Abstract
The respiratory and cardiovascular systems are highly intertwined, both anatomically and physiologically. Respiratory and cardiovascular neurons are often co-localized in the same brainstem regions, and this is particularly evident in the ventral medulla which contains pre-sympathetic neurons in the rostral ventrolateral medulla, premotor parasympathetic cardioinhibitory neurons in the nucleus ambiguus, and the ventral respiratory group, which includes the pre-Botzinger complex. Anatomical studies of respiratory and cardiovascular neurons have demonstrated that many of these neurons have projections and axon collateral processes which extend into their neighboring cardiorespiratory regions providing an anatomical substrate for cardiorespiratory interactions. As other reports in this Special Issue of Respiratory Physiology & Neurobiology focus on interactions between the respiratory network and baroreceptors, neurons in the nucleus tractus solitarius, presympathetic neurons and sympathetic activity, this report will focus on the respiratory modulation of parasympathetic activity and the neurons that generate parasympathetic activity to the heart, cardiac vagal neurons.
1. Anatomy and Role of Parasympathetic Innervation of the Heart
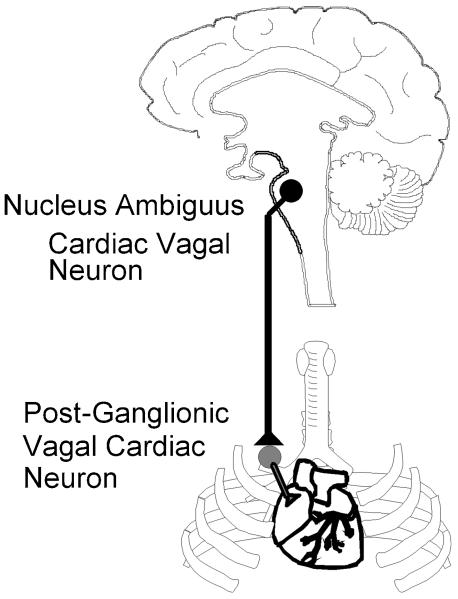
The location of pre- and post- ganglionic vagal cardioinhibitory neurons are illustrated in Figure 1. Preganglionic cardiac vagal neurons, whose cell bodies are located mostly in the nucleus ambiguus, and to a lesser extent in the dorsal motor nucleus of the vagus, send their fibers in the vagi nerves to cardiac ganglia within the connective and fatty tissue that surround the right atrium and vena cava (Machado et al. 1988; Loewy et al. 1990b; Mendelowitz et al. 1991; Cheng et al. 2000). Postganglionic fibers emerge from these ganglia to innervate the nearby sinoatrial and atrioventricular nodes of the heart (Rardon et al. 1983; Pardini et al. 1987).
Figure 1.
Premotor cardiac vagal neurons originate primarily in the nucleus ambiguus in the ventral brainstem. The axons of these cardioinhibitory preganglionic parasympathetic neurons are within the vagi nerves and synapse upon postganglionic neurons in cardiac ganglia that are in fat pads at the base of the heart. Upon excitation from preganglionic cardiac vagal neurons postganglionic neurons inhibit and control the activity of cardiac pacemaker cells in the sino-atrial node of the heart.
Heart rate is dominated by the activity of the cardioinhibitory parasympathetic nervous system. In conscious and anesthetized animals there is a tonic level of parasympathetic nerve firing and little, if any, sympathetic activity at rest (humans (Pickering et al. 1972), dogs (Scher et al. 1970), cats (Kunze 1972), rats (Coleman 1980; Stornetta et al. 1987)). During increases in arterial pressure the initial reflex induced slowing of the heart is caused primarily, if not exclusively, by increases in cardiac vagal nerve activity(Scher et al. 1970; Stornetta et al. 1987). During decreases in arterial pressure the baroreflex induced tachycardia is caused mostly by decreases in parasympathetic, in addition to increases in sympathetic nerve activity (Scher et al. 1970; Spyer 1981; Spyer et al. 1988). When both parasympathetic and sympathetic activities are present, parasympathetic activity generally dominates the control of heart rate. Increases in parasympathetic activity evoke a bradycardia that is more pronounced when there is a high level of sympathetic firing (Levy et al. 1969). When there is a moderate or high level of parasympathetic activity, changes in sympathetic firing elicit negligible changes in heart rate (Levy et al. 1969).
Several disease states are associated with dysregulation of parasympathetic outflow to the heart. Hypertension, diabetes, hypothyroidism and coronary artery disease are associated with decreased parasympathetic activity (Inukai et al. 1990; Ajiki et al. 1993; Oka et al. 1996; Julius et al. 1998; Motte et al. 2005; Evrengul et al. 2006). Parasympathetic withdrawal is associated with ventricular arrhythmias and sudden cardiac death (Hull et al. 1995). Congestive heart failure is associated with heightened sympathetic and diminished parasympathetic outflow (Ferguson et al. 1990). Further, diminished heart rate recovery after exercise due to blunted parasympathetic outflow is an independent predictor of mortality in chronic heart failure (Nishime et al. 2000). Re-establishment of parasympathetic outflow is associated with increased recovery in ischemia and reperfusion induced arrhythmias, as well as myocardial infarction, and restoring proper parasympathetic outflow is suggested as a therapeutic target to reduce mortality and sudden death (Eckberg et al. 1971; La Rovere et al. 1988; Vanoli et al. 1991; Routledge et al. 2002).
2. Neurophysiology Of Cardiac Vagal Activity
It is widely accepted that parasympathetic activity originates from the central nervous system rather than from peripheral ganglia. Preganglionic cardiac vagal neurons exclusively generate parasympathetic activity to the heart and are tonically active with a firing pattern that is cardiac pulse synchronous (Heymans 1958; Kunze 1972; Spyer 1981; Gilbey et al. 1984; Loewy A.D. 1990). Section of the preganglionic cardiac vagal fibers, leaving only postganglionic innervation intact, releases the heart from parasympathetic inhibition (Heymans 1958).
However, in the absence of synaptic activity, cardiac vagal neurons in the nucleus ambiguus are silent and do not exhibit spontaneous activity. Cardiac vagal neurons do not display any pacemaker-like properties such as repetitive or phasic depolarizations or action potentials (Mendelowitz 1996). However, only a small depolarizing current (100pA) is needed to evoke repetitive firing in cardiac vagal neurons, and this activity occurs with little delay and minimal spike frequency adaptation during any maintained depolarizing currents (Mendelowitz 1996). The voltage gated currents and firing characteristics of cardiac vagal neurons enable them to follow fast synaptic drive closely as well as integrate long-lasting modulatory influences (Mendelowitz 1996; Mihalevich et al. 1996). Hyperpolarization of these neurons prior to depolarization relieves the inactivation of a 4-aminopyridine sensitive potassium channel. Therefore, hyperpolarizing synaptic inputs could inhibit cardiac vagal neuron activity both directly, and by activation of this K+ current (Mendelowitz 1996; Mihalevich et al. 1996).
The absence of pacemaker activity in cardiac vagal neurons in-vitro is consistent with results from in-vivo studies. In the relatively few in-vivo studies that have successfully identified and examined cardiac vagal neurons with extracellular electrodes, the great majority (identified by antidromic stimulation) did not fire spontaneously (McAllen et al. 1978; Nosaka et al. 1979; Jordan et al. 1982; Gilbey et al. 1984). In the only in-vivo study in which intracellular recordings were successful (and in only 2 neurons) cardiac vagal neurons were silent (Gilbey et al. 1984). The lack of ongoing cardiac vagal activity in these anesthetized in-vivo studies is somewhat unexpected since in conscious animals and humans (rats (Coleman 1980; Stornetta et al. 1987; Spyer et al. 1988), dogs (Scher et al. 1970), cats (Kunze 1972), humans (Pickering et al. 1972)) there is a high level of tonic cardiac vagal activity. However in the in-vivo experiments excitatory pathways to cardiac vagal neurons may have been inhibited due to the trauma of the acute open-chest surgery, or anesthesia, which, in general, inhibits excitatory and augments inhibitory pathways (Wakamori et al. 1991). The tonic parasympathetic activity that is present in unanesthetized animals is therefore likely maintained to a large extent by an excitatory synaptic input that is reduced or absent in anesthetized in-vivo preparations.
The cardiovascular control and activation of cardiac vagal neurons in the brainstem is strongly influenced by the activation and modulation of glutamatergic and GABAergic synaptic pathways to cardiac vagal neurons. Stimulation of the nucleus tractus solitarius (NTS) evokes a glutamatergic pathway that activates both NMDA and non-NMDA postsynaptic currents in cardiac vagal neurons (Neff et al. 1998; Mendelowitz 1999). This pathway likely constitutes the essential link between increases in blood pressure and afferent baroreceptor activity, which activates neurons in the NTS, and the reflex compensatory decrease in heart rate caused by increases in efferent cardioinhibitory cardiac vagal activity.
GABAergic neurons that project to cardiac vagal neurons have recently been localized to populations within specific areas immediately medial and ventral to the nucleus ambiguus, and in close proximity to the NTS (Frank et al. 2009). The GABAergic pathway from the NTS can be activated upon both electrical and photo-uncaging excitation of these neurons (Wang et al. 2001a; Frank et al. 2009). Stimulation of afferents in the central end of a sectioned vagus nerve evokes both GABAergic and glutamatergic responses in cardiac vagal neurons (Evans et al. 2003). Capsaicin, which inactivates C-fibers, increased the latency of the GABAergic response without changing the latency of the glutamatergic responses (Evans et al. 2003). It is likely that this inhibitory GABAergic pathway evoked from vagal nerve or NTS stimulation is involved in patterning cardiac vagal activity which is bursting and synchronous with the cardiac cycle.
3 Respiratory Modulation of Parasympathetic Cardiac Vagal Neurons
Cardiac vagal neurons are profoundly influenced by inputs from the respiratory system. There are two well-known physiological interactions between the respiratory system and cardiac vagal neuron activity. The respiratory system influences cardiovascular reflexes by modulating the baroreceptor and chemoreceptor input to cardiac vagal neurons. In both animals and humans, the baroreceptor and chemoreceptor reflexes are inhibited during inspiration, and are facilitated during post-inspiration and expiration, or during a maintained phase of post-inspiration and apnea (Davidson et al. 1976; Eckberg et al. 1977; McAllen et al. 1978). This respiratory modulation of both reflexes persists after pulmonary denervation, as well as ventilatory paralysis, suggesting that this “gating” of the baroreceptor and chemoreceptor reflexes occurs within the brainstem (Loewy et al. 1990a; Eckberg 2003). A number of studies indicate respiratory inputs do not alter baroreceptor and chemoreceptor synapses at their first synapse in the nucleus tractus solitarius (NTS) (Spyer 1981; Spyer et al. 1988) suggesting that respiratory influences on baroreceptor and chemoreceptor reflexes occur beyond the NTS in these brainstem reflex pathways.
In addition to respiratory modulation of baro- and chemo-reflexes, the most ubiquitous cardiorespiratory interaction is respiratory sinus arrhythmia. During each respiratory cycle the heart beat slows during expiration and increases during inspiration. Respiratory sinus arrhythmia helps match pulmonary blood flow to lung inflation and maintain the appropriate diffusion gradient for oxygen in the lungs(Anrep et al. 1936a).
Many factors influence the changes in heart rate in response to respiration. These include sensory input related to lung inflation, changes in the activity of atrial stretch-sensitive receptors (due to variations in venous return produced by intrathoracic pressure changes), and activation of baroreceptors in the aortic arch and carotid sinus (also due to variations in venous return)(Anrep et al. 1936a; Anrep et al. 1936b; Richter et al. 1990; Berne et al. 1997). While feedback from pulmonary stretch receptors, direct respiratory related changes in venous return and cardiac stretch can evoke respiratory related changes in heart rate, the dominant source of respiratory sinus arrhythmia originates from the brainstem (Anrep GV 1935). Respiratory sinus arrhythmia persists when the lungs are stationary (caused by muscle paralysis or constant flow ventilation), and the respiratory modulation of heart rate remains synchronized with brainstem respiratory rhythms even if artificial ventilation of the lungs, and chemoreceptor activation, occur at different intervals (Spyer et al. 1988; Daly 1991; Elghozi et al. 1991; Hrushesky 1991; Shykoff et al. 1991). In both animals and humans respiratory sinus arrhythmia is mediated via cardiac vagal activity. Respiratory sinus arrhythmia persists in animals upon sectioning sympathetic pathways, and in quadriplegic patients with spinal cord injury and sympathetic dysfunction (Inoue et al. 1990; Daly 1991; Elghozi et al. 1991; Hrushesky 1991; Shykoff et al. 1991). Furthermore, respiratory sinus arrhythmia persists after blocking sympathetic activity to the heart by administration of the beta-adrenergic antagonist propanolol, and after sectioning of sympathetic fibers (Levy et al. 1969; Kollai et al. 1979; Hrushesky 1991). Blocking parasympathetic activity with atropine, however, significantly reduces respiratory sinus arrhythmia, indicating that this cardiorespiratory interaction is predominantly mediated by the activity of cardiac vagal neurons (Warner et al. 1986; Hrushesky 1991; Berne et al. 1997).
It is well established in many species (including neonatal(Hathorn 1987) and adult humans(Hirsch et al. 1981; Eckberg 1983), baboons(Myers et al. 1990), dogs(Hayano et al. 1996), seals(Castellini et al. 1994), and rabbits(Jordan et al. 1982)) that heart rate increases during inspiration and decreases during post-inspiration/expiration. However, surprisingly a recent study proposed that rats have an inverted respiratory sinus arrhythmia. This conclusion was based on the in-vivo observation that cardiac vagal neurons fired during inspiration in urethane anesthetized rats (Rentero et al. 2002). However more recent results have resolved this apparent controversy. Heart rate increases during inspiration in conscious, freely moving rats, similar to the pattern of respiratory sinus arrhythmia in other mammals, including humans (Bouairi et al. 2004). However the anesthetic urethane alters this cardiorespiratory interaction and produces an inversion of normal respiratory sinus arrhythmia (Bouairi et al. 2004). Other anesthetics also blunt respiratory sinus arrhythmia, raising the concern that respiratory modulation of parasympathetic cardiac vagal neurons is highly susceptible and should be studied without the confounding effects of anesthetics (Bouairi et al. 2004).
To mediate respiratory sinus arrhythmia cardiac vagal neurons fire most rapidly in post-inspiration, and are often silent in inspiration (Kunze 1972; McAllen et al. 1978; Spyer 1981). Unfortunately obtaining information concerning the transmitters and neurons responsible for this cardiorespiratory interaction in-vivo is extremely difficult. The low probability of finding and recording from the sparse number of cardiac vagal neurons (~200/animal) and the difficulty of the necessary task of identifying these neurons by antidromic stimulation of the cardiac branch of the vagus nerve make in-vivo recordings particularly challenging. In the only in-vivo study in which intracellular recordings were successful (and in only 2 neurons) cardiac vagal neurons received inhibitory synaptic input during inspiration (Gilbey et al. 1984). During inspiration input resistance decreased and injection of chloride reversed this hyperpolarization (Gilbey et al. 1984). This inhibitory chloride conductance would most likely be caused by activation of postsynaptic GABA or glycine receptors during inspiration. Paradoxically, however, in a review by one of these investigators it is stated “the inspiratory related inhibition of cardioinhibitory neurons is not antagonized by the iontophoretic application of either bicuculline or the glycine antagonist strychnine” (Loewy A.D. 1990). This seemingly conflicting data could be due to the small sample size, inability of microinjections to distinguish direct effects on cardiac vagal neurons from actions on presynaptic terminals or local interneurons, and anesthetic modulation of the respiratory activity and/or neurotransmission to cardiac vagal neurons.
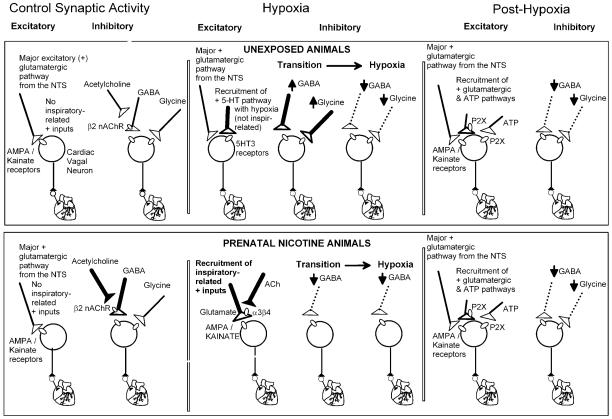
More recent in-vitro work has characterized the respiratory inputs to cardiac vagal neurons that mediate respiratory sinus arrhythmia. In brain slices that generate rhythmic, inspiratory phase motor discharge, the frequency of both spontaneous GABAergic and glycinergic synaptic events in cardiac vagal neurons significantly increase with each inspiratory burst (Neff et al. 2003). The GABA mediated inhibition of cardiac vagal neurons during inspiration is inhibited by curare, indicating that the increase in GABAergic frequency is mediated by the activation of nicotinic receptors (Neff et al. 2003). This increase in GABAergic frequency is unaffected by α-Bungarotoxin, demonstrating that it was not mediated by the activation of α7 nicotinic receptors (Neff et al. 2003). A β2-selective concentration of dihydro-beta-erithroidine (DHβE), however, abolishes the GABAergic inhibition of cardiac vagal neurons during inspiration, indicating that activation of β2 containing nicotinic receptors by endogenous acetylcholine drives the GABAergic inhibition in this cardiorespiratory interaction (Neff et al. 2003). The facilitation of GABAergic inhibition of cardiac vagal neurons by activation of nicotinic receptors is consistent with previous work which has shown that spontaneous GABAergic inhibition of cardiac vagal neurons is enhanced by the activation of β2 receptors presumably located in the presynaptic terminals of GABAergic neurons(Wang et al. 2001b; Wang et al. 2003). This study also indicates that the nicotinic receptors responsible for the increased GABAergic activity during inspiration are in close proximity to the cardiac vagal neurons since focal application of the nicotinic antagonist DHβE abolished this increase. In contrast, greater concentrations of DHβE did not significantly alter the respiratory related increase in glycinergic synaptic frequency in cardiac vagal neurons (Neff et al. 2003). Interestingly, previous studies have shown that spontaneous glycinergic inputs to cardiac vagal neurons are also enhanced by activation of β2 nicotinic receptors in glycinergic presynaptic terminals(Wang et al. 2003). This suggests that although glycinergic inputs to cardiac vagal neurons likely possess presynaptic nicotinic receptors, these receptors are apparently not involved with the inspiratory evoked increase in glycinergic synaptic input to cardiac vagal neurons. Figure 2, top, illustrates many of the most important and endogenously active synaptic pathways to cardiac vagal neurons and the pre- and post-synaptic receptors activated.
Figure 2.
As shown in the top panel, under unstressed conditions the major synaptic pathways to cardiac vagal neurons include an excitatory (denoted as +) glutamatergic pathway from the Nucleus Tractus Solitarius (NTS), likely essential for the baroreceptor reflex, and inspiratory-evoked inhibitory GABAergic and glycinergic neurotransmission that likely mediates respiratory sinus arrhythmia. The increase in GABAergic, but not glycinergic activity during inspiratory activity is mediated by presynaptic β2 containing nicotinic receptors (nAChR). In response to hypoxia there is a biphasic change in GABAergic and glycinergic neurotransmission to cardiac vagal neurons with an initial increase, (illustrated by bolder lines) followed by a depression (illustrated by dashed lines) of inhibitory input. Hypoxia also recruits a serotonergic pathway that activates postsynaptic 5-HT3 receptors in cardiac vagal neurons, this serotonergic pathway is spontaneously active but not inspiratory related. In the recovery from hypoxia the depression (dashed lines) of inhibitory GABAergic and glycinergic inputs persist. In addition, inspiratory-evoked excitatory pathways are recruited that are both glutamatergic and purinergic.
After exposure to prenatal nicotine many of the activated receptors and synaptic pathways to cardiac vagal neurons are altered, as shown in the bottom panel. Prenatal nicotine exposure potentiates the GABAergic, but not glycinergic neurotransmission to cardiac vagal neurons (as illustrated by bolder lines). In contrast to unexposed animals in which hypoxia evokes a biphasic change in the frequency of inhibitory neurotransmission, prenatal nicotine exposure transforms this response to hypoxia from biphasic to a precipitous decrease in spontaneous GABAergic activity (dashed lines). An inspiratory related glutamatergic excitation is recruited during hypoxia and hypoxia/hypercapnia in prenatal nicotine exposed animals which is dependent upon activation of presynaptic α3β4 nicotinic receptors (bold lines), that is not present in unexposed animals. This long lasting inspiratory related excitation persists throughout recovery from hypoxia/hypercapnia in prenatal nicotine exposed animals and it is dependent upon activation of both glutamatergic and purinergic receptors. Thus, following hypoxia, prenatal nicotine exposure exaggerates the activity in cardiac vagal neurons as excitatory glutamatergic and purinergic pathways are facilitated while inhibitory GABAergic and glycinergic neurotransmission is diminished.
3.1 Respiratory Influences on Cardiac Vagal Neurons During Hypoxia
3.1.1 Acute hypoxia
Challenges such as hypoxia evoke strong coordinated responses from the respiratory and cardiovascular systems. Hypoxia initially elicits a transient increase, followed by a sustained decrease in respiratory frequency, and eventually cessation of breathing (Guntheroth et al. 1975). In addition, respiration changes from the normal eupnic pattern of breathing to gasping in response to hypoxia, which increases the chance of autoresuscitation (Guntheroth et al. 1975).
The heart rate also exhibits a biphasic response to hypoxia. Hypoxia evokes a transient tachycardia, followed by a parasympathetically mediated bradycardia, and ultimately, cessation of cardiac contractions (Taylor et al. 1982; Schuen et al. 1997; Deshpande et al. 1999). Studies in humans have shown that hypoxia induced bradycardia can be blocked by atropine and is absent in heart transplant recipients (Berk et al. 1977; Somers et al. 1992; Madden et al. 1997; Baird 2004; Martin et al. 2004). In a wide variety of animals tested application of atropine to block parasympathetic outflow prevents bradycardia during hypoxia (de Burgh Daly et al. 1977; Daly et al. 1978; Cohn et al. 1980; Lewis et al. 1980; Przybylski et al. 1980; Ikenoue et al. 1981; Hayashi et al. 1982; O'Donnell et al. 1992; Yu et al. 1998). Further, vagotomy prevents bradycardia during hypoxia (Boddy et al. 1974; Przybylski et al. 1980). Bradycardia during hypoxia increases animal survival, as atropine sharply decreases survival under hypoxia (Scremin et al. 1980). The changes in parasympathetic cardiac activity in response to hypoxia are due to changes in medullary activity since the discharge of cardiac efferent fibers in the central end of the transected vagus nerve is increased during hypoxia (Potter et al. 1986). Although peripheral chemoreceptors may also be involved, the hypoxia induced bradycardia persists after section of both the carotid sinus and aortic nerves indicating chemoreceptors within the central nervous system can activate pathways that increase the activity of cardiac vagal neurons (Serani et al. 1983).
Recent work has delineated the changes in synaptic neurotransmission to cardiac vagal neurons evoked during hypoxia. Hypoxia evokes a biphasic change in inhibitory neurotransmission to cardiac vagal neurons (Neff et al. 2004). In response to hypoxia, GABAergic inhibition of cardiac vagal neurons changes in a biphasic fashion, initially increasing and then significantly decreasing during hypoxia (Neff et al. 2004). Similarly, in response to hypoxia there is a biphasic change in glycinergic inhibition comprised initially of an increase followed by a depression of spontaneous and inspiratory evoked glycinergic activity (Neff et al. 2004), see figure 2.
In addition to changes in GABA neurotransmission, serotonin (5-hydroxytryptamine, 5-HT) receptors and signaling in the brainstem play an important role in central cardiorespiratory responses to hypoxia. Hypoxia induces Fos-like immunoreactivity in 5-HT neurons in the nucleus raphe pallidus, the nucleus raphe magnus, and along the ventral medullary surface (Erickson et al. 1994; Teppema et al. 1997). Within the ventral respiratory group 5-HT levels significantly increase and reach a maximum after 9 minutes of hypoxia and then gradually decline posthypoxia (Richter et al. 1999). 5-HT acting on 5-HT1A receptors in the nucleus raphe magnus plays no role under normal conditions but modulates breathing during hypoxia (Nucci et al. 2008). In the anteroventral preoptic region both 5-HT1A and 5-HT7 receptors are involved in the inhibitory modulation of the hypoxic ventilatory response (Gargaglioni et al. 2006). Activation of central 5-HT2A receptors is required to sustain hypoxic gasping and to restore respiratory activity during posthypoxia (Tryba et al. 2006; St-John et al. 2007). Central 5-HT2A receptors are also critical for long-term facilitation in respiratory activity followed by intermittent hypoxia (Fuller et al. 2001; McGuire et al. 2004; Tryba et al. 2006).
5-HT receptors and pathways are recruited in the response of cardiac vagal neurons to hypoxia. Within the nucleus ambiguus premotor neurons receive a large number of axosomatic 5-HT contacts, and the 5-HT synaptic density on neurons in the nucleus ambiguus is among the highest in the brainstem (Takeuchi et al. 1983). 5-HT fibers also specifically surround cardiac vagal neurons, which have been described as “ensheathed in 5-HT immunoreactive axonal boutons” (Izzo et al. 1993). Under normoxic conditions excitatory synaptic inputs to cardiac vagal neurons are nearly completely blocked by application of NMDA and AMPA/kainate glutamatergic receptor antagonists, while blocking 5-HT3 and purinergic receptors has no effect (Dergacheva et al. 2009b). However, hypoxia recruits an additional 5-HT-mediated excitation of cardiac vagal neurons which can be blocked with the 5-HT3 receptor antagonist, ondansetron. This serotonergic pathway is spontaneously active and is not inspiratory related. This direct activation of 5-HT3 receptors on cardiac vagal neurons, in combination with glutamatergic receptor activation, provides excitatory input and helps maintains parasympathetic cardiac activity during hypoxia (Dergacheva et al. 2009b), see figure 2. In addition, during hypoxia 5-HT2 receptors on cardiac vagal neurons are also recruited and act to sustain excitation of cardiac vagal neuron activity during hypoxia via facilitation of 5-HT3 receptor activation (Dergacheva et al. 2009a). 5-HT receptors may also be responsible for the withdrawal of GABAergic and glycinergic neurotransmission to cardiac vagal neurons during hypoxia. Although this has not yet been tested, previous work has demonstrated that activation of 5-HT1A/7 receptors, as well as repetitive activation of 5-HT2B receptors exerts an inhibitory action on both spontaneous and inspiratory-related GABAergic inputs to cardiac vagal neurons (Dergacheva et al. 2007; Wang et al. 2007). Therefore, a combination of two mechanisms: disinhibition of cardiac vagal neurons via withdrawal of GABAergic and glycinergic neurotransmission, and excitation of cardiac vagal neurons via activation of postsynaptic glutamatergic and 5-HT3 receptors is likely important in the increase in cardiac vagal neuron activity and the resultant bradycardia during hypoxia.
Hypoxia not only evokes a biphasic tachycardia followed by a bradycardia, but in the recovery following hypoxia a strong bradycardia persists during the recovery period (Pichot et al. 2000; Roche et al. 2002). Pronounced excitatory inspiratory-related synaptic pathways are recruited to excite cardiac vagal neurons post hypoxia (Evans et al. 2005). During recovery from hypoxia spontaneous and respiratory-related excitatory events are generated mainly by the recruitment of glutamatergic and purinergic pathways (Griffioen et al. 2007a; Dergacheva et al. 2009b), see figure 2. 5-HT2 receptors not only play a role by maintaining excitatory neurotransmission to cardiac vagal neurons during hypoxia, as previously discussed, but activation of 5HT2 receptors diminishes the subsequent inspiratory-related excitatory neurotransmission to cardiac vagal neurons that is recruited during the recovery from hypoxia likely by exerting an inhibitory action on inspiratory-related purinergic signaling (Dergacheva et al. 2009a).
Brief and intermittent hypoxic episodes incrementally recruit a respiratory-related glutamatergic neurotransmission that occurs during respiratory bursts and becomes increasingly robust in successive hypoxic episodes (Griffioen et al. 2007b). Likely as a result of the successive recovery periods that occur with repeated periods of hypoxia reactive oxygen species are produced (Griffioen et al. 2007b). Inspiratory-synchronous glutamatergic inputs to cardiac vagal neurons during repeated bouts of hypoxia are blocked by application of reactive oxygen species scavengers, and visualization of reactive oxygen species generation indicates they are incrementally produced in the ventrolateral medulla during each hypoxic period (Griffioen et al. 2007b).
The reactive oxygen species-dependent cardiorespiratory plasticity induced by intermittent hypoxia likely involves serotonergic synaptic mechanisms. Central respiratory responses to repeated hypoxia are serotonin dependent, and the respiratory plasticity evoked by intermittent hypoxia can be mimicked with intermittent application of serotonin 5HT2A agonists (Feldman et al. 2003; Bocchiaro et al. 2004). Taken together with the serotonin-dependent increases in glutamatergic neurotransmission to cardiac vagal neurons elicited during single hypoxic insults, this suggests that reactive oxygen species may enhance serotonergic pathways to induce inspiratory-related excitatory inputs during repeated hypoxias.
3.1.2 Chronic Intermittent Hypoxia
Obstructive Sleep Apnea (OSA), and accompanying chronic intermittent hypoxia, is a significant health risk occurring in as many as ~24% of males and 9% of females (between 30-60 years of age) within the United States population (Bazzano et al. 2007; Punjabi 2008). Severe OSA increases cardiovascular mortality 4 fold, and even when corrected for other risk factors severe OSA increases cardiovascular mortality 3 fold (Marin et al. 2005). Obstructive sleep apnea can participate in both the initiation and progression of several cardiovascular diseases including hypertension, arrhythmias, myocardial ischemia and stroke (Bradley et al. 2009; Kato et al. 2009). Treatment of OSA is primarily continuous positive airway pressure (CPAP), and while this treatment is marginally effective in reducing elevated arterial pressure (~2 mmHg) (Bazzano et al. 2007), and partially restoring baroreflex sensitivity (Parati et al. 2007), CPAP is intrusive, poorly tolerated and often discontinued despite the continued health risks of OSA (Bazzano et al. 2007).
Chronic intermittent hypoxia, in both animal models and humans, decreases baroreflex sensitivity, elevates blood pressure and sympathetic activity, and diminishes parasympathetic activity to the heart (Carlson et al. 1996; Bonsignore et al. 2002; Narkiewicz et al. 2003; Bonsignore et al. 2006; Lai et al. 2006). While chronic intermittent hypoxia decreases the baroreflex control of heart rate and diminishes parasympathetic activity to the heart, these changes are not due to changes in parasympathetic innervation of the sinoatrial node or function within the cardiac ganglia, but are rather most likely due to changes in the activity of premotor parasympathetic cardiac vagal neurons in the brainstem. Although anatomical work has shown a decrease in efferent cardiac vagal innervation, ganglia size and density of axonal terminals within the cardiac ganglia after chronic intermittent hypoxia (Soukhova-O'Hare et al. 2006; Lin et al. 2008) heart rate responses to vagal efferent stimulation is not diminished, but rather enhanced (Gu et al. 2007; Lin et al. 2007; Yan et al. 2008). These results indicate a central dysregulation of premotor cardiac vagal neuronal activity, but not cardiac ganglia function, is responsible for the impaired parasympathetic control of heart rate that occurs with chronic intermittent hypoxia. The bradycardia evoked upon microinjection of glutamate (Yan et al. 2008) as well as NMDA and AMPA (Yan et al. 2009) into the nucleus ambiguus is diminished by chronic intermittent hypoxia. However beyond alterations in glutamate receptor density little is known how chronic intermittent hypoxia impairs cardiac vagal neuron function, and whether these changes include alterations in GABA, glycine, glutamate, 5-HT and purinergic pathways and receptors known to be essential in the responses of cardiac vagal neurons to acute hypoxic challenges.
4. Prenatal Nicotine Exposure Alters Respiratory Related Cardiac Vagal Neuron Activity
The reduction in heart rate and respiratory frequency in response to hypoxia normally serves to reduce the metabolic demand of the cardiac and respiratory muscles, and thus prolong survival under hypoxic conditions(Schuen et al. 1997). Exaggeration of this protective response to hypoxia, however, could be detrimental. Sudden Infant Death Syndrome (SIDS) is the leading cause of infant death in the postneonatal period (between 28 days and 11 months after birth) and occurs in about 0.5 per 1000 births in the U.S. (Anderson 2002). Infants that succumb to SIDS typically experience a severe bradycardia which precedes or is accompanied by a centrally mediated, life-threatening apnea(Meny et al. 1994; Nachmanoff et al. 1998; Poets et al. 1999; Fewell et al. 2001). Cardiorespiratory monitor recordings from SIDS victims reveal that a likely trigger for apnea and bradycardia is a period of hypoxemia and gasping (Meny et al. 1994; Poets et al. 1999). Although the causes of the apnea and bradycardia prevalent in SIDS victims are unknown, it has been hypothesized that these fatal events are augmented cardiorespiratory responses to hypoxia and/or hypercapnia (Meny et al. 1994).
Chronic fetal nicotine exposure by maternal cigarette smoking is highly correlated with SIDS and increases the risk of SIDS by 2-4 times (Haglund et al. 1990; Mitchell et al. 1993). Maternal smoking has substantial cardiorespiratory effects, including acute fetal tachycardia(Sontag et al. 1935; Hellman et al. 1961) and increased incidence of fetal apnea (Gennser et al. 1975). Nicotine, one of the major pharmacological components of cigarette smoke, has been proposed to be a major link between maternal smoking and SIDS(Slotkin et al. 1997; Nachmanoff et al. 1998; Bamford et al. 1999; St-John et al. 1999). Nicotine readily crosses the placental barrier and has been found in the blood and pericardial fluid of SIDS infants(Milerad et al. 1994). Exposure to prenatal nicotine impairs the ability of rat pups to autoresuscitate following repeated bouts of hypoxia (Fewell et al. 1998; Slotkin et al. 2005). Rat pups exposed to prenatal nicotine during gestation become apneic more rapidly than unexposed animals(Fewell et al. 2001). In addition, neonatal rats exposed to nicotine in utero exhibit a more rapid and substantial bradycardia during hypoxia(Slotkin et al. 1997). These augmented cardiorespiratory responses to hypoxia bear a striking resemblance to the life-threatening cardiorespiratory events observed in SIDS victims.
Prenatal nicotine exposure alters GABAergic, but not glycinergic neurotransmission in the inhibitory brainstem synaptic pathways to cardiac vagal neurons, see figure 2. In particular, prenatal nicotine exposure potentiates the inspiratory-related GABAergic, but not glycinergic, neurotransmission to cardiac vagal neurons (Neff et al. 2004; Huang et al. 2006). The enhanced GABAergic neurotransmission to cardiac vagal neurons following prenatal nicotine exposure is mediated by β2-containing nicotinic receptors.
Perhaps even more relevant to the reduced ability to autoresuscitate in response to challenges such as hypoxia, prenatal nicotine alters the respiratory control of cardiac vagal neurons during hypoxic challenge. In contrast to unexposed animals in which hypoxia evokes a biphasic change in the frequency of inhibitory neurotransmission to cardiac vagal neurons, prenatal nicotine exposure transforms this response to hypoxia from biphasic to a precipitous decrease in spontaneous GABAergic activity (Neff et al. 2004), see figure 2. This enhanced withdrawal of inhibitory GABAergic synaptic inputs to cardiac vagal neurons would result in a more rapid hypoxia-induced increase in parasympathetic outflow to the heart, and thus a more rapid and substantial bradycardia in response to hypoxia. Consistent with this hypothesis animals exposed to nicotine prenatally do not experience an initial tachycardia when exposed to hypoxia, but rather, a rapid and dramatic bradycardia, which is significantly greater than the mild bradycardia observed in unexposed animals (Slotkin et al. 1997).
In addition to alterations in inhibitory neurotransmission, there are significant changes in excitatory neurotransmission to cardiac vagal neurons. A long-lasting inspiratory related excitation is recruited during both hypoxia and hypoxia/hypercapnia, and persists throughout recovery from hypoxia/hypercapnia in prenatal nicotine exposed animals (Evans et al. 2005; Kamendi et al. 2009). This glutamatergic neurotransmission is facilitated by non-β2 containing nicotinic receptors. A small portion of this glutamatergic excitation can be blocked with antagonists targeting α7 nicotinic receptors (Huang et al. 2007). In addition, application of the selective α3β4 nicotinic receptor blocker (α-conotoxin AuIB) also prevents the increase in inspiratory related excitation of cardiac vagal neurons and abolished spontaneous EPSCs in cardiac vagal neurons during hypoxia/hypercapnia (Kamendi et al. 2009), see figure 2. These results suggest that following prenatal nicotine exposure there is a novel recruitment of a glutamatergic pathway to cardiac vagal neurons during hypoxia/hypercapnia that is not present in unexposed animals, and that endogenous activation of α7 and α3β4 nicotinic receptors mediates this glutamate neurotransmission to cardiac vagal neurons. A combination of hypoxia evoked recruitment of an excitatory pathway, together with exaggerated inhibition of GABAergic neurotransmission to cardiac vagal neurons would predict a much stronger excitation of cardiac vagal neurons and a larger bradycardia in animals exposed to prenatal nicotine. These changes closely predict, and provide a neurochemical mechanism for the substantial and potentially lethal potentiation of the hypoxia-induced bradycardia observed in rats prenatally exposed to nicotine (Slotkin et al. 1997). Further, these results propose cellular mechanisms by which prenatal nicotine could increase SIDS incidence.
5. Summary
Over the past 10 years there have been many significant advances in our understanding of the respiratory modulation of cardiac vagal neurons in the brainstem. Cardiac vagal neurons do not possess pacemaker activity, but rather receive excitatory influences from other brainstem regions, predominantly the NTS. Respiratory modulated changes in parasympathetic cardiac activity that mediate respiratory sinus arrhythmia are not via changes in excitatory synaptic inputs, but rather increases in inhibitory GABAergic and glycinergic neurotransmission to cardiac vagal neurons during inspiratory activity. Challenges such as hypoxia not only alter inspiratory related GABAergic and glycinergic inputs, but also recruit additional excitatory pathways from glutamatergic, purinergic and serotonergic neurons to excite cardiac vagal neurons and induce bradycardia during and post hypoxia. Prenatal nicotine exposure initiates and augments the recruitment of many of these excitatory pathways, and also diminishes the GABAergic inhibitory neurotransmission to cardiac vagal neurons. This change in the pattern of cardiorespiratory network function likely mediates the diminished ability to recover from hypoxic challenges following prenatal nicotine exposure. Further work is necessary to determine, at the cellular level, alterations in receptor function and synaptic transmission to cardiac vagal neurons that impair parasympathetic activity to the heart in chronic cardiorespiratory diseases such as heart failure and OSA. Identification of these changes will provide a clear understanding of parasympathetic dysfunction in these diseases as well as provide future targets for restoring cardiac vagal neuron activity to increase survival.
Acknowledgements
Supported by NIH grants HL49965, HL59895 and HL72006 to D.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajiki K, Murakawa Y, Yanagisawa-Miwa A, Usui M, Yamashita T, Oikawa N, Inoue H. Autonomic nervous system activity in idiopathic dilated cardiomyopathy and in hypertrophic cardiomyopathy. Am J Cardiol. 1993;71:1316–1320. doi: 10.1016/0002-9149(93)90547-p. [DOI] [PubMed] [Google Scholar]
- Anderson RN. Deaths: Leading Causes for 2000. National Vital Statistics Reports. 2002;50 [PubMed] [Google Scholar]
- Anrep G, Pascual F, Rossler R. Respiratory Variations of the Heart Rate II--The Central Mechanism of the Respiratory Sinus Arrythmia and the Inter-relations Between the Central and the Reflex Mechanisms. Proc Royal Soc. 1936a;119:218–232. [Google Scholar]
- Anrep G, Pascual F, Rossler R. Respiratory Variations of the Heart Rate I--The Reflex Mechanism of the Respiratory Sinus Arrhythmia. Proc Royal Soc. 1936b;119:191–217. [Google Scholar]
- Anrep GV PW, Rossler R. Respiratory Variations of the Heart Rate. II The Central Mechanism of the respiratory arrhythmia and the interrelationships between the central and reflex mechanisms. Proc. Roy. Soc. 1935;119:218–231. [Google Scholar]
- Baird TM. Clinical correlates, natural history and outcome of neonatal apnoea. Semin Neonatol. 2004;9:205–211. doi: 10.1016/j.siny.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Bamford O, Carroll J. Dynamic ventilatory responses in rats: normal development and effects of prenatal nicotine exposure. Respiration Physiology. 1999;117:29–40. doi: 10.1016/s0034-5687(99)00054-7. [DOI] [PubMed] [Google Scholar]
- Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–423. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- Berk JL, Levy MN. Profound reflex bradycardia produced by transient hypoxia or hypercapnia in man. Eur Surg Res. 1977;9:75–84. doi: 10.1159/000127928. [DOI] [PubMed] [Google Scholar]
- Berne RM, Levy MN. Cardiovascular physiology. Mosby; St. Louis: 1997. [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci U S A. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy K, Dawes GS, Fisher R, Pinter S, Robinson JS. Foetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep. J Physiol. 1974;243:599–618. doi: 10.1113/jphysiol.1974.sp010768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignore MR, Parati G, Insalaco G, Castiglioni P, Marrone O, Romano S, Salvaggio A, Mancia G, Bonsignore G, Di Rienzo M. Baroreflex control of heart rate during sleep in severe obstructive sleep apnoea: effects of acute CPAP. Eur Respir J. 2006;27:128–135. doi: 10.1183/09031936.06.00042904. [DOI] [PubMed] [Google Scholar]
- Bonsignore MR, Parati G, Insalaco G, Marrone O, Castiglioni P, Romano S, Di Rienzo M, Mancia G, Bonsignore G. Continuous positive airway pressure treatment improves baroreflex control of heart rate during sleep in severe obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2002;166:279–286. doi: 10.1164/rccm.2107117. [DOI] [PubMed] [Google Scholar]
- Bouairi E, Neff R, Evans C, Gold A, Andresen MC, Mendelowitz D. Respiratory Sinus Arrhythmia in Freely Moving and Anesthetized Rats. J Appl Physiol. 2004;97:1431–1436. doi: 10.1152/japplphysiol.00277.2004. [DOI] [PubMed] [Google Scholar]
- Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- Carlson JT, Hedner JA, Sellgren J, Elam M, Wallin BG. Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154:1490–1496. doi: 10.1164/ajrccm.154.5.8912770. [DOI] [PubMed] [Google Scholar]
- Castellini MA, Rea LD, Sanders JL, Castellini JM, Zenteno-Savin T. Developmental changes in cardiorespiratory patterns of sleep-associated apnea in northern elephant seals. Am J Physiol. 1994;267:R1294–1301. doi: 10.1152/ajpregu.1994.267.5.R1294. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Powley TL. Nucleus Ambiguus Projections to Cardiac Ganglia of Rat Atria: An Anterograde Tracing Study. J Comp Neuro. 2000;424:588–606. [PubMed] [Google Scholar]
- Cohn HE, Piasecki GJ, Jackson BT. The effect of fetal heart rate on cardiovascular function during hypoxemia. Am J Obstet Gynecol. 1980;138:1190–1199. doi: 10.1016/s0002-9378(16)32791-0. [DOI] [PubMed] [Google Scholar]
- Coleman TG. Arterial baroreflex control of heart rate in the conscious rat. Am J Physiol. 1980;238:H515–520. doi: 10.1152/ajpheart.1980.238.4.H515. [DOI] [PubMed] [Google Scholar]
- Daly MB, Korner PI, Angell-James JE, Oliver JR. Cardiovascular-respiratory reflex interactions between carotid bodies and upper-airways receptors in the monkey. Am J Physiol. 1978;234:H293–299. doi: 10.1152/ajpheart.1978.234.3.H293. [DOI] [PubMed] [Google Scholar]
- Daly MD. Some reflex cardioinhibitory responses in the cat and their modulation by central inspiratory neuronal activity. J Physiol. 1991;439:559–577. doi: 10.1113/jphysiol.1991.sp018682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson NS, Goldner S, McCloskey DI. Respiratory modulation of barareceptor and chemoreceptor reflexes affecting heart rate and cardiac vagal efferent nerve activity. J Physiol. 1976;259:523–530. doi: 10.1113/jphysiol.1976.sp011480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Burgh Daly M, Elsner R, Angell-James JE. Cardiorespiratory control by carotid chemoreceptors during experimental dives in the seal. Am J Physiol. 1977;232:H508–516. doi: 10.1152/ajpheart.1977.232.5.H508. [DOI] [PubMed] [Google Scholar]
- Dergacheva O, Griffioen KJ, Wang X, Kamendi H, Gorini C, Mendelowitz D. 5-HT(2) receptor subtypes mediate different long-term changes in GABAergic activity to parasympathetic cardiac vagal neurons in the nucleus ambiguus. Neuroscience. 2007;149:696–705. doi: 10.1016/j.neuroscience.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Kamendi H, Wang X, Pinol RA, Frank J, Gorini C, Jameson H, Lovett-Barr MR, Mendelowitz D. 5-HT2 receptors modulate excitatory neurotransmission to cardiac vagal neurons within the nucleus ambiguus evoked during and after hypoxia. Neuroscience. 2009a;164:1191–1198. doi: 10.1016/j.neuroscience.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Kamendi H, Wang X, Pinol RM, Frank J, Jameson H, Gorini C, Mendelowitz D. The role of 5-HT3 and other excitatory receptors in central cardiorespiratory responses to hypoxia: implications for sudden infant death syndrome. Pediatr Res. 2009b;65:625–630. doi: 10.1203/PDR.0b013e3181a16e9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande P, Khurana A, Hansen P, Wilkins D, Thach BT. Failure of autoresuscitation in weanling mice: significance of cardiac glycogen and heart rate regulation. J Appl Physiol. 1999;87:203–210. doi: 10.1152/jappl.1999.87.1.203. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Human sinus arrhythmia as an index of vagal cardiac outflow. J Appl Physiol. 1983;54:961–966. doi: 10.1152/jappl.1983.54.4.961. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. The human respiratory gate. J Physiol. 2003;548:339–352. doi: 10.1113/jphysiol.2003.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med. 1971;285:877–883. doi: 10.1056/NEJM197110142851602. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Orshan CR. Respiratory and baroreceptor reflex interactions in man. J Clin Invest. 1977;59:780–785. doi: 10.1172/JCI108699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elghozi JL, Laude D, Girard A. Effects of respiration on blood pressure and heart rate variability in humans. Clin Exp Pharmacol Physiol. 1991;18:735–742. doi: 10.1111/j.1440-1681.1991.tb01391.x. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Evans C, Baxi S, Neff RA, Venkatesan P, Mendelowitz D. Synaptic activation of cardiac vagal neurons by capsaicin sensitive and insensitive sensory neurons. Brain Res. 2003;979:210–215. doi: 10.1016/s0006-8993(03)02937-8. [DOI] [PubMed] [Google Scholar]
- Evans C, Wang J, Neff R, Mendelowitz D. Hypoxia recruits a respiratory-related excitatory pathway to brainstem premotor cardiac vagal neurons in animals exposed to prenatal nicotine. Neuroscience. 2005;133:1073–1079. doi: 10.1016/j.neuroscience.2005.03.053. [DOI] [PubMed] [Google Scholar]
- Evrengul H, Tanriverdi H, Kose S, Amasyali B, Kilic A, Celik T, Turhan H. The relationship between heart rate recovery and heart rate variability in coronary artery disease. Ann Noninvasive Electrocardiol. 2006;11:154–162. doi: 10.1111/j.1542-474X.2006.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DW, Berg WJ, Sanders JS. Clinical and hemodynamic correlates of sympathetic nerve activity in normal humans and patients with heart failure: evidence from direct microneurographic recordings. J Am Coll Cardiol. 1990;16:1125–1134. doi: 10.1016/0735-1097(90)90544-y. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Smith FG. Perinatal nicotine exposure impairs ability of newborn rats to autoresuscitate from apnea during hypoxia. J Appl Physiol. 1998;85:2066–2074. doi: 10.1152/jappl.1998.85.6.2066. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Smith FG, Vienna KYN. Prenatal exposure to nicotine impairs protective responses of rat pups to hypoxia in an age dependent manner. Respiration Physiology. 2001;127:61–73. doi: 10.1016/s0034-5687(01)00232-8. [DOI] [PubMed] [Google Scholar]
- Frank JG, Jameson HS, Gorini C, Mendelowitz D. Mapping and identification of GABAergic neurons in transgenic mice projecting to cardiac vagal neurons in the nucleus ambiguus using photo-uncaging. J Neurophysiol. 2009;101:1755–1760. doi: 10.1152/jn.91134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Physiological and Genomic Consequences of Intermittent Hypoxia Selected Contribution: Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Gargaglioni LH, Bicego KC, Nucci TB, Branco LG. Serotoninergic receptors in the anteroventral preoptic region modulate the hypoxic ventilatory response. Respir Physiol Neurobiol. 2006;153:1–13. doi: 10.1016/j.resp.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Gennser G, Marsal K, Brantmark B. Maternal smoking and fetal breathing movements. Am J Obstet Gynecol. 1975;123:861–867. doi: 10.1016/0002-9378(75)90863-7. [DOI] [PubMed] [Google Scholar]
- Gilbey MP, Jordan D, Richter DW, Spyer KM. Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in the cat. J Physiol. 1984;356:65–78. doi: 10.1113/jphysiol.1984.sp015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffioen KJ, Gorini C, Jameson H, Mendelowitz D. Purinergic P2X receptors mediate excitatory transmission to cardiac vagal neurons in the nucleus ambiguus after hypoxia. Hypertension. 2007a;50:75–81. doi: 10.1161/HYPERTENSIONAHA.106.086140. [DOI] [PubMed] [Google Scholar]
- Griffioen KJ, Kamendi HW, Gorini CJ, Bouairi E, Mendelowitz D. Reactive oxygen species mediate central cardiorespiratory network responses to acute intermittent hypoxia. J Neurophysiol. 2007b;97:2059–2066. doi: 10.1152/jn.00975.2006. [DOI] [PubMed] [Google Scholar]
- Gu H, Lin M, Liu J, Gozal D, Scrogin KE, Wurster R, Chapleau MW, Ma X, Cheng ZJ. Selective impairment of central mediation of baroreflex in anesthetized young adult Fischer 344 rats after chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H2809–2818. doi: 10.1152/ajpheart.00358.2007. [DOI] [PubMed] [Google Scholar]
- Guntheroth WG, Kawabori I. Hypoxic apnea and gasping. J Clin Invest. 1975;56:1371–1377. doi: 10.1172/JCI108217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund B, Cnattingius S. Cigarette smoking as a risk factor for sudden infant death syndrome: a population-based study. Am J Public Health. 1990;80:29–32. doi: 10.2105/ajph.80.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathorn MK. Respiratory sinus arrhythmia in new-born infants. J Physiol. 1987;385:1–12. doi: 10.1113/jphysiol.1987.sp016480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano J, Yasuma F, Okada A, Mukai S, Fujinami T. Respiratory sinus arrhythmia. A phenomenon improving pulmonary gas exchange and circulatory efficiency. Circulation. 1996;94:842–847. doi: 10.1161/01.cir.94.4.842. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Nagasaka T. Hypoxic tachycardia in hypoxia-acclimated rats. Jpn J Physiol. 1982;32:149–152. doi: 10.2170/jjphysiol.32.149. [DOI] [PubMed] [Google Scholar]
- Hellman LM, Johnson HL, Tolles WE, Jones EH. Some factors affecting the fetal heart rate. Am J Obstet Gynecol. 1961;82:1055–1063. doi: 10.1016/s0002-9378(16)36198-1. [DOI] [PubMed] [Google Scholar]
- Heymans C.a.N., E. Reflexogenic Areas of the Cardiovascular System. Churchill; London: 1958. [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Bishop B. Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. Am J Physiol. 1981;241:H620–629. doi: 10.1152/ajpheart.1981.241.4.H620. [DOI] [PubMed] [Google Scholar]
- Hrushesky WJ. Quantitative respiratory sinus arrhythmia analysis. A simple noninvasive, reimbursable measure of cardiac wellness and dysfunction. Ann N Y Acad Sci. 1991;618:67–101. doi: 10.1111/j.1749-6632.1991.tb27238.x. [DOI] [PubMed] [Google Scholar]
- Huang ZG, Griffioen KJ, Wang X, Dergacheva O, Kamendi H, Gorini C, Bouairi E, Mendelowitz D. Differential control of central cardiorespiratory interactions by hypercapnia and the effect of prenatal nicotine. J Neurosci. 2006;26:21–29. doi: 10.1523/JNEUROSCI.4221-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZG, Griffioen KJ, Wang X, Dergacheva O, Kamendi H, Gorini C, Mendelowitz D. Nicotinic receptor activation occludes purinergic control of central cardiorespiratory network responses to hypoxia/hypercapnia. J Neurophysiol. 2007;98:2429–2438. doi: 10.1152/jn.00448.2007. [DOI] [PubMed] [Google Scholar]
- Hull SS, Jr., Vanoli E, Adamson PB, De Ferrari GM, Foreman RD, Schwartz PJ. Do increases in markers of vagal activity imply protection from sudden death? The case of scopolamine. Circulation. 1995;91:2516–2519. doi: 10.1161/01.cir.91.10.2516. [DOI] [PubMed] [Google Scholar]
- Ikenoue T, Martin CB, Jr., Murata Y, Ettinger BB, Lu PS. Effect of acute hypoxemia and respiratory acidosis on the fetal heart rate in monkeys. Am J Obstet Gynecol. 1981;141:797–806. doi: 10.1016/0002-9378(81)90707-9. [DOI] [PubMed] [Google Scholar]
- Inoue K, Miyake S, Kumashiro M, Ogata H, Yoshimura O. Power spectral analysis of heart rate variability in traumatic quadriplegic humans. Am J Physiol. 1990;258:H1722–1726. doi: 10.1152/ajpheart.1990.258.6.H1722. [DOI] [PubMed] [Google Scholar]
- Inukai T, Kobayashi I, Kobayashi T, Ishii A, Yamaguchi T, Yamaguchi Y, Iwashita A, Ohshima K, Shimomura Y, Kobayashi S. Parasympathetic nervous system activity in hypothyroidism determined by R-R interval variations on electrocardiogram. J Intern Med. 1990;228:431–434. doi: 10.1111/j.1365-2796.1990.tb00259.x. [DOI] [PubMed] [Google Scholar]
- Izzo PN, Deuchars J, Spyer KM. Localization of cardiac vagal preganglionic motoneurones in the rat: immunocytochemical evidence of synaptic inputs containing 5-hydroxytryptamine. J Comp Neurol. 1993;327:572–583. doi: 10.1002/cne.903270408. [DOI] [PubMed] [Google Scholar]
- Jordan D, Khalid ME, Schneiderman N, Spyer KM. The location and properties of preganglionic vagal cardiomotor neurones in the rabbit. Pflugers Arch. 1982;395:244–250. doi: 10.1007/BF00584817. [DOI] [PubMed] [Google Scholar]
- Julius S, Valentini M. Consequences of the increased autonomic nervous drive in hypertension, heart failure and diabetes. Blood Press Suppl. 1998;3:5–13. doi: 10.1080/080370598438410-1. [DOI] [PubMed] [Google Scholar]
- Kamendi HW, Cheng Q, Dergacheva O, Gorini C, Jameson HS, Wang X, McIntosh JM, Mendelowitz D. Abolishment of serotonergic neurotransmission to cardiac vagal neurons during and after hypoxia and hypercapnia with prenatal nicotine exposure. J Neurophysiol. 2009;101:1141–1150. doi: 10.1152/jn.90680.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Adachi T, Koshino Y, Somers VK. Obstructive sleep apnea and cardiovascular disease. Circ J. 2009;73:1363–1370. doi: 10.1253/circj.cj-09-0364. [DOI] [PubMed] [Google Scholar]
- Kollai M, Koizumi K. Reciprocal and non-reciprocal action of the vagal and sympathetic nerves innervating the heart. J Auton Nerv Syst. 1979;1:33–52. doi: 10.1016/0165-1838(79)90004-3. [DOI] [PubMed] [Google Scholar]
- Kunze DL. Reflex discharge patterns of cardiac vagal efferent fibres. J Physiol. 1972;222:1–15. doi: 10.1113/jphysiol.1972.sp009784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rovere MT, Specchia G, Mortara A, Schwartz PJ. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction. A prospective study. Circulation. 1988;78:816–824. doi: 10.1161/01.cir.78.4.816. [DOI] [PubMed] [Google Scholar]
- Lai CJ, Yang CC, Hsu YY, Lin YN, Kuo TB. Enhanced sympathetic outflow and decreased baroreflex sensitivity are associated with intermittent hypoxia-induced systemic hypertension in conscious rats. J Appl Physiol. 2006;100:1974–1982. doi: 10.1152/japplphysiol.01051.2005. [DOI] [PubMed] [Google Scholar]
- Levy MN, Zieske H. Autonomic control of cardiac pacemaker activity and atrioventricular transmission. J Appl Physiol. 1969;27:465–470. doi: 10.1152/jappl.1969.27.4.465. [DOI] [PubMed] [Google Scholar]
- Lewis AB, Donovan M, Platzker AC. Cardiovascular responses to autonomic blockade in hypoxemic fetal lambs. Biol Neonate. 1980;37:233–242. doi: 10.1159/000241281. [DOI] [PubMed] [Google Scholar]
- Lin M, Ai J, Li L, Huang C, Chapleau MW, Liu R, Gozal D, Wead WB, Wurster RD, Cheng ZJ. Structural remodeling of nucleus ambiguus projections to cardiac ganglia following chronic intermittent hypoxia in C57BL/6J mice. J Comp Neurol. 2008;509:103–117. doi: 10.1002/cne.21732. [DOI] [PubMed] [Google Scholar]
- Lin M, Liu R, Gozal D, Wead WB, Chapleau MW, Wurster R, Cheng ZJ. Chronic intermittent hypoxia impairs baroreflex control of heart rate but enhances heart rate responses to vagal efferent stimulation in anesthetized mice. Am J Physiol Heart Circ Physiol. 2007;293:H997–1006. doi: 10.1152/ajpheart.01124.2006. [DOI] [PubMed] [Google Scholar]
- Loewy A.D. SKM. Central Regulation of Autonomic Functions. Oxford University Press; 1990. [Google Scholar]
- Loewy AD, Spyer KM. Central Regulation of Autonomic Functions. Oxford University Press; 1990a. [Google Scholar]
- Loewy AD, Spyer KM. Vagal Preganglionic Neurons. In: Spyer KM, editor. Central Regulation of Autonomic Functions. Oxford University Press; New York: 1990b. pp. 68–87. [Google Scholar]
- Machado BH, Brody MJ. Role of the nucleus ambiguus in the regulation of heart rate and arterial pressure. Hypertension. 1988;11:602–607. doi: 10.1161/01.hyp.11.6.602. [DOI] [PubMed] [Google Scholar]
- Madden BP, Shenoy V, Dalrymple-Hay M, Griffiths T, Millard J, Backhouse L, Clarke J, Murday A. Absence of bradycardic response to apnea and hypoxia in heart transplant recipients with obstructive sleep apnea. J Heart Lung Transplant. 1997;16:394–397. [PubMed] [Google Scholar]
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5(Suppl A):S377–382. doi: 10.1016/s1526-0542(04)90067-x. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Spyer KM. The baroreceptor input to cardiac vagal motoneurones. J Physiol. 1978;282:365–374. doi: 10.1113/jphysiol.1978.sp012469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R334–341. doi: 10.1152/ajpregu.00463.2003. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D. Firing properties of identified parasympathetic cardiac neurons in nucleus ambiguus. Am J Physiol. 1996;271:H2609–2614. doi: 10.1152/ajpheart.1996.271.6.H2609. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D. Advances in Parasympathetic Control of Heart Rate and Cardiac Function. News Physiol Sci. 1999;14:155–161. doi: 10.1152/physiologyonline.1999.14.4.155. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D, Kunze DL. Identification and dissociation of cardiovascular neurons from the medulla for patch clamp analysis. Neurosci Lett. 1991;132:217–221. doi: 10.1016/0304-3940(91)90305-d. [DOI] [PubMed] [Google Scholar]
- Meny RG, Carroll JL, Carbone MT, Kelly DH. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics. 1994;93:44–49. [PubMed] [Google Scholar]
- Mihalevich M, Neff RA, Mendelowitz D. Voltage-gated currents in identified parasympathetic cardiac neurons in the nucleus ambiguus. Brain Res. 1996;739:258–262. doi: 10.1016/s0006-8993(96)00868-2. [DOI] [PubMed] [Google Scholar]
- Milerad J, Rajs J, Gidlund E. Nicotine and cotinine levels in pericardial fluid in victims of SIDS. Acta Paediatr. 1994;83:59–62. doi: 10.1111/j.1651-2227.1994.tb12953.x. [DOI] [PubMed] [Google Scholar]
- Mitchell EA, Ford RP, Stewart AW, Taylor BJ, Becroft DM, Thompson JM, Scragg R, Hassall IB, Barry DM, Allen EM, et al. Smoking and the sudden infant death syndrome. Pediatrics. 1993;91:893–896. [PubMed] [Google Scholar]
- Motte S, Mathieu M, Brimioulle S, Pensis A, Ray L, Ketelslegers JM, Montano N, Naeije R, van de Borne P, Entee KM. Respiratory-related heart rate variability in progressive experimental heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H1729–1735. doi: 10.1152/ajpheart.01129.2004. [DOI] [PubMed] [Google Scholar]
- Myers MM, Fifer W, Haiken J, Stark RI. Relationships between breathing activity and heart rate in fetal baboons. Am J Physiol. 1990;258:R1479–1485. doi: 10.1152/ajpregu.1990.258.6.R1479. [DOI] [PubMed] [Google Scholar]
- Nachmanoff DB, Panigrahy A, Filiano JJ, Mandell F, Sleeper LA, Valdes-Dapena M, Krous HF, White WF, Kinney HC. Brainstem 3H-nicotine receptor binding in the sudden infant death syndrome. J Neuropathol Exp Neurol. 1998;57:1018–1025. doi: 10.1097/00005072-199811000-00004. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177:385–390. doi: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- Neff RA, Mihalevich M, Mendelowitz D. Stimulation of NTS activates NMDA and non-NMDA receptors in rat cardiac vagal neurons in the nucleus ambiguus. Brain Res. 1998;792:277–282. doi: 10.1016/s0006-8993(98)00149-8. [DOI] [PubMed] [Google Scholar]
- Neff RA, Simmens SJ, Evans C, Mendelowitz D. Prenatal nicotine exposure alters central cardiorespiratory responses to hypoxia in rats: implications for sudden infant death syndrome. J Neurosci. 2004;24:9261–9268. doi: 10.1523/JNEUROSCI.1918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff RA, Wang J, Baxi S, Evans C, Mendelowitz D. Respiratory sinus arrhythmia: endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardioinhibitory parasympathetic neurons. Circ Res. 2003;93:565–572. doi: 10.1161/01.RES.0000090361.45027.5B. [DOI] [PubMed] [Google Scholar]
- Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. Jama. 2000;284:1392–1398. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- Nosaka S, Yamamoto T, Yasunaga K. Localization of vagal cardioinhibitory preganglionic neurons with rat brain stem. J Comp Neurol. 1979;186:79–92. doi: 10.1002/cne.901860106. [DOI] [PubMed] [Google Scholar]
- Nucci TB, Branco LG, Gargaglioni LH. 5-HT1A, but not 5-HT2 and 5-HT7, receptors in the nucleus raphe magnus modulate hypoxia-induced hyperpnoea. Acta Physiol (Oxf) 2008;193:403–414. doi: 10.1111/j.1748-1716.2008.01853.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell CP, Bower EA. Heart rate changes evoked by hypoxia in the anaesthetized, artificially ventilated cat. Exp Physiol. 1992;77:271–283. doi: 10.1113/expphysiol.1992.sp003587. [DOI] [PubMed] [Google Scholar]
- Oka H, Mochio S, Sato K, Sato H, Katayama K. Prolongation of QTc interval and autonomic nervous dysfunction in diabetic patients. Diabetes Res Clin Pract. 1996;31:63–70. doi: 10.1016/0168-8227(96)01194-1. [DOI] [PubMed] [Google Scholar]
- Parati G, Lombardi C, Narkiewicz K. Sleep apnea: epidemiology, pathophysiology, and relation to cardiovascular risk. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1671–1683. doi: 10.1152/ajpregu.00400.2007. [DOI] [PubMed] [Google Scholar]
- Pardini BJ, Patel KP, Schmid PG, Lund DD. Location, distribution and projections of intracardiac ganglion cells in the rat. J Auton Nerv Syst. 1987;20:91–101. doi: 10.1016/0165-1838(87)90106-8. [DOI] [PubMed] [Google Scholar]
- Pichot V, Roche F, Gaspoz JM, Enjolras F, Antoniadis A, Minini P, Costes F, Busso T, Lacour JR, Barthelemy JC. Relation between heart rate variability and training load in middle-distance runners. Med Sci Sports Exerc. 2000;32:1729–1736. doi: 10.1097/00005768-200010000-00011. [DOI] [PubMed] [Google Scholar]
- Pickering TG, Gribbin B, Petersen ES, Cunningham DJ, Sleight P. Effects of autonomic blockade on the baroreflex in man at rest and during exercise. Circ Res. 1972;30:177–185. doi: 10.1161/01.res.30.2.177. [DOI] [PubMed] [Google Scholar]
- Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and Other Cardiorespiratory Patterns during Sudden Infant Deaths. Pediatr Res. 1999;45:350–354. doi: 10.1203/00006450-199903000-00010. [DOI] [PubMed] [Google Scholar]
- Potter EK, McCloskey DI. Effects of hypoxia on cardiac vagal efferent activity and on the action of the vagus nerve at the heart in the dog. J Auton Nerv Syst. 1986;17:325–329. doi: 10.1016/0165-1838(86)90098-6. [DOI] [PubMed] [Google Scholar]
- Przybylski J, Trzebski A, Przybyszewski A. Circulatory responses to acute hypoxia in spontaneously hypertensive and normotensive rats. Acta Physiol Pol. 1980;31:463–468. [PubMed] [Google Scholar]
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rardon DP, Bailey JC. Parasympathetic effects on electrophysiologic properties of cardiac ventricular tissue. J Am Coll Cardiol. 1983;2:1200–1209. doi: 10.1016/s0735-1097(83)80351-9. [DOI] [PubMed] [Google Scholar]
- Rentero N, Cividjian A, Trevaks D, Pequignot JM, Quintin L, McAllen RM. Activity patterns of cardiac vagal motoneurons in rat nucleus ambiguus. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1327–1334. doi: 10.1152/ajpregu.00271.2002. [DOI] [PubMed] [Google Scholar]
- Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol. 1999;514(Pt 2):567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Cardiorespiratory Control. In: Spyer KM, editor. Central Regulation of Autonomic Function. Oxford University Press; New York: 1990. pp. 189–207. [Google Scholar]
- Roche F, Reynaud C, Garet M, Pichot V, Costes F, Barthelemy JC. Cardiac baroreflex control in humans during and immediately after brief exposure to simulated high altitude. Clin Physiol Funct Imaging. 2002;22:301–306. doi: 10.1046/j.1475-097x.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- Routledge HC, Chowdhary S, Townend JN. Heart rate variability--a therapeutic target? J Clin Pharm Ther. 2002;27:85–92. doi: 10.1046/j.1365-2710.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- Scher AM, Young AC. Reflex control of heart rate in the unanesthetized dog. Am J Physiol. 1970;218:780–789. doi: 10.1152/ajplegacy.1970.218.3.780. [DOI] [PubMed] [Google Scholar]
- Schuen JN, Bamford OS, Carroll JL. The cardiorespiratory response to anoxia: normal development and the effect of nicotine. Respiration Physiology. 1997;109:231–239. doi: 10.1016/s0034-5687(97)00052-2. [DOI] [PubMed] [Google Scholar]
- Scremin AM, Scremin OU, Brechner T. Survival under hypoxia. Age dependence and effect of cholinergic drugs. Stroke. 1980;11:548–552. doi: 10.1161/01.str.11.5.548. [DOI] [PubMed] [Google Scholar]
- Serani A, Lavados M, Zapata P. Cardiovascular responses to hypoxia in the spontaneously breathing cat: reflexes originating from carotid and aortic bodies. Arch Biol Med Exp (Santiago) 1983;16:29–41. [PubMed] [Google Scholar]
- Shykoff BE, Naqvi SS, Menon AS, Slutsky AS. Respiratory sinus arrhythmia in dogs. Effects of phasic afferents and chemostimulation. J Clin Invest. 1991;87:1621–1627. doi: 10.1172/JCI115176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Saleh JL, McCook EC, Seidler FJ. Impaired cardiac function during postnatal hypoxia in rats exposed to nicotine prenatally: implications for perinatal morbidity and mortality, and for sudden infant death syndrome. Teratology. 1997;55:177–184. doi: 10.1002/(SICI)1096-9926(199703)55:3<177::AID-TERA2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Qiao D, Aldridge JE, Tate CA, Cousins MM, Proskocil BJ, Sekhon HS, Clark JA, Lupo SL, Spindel ER. Effects of prenatal nicotine exposure on primate brain development and attempted amelioration with supplemental choline or vitamin C: neurotransmitter receptors, cell signaling and cell development biomarkers in fetal brain regions of rhesus monkeys. Neuropsychopharmacology. 2005;30:129–144. doi: 10.1038/sj.npp.1300544. [DOI] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Mark AL, Abboud FM. Parasympathetic hyperresponsiveness and bradyarrhythmias during apnoea in hypertension. Clin Auton Res. 1992;2:171–176. doi: 10.1007/BF01818958. [DOI] [PubMed] [Google Scholar]
- Sontag LW, Wallace RF. The effect of cigaret smoking during pregnancy upon the fetal heart rate. Am J Obstet Gynecol. 1935;29:77–83. [Google Scholar]
- Soukhova-O'Hare GK, Cheng ZJ, Roberts AM, Gozal D. Postnatal intermittent hypoxia alters baroreflex function in adult rats. Am J Physiol Heart Circ Physiol. 2006;290:H1157–1164. doi: 10.1152/ajpheart.00767.2005. [DOI] [PubMed] [Google Scholar]
- Spyer KM. Neural organisation and control of the baroreceptor reflex. Rev Physiol Biochem Pharmacol. 1981;88:24–124. [PubMed] [Google Scholar]
- Spyer KM, Gilbey MP. Cardiorespiratory interactions in heart-rate control. Ann N Y Acad Sci. 1988;533:350–357. doi: 10.1111/j.1749-6632.1988.tb37263.x. [DOI] [PubMed] [Google Scholar]
- St-John WM, Leiter JC. Maternal nicotine depresses eupneic ventilation of neonatal rats. Neurosci Lett. 1999;267:206–208. doi: 10.1016/s0304-3940(99)00364-x. [DOI] [PubMed] [Google Scholar]
- St-John WM, Leiter JC. Maintenance of gasping and restoration of eupnea after hypoxia is impaired following blockers of {alpha}-1 adrenergic receptors and serotonin 5HT2 receptors. J Appl Physiol. 2007 doi: 10.1152/japplphysiol.00599.2007. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Guyenet PG, McCarty RC. Autonomic nervous system control of heart rate during baroreceptor activation in conscious and anesthetized rats. J Auton Nerv Syst. 1987;20:121–127. doi: 10.1016/0165-1838(87)90109-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Kojima M, Matsuura T, Sano Y. Serotonergic innervation on the motoneurons in the mammalian brainstem. Light and electron microscopic immunohistochemistry. Anat Embryol (Berl) 1983;167:321–333. doi: 10.1007/BF00315670. [DOI] [PubMed] [Google Scholar]
- Taylor EW, Butler PJ. Nervous control of heart rate: activity in the cardiac vagus of the dogfish. J Appl Physiol. 1982;53:1330–1335. doi: 10.1152/jappl.1982.53.6.1330. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Jr., Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- Wakamori M, Ikemoto Y, Akaike N. Effects of two volatile anesthetics and a volatile convulsant on the excitatory and inhibitory amino acid responses in dissociated CNS neurons of the rat. J Neurophysiol. 1991;66:2014–2021. doi: 10.1152/jn.1991.66.6.2014. [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Mendelowitz D. Characteristics of spontaneous and evoked GABAergic synaptic currents in cardiac vagal neurons in rats. Brain Res. 2001a;889:78–83. doi: 10.1016/s0006-8993(00)03112-7. [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Mendelowitz D. Characteristics of spontaneous and evoked GABAergic synaptic currents in cardiac vagal neurons in rats. Brain Res. 2001b;889:78–83. doi: 10.1016/s0006-8993(00)03112-7. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang X, Irnaten M, Venkatesan P, Evans C, Baxi S, Mendelowitz D. Endogenous acetylcholine and nicotine activation enhances GABAergic and glycinergic inputs to cardiac vagal neurons. J Neurophysiol. 2003;89:2473–2481. doi: 10.1152/jn.00934.2002. [DOI] [PubMed] [Google Scholar]
- Wang X, Dergacheva O, Kamendi H, Gorini C, Mendelowitz D. 5-Hydroxytryptamine 1A/7 and 4alpha receptors differentially prevent opioid-induced inhibition of brain stem cardiorespiratory function. Hypertension. 2007;50:368–376. doi: 10.1161/HYPERTENSIONAHA.107.091033. [DOI] [PubMed] [Google Scholar]
- Warner MR, deTarnowsky JM, Whitson CC, Loeb JM. Beat-by-beat modulation of AV conduction. II. Autonomic neural mechanisms. Am J Physiol. 1986;251:H1134–1142. doi: 10.1152/ajpheart.1986.251.6.H1134. [DOI] [PubMed] [Google Scholar]
- Yan B, Li L, Harden SW, Gozal D, Lin Y, Wead WB, Wurster RD, Cheng ZJ. Chronic intermittent hypoxia impairs heart rate responses to AMPA and NMDA and induces loss of glutamate receptor neurons in nucleus ambiguous of F344 rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R299–308. doi: 10.1152/ajpregu.90412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Soukhova-O'Hare GK, Li L, Lin Y, Gozal D, Wead WB, Wurster RD, Cheng ZJ. Attenuation of heart rate control and neural degeneration in nucleus ambiguus following chronic intermittent hypoxia in young adult Fischer 344 rats. Neuroscience. 2008;153:709–720. doi: 10.1016/j.neuroscience.2008.01.066. [DOI] [PubMed] [Google Scholar]
- Yu ZY, Lumbers ER, Gibson KJ, Stevens AD. Effects on hypoxaemia on foetal heart rate, variability and cardiac rhythm. Clin Exp Pharmacol Physiol. 1998;25:577–584. doi: 10.1111/j.1440-1681.1998.tb02255.x. [DOI] [PubMed] [Google Scholar]