed to mammary tumors and the development of papillomas (Bhoumik et al., 2008; Maekawa et al., 2007). Our characterization of ATF2 in v-Rel-mediated transformation similarly suggests opposing roles for ATF2 in oncogenesis. The modest increase in ATF2 expression observed in v-Rel transformed cells promotes oncogenesis, as demonstrated by reduced colony formation of cells expressing shRNAs against ATF2. However, enhanced expression of ATF2 inhibits transformation by v-Rel. These results suggest that there is a delicate balance between the oncogenic and tumor suppressor functions of ATF2.
While dissecting the dual role of ATF2 in the context of v-Rel transformation, we observed elevated levels of phosphorylated ATF2 at Thr69 and Thr71 in cells overexpressing ATF2, which was solely dependent on the activity of p38 MAPK (Figure 5). In addition, overexpression of ATF2 resulted in increased p38 activity in both fibroblast and lymphoid cells. These results suggest a positive feedback mechanism whereby ATF2 induces p38 activity to further induce its own activity. Although negative feedback regulation of p38 has been reported, this is the first report of a downstream effector of p38 positively regulating its activity (Breitwieser et al., 2007). Interestingly, deletion of the transcriptional activation domain of ATF2 in an ATF7-null background results in the activation of p38 through the downregulation of dual specificity phosphatases (DUSP) (Breitwieser et al., 2007). Whether p38 activation induced by overexpression of ATF2 also occurs through altered expression of DUSP family members remains to be determined.
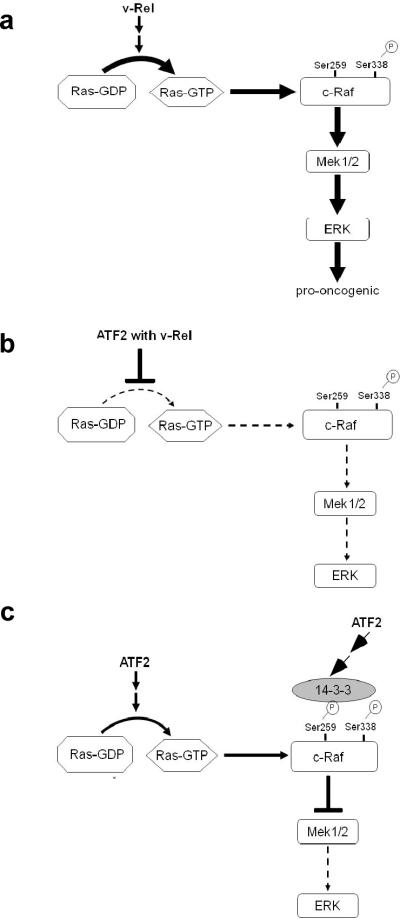
While ATF2 is able to activate p38, our novel observations of the effect of ATF2 on the ERK pathway provided insight into its transformation-inhibiting potential. We observed decreased levels of active, phosphorylated ERK in cells overexpressing ATF2 (Figure 5). Activation of the ERK pathway has a well-defined role in oncogenesis, and our results support a model in which ATF2 inhibits transformation by blocking v-Rel-mediated activation of the Ras-Raf-MEK-ERK pathway (Figure 7). v-Rel induced the activation of Ha-Ras and its downstream effector pathway components c-Raf, MEK1/2, and ERK (Figure 6 and 7a). However, the expression of ATF2 in the presence of v-Rel blocked activation of this pathway (Figure 7b). Reducing ATF2 expression by shRNAs had the opposite effect, derepressing the activity of c-Raf, MEK1/2, and ERK, which further corroborated the inhibitory effects of ATF2 on this pathway (Figure 6). Furthermore, the expression of a DN-Ras mutant decreased the level of active, phosphorylated ERK in fibroblast and lymphoid cells transformed by v-Rel and blocked the ability of a v-Rel transformed cell line to form colonies in soft agar. Although we cannot rule out the possibility that DN-Ras inhibits other Ha-Ras effector pathways, we have observed a similar reduction in colony formation of v-Rel transformed cells exposed to chemical inhibitors of the ERK pathway (J. Kralova et al, submitted manuscript). Furthermore, analysis of the JNK MAPK and PI3K/Akt pathways, known effectors of Ha-Ras, revealed no change in their activation states with the expression of DN-Ras (Supplemental Figure 3) (Luo et al., 2003; Minden et al., 1995).
Figure 7.
Model for the regulation of Ras-Raf-MEK-ERK pathway by v-Rel and ATF2. (a) v-Rel expression in fibroblast and lymphoid cells results in the induction of Ha-Ras activity, resulting in the phosphorylation and activation of c-Raf, MEK1/2, and ERK. The activation of this pathway by v-Rel plays a key role in transformation by v-Rel. (b) The co-expression of ATF2 with v-Rel blocks v-Rel-mediated activation of the Ras-Raf-MEK-ERK pathway. (c) The ectopic expression of ATF2 alone in DT40 cells induces Ha-Ras activity. However, the activation of the downstream MEK-ERK pathway is blocked by the recruitment of the inhibitory protein 14-3-3 due to the phosphorylation of c-Raf at Ser259.
Our results also suggest that ATF2 can regulate signaling pathways in a cell-type specific and/or context-dependent manner. Differences were found at the stage at which ATF2 regulated the Ras/ERK signaling pathway in fibroblast and lymphoid cells. In fibroblasts, the overexpression of ATF2 (in the absence or presence of v-Rel) blocked the activation of c-Raf, MEK1/2, and ERK. However, in the DT40 B-cell line, overexpression of ATF2 in the absence of v-Rel increased Ha-Ras activity and phosphorylated c-Raf (Ser338) levels whereas phospho-MEK1/2 and phospho-ERK levels were found to be decreased. Our results suggest that negative regulation of c-Raf activity is responsible for the reduced levels of phosphorylated MEK1/2 and ERK. While phosphorylation of c-Raf at Ser338 is involved in positive regulation, its phosphorylation at Ser259 is known to be inhibitory, preventing the activation of downstream effectors (Chong et al., 2001). Examination of the phosphorylation status of c-Raf revealed higher levels of phosphorylation at Ser259 in DT40 cells overexpressing ATF2 alone relative to control cells, suggesting that ATF2 can inhibit c-Raf activity through recruitment of 14-3-3 proteins (Figure 6c and 7c). This event may be cell-type specific, since the analysis in fibroblasts did not reveal any differences in the phosphorylation status of c-Raf at Ser259 in cells overexpressing ATF2 alone. Since DT40 cells are transformed by the insertional activation of c-myc by avian leukosis virus, the differential effects of ATF2 on the Ras-Raf-MEK-ERK pathway in this cell type may be due to the activity of c-Myc (Ewert and Duhadaway, 1999). Taken together, our results suggest a model in which the overexpression of ATF2 inhibits v-Rel-mediated activation of the Ras-Raf-MEK-ERK pathway, and depending on the cellular context, may do so at different stages of the pathway.
Our studies demonstrate a clear role for c-Jun and c-Fos in the v-Rel transformation process. These are the only examples of genes differentially activated by v-Rel and c-Rel that have been shown to enhance transformation by c-Rel. However, experiments with ATF2 revealed that different AP-1 proteins can have distinct and often complex roles in transformation. Our results indicate that ATF2 can regulate the Ras-Raf-MEK-ERK pathway at multiple steps. The work of others suggests that Ras pathway inhibition by ATF2 is likely not restricted to the avian system. ATF2-null keratinocytes are more susceptible to transformation by oncogenic Ras than wild-type cells (Bhoumik et al., 2008). Further examination of the mechanism(s) of ATF2 regulation of Ras activity will likely provide greater insight into the role of ATF2 in oncogenesis.
Materials and Methods
Cell culture
Primary CEFs were prepared from 10–11 day old embryos (Charles River SPAFAS, Wilmington, MA) and grown in Dulbecco's modified Eagle's Medium (DMEM) supplemented with 5% newborn calf serum (Atlanta Biologicals, Lawrenceville, GA), 5% chicken serum, and 1% Antibiotic-Antimycotic (Invitrogen Corporation, Carlsbad, CA). Cells were grown at 37°C and 8% CO2. The DT40 and DT95 B-cell lines, transformed by the avian leukosis virus, and the v-Rel transformed 160/2 cell lines were grown in the same conditions. Retroviral stocks were prepared using DMEM supplemented with 5% fetal calf serum instead of 5% newborn calf serum.
Retroviral vectors
Four retroviral vector systems were employed in these studies. The REV-based retroviral vectors pREV-C and pREV-TW contain the coding regions of c-Rel and v-Rel, respectively (Nehyba et al., 1997). The bicistronic spleen necrosis virus-based pBIS retroviral vector has been previously described (Majid et al., 2006). The ORFs encoding c-Rel and v-Rel were cloned into pBIS to form the pBIS-C and pBIS-V vector, respectively. c-jun, c-fos, and ATF2 were cloned into the pBIS, pBIS-C, and pBIS-V retroviral vectors and Mo, fra-2, and antisense ATF2 into the pBIS and pBIS-V retroviral vectors.
To generate the M0 (E264K) mutant of avian c-Jun, a point mutation was introduced into c-Jun using the Quik-Change Site-Directed Mutagenesis Kit (Stratagene, LaJolla, CA). The primer designed was as follows: 5'-GGAAAGGATTGCC AGGTTGaAAGAAAAAGTGAAAAC-3'. Lower case letter indicates position of the mutagenic nucleotide. The mutagenized sequence of M0 was confirmed at the DNA Sequencing Facility at the University of Texas at Austin.
The Rous sarcoma virus (RSV)-based shRNA vector system is composed of pRFPRNAiC and RCASARNAi (Das et al., 2006). Gene specific oligonucleotides were first cloned into pRFPRNAiC. A NotI-ClaI restriction fragment containing this cassette downstream of the chicken U6 promoter was excised and cloned into RCASARNAi to generate a retroviral vector expressing each shRNA. The oligos encoding the shRNA hairpins for each gene are described in Suppemental Materials and Methods.
The RSV-based retroviral vector system, DS, which is composed of the pTZDS-XB and pREP-A plasmids, was employed to express wild-type Ha-Ras and a dominant negative Ha-Ras mutant (Howe and Marshall, 1993; Hrdlickova et al., 2001). The chicken Ha-Ras gene was mutated to encode a dominant negative Ha-Ras protein (S17N) using the Quik-Change Site Directed Mutagenesis Kit (Stratagene).
Western blot analysis
Proteins from whole cell lysates were resolved by 10% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to Optitran nitrocellulose membranes (Schleicher and Schuell, Keene NH). For the detection of v-Rel and c-Rel, the HY87 monoclonal antibody was used (Hrdlickova et al., 1994). Primary antibodies purchased from Santa Cruz Biotechnology (Santa Cruz, CA) were used to detect avian c-Fos (sc-253), c-Jun (sc-45), Fra2 (sc-604), and ATF2 (sc-6233). Primary antibodies were obtained from Cell Signaling Technology (Danvers, MA) to detect the following avian proteins: phospho-ATF2 (cat. 9225), total ERK (cat. 1902), phospho-ERK (cat. 9191), total JNK (cat. 9258), phospho-JNK (cat. 4668), total p38 (cat. 9219), phospho-p38 (cat. 9216), total MEK1/2 (cat. 9126), phospho-MEK1/2 (cat. 9121), total Raf (cat. 9422), phospho-Raf (cat. 9421 & 9427), total MAPKAP2 (cat. 3042), phospho-MAPKAP2 (cat. 3007), total Akt (cat. 9272), and phospho-Akt (cat. 9271). The levels of GTP-bound Ha-Ras were determined using the Active Ras Pull-Down and Detection Kit (Thermo Scientific, Waltham, MA) according to the manufacturer's directions. The monoclonal antibody from this kit was also used for the detection of total Ha-Ras. Secondary antibodies employed for this study were horseradish peroxidase-conjugated donkey anti-rabbit IgG and donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Protein expression was visualized by Western lighting chemiluminescence reagent (Perkin-Elmer Life and Analytical Sciences, Wellesley, MA) or by SuperSignal West Dura reagent (Thermo Scientific).
In vitro transformation assays
In vitro transformation assays were performed as previously described (Liss and Bose, 2002). Briefly, primary splenic lymphocytes were isolated from spleens of three-week old chickens, purified by differential gradient centrifugation, and infected with BIS retroviruses Cells were resuspended in DMEM media containing 15% fetal bovine serum, 5% chicken serum, and 1% Antibiotic-Antimycotic 24 hours after infection and incubated for an additional two days before plating in soft agar. For liquid transformation assays, splenic lymphocytes were infected and, a day later, resuspended in fresh DMEM as described above. After an additional 3 days, cells from each infection underwent eight 1:2 serial dilutions. 160 μl of each dilution was aliquoted into 12 wells in a 96-well plate. For both in vitro and liquid transformation assays, plates were kept at 37°C and 8% CO2 and colonies scored 10 days later. Liquid transformation efficiency was calculated by multiplying the number of wells showing visible growth for a specific dilution by the reciprocal of that dilution.
Soft agar colony assays of v-Rel-transformed cell lines
Plating of v-Rel transformed cells in soft agar has been previously described (Majid et al., 2006). The 160/2 v-Rel-transformed cell line was infected with empty RCAS vector or one encoding shRNAs specific for luciferase, c-Jun, c-Fos, or ATF2 at an MOI of 7–10. Viable cells were counted 10 days post-infection and plated in 0.35% soft agar (DMEM containing 0.35% Noble Agar (Becton, Dickinson and Company, Sparks, MD), 5% FCS, 5% CS, and 1% Antibiotic-Antimycotic). Cells were plated at a concentration of 1.5 × 104 cells/plate. Plates were kept at 37°C and 8% CO2, and colonies were scored 7–10 days post-plating.
Electrophoretic Mobility Shift Assays
Nuclear extracts from DT40 cells were prepared as previously described (Dyer and Herzog, 1995). In vitro translations were performed using the T7 polymerase based TNT Coupled Reticulocyte Lysate System (Promega, Madison WI) according to the manufacturer's directions. Genes encoding c-Fos, c-Jun, M0 and ATF2 were expressed from the pBluescript expression vector. Parallel reactions expressing these proteins alone or in combination were performed in the presence or absence of [35S]-methionine. Proteins containing [35S]-methionine were resolved by 10% SDS-PAGE and quantitated by phosphorimager analysis and normalized to the number of methionines. The amounts of DNA were adjusted so that translated c-Fos and ATF2 were in two-fold excess relative to c-Jun or M0. Parallel translations performed in the absence of [35S]-methionine were employed in EMSA reactions.
For electrophoretic mobility shift assays, 5 μg of nuclear extract or 2 μl of in vitro translated protein was incubated for 15 min at room temperature in a total reaction volume of 25 μl containing 20 mM Tris pH 7.5, 75 mM KCl, 40 μM EDTA, 5% glycerol, 1 mM dithiothreitol, 100 μg BSA/ml, and 1 μg of poly[dI/dC]. [32P]dCTP-labeled Coll or Jun2 DNA probes (50,000 cpm) were then added to the reaction mixture which was incubated for an additional 20 min at room temperature. For supershift analysis, 4 μg of the appropriate antibody or normal rabbit serum was added and incubated on ice for 45 min before the addition of radiolabeled oligonucleotide. Antibodies for supershifts were obtained from Santa Cruz Biotechnology and are listed as follows: c-Jun (sc-45X), c-Fos (sc-253X), Fra2 (sc-604X), and ATF2 (sc-6233X). Samples were analyzed by electrophoresis on a 5% nondenaturing polyacrylamide gel in 0.25× TBE buffer. Gels were dried and DNA-protein complexes visualized by autoradiography.
The top strands of the oligonucleotide sequence used for probes are listed as follows: Coll: 5'-AGCTAGCAtgagtcaGACAC-3; Jun2: 5'-AGCTAGCAttacctcatCCC-3'. The lower case letters represent nucleotides specific for Jun:Fos binding (Coll) and Jun:ATF2 binding (Jun2). The bottom strands of the probe were synthesized by annealing a 9-bp oligonucleotide complementary to the 3' end of the top strand and extending with Klenow in the presence of [32P]dCTP (Liss and Bose, 2002).
Supplementary Material
Supplemental Figure 1. Alterations in the expression and activity of AP-1 family members in response to Rel proteins. CEFs, DT40, and DT95 cells were infected with REV-C expressing c-Rel (C), REV-TW expressing v-Rel (V), or helper virus alone (H). RNA (10 μg) and whole cell lysates (25–50 μg) from infected cells were employed in Northern (a) and Western (b) blot analyses, respectively. The detected genes and proteins are indicated to the right of each panel. (c) Activation of AP-1 dependent luciferase reporter constructs containing AP-1 binding sites from the collagenase promoter (5×coll) or c-jun promoter (5×jun2). CEFs were transiently transfected with the AP-1 reporter constructs and the expression vector Rc/RSV encoding c-Rel or v-Rel. Cell lysates were prepared 32–36 hours after transfection and luciferase assays performed. The average and standard deviation of at least four independent experiments is shown.
Supplemental Figure 2. Regulation of c-fos and ATF2 promoters by v-Rel. (a) Deletion constructs of the c-fos promoter were subcloned into the pGL3-Basic reporter vector. Diagrams of the reporter constructs are shown to the left of the luciferase assay results. The relative position of the predicted transcription start site is indicated by an arrow. CEF cultures were transfected with c-fos reporter constructs, the pRL-TK coreporter vector, and an empty expression plasmid (pCI) as a control, or pCI encoding v-Rel or c-Rel. Cells were harvested 32 hours after transfection and analyzed for luciferase activity. Luciferase activity from the c-fos promoter constructs was normalized for transfection efficiency by measuring pRL-TK coreporter activity. The results are presented as fold activation relative to results obtained with an empty expression plasmid control. The average and standard deviation of at least three independent experiments is shown. (b) The sequence of the serum response element and κB site located between −312 and −267 of the c-fos promoter is shown. (c) Nuclear extracts were isolated from CEF cultures 7–10 days after infection with the helper virus CSV (H) or viruses expressing c-Rel (C) or v-Rel (V). EMSAs were performed using probes encompassing the sequences shown in panel b. Nuclear extracts from CEF cultures transformed by v-Rel were incubated with normal rabbit serum (NRS) or antisera specific for Elk-1, serum response factor (SRF), or v-Rel prior to the addition probe. Binding complexes containing SRF and v-Rel are indicated. (d) v-Rel, SRF, and c-Jun directly bind the c-fos promoter in v-Rel transformed cells. Protein-DNA complexes from v-Rel transformed CEF cultures were crosslinked with formaldehyde, DNA sheared by sonication, and employed in chromatin immunoprecipitation experiments with control normal rabbit serum (NRS) and antibodies specific to Elk-1, SRF, c-Jun, and v-Rel. Input and precipitated DNA was amplified by PCR using primers flanking −312 and −267 of the c-fos promoter, the κB sites within the ikba promoter (positive control), or a region within the collagenase promoter (COLA). (e) v-Rel binds to two κB sites in the proximal promoter of ATF2. Nuclear extracts were isolated from CEF cultures 7–10 days after infection with the helper virus CSV (H) or viruses expressing v-Rel (V). EMSAs were performed using probes encompassing two putative κB sites on the ATF2 promoter at −373 and −430. Analysis was restricted to κB sites identified within 500 base pairs of the ATF2 transcription start site. Supershift assays were also performed by incubating nuclear extracts from CEF cultures transformed by v-Rel with normal rabbit serum (NRS) or antisera specific to v-Rel prior to addition of the probe containing the putatitve κB sites.
Supplemental Figure 3. Ha-Ras does not regulate JNK or AKT activity in v-Rel transformed cells. 160/2 cells were infected with control DS virus or viruses expressing wild-type Ha-Ras (WT) or DN-Ras (DN). Whole cell lysates were prepared and analyzed by Western blot for the levels of total and phosphorylated JNK and AKT.
Acknowledgements
We thank Ashley Sims for her technical assistance and Marc Castellazzi for providing the Coll and Jun2 luciferase reporter vectors.
This study was supported by Public Health Service grants CA33192 and CA098151 from the National Cancer Institute.
Footnotes
Conflict of Interest The authors declare no conflict of interest.
References
- Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55:875–85. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Angel P, Imagawa M, Chiu R, Stein B, Imbra RJ, Rahmsdorf HJ, et al. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49:729–39. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Bhoumik A, Fichtman B, Derossi C, Breitwieser W, Kluger HM, Davis S, et al. Suppressor role of activating transcription factor 2 (ATF2) in skin cancer. Proc Natl Acad Sci USA. 2008;105:1674–9. doi: 10.1073/pnas.0706057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser W, Lyons S, Flenniken AM, Ashton G, Bruder G, Willington M, et al. Feedback regulation of p38 activity via ATF2 is essential for survival of embryonic liver cells. Genes Dev. 2007;21:2069–82. doi: 10.1101/gad.430207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary A, King WG, Mattaliano MD, Frost JA, Diaz B, Morrison DK, et al. Phosphatidylinositol 3-kinase regulates Raf1 through Pak phosphorylation of serine 338. Curr Biol. 2000;10:551–4. doi: 10.1016/s0960-9822(00)00475-9. [DOI] [PubMed] [Google Scholar]
- Chen FE, Ghosh G. Regulation of DNA binding by Rel/NF-κB transcription factors: structural views. Oncogene. 1999;18:6845–52. doi: 10.1038/sj.onc.1203224. [DOI] [PubMed] [Google Scholar]
- Chin M, Herscovitch M, Zhang N, Waxman DJ, Gilmore TD. Overexpression of an activated REL mutant enhances the transformed state of the human B-lymphoma BJAB cell line and alters its gene expression profile. Oncogene. 2009;28:2100–11. doi: 10.1038/onc.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong H, Lee J, Guan KL. Positive and negative regulation of Raf kinase activity and function by phosphorylation. Embo J. 2001;20:3716–27. doi: 10.1093/emboj/20.14.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G, Gilmore TD. Mutations in the NF-κB signaling pathway: implications for human disease. Oncogene. 2006;25:6831–43. doi: 10.1038/sj.onc.1209939. [DOI] [PubMed] [Google Scholar]
- Das RM, Van Hateren NJ, Howell GR, Farrell ER, Bangs FK, Porteous VC, et al. A robust system for RNA interference in the chicken using a modified microRNA operon. Dev Biol. 2006;294:554–63. doi: 10.1016/j.ydbio.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Dyer RB, Herzog NK. Isolation of intact nuclei for nuclear extract preparation from a fragile B-lymphocyte cell line. Biotechniques. 1995;19:192–5. [PubMed] [Google Scholar]
- Ewert D, Duhadaway J. Inhibition of apoptosis by Marek's disease viruses. Acta Virol. 1999;43:133–5. [PubMed] [Google Scholar]
- Fujii M, Minamino T, Nomura M, Miyamoto K, Tanaka J, Seiki M. v-Rel activates the proto-oncogene c-jun promoter: a correlation with its transforming activity. Leukemia. 1997;11(Suppl 3):402–4. [PubMed] [Google Scholar]
- Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, et al. NF-κB and AP-1 connection: mechanism of NF-κB-dependent regulation of AP-1 activity. Mol Cell Biol. 2004;24:7806–19. doi: 10.1128/MCB.24.17.7806-7819.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore TD. Multiple mutations contribute to the oncogenicity of the retroviral oncoprotein v-Rel. Oncogene. 1999;18:6925–37. doi: 10.1038/sj.onc.1203222. [DOI] [PubMed] [Google Scholar]
- Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–4. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117:5965–73. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- Howe LR, Marshall CJ. Identification of amino acids in p21ras involved in exchange factor interaction. Oncogene. 1993;8:2583–90. [PubMed] [Google Scholar]
- Hrdlickova R, Nehyba J, Bose HR., Jr. Interferon regulatory factor 4 contributes to transformation of v-Rel-expressing fibroblasts. Mol Cell Biol. 2001;21:6369–86. doi: 10.1128/MCB.21.19.6369-6386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdlickova R, Nehyba J, Humphries EH. v-rel induces expression of three avian immunoregulatory surface receptors more efficiently than c-rel. J Virol. 1994;68:308–19. doi: 10.1128/jvi.68.1.308-319.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaitzidis D, Davis RE, Rosenwald A, Staudt LM, Gilmore TD. The human B-cell lymphoma cell line RC-K8 has multiple genetic alterations that dysregulate the Rel/NF-κB signal transduction pathway. Oncogene. 2002;21:8759–68. doi: 10.1038/sj.onc.1206033. [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–6. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Kralova J, Liss AS, Bargmann W, Bose HR., Jr. AP-1 factors play an important role in transformation induced by the v-rel oncogene. Mol Cell Biol. 1998;18:2997–3009. doi: 10.1128/mcb.18.5.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JS, Vijayanathan V, Thomas TJ, Pestell RG, Albanese C, Gallo MA, et al. Activation of cyclin D1 by estradiol and spermine in MCF-7 breast cancer cells: a mechanism involving the p38 MAP kinase and phosphorylation of ATF-2. Oncol Res. 2005;15:113–28. doi: 10.3727/096504005776367924. [DOI] [PubMed] [Google Scholar]
- Liss AS, Bose HR. In: Encyclopedia of Virology. Mahy BWJ, Van Regenmortel MHV, editors. 2008. pp. 412–419. [Google Scholar]
- Liss AS, Bose HR., Jr. Mutational analysis of the v-Rel dimerization interface reveals a critical role for v-Rel homodimers in transformation. J Virol. 2002;76:4928–39. doi: 10.1128/JVI.76.10.4928-4939.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–62. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Shinagawa T, Sano Y, Sakuma T, Nomura S, Nagasaki K, et al. Reduced levels of ATF-2 predispose mice to mammary tumors. Mol Cell Biol. 2007;27:1730–44. doi: 10.1128/MCB.01579-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid SM, Liss AS, You M, Bose HR. The suppression of SH3BGRL is important for v-Rel-mediated transformation. Oncogene. 2006;25:756–68. doi: 10.1038/sj.onc.1209107. [DOI] [PubMed] [Google Scholar]
- Mathas S, Hinz M, Anagnostopoulos I, Krappmann D, Lietz A, Jundt F, et al. Aberrantly expressed c-Jun and JunB are a hallmark of Hodgkin lymphoma cells, stimulate proliferation and synergize with NF-κB. EMBO J. 2002;21:4104–13. doi: 10.1093/emboj/cdf389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathas S, Johrens K, Joos S, Lietz A, Hummel F, Janz M, et al. Elevated NF-κB p50 complex formation and Bcl-3 expression in classical Hodgkin, anaplastic large-cell, and other peripheral T-cell lymphomas. Blood. 2005;106:4287–93. doi: 10.1182/blood-2004-09-3620. [DOI] [PubMed] [Google Scholar]
- Minden A, Lin A, Claret FX, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–57. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- Morton S, Davis RJ, Cohen P. Signalling pathways involved in multisite phosphorylation of the transcription factor ATF-2. FEBS Lett. 2004;572:177–83. doi: 10.1016/j.febslet.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Nehyba J, Hrdlickova R, Bose HR., Jr. Differences in κB DNA-binding properties of v-Rel and c-Rel are the result of oncogenic mutations in three distinct functional regions of the Rel protein. Oncogene. 1997;14:2881–97. doi: 10.1038/sj.onc.1201150. [DOI] [PubMed] [Google Scholar]
- Rassidakis GZ, Thomaides A, Atwell C, Ford R, Jones D, Claret FX, et al. JunB expression is a common feature of CD30+ lymphomas and lymphomatoid papulosis. Mod Pathol. 2005;18:1365–70. doi: 10.1038/modpathol.3800419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–47. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- Ricote M, Garcia-Tunon I, Bethencourt F, Fraile B, Onsurbe P, Paniagua R, et al. The p38 transduction pathway in prostatic neoplasia. J Pathol. 2006;208:401–7. doi: 10.1002/path.1910. [DOI] [PubMed] [Google Scholar]
- Robbins DJ, Cheng M, Zhen E, Vanderbilt CA, Feig LA, Cobb MH. Evidence for a Ras-dependent extracellular signal-regulated protein kinase (ERK) cascade. Proc Natl Acad Sci USA. 1992;89:6924–8. doi: 10.1073/pnas.89.15.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian E. AP-1 - The Jun proteins: Oncogenes or tumor suppressors in disguise? Cell Signal. 2010;22:894–9. doi: 10.1016/j.cellsig.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Stephens RM, Rice NR, Hiebsch RR, Bose HR, Jr., Gilden RV. Nucleotide sequence of v-rel: the oncogene of reticuloendotheliosis virus. Proc Natl Acad Sci USA. 1983;80:6229–33. doi: 10.1073/pnas.80.20.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, King AJ, Diaz HB, Marshall MS. Regulation of the protein kinase Raf-1 by oncogenic Ras through phosphatidylinositol 3-kinase, Cdc42/Rac and Pak. Curr Biol. 2000;10:281–4. doi: 10.1016/s0960-9822(00)00359-6. [DOI] [PubMed] [Google Scholar]
- van Dam H, Castellazzi M. Distinct roles of Jun:Fos and Jun:ATF dimers in oncogenesis. Oncogene. 2001;20:2453–64. doi: 10.1038/sj.onc.1204239. [DOI] [PubMed] [Google Scholar]
- Vlahopoulos SA, Logotheti S, Mikas D, Giarika A, Gorgoulis V, Zoumpourlis V. The role of ATF-2 in oncogenesis. Bioessays. 2008;30:314–27. doi: 10.1002/bies.20734. [DOI] [PubMed] [Google Scholar]
- Zoumpourlis V, Papassava P, Linardopoulos S, Gillespie D, Balmain A, Pintzas A. High levels of phosphorylated c-Jun, Fra-1, Fra-2 and ATF-2 proteins correlate with malignant phenotypes in the multistage mouse skin carcinogenesis model. Oncogene. 2000;19:4011–21. doi: 10.1038/sj.onc.1203732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Alterations in the expression and activity of AP-1 family members in response to Rel proteins. CEFs, DT40, and DT95 cells were infected with REV-C expressing c-Rel (C), REV-TW expressing v-Rel (V), or helper virus alone (H). RNA (10 μg) and whole cell lysates (25–50 μg) from infected cells were employed in Northern (a) and Western (b) blot analyses, respectively. The detected genes and proteins are indicated to the right of each panel. (c) Activation of AP-1 dependent luciferase reporter constructs containing AP-1 binding sites from the collagenase promoter (5×coll) or c-jun promoter (5×jun2). CEFs were transiently transfected with the AP-1 reporter constructs and the expression vector Rc/RSV encoding c-Rel or v-Rel. Cell lysates were prepared 32–36 hours after transfection and luciferase assays performed. The average and standard deviation of at least four independent experiments is shown.
Supplemental Figure 2. Regulation of c-fos and ATF2 promoters by v-Rel. (a) Deletion constructs of the c-fos promoter were subcloned into the pGL3-Basic reporter vector. Diagrams of the reporter constructs are shown to the left of the luciferase assay results. The relative position of the predicted transcription start site is indicated by an arrow. CEF cultures were transfected with c-fos reporter constructs, the pRL-TK coreporter vector, and an empty expression plasmid (pCI) as a control, or pCI encoding v-Rel or c-Rel. Cells were harvested 32 hours after transfection and analyzed for luciferase activity. Luciferase activity from the c-fos promoter constructs was normalized for transfection efficiency by measuring pRL-TK coreporter activity. The results are presented as fold activation relative to results obtained with an empty expression plasmid control. The average and standard deviation of at least three independent experiments is shown. (b) The sequence of the serum response element and κB site located between −312 and −267 of the c-fos promoter is shown. (c) Nuclear extracts were isolated from CEF cultures 7–10 days after infection with the helper virus CSV (H) or viruses expressing c-Rel (C) or v-Rel (V). EMSAs were performed using probes encompassing the sequences shown in panel b. Nuclear extracts from CEF cultures transformed by v-Rel were incubated with normal rabbit serum (NRS) or antisera specific for Elk-1, serum response factor (SRF), or v-Rel prior to the addition probe. Binding complexes containing SRF and v-Rel are indicated. (d) v-Rel, SRF, and c-Jun directly bind the c-fos promoter in v-Rel transformed cells. Protein-DNA complexes from v-Rel transformed CEF cultures were crosslinked with formaldehyde, DNA sheared by sonication, and employed in chromatin immunoprecipitation experiments with control normal rabbit serum (NRS) and antibodies specific to Elk-1, SRF, c-Jun, and v-Rel. Input and precipitated DNA was amplified by PCR using primers flanking −312 and −267 of the c-fos promoter, the κB sites within the ikba promoter (positive control), or a region within the collagenase promoter (COLA). (e) v-Rel binds to two κB sites in the proximal promoter of ATF2. Nuclear extracts were isolated from CEF cultures 7–10 days after infection with the helper virus CSV (H) or viruses expressing v-Rel (V). EMSAs were performed using probes encompassing two putative κB sites on the ATF2 promoter at −373 and −430. Analysis was restricted to κB sites identified within 500 base pairs of the ATF2 transcription start site. Supershift assays were also performed by incubating nuclear extracts from CEF cultures transformed by v-Rel with normal rabbit serum (NRS) or antisera specific to v-Rel prior to addition of the probe containing the putatitve κB sites.
Supplemental Figure 3. Ha-Ras does not regulate JNK or AKT activity in v-Rel transformed cells. 160/2 cells were infected with control DS virus or viruses expressing wild-type Ha-Ras (WT) or DN-Ras (DN). Whole cell lysates were prepared and analyzed by Western blot for the levels of total and phosphorylated JNK and AKT.