Abstract
Background
The aim of the study was to evaluate gender differences and the effect of menstrual cycle and menopausal status on irritable bowel syndrome (IBS) symptoms.
Methods
We performed a systematic review of MEDLINE to search for studies comparing IBS symptoms between gender, menstrual cycle phases, and menopausal states in IBS and/or healthy individuals. We performed meta-analyses to compare the relative risk (RR) of individual IBS symptoms between men and women.
Results
Twenty-two studies measured gender differences in IBS symptoms. Women were more likely to report abdominal pain (RR=1.12, CI [1.02, 1.22]) and constipation-related symptoms (RR=1.12, CI [1.02, 1.23]) than men (all p<0.05). However, men with IBS were more likely to report the diarrhea-related symptoms than women with IBS (RR=0.84, CI [0.75, 0.94], p<0.05). A systematic review of 13 studies demonstrated that both IBS and healthy women reported increased IBS symptoms during menses vs. other phases. There were insufficient data to determine the effect of menopause and hormone supplementation on IBS symptoms.
Conclusion
In the general and IBS populations, gender differences in IBS symptoms exist although these differences are modest. Studies suggest that female sex hormones influence the severity of IBS symptoms, but more studies are needed.
Keywords: irritable bowel syndrome, gender, abdominal pain, bloating, constipation, meta-analysis
INTRODUCTION
Irritable bowel syndrome (IBS) is characterized by recurrent abdominal pain or discomfort associated with a change in bowel habits.1 The prevalence of IBS ranges from 6-22% in Western countries, although the prevalence in Eastern countries tends to be lower and ranges from 2-17%.2 IBS has a female predominance with a female-to-male ratio of 2-2.5:1 in those who seek health care. The female predominance is less apparent in the general population, which suggests that women with IBS are more likely to seek healthcare for their symptoms.3-7 However, some Asian studies fail to report significant gender differences in the prevalence of IBS, suggesting that cultural differences may also play a role in IBS symptom reporting.8, 9
Gender differences in IBS are evident by sub-classification, non-gastrointestinal (GI) symptoms, pathophysiologic responses, and treatment response.10 Specifically, female predominance is particularly apparent in the IBS with constipation (IBS-C) subtype compared to IBS with diarrhea (IBS-D) and alternating or mixed pattern (IBS-M).11 Although gender differences in pathophysiologic studies (e.g., GI transit, rectal perception, brain activation patterns) have been reported in IBS and healthy controls,12, 13 there are also conflicting reports that failed to identify differences between men and women.14 Response to some IBS treatments, such as serotonergic agents, appears to be more robust in women than men.12, 15-17 However, these findings may be due to an inadequate number of men in IBS studies rather than a true gender difference in non-study populations. In fact, there have been inadequate efforts to enroll sufficient numbers of men in many IBS studies and inadequate attempts to control for menstrual cycle phase among women in these trials.10 In addition, gender differences and the effect of female sex hormones have largely been understudied in IBS. A recent review suggested that a strong relationship between menstrual cycle and bowel symptoms exists and that this may be due to effects of ovarian hormones on visceral pain sensitivity and bowel function.18
It is important to determine whether there are true symptom differences between genders in IBS because this information can potentially impact our understanding of the pathophysiology of IBS and influence research study design, drug development, and treatment. Two previous reviews assessed gender differences in the diagnosis of IBS. One review was conducted in developing countries,19 while the other was a systematic review of studies conducted in community populations.2 Both reviews concluded that most Western studies supported a female predominance of IBS. However, approximately half of the studies conducted in Eastern countries reported a female predominance while the other half did not. Irrespective of whether the studies were conducted in Western or Eastern populations, the female-to-male ratio was dependent on the diagnostic criteria, i.e., a greater female predominance was seen when Manning criteria was used vs. Rome criteria.2
In order to better understand gender differences in IBS, we performed a systematic review and meta-analysis of the literature to evaluate gender differences in individual IBS symptoms and the role of menstrual cycle, menopausal status and hormone supplementation on these symptoms. We hypothesized that the pooled data would reveal that women more frequently report abdominal pain and non-pain related symptoms associated with constipation such as hard stools, bloating, abdominal distension, and straining than men, and that men more often report symptoms associated with diarrhea such as loose stools and increased stool frequency. In addition, we hypothesized that the pooled data would reveal a higher prevalence of IBS symptoms at times when ovarian hormones are low, i.e. at the onset of menses in premenopausal women and during menopause, and these symptoms decrease with hormone supplementation.
METHODS
Search Strategy
The analysis for this study was based on a broader search for articles pertaining to dyspepsia and/or IBS. We conducted a comprehensive search for English-language studies in MEDLINE published up to June 2010, which examined gender differences in IBS and/or the effect of menstrual cycle, menopausal status, or hormone supplement on GI symptoms. The keywords and search strings used to perform the search with Reference Manager Software are indicated in Table 1. In addition, we performed manual searches of reference lists from relevant papers to identify other manuscripts which may have been missed by the search strategy.
Table 1.
Systematic review search strategy
| Group | Search Terms | Significance of Grouping |
|---|---|---|
| 1 | MEDLINE | Targeted Bibliographic Database |
| 2 | Irritable bowel syndrome [MeSH Terms] OR
irritable bowel OR gastrointestinal transit [MeSH Terms] OR
intestinal transit OR functional gastrointestinal disorder* OR
gastrointestinal motility [MeSH Terms] OR visceral hyperalgesia OR
functional colonic diseases OR colonic diseases OR gastrointestinal
diseases OR gastrointestinal transit OR intestinal
pseudo-obstruction OR dyspepsia Field: title/ abstract |
Targeted Topic Focus |
| 3 | Gender OR menstrual cycle [MeSH Terms] OR
estrogen* OR progesterone [MeSH Terms] OR menstruation [MeSH Terms]
OR luteal phase [MeSH Terms] OR oral contraceptives [MeSH Terms] OR
testosterone [MeSH Terms] OR sex characteristics [MeSH Terms] OR sex
hormones OR gonadal steroid hormones OR estradiol congeners OR
contraceptive agents OR contraceptives, oral, hormonal [MeSH Terms]
OR contraceptives, oral, sequential [MeSH Terms] OR contraceptives,
oral [MeSH Terms] OR menopause Field: text word |
Targeted Content Keywords |
| 4 | Review OR letter OR news OR
editorial Field: Publication Type NOT Cat OR mouse OR rat OR feline OR porcine OR canine OR dog OR bovine OR cow OR horse Field: text word |
Excluded study types and content |
The four search groups were combined as follows: (1 AND 2 AND 3 NOT 4).
MeSH=medical subject heading. Asterisk (*) indicates keyword truncation
Screening Strategy
We excluded titles if they did not meet the following inclusion criteria: (1) written in English, (2) investigation of humans, (3) examination of IBS or functional dyspepsia symptoms, (4) assessment of IBS or functional dyspepsia symptoms in relation to menstrual cycle, hormone supplement, and/or gender, and (5) study population consisted of the general population, and/or patient with dyspepsia and/or IBS. The analysis for this study was based on a broader search for articles pertaining to IBS and/or dyspepsia. Two reviewers then independently assessed the relevancy of the abstracts for the remaining articles. We excluded abstracts that did not meet these criteria. Next, the two reviewers independently assessed the manuscripts of the remaining abstracts based on the pre-specified inclusion criteria. Additionally, we manually reviewed the reference lists of identified studies to evaluate for extant literature not captured by the search strategy. In three cases, we contacted authors to obtain manuscripts. We applied Cohen's kappa statistic to assess inter-rater agreement and a third investigator resolved disagreements when necessary.
Data abstraction
We created a standardized data abstraction form in order to summarize information germane to the aims of this study. We compiled and summarized abstracted data using pre-specified evidence tables.
Quality Assessment (QA)
The Downs and Black (D&B) checklist was used to evaluate the quality of all studies (Appendix).20 The D&B checklist was developed to assess the methodological quality of randomized and non-randomized studies of health interventions. Of the few checklists available that assess the quality of both non-randomized and randomized studies, the D&B checklist provides good test-retest reliability, inter-rater reliability, and criterion validity. Several systematic reviews and meta-analyses have used modified versions of this checklist to assess study quality.21-23 The checklist is composed of 26 items subdivided into five components: reporting, internal validity, confounding, external validity, and power. Because 11 items in the original list were specific to randomized studies (intervention, randomization, and power calculation), these items were not included in the assessment of quality of non-randomized studies. The maximum QA score on the quality scales was 17 for non-randomized studies and 32 for randomized studies. In order to more effectively compare the quality of the studies we subjectively categorized the QA scores. For non-randomized studies, the quality was rated as poor, intermediate, and good if the QA score was 0-6, 7-11, and 12-17, respectively. For randomized studies, the quality was rated as poor, intermediate and good if the QA score was 0-10, 11-20, 21-32, respectively.
Statistical analysis
We performed statistical analysis only on those studies investigating gender differences in IBS symptoms, and performed meta-analysis only for IBS symptoms that were assessed in ≥ 3 studies. We reported summary statistics as relative risk (RR) favoring women. All data were fitted into a 2×2 matrix in order to calculate the summary effect using the Mantel-Haenszel Method. In cases where none of the subjects reported a particular symptom, 1.0 was added to each cell of the 2×2 matrix.
We used Cochrane's Q statistic to test for heterogeneity and adopted a P value of greater than 0.10 for the Q statistic as evidence for homogeneity.24 If the data were homogeneous, we then selected a fixed-effects model.24 If the data were heterogeneous, we then performed both a fixed and random-effects model.24
We performed a qualitative appraisal of publication bias by constructing a funnel plot and observing for evidence of asymmetry,24 and performed a quantitative appraisal for publication bias by conducting an Egger's test.24 We assumed there was evidence for publication bias if there was a qualitative lack of small “negative” studies on the funnel plot, or if the P value for the Egger's test was > 0.10.24
All statistical analysis was conducted using Stata™ statistical software version 8.0 (Stata Corp LP, College Station, TX, USA).
RESULTS
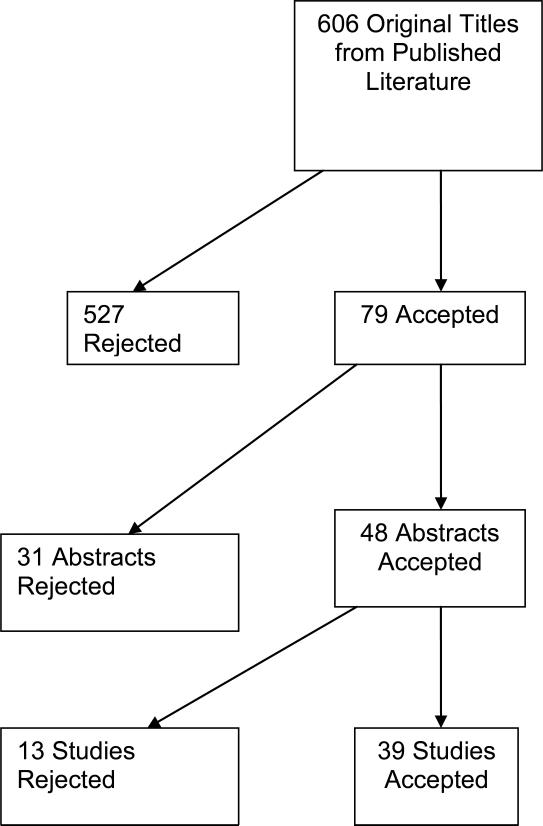
Of the 599 studies identified by the defined search strategy, 39 studies were included in our systematic review (Figure 1).9, 25-60 There was sufficient inter-rater agreement among the studies selected (abstract κ=0.73 and content κ=0.83 selection).61
Figure 1.
Results of the literature search are shown.
Gender differences in IBS symptoms
Table 2 shows the general characteristics of the 22 studies (9 surveyed the general population sample, 12 studied an IBS only sample, and 1 studied both general population and IBS) which compared prevalence rates of IBS symptoms in men and women.9, 25-36, 47, 51-54, 56, 58-60 Fourteen studies were considered good quality and eight were considered of intermediate quality.
Table 2.
Evidence table of studies comparing sex differences in IBS symptoms
| Study | General Population N, (% F) | IBS N, (% F) | Population | Study Design | Setting | Country | IBS diagnostic criteria | QA |
|---|---|---|---|---|---|---|---|---|
| Bouchoucha26 | 134 (42.5) | 752 (74.2) | IBS and Controls | Cross-sectional | 2° Care | France | Rome I and II | 11 |
| Kim51 | 1717 (56.5) | N/A | General Population | Cross-sectional | University | Korea | N/A | 15 |
| Sandler52 | 2510 (62.0) | N/A | General population | Cross-sectional | N/A | USA | N/A | 14 |
| Shen58 | 491 (50.9) | N/A | General population | Cross-sectional | University | Chinese | N/A | 8 |
| Talley27 | 835 (54.0) | N/A | General population | Cross-sectional | N/A | USA | N/A | 13 |
| Talley28 | 726 (53.7) | N/A | General population | Cross-sectional | N/A | Australia | N/A | 13 |
| Tan53 | 533 (57.0) | N/A | General population | Cross-sectional | University | Malaysia | N/A | 15 |
| Taub29 | 1344 (61.6) | N/A | General population | Cross-sectional | N/A | USA | N/A | 12 |
| Tuteja30 | 723 (60.7) | N/A | General population | Cross-sectional | N/A | USA | N/A | 15 |
| Zuckerman31 | 405 (53.6) | N/A | General population | Cross-sectional | N/A | Vietnam | N/A | 13 |
| Celebi54 | N/A | 111 (64.0) | IBS | Cross-sectional | 1° Care | Turkey | Rome II | 15 |
| Barakzai47 | N/A | 139 (71.4) | IBS | Retrospective | 1° Care | USA (Mexican-Americans) | Rome II criteria met in 58% of women and 79% men | 11 |
| Han9 | N/A | 70 (45.7) | IBS | Cross-sectional | Community | Korea | Rome II | 15 |
| Lee, OY32 | N/A | 714 (66.8) | IBS | Cross-sectional | Advertisement and 3° Care | USA | Rome | 13 |
| Lu33 | N/A | 447 (40.5) | IBS | Cross-sectional | 1° Care | China | Rome II | 11 |
| Masud25 | N/A | 593 (61.3) | IBS | Cross-sectional | Community | Bangladesh | Rome | 13 |
| Perveen56 | N/A | 116 (42.2) | IBS | Cross-sectional | Community | Bangladesh | Rome II | 10 |
| Ringel60 | N/A | 337 (70.9) | IBS | Cross-sectional | Community | USA | Modified Rome II | 11 |
| Schmulson59 | N/A | 295 (67.8) | IBS | Cross-sectional | Advertisement and 2° Care | Mexico | Rome II | 11 |
| Si34 | None | 662 (52.9) | IBS | Cross-sectional | 2° Care | China | Rome II | 15 |
| Smith35 | None | 97 (62.9) | IBS | Prospective | 2° Care | USA | Modified Manning | 9 |
| Thompson36 | None | 156 (83.3) | IBS | Cross-sectional | 1° and 2° Care | USA | 90.4% met Manning criteria and 68.6% met Rome criteria | 14 |
Abbreviations: QA: quality assessment; N/A: Not applicable; 1°: primary; 2°: secondary; 3°: tertiary
IBS diagnostic symptoms
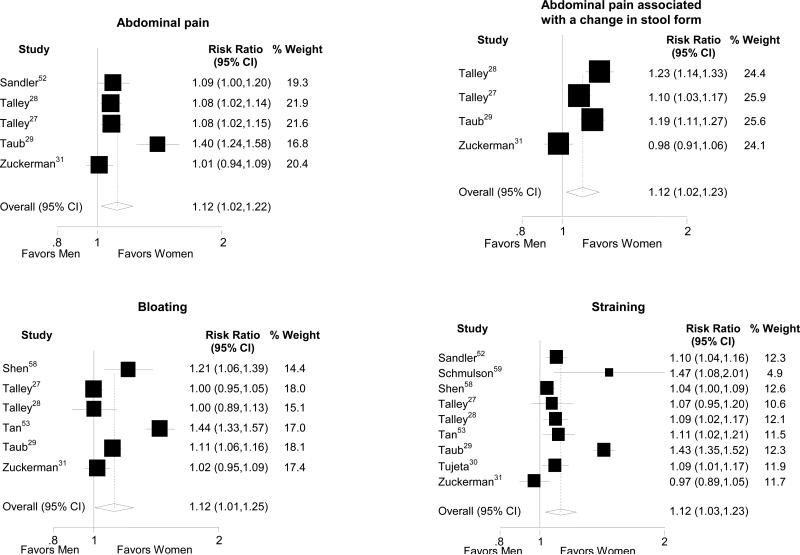
In the general population, women were more likely to report abdominal pain and pain-related IBS diagnostic symptoms (Table 3, Figure 3).27-29, 31, 34-36, 51, 58 In contrast, in the IBS patient population, the prevalence of the pain-related symptoms did not differ between men and women.
Table 3.
Relative risk (RR) of IBS symptoms in women versus men in both the general and IBS patient populations
| General Population | IBS Only | |||||
|---|---|---|---|---|---|---|
| RR favoring women (95% CI) | N of studies | Heterogeneity | RR favoring women (95% CI) | N of studies | Heterogeneity | |
| IBS Diagnostic Symptoms | ||||||
| Pain | 1.12 (1.02,1.22)* | 5 | P<0.001 | N/A | 1 | |
| Pain relieved with defecation | 1.13 (0.97,1.31) | 6 | P<0.001 | 1.00 (0.80,1.25) | 5 | P=0.012 |
| Pain associated with change in frequency | 1.08 (1.03,1.14)* | 6 | P=0.005 | N/A | 1 | |
| Pain associated with change in form | 1.12 (1.02,1.23)* | 6 | P<0.001 | 0.96 (0.82,1.12) | 4 | P=0.69 |
| Supportive Symptoms | ||||||
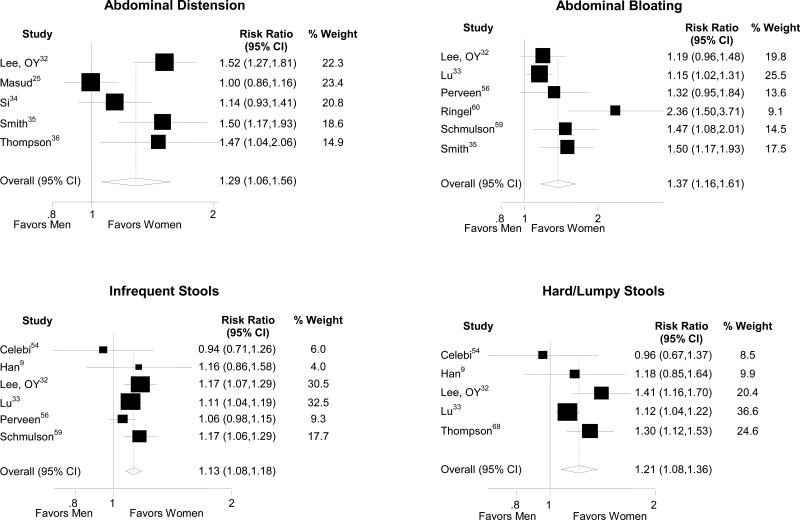
| Distension | N/A | 2 | 1.29 (1.06,1.56)* | 5 | P=0.002 | |
| Bloating | 1.12 (1.01,1.25)* | 9 | P<0.001 | 1.37 (1.16,1.61)* | 6 | P=0.028 |
| Incomplete Evacuation | 1.03 (0.93,1.13) | 6 | P<0.001 | 1.15 (0.97,1.37) | 7 | P=0.300 |
| Infrequent stools | 1.06 (0.99,1.14) | 5 | P<0.001 | 1.13 (1.08,1.18)* | 6 | P=0.387 |
| Lumpy/Hard stools | 0.98 (0.88,1.10) | 4 | P<0.001 | 1.21 (1.08,1.36)* | 5 | P=0.089 |
| Straining | 1.12 (1.03,1.23)* | 4 | P<0.001 | 1.01 (0.93,1.09) | 5 | P=0.284 |
| Mucus | 1.02 (0.97,1.07) | 5 | P<0.001 | 1.07 (0.96,1.19) | 8 | P=0.002 |
| Urgency | 1.02 (0.93,1.11) | 4 | P<0.001 | 1.04 (0.95,1.15) | 6 | P=0.249 |
| Loose/Watery stools | 0.97 (0.92,1.01) | 5 | P=0.002 | 0.84 (0.75,0.94)* | 5 | P=0.420 |
| Frequent stools | 1.00 (0.98,1.01) | 4 | P=0.635 | 0.88 (0.80,0.96)* | 5 | P=0.014 |
Abbreviations: RR: relative risk; CI: confidence interval; N= number; N/A: Not applicable due to insufficient number of studies to perform meta-analysis.
P < 0.05
Figure 3.
Forest plots of the risk ratios and 95% confidence intervals for gender differences in general population studies evaluating abdominal pain, bloating, and straining are shown.
Supportive symptoms
Overall, women were more likely to report supportive symptoms of IBS than men (Table 3). In the general population, gender differences were seen in predominantly constipation-related symptoms including abdominal distension, bloating, and straining (Figure 3). The greatest gender disparities were seen with bloating and pain. Similarly, women with IBS demonstrated considerably higher risk for constipation-related symptoms including abdominal distension, bloating, infrequent stools and hard stools than men with IBS (Figure 4). Men with IBS were significantly more likely to report the diarrhea-related symptoms of loose/watery stools and increased stool frequency than women with IBS (Table 3).
Figure 4.
Forest plots of the risk ratios and 95% confidence intervals for gender differences in IBS only studies evaluating bloating, distension, hard/lumpy stools, and infrequent stools are shown.
Assessment for publication bias
Only two symptom comparisons surveyed in the general population showed a potential publication bias. Funnel plots suggest a lack of small, positive studies for abdominal pain associated with change in stool frequency and a lack of small, negative studies for frequent stools.
Menstrual cycle effect on IBS symptoms
Table 4 provides general information on the 13 studies that assessed the effect of menstrual cycle on IBS symptoms.32, 37-45, 62, 63 Study methodology varied with regards to study design and determination of menstrual cycle phase with seven studies of good quality and five of intermediate quality. Six studies assessed symptoms prospectively using daily diaries, one surveyed current symptom severity score, and the remaining studies used symptom recall. Only two studies used ovulation kits to document menstrual cycle phase.
Table 4.
Evidence table of studies comparing menstrual cycle effect on IBS symptoms
| Study | Population (N) | Study Design | Setting | Menstrual Cycle Assessment | IBS diagnostic criteria | Findings | QA |
|---|---|---|---|---|---|---|---|
| Jackson41 | Healthy Controls (20) | Prospective (daily diary) | Not specified | Menses: day 1-4; Follicular: day 8-10 Luteal: day 18-20 Pre-menstrual: day 24-28 | N/A | Stool form was significantly looser at menses compared to luteal phase; There was not a significant phase effect on number of stools per day | 9 |
| Hinds62 | Healthy Controls (25) | Retrospective | Not specified | Recall | N/A | 96% reported change in bowel habit before and during menses; 72% reported loose stools during menses; 32% reported constipation the week prior to menses | 9 |
| Simmons44 | Healthy Controls (7) | Prospective (daily diary) | Not specified | Basal body temperature | N/A | No significant phase effect on stool form; 1 of 7 subjects reported constipation before or during menses; 2 of 7 subjects reported diarrhea at the beginning of menses | 11 |
| Lee, SY43 | GI Clinic patients (193) | Cross-sectional (survey) | 3° care | Menstrual: day 0-6; Proliferative: day 7-14; Secretory: after day 15 | Rome II | 50.8% of women met diagnostic criteria for IBS. No significant phase effect for abdominal pain, constipation, diarrhea, abdominal distension, tenesmus | 13 |
| Altman63 | IBS (114) | Prospective (daily diary) | 1° care and community | Menses: day 1; Luteal phase: 7-10 days prior to onset of menses | Diagnosis made by health care provider; criteria not specified | No significant phase effect on abdominal or stomach pain | 13 |
| Chang37 | IBS (380) | Retrospective | 3° care and community | Recall | Rome I | 40% of bloating patients and 43% of bloating + distension patients reported that bloating was related to menstrual cycle | 15 |
| Houghton40 | IBS (29) | Prospective (daily diary) | Not specified | Menses: day 1-4; Follicular: day 8-10; Luteal: day 18-20; Pre-menstrual: day 24-28 | Rome I | There were significant phase effects on abdominal pain, bloating, stool frequency, and loose stools | 11 |
| Lee, OY32 | IBS (477) | Retrospective | 3° care and community | Recall | Rome | Overall 40% of women and 50.8% of women <45 yrs of age reported menstrual cycle-related worsening of symptoms | 14 |
| Heitkemper38 | IBS (149) Healthy Controls (42) | Prospective (daily diary) | Community | Ovulation kit, Counting days | Rome I | Severity of bloating, and % days with loose stools (latter in IBS only) significantly increased during menses vs. other phases; No significant phase effect on abdominal pain, intestinal gas, constipation, diarrhea, or hard stools | 16 |
| Heitkemper39 | IBS (44) Healthy Controls (25) | Prospective (daily diary) | Community | Ovulation kit | Rome | There was a significant phase effect on bloating and stool consistency; There was no phase effect on abdominal pain, intestinal gas, or stool frequency | 15 |
| Houghton57 | IBS-D (39) Healthy Controls (19) | Cross-sectional | University clinic, general practices, advertiseme nt | Luteal phase (days 18-20 of cycle) or taking OCP were classified as High Progesterone/estrogen; Menses (days 2-3) was classified as Low Progesterone/estrogen: menses (days 2-3 of cycle) | Rome II | No differences in severity of pain, bloating, urgency, or overall symptoms between high and low progesterone/estrogen conditions in IBS-D women or healthy women. | 11 |
| Kane42 | IBS (46) Healthy Controls (90) | Retrospective | 2° care and community | Recall | Rome | Increased diarrhea was reported significantly more often during premenstrual period and menses in IBS vs. controls. Increased constipation was less often reported than diarrhea but was significantly more prevalent in IBS vs. controls during menses. There was a significant menstrual cycle effect on abdominal pain in IBS | 16 |
| Whitehead45 | IBS (72) Healthy Controls (234) | Retrospective | 1° and 2° care | Recall | Manning | IBS were more likely than controls to report menses-related increases in gas (39-48% vs. 14%), diarrhea (29-32% vs. 19%), and constipation (18-24% vs. 11%). | 11 |
Abbreviations: QA: quality assessment; GI: Gastrointestinal; N/A: Not applicable; 1°: primary; 2°: secondary; 3°: tertiary; OCP: oral contraceptive agent
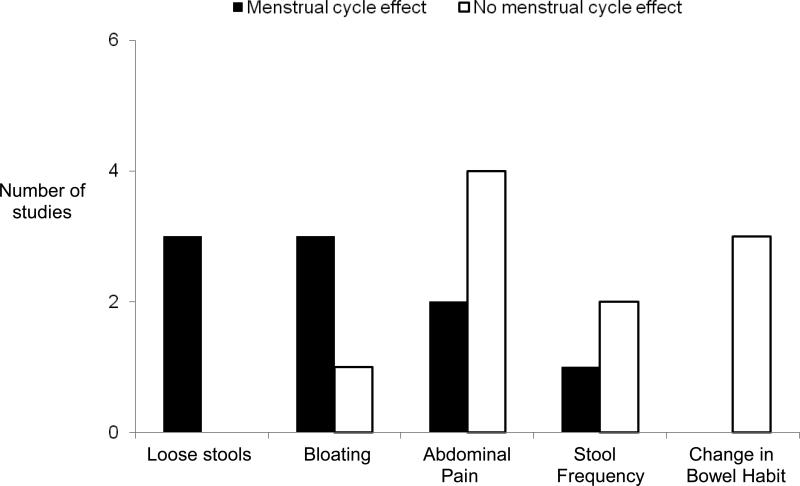
All but three studies stated that women (average 40-60%) reported increased GI symptoms at time of menses compared to other phases.32, 37-42, 44, 45, 62, 63 The symptoms for which most studies showed a significant menstrual cycle effect were (in descending order): loose stools, bloating, abdominal pain, stool frequency, and other changes in bowel habit (Figure 2, Table 4). In general, increased diarrhea was reported at the time of menses by more women than increased constipation. Although menstrual cycle effects on symptoms were similar in healthy women and IBS women, symptom severity was greater in women with IBS.
Figure 2.
The number of studies that tested for a significant effect of IBS symptoms on menstrual cycle is presented. The p-value of significance was specific to each study. One study was not included in the figure because statistical analysis of menstrual cycle effect on bloating was not performed.37 Another study was not included because it did not specifically evaluate a menstrual cycle effect on these symptoms.45
If only the seven higher quality studies were assessed, five reported a menstrual cycle effect on IBS symptoms at time of menses, while two did not. Bloating, gas and bowel habit changes (diarrhea more than constipation) were reported to increase at time of menses. Only 3 of these studies assessed symptoms prospectively and were from a single center.
Effect of menopausal status on IBS symptoms
Three retrospective and one prospective survey compared IBS symptoms in premenopausal vs. postmenopausal women32, 37, 46, 55 and were considered to be of high quality. Sample sizes for premenopausal women ranged from 58-89 subjects and postmenopausal women ranged from 55-170 subjects. Amongst the three studies restricted to IBS,32, 37, 55 nausea was the only symptom reported more frequently by premenopausal women than postmenopausal women.32 In the study by Cain et al.,55 various GI symptoms were reported less frequently by premenopausal women than postmenopausal women, however these differences were not significant after adjusting for age. Amongst healthy women, gaseousness and excessive flatulence were the only GI symptoms that were significantly more prevalent in postmenopausal women.
Effect of hormone supplementation on IBS symptoms
Table 5 shows the results from four studies which evaluated the effect of hormone supplementation on IBS symptoms in pre- or post-menopausal women. Two of the studies were randomized controlled trials; one evaluating the effect of estradiol or progesterone in postmenopausal women50 was of high quality, and the other evaluating a gondotropin-releasing hormone (GnRH) agonist in premenopausal women49 and was of intermediate quality. The other two studies were not randomized controlled trials. One consisted of a retrospective chart review48 and the other was a prospective study,39 but both achieved criteria for good quality.
Table 5.
Evidence table of studies comparing hormone supplement effect on IBS
| Study | Treatment groups (N) | Population | Menopausal status | Study Design | Age (yrs) | Setting | IBS diagnostic criteria | Symptoms | QA |
|---|---|---|---|---|---|---|---|---|---|
| Ruigomez48 | IBS HRT users (405); IBS HRT never users (255) | IBS | Not specified | Prospective | 50 -69 | 1° care | Not specified | IBS symptoms were similar among users of HRT and HRT never users (data not provided) | 15 |
| Palomba49 | Leuprolide acetate depot (LAD) plus tibolone (36); LAD plus placebo (37); Placebo (39) | IBS | Pre-menopausal | RCT | 25.5 (mean) | 2° care | Rome II | There was an improvement from baseline and vs. placebo in abdominal discomfort and distension, nausea, frequency and consistency, diarrhea, constipation, and bloating for both treatment groups | 17 |
| Gonenne50 | Placebo (12), Progesterone (13), Estradiol (12), Combined progesterone and Estradiol (12) | Controls | Post-menopausal | RCT | 40-65 | Not specified | N/A | Treatment effect of looser stool consistency scores with progesterone alone, estradiol alone, and progesterone + estradiol; Greater ease of passage scores for estradiol alone; No treatment effect on stool frequency, stool consistency, or sense of incomplete passage | 24 |
| Heitkemper38 | IBS oral contraceptive (56); IBS non-OCP (93) | IBS | Pre-menopausal | Prospective | 32.5 (mean) | General population | Rome I | OCP users reported less severe abdominal pain than non-OCP users but this difference did not maintain significance after multiple comparisons. There were no significant differences in other individual GI symptoms. | 16 |
Abbreviations: RCT: randomized controlled trial; 1°: primary; 2°: secondary; 3°: tertiary; HRT: hormone replacement therapy; OCP: oral contraceptive agent; N/A: not applicable
Two studies investigated the effect of hormone replacement therapy (HRT) on IBS symptoms in women over the age of 50 who were presumably postmenopausal.48, 50 Ruigomez et al.48 found that women who use HRT are more likely to develop IBS than women who do not; however, the prevalence and severity of IBS symptoms were similar among non-HRT users and HRT users. Gonenne et al.50 found that postmenopausal healthy women who were given estradiol or progesterone therapy alone for 7 days were more likely to have looser stools and greater ease of passage than those on placebo.
Two studies evaluated the effect of hormone supplementation on IBS symptoms in premenopausal women. One study assessed the effect of oral contraceptive pills (OCP), while the other assessed the effect of a GnRH agonist.38, 49 Heitkemper et al.38 found that OCP use by women with IBS was associated with lower abdominal pain severity compared to non-OCP users but this difference did not maintain significance after correcting for multiple comparisons. Palomba et al.49 found that treatment with a GnRH agonist improved the severity of IBS symptoms compared to placebo.
DISCUSSION
Due to conflicting data in the literature regarding gender differences in IBS, we performed a systematic review to investigate if there are differences in IBS symptoms between genders, and further evaluated the literature regarding the relationship between IBS symptoms, menstrual cycle phase, and menopause. Our study has four main findings: 1) women experience a greater prevalence of IBS symptoms than men, particularly constipation-related symptoms, 2) women appear to have more frequent and severe IBS symptoms during menses compared to other phases of the menstrual cycle, 3) the effect of hormonal therapy on IBS symptoms cannot be determined based on limited available data, and 4) there is a lack of studies comparing IBS symptoms in pre- and post-menopausal women.
For the most part, the occurrence and diagnosis of IBS is more common in women than men.6, 29, 64 Female gender is a significant independent risk factor for the development of IBS,65 including post-infectious IBS.66 In cross-sectional surveys conducted in the U.S.,67 Canada,68 and Israel,69 women reported IBS symptoms 1.5 to 2 times more commonly than men. Greater health care seeking and referral for IBS in women may in part be due to increased IBS severity32, 70, 71 and greater impact of symptoms on health related quality of life in women with IBS compared to men.70, 72, 73 However, population studies conducted in Asia suggest that the prevalence of IBS in men and women are similar.19, 74-76 This difference may be largely due to cultural differences. In Asia, men see physicians as much and more often than women possibly for cultural and economic reasons.76-79
Gender differences in IBS symptoms
Our meta-analysis reveals that women more frequently report individual IBS symptoms than men. In general population studies, which included individuals with IBS, women reported a greater prevalence of the IBS diagnostic (i.e., pain-related) symptoms than men. These findings are consistent with studies which showed an enhanced perception of pain or discomfort to distension in the colon and rectum in women vs. men.80, 81
Overall, women had a greater prevalence of constipation-associated symptoms, particularly bloating and abdominal distension, associated than men. Men had a greater prevalence of diarrhea-associated symptoms of loose/watery stools and increased stool frequency in the IBS only studies, but not in the general population studies. This is in line with studies demonstrating a female predominance in IBS-C67, 82, 83 and chronic constipation.82, 84 Several studies have found that women have slower colonic transit than men.85-87
The generally higher IBS symptom reporting in women than men may be due to several reasons. Since women with IBS tend to have significantly more healthcare visits than men,88 studies which used healthcare-based recruitment methods may underestimate the symptom prevalence in men. Another plausible explanation is that women tend to recall their symptoms better than men.89 However, this is not likely to explain why constipation-associated symptoms were reported more commonly in women than men, while diarrhea-associated symptoms were reported more often in men. Gender differences have been demonstrated in GI function, including transit time, visceral perception, brain activation patterns and colonic mucosal mast cell count12, 13, 81, 90, 91 which can conceivably contribute to the greater prevalence of IBS symptoms in women and the gender differences in bowel habits.
The majority of Western studies reported a higher prevalence of individual IBS symptoms in women compared to men.26, 27, 29, 30, 35, 36 Similarly, eight of the ten Eastern studies found that more women reported individual IBS symptoms than men,9, 31, 33, 34, 51, 53, 56, 58 however one of these studies also reported higher prevalences of loose stools and increased stool frequency in men.33 These studies were conducted mainly in community and university clinic populations, although one surveyed secondary clinic patients. Interestingly, two studies did not find a gender difference in individual symptoms but reported a higher ratio of women-to-men with Rome positive IBS (1.3-1.8:1).25, 54 One of these studies was conducted in a rural community in Bangladesh25 and the other in an urban community in Turkey.54 Thus, this meta-analysis supports a higher overall prevalence of IBS symptoms in women than men in Western and Eastern countries although the differences are relatively modest. This review overcomes the limitations of previous epidemiological investigations,9, 26-28, 30, 31, 33-36, 47, 92 because information from studies conducted in both Eastern and Western populations are included. However, additional studies are needed to investigate how the interactions between genetic, environmental and/or cultural factors contribute to gender differences in IBS symptoms and may differ across diverse cultures.
The relatively small, but significant gender differences in the prevalence of individual IBS symptoms suggests that gender effects may be confounded by other factors that significantly influence the presence of IBS symptoms. These include psychological and social factors that can affect symptom reporting, health care seeking and global outcomes in IBS.93 For example, studies have reported that a previous history of abuse and other traumatic events or stressors are associated with more severe pain and greater symptom severity in IBS.94-97
Limitations of our meta-analysis include the extensive heterogeneity of the studies, which may in part explain the low RR estimates. For example, the diagnostic criteria used for IBS varied between studies. This is notable because gender differences in the prevalence of IBS vary according to criteria used.98 In a population-based, cross-sectional survey study in Olmstead County, Minnesota, there was a greater prevalence of women with IBS if the Manning criteria were used, but a higher prevalence of men with IBS if the Rome criteria were used.99
Menstrual cycle effect on IBS symptoms
Our systematic review found that IBS symptoms are heightened at time of menses. Enhanced visceral perception at menses is supported by the finding of decreased sensory thresholds to rectal distension compared to other phases of the menstrual cycle.40 One plausible mechanism that is supported by some animal and human studies is that declining or low ovarian hormone levels at time of menses may underlie the increased GI symptoms and discomfort across the menstrual cycle.100 A study by Laessle and colleagues101 showed that progesterone levels negatively correlated with pain-related symptoms (i.e., back pain and headache) which supports that lower levels of female sex hormones are associated with greater somatic pain. It is conceivable that women have more prevalent and severe IBS symptoms at time of menses when progesterone (and estrogen) decline from high to low levels. There are other possible mechanisms involved in GI function to explain these findings. For example, gender differences in post-prandial serotonin levels exist in IBS-D102, 103 and in colonic mucosal mast cell counts in IBS.91 Both have been found to correlate with IBS symptoms and potentially a key role in the pathophysiology of IBS.104 Additionally, mast cell secretion is affected by both estrogen and progesterone.105
While more studies reported increased diarrhea at time of menses than increased constipation, there were two retrospective recall studies which found that some women reported increased diarrhea and others reported increased constipation (Table 4).42, 45 It is possible that an increase in a particular bowel habit would be reported by IBS patients with that predominant bowel subtype. For example, women with IBS-D may be more likely to report increased diarrhea than constipation, while IBS-C patients may be more likely to report increased constipation. However, this data was not available in the studies to determine if this could explain these findings.
Despite a relatively adequate number of studies, methodologic limitations in these studies could have affected the results. Half of the studies were based on symptom recall. Since women are likely to report a greater severity of symptoms retrospectively than prospectively,106 the retrospective studies may overestimate the effect of menstrual cycle on IBS symptoms and are less accurate than prospective assessment. In addition, confirmation of menstrual cycle phase should be performed using ovulation kits although this was only done in two of the 12 studies. A review of the effect of menstrual cycle phase on experimental pain response found a lack of standardized operational definitions for identifying menstrual cycle phases.107 More studies with an optimal study design (e.g., use of ovulation kits, prospective daily symptom assessment, sufficient sample sizes) are needed to truly assess the validity of the menstrual cycle effect on IBS symptoms.
Menopausal status effect on IBS symptoms
Due to the small number of studies that compared GI symptoms in pre- and post-menopausal women, there is insufficient evidence to determine the effect of menopausal status on IBS symptoms. However, the limited data suggest that premenopausal women with IBS are more likely to experience nausea than postmenopausal women with IBS. This finding is supported by studies which have shown that nausea and vomiting that occurred postoperatively,108, 109 and during pregnancy,110, 111 are associated with high levels estrogen and/or progesterone levels.
In one study, healthy post-menopausal women reported gas and excessive flatulence significantly more than pre-menopausal women.46 To our knowledge, there have not been studies that have compared intestinal gas clearance or colon transit times in pre- and post-menopausal women.
Effect of hormone supplement on IBS symptoms
The available studies provided limited information and therefore a conclusion on the effects of hormone supplementation cannot be definitively determined. However, the available evidence suggests that use of OCPs and a GnRH agonist by premenopausal women may be protective of IBS symptoms, although further studies are needed. These medications act differently on the hypothalamic-pituitary-gonadal axis. OCPs prevent ovulation because the progesterone derivative inhibits the release of GnRH by the hypothalamus thereby decreasing the release of follicular stimulating hormone (FSH) and luteinizing hormone (LH) by the anterior pituitary, and estrogen inhibits follicular development. In contrast, leuprolide, the GnRH agonist, stimulates the release of FSH and LH, which suppress the secretion of ovarian hormones. Undoubtedly, more studies are needed to conclusively determine the effect of hormone supplements on IBS symptoms.
Conclusion
Our study demonstrates that women overall have a greater prevalence of IBS symptoms than men, particularly those associated with constipation. However, within the IBS patient group, men have more diarrhea symptoms than women. There are some limitations in the quality, methodology and number of studies evaluating the effect of menstrual cycle, hormone supplementation and menopausal status on IBS symptoms. Notably, most studies relied on symptom recall. Nonetheless, existing data raise the possibility that there may be a female sex hormone effect on IBS symptoms as well as on GI function. It is plausible that this may contribute to the increased prevalence and greater vulnerability to develop IBS, the higher prevalence of constipation symptoms, and the increased severity of symptoms at time of menses in women. In addition to the need for more, well-designed studies, there should be a greater attempt to recruit adequate and comparable numbers of men and women with and without IBS and attention should be paid to assessing and controlling for menstrual cycle phase and menopausal status in clinical study design since they can potentially affect study results.
Acknowledgements
The authors would like to thank Melissa Alberto and Teresa Olivas for their assistance in the preparation of the manuscript.
Declaration of funding interests:
This study was funded by NIH grants # P50 DK64539 and R24 AT002681.
Abbreviations
- IBS
irritable bowel syndrome
- IBS-C
IBS with constipation
- IBS-D
IBS with diarrhea
- IBS-M
IBS with alternating bowel habits or mixed pattern
- RR
relative risk
- CI
confidence interval
- QA
quality assessment
- GnRH
gondotropin-releasing hormone
- HRT
hormone replacement therapy
- OCP
oral contraceptive agent
- RCT
randomized controlled trial
- 1°
primary
- 2°
secondary
- 3°
tertiary
- N/A
not applicable
Appendix
Appendix.
Down and Black checklist for measuring study quality.
| Assessment | Scoring |
|---|---|
| Reporting | |
| 1. Is the hypothesis/aim/objective of the study clearly described? | Yes=1 No=0 |
| 2. Are the main outcomes to be measured clearly described in the Introduction or Methods section? | Yes=1 No=0 |
| 3. Are the characteristics of the patients included in the study clearly described? | Yes=1 No=0 |
| 4. Are the interventions of interest clearly described? | Yes=1 No=0 |
| 5. Are the distributions of principal confounders in each group of subjects to be compared clearly described? | Yes =2 Partially=1 No =0 |
| 6. Are the main findings of the study clearly described? | Yes=1 No=0 |
| 7. Does the study provide estimates of the random variability in the data for the main outcomes? | Yes=1 No=0 |
| 8. Have all important adverse events that may be a consequence of the intervention been reported? | Yes=1 No=0 |
| 9. Have the characteristics of patients lost to follow-up been described? | Yes=1 No=0 |
| 10. Have actual probability values been reported (e.g. 0.035 rather than <0.05) for the main outcomes except where the probability value is less than 0.001? | Yes=1 No=0 |
| External validity | |
| 11. Were the subjects asked to participate in the study representative of the entire population from which they were recruited? | Yes=1 No=0 Unable to determine = 0 |
| 12. Were those subjects who were prepared to participate representative of the entire population from which they were recruited? | Yes=1 No=0 Unable to determine = 0 |
| 13. Were the staff, places, and facilities where the patients were treated, representative of the treatment the majority of patients receive? | Yes=1 No=0 Unable to determine = 0 |
| Internal validity – bias | |
| 14. Was an attempt made to blind study subjects to the intervention they have received? | Yes=1 No=0 Unable to determine = 0 |
| 15. Was an attempt made to blind those measuring the main outcomes of the intervention? | Yes=1 No=0 Unable to determine = 0 |
| 16. If any of the results of the study were based on “data dredging,” was this made clear? | Yes=1 No=0 Unable to determine = 0 |
| 17. In trials and cohort studies, do the analyses adjust for different lengths of follow-up of patients, or in case-control studies, is the time period between the intervention and outcome the same for cases and controls? | Yes=1 No=0 Unable to determine = 0 |
| 18. Were the statistical tests used to assess the main outcomes appropriate? | Yes=1 No=0 Unable to determine = 0 |
| 19. Was compliance with the intervention/s reliable? | Yes=1 No=0 Unable to determine = 0 |
| 20. Were the main outcome measures used accurate (valid and reliable)? | Yes=1 No=0 Unable to determine = 0 |
| Internal validity-confounding (selection bias) | |
| 21. Were the patients in different intervention groups (trials and cohort studies) or were the cases and controls (case-control studies) recruited from the same population? | Yes=1 No=0 Unable to determine = 0 |
| 22. Were study subjects in different intervention groups (trials and cohort studies) or were the cases and controls (case-control studies) recruited over the same period of time? | Yes=1 No=0 Unable to determine = 0 |
| 23. Were study subjects randomized to intervention groups? | Yes=1 No=0 Unable to determine = 0 |
| 24. Was the randomized intervention assignment concealed from both patients and health care staff until recruitment was complete and irrevocable? | Yes=1 No=0 Unable to determine = 0 |
| 25. Was there adequate adjustment for confounding in the analyses from which the main findings were drawn? | Yes=1 No=0 Unable to determine = 0 |
| 26. Were losses of patients to follow-up taken into account? | Yes=1 No=0 Unable to determine = 0 |
| Power | |
| 27. Did the study have sufficient power to detect a clinically important effect where the probability value for a difference being due to chance is less than 5%? | <n1=0 n1-n2=1 n3-n4=2 n5-n6=3 n7-n8=4 n8+=5 |
REFERENCES
- 1.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Kang JY. Systematic review: the influence of geography and ethnicity in irritable bowel syndrome. Aliment Pharmacol Ther. 2005;21:663–76. doi: 10.1111/j.1365-2036.2005.02396.x. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri M, Choi MG. Review article: Irritable bowel syndrome. Alimentary Pharmacology and Therapeutics. 1997;11:3–15. doi: 10.1046/j.1365-2036.1997.84256000.x. [DOI] [PubMed] [Google Scholar]
- 4.Talley NJ. Irritable bowel syndrome: definition, diagnosis and epidemiology. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:371–84. doi: 10.1053/bega.1999.0033. [DOI] [PubMed] [Google Scholar]
- 5.Thompson DG. GLP-1 and the gut. Gut. 2000;46:591. doi: 10.1136/gut.46.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller-Lissner SA, Bollani S, Brummer RJ, Coremans G, Dapoigny M, Marshall JK, Muris JW, Oberndorff-Klein Wolthuis A, Pace F, Rodrigo L, Stockbrugger R, Vatn MH. Epidemiological aspects of irritable bowel syndrome in Europe and North America. Digestion. 2001;64:200–4. doi: 10.1159/000048862. [DOI] [PubMed] [Google Scholar]
- 7.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 8.Husain N, Chaudhry IB, Jafri F, Niaz SK, Tomenson B, Creed F. A population-based study of irritable bowel syndrome in a non-Western population. Neurogastroenterol Motil. 2008;20:1022–9. doi: 10.1111/j.1365-2982.2008.01143.x. [DOI] [PubMed] [Google Scholar]
- 9.Han SH, Lee OY, Bae SC, Lee SH, Chang YK, Yang SY, Yoon BC, Choi HS, Hahm JS, Lee MH, Lee DH, Kim TH. Prevalence of irritable bowel syndrome in Korea: population-based survey using the Rome II criteria. J Gastroenterol Hepatol. 2006;21:1687–92. doi: 10.1111/j.1440-1746.2006.04269.x. [DOI] [PubMed] [Google Scholar]
- 10.Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123:1686–1701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- 11.Talley NJ, Zinsmeister AR, Melton Iii LJ. Irritable bowel syndrome in a community: symptom syubgroups, risk factors, and health care utilization. American Journal of Epidemiology. 1995;142:76–83. doi: 10.1093/oxfordjournals.aje.a117548. [DOI] [PubMed] [Google Scholar]
- 12.Viramontes BE, Camilleri M, McKinzie S, Pardi DS, Burton D, Thomforde GM. Gender-related differences in slowing colonic transit by a 5-HT 3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. American Journal of Gastroenterology. 2001;96:2671–2679. doi: 10.1111/j.1572-0241.2001.04138.x. [DOI] [PubMed] [Google Scholar]
- 13.Rao SS, Sadeghi P, Beaty J, Kavlock R, Ackerson K. Ambulatory 24-h colonic manometry in healthy humans. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2001;280:G629–G639. doi: 10.1152/ajpgi.2001.280.4.G629. [DOI] [PubMed] [Google Scholar]
- 14.Soffer EE, Kongara K, Achkar JP, Gannon J. Colonic motor function in humans is not affected by gender. Digestive Diseases and Sciences. 2000;45:1281–1284. doi: 10.1023/a:1005535432163. [DOI] [PubMed] [Google Scholar]
- 15.Drossman DA, Chey W, Panas R, Wahle A, Scott C, Ueno R. Lubiprostone significantly improves symptom relief rates in adults with irritable bowel syndrome and constipation (IBS-C): Data from two twelve week, randomized, placebo controlled double blind trials. Gastroenterology. 2007;132:2586–2587. [Google Scholar]
- 16.Chang L, Ameen VZ, Dukes GE, McSorley DJ, Carter EG, Mayer EA. A dose-ranging, phase II study of the efficacy and safety of alosetron in men with diarrhea-predominant IBS. American Journal of Gastroenterology. 2005;100:115–123. doi: 10.1111/j.1572-0241.2005.40365.x. [DOI] [PubMed] [Google Scholar]
- 17.Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545–55. doi: 10.1016/j.cgh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heitkemper MM, Chang L. Do fluctuations in ovarian hormones affect gastrointestinal symptoms in women with irritable bowel syndrome? Gend Med. 2009;6(Suppl 2):152–67. doi: 10.1016/j.genm.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gwee KA. Irritable bowel syndrome in developing countries - a disorder of civilization or colonization? Neurogastroenterology and Motility. 2005;17:317–324. doi: 10.1111/j.1365-2982.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 20.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMillan AM, Landorf KB, Barrett JT, Menz HB, Bird AR. Diagnostic imaging for chronic plantar heel pain: a systematic review and meta-analysis. J Foot Ankle Res. 2009;2:32. doi: 10.1186/1757-1146-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tonelli M, Wiebe N, Hemmelgarn B, Klarenbach S, Field C, Manns B, Thadhani R, Gill J. Trace elements in hemodialysis patients: a systematic review and meta-analysis. BMC Med. 2009;7:25. doi: 10.1186/1741-7015-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egger M, Altman DG, Smith GD. Systematic reviews in health care: meta-analysis in context. BMJ Publishing Group. 2001 [Google Scholar]
- 25.Masud MA, Hasan M, Khan AK. Irritable bowel syndrome in a rural community in Bangladesh: prevalence, symptoms pattern, and health care seeking behavior. Am J Gastroenterol. 2001;96:1547–52. doi: 10.1111/j.1572-0241.2001.03760.x. [DOI] [PubMed] [Google Scholar]
- 26.Bouchoucha M, Devroede G, Dorval E, Faye A, Arhan P, Arsac M. Different segmental transit times in patients with irritable bowel syndrome and “normal” colonic transit time: Is there a correlation with symptoms? Techniques in Coloproctology. 2006;10:287–296. doi: 10.1007/s10151-006-0295-9. [DOI] [PubMed] [Google Scholar]
- 27.Talley NJ, Zinsmeister AR, Van Dyke C, Melton LJ. Epidemiology of colonic symptoms and irritable bowel syndrome. Gastroenterology. 1991;101:927–934. doi: 10.1016/0016-5085(91)90717-y. [DOI] [PubMed] [Google Scholar]
- 28.Talley NJ, Boyce P, Jones M. Identification of distinct upper and lower gastrointestinal symptom groupings in an urban population. Gut. 1998;42:690–695. doi: 10.1136/gut.42.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taub E, Cuevas JL, Cook EW, Crowell M, Whitehead WE. Irritable bowel syndrome defined by factor analysis. Gender and race comparisons. Digestive Diseases and Sciences. 1995;40:2647–2655. doi: 10.1007/BF02220455. [DOI] [PubMed] [Google Scholar]
- 30.Tuteja AK, Talley NJ, Joos SK, Tolman KG, Hickam DH. Abdominal bloating in employed adults: prevalence, risk factors, and association with other bowel disorders. Am J Gastroenterol. 2008;103:1241–8. doi: 10.1111/j.1572-0241.2007.01755.x. [DOI] [PubMed] [Google Scholar]
- 31.Zuckerman MJ, Nguyen G, Ho H, Nguyen L, Gregory GG. A survey of irritable bowel syndrome in Vietnam using the Rome criteria. Dig Dis Sci. 2006;51:946–51. doi: 10.1007/s10620-005-9005-0. [DOI] [PubMed] [Google Scholar]
- 32.Lee OY, Mayer EA, Schmulson M, Chang L, Naliboff B. Gender-related differences in IBS symptoms. American Journal of Gastroenterology. 2001;96:2184–2193. doi: 10.1111/j.1572-0241.2001.03961.x. [DOI] [PubMed] [Google Scholar]
- 33.Lu CL, Chang FY, Lang HC, Chen CY, Luo JC, Lee SD. Gender difference on the symptoms, health-seeking behaviour, social impact and sleep quality in irritable bowel syndrome: a Rome II-based survey in an apparent healthy adult Chinese population in Taiwan. Aliment Pharmacol Ther. 2005;21:1497–505. doi: 10.1111/j.1365-2036.2005.02512.x. [DOI] [PubMed] [Google Scholar]
- 34.Si JM, Wang LJ, Chen SJ, Sun LM, Dai N. Irritable bowel syndrome consulters in Zhejiang province: the symptoms pattern, predominant bowel habit subgroups and quality of life. World J Gastroenterol. 2004;10:1059–64. doi: 10.3748/wjg.v10.i7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith RC, Greenbaum DS, Vancouver JB, Henry RC, Reinhart MA, Greenbaum RB, Dean HA, Mayle JE. Gender differences in Manning criteria in the irritable bowel syndrome. Gastroenterology. 1991;100:591–595. doi: 10.1016/0016-5085(91)80002-q. [DOI] [PubMed] [Google Scholar]
- 36.Thompson WG. Gender differences in irritable bowel syndromes. European Journal of Gastroenterology and Hepatology. 1997;9:299–302. doi: 10.1097/00042737-199703000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Chang L, Lee OY, Naliboff B, Schmulson M, Mayer EA. Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. American Journal of Gastroenterology. 2001;96:3341–3347. doi: 10.1111/j.1572-0241.2001.05336.x. [DOI] [PubMed] [Google Scholar]
- 38.Heitkemper MM, Cain KC, Jarrett ME, Burr RL, Hertig V, Bond EF. Symptoms across the menstrual cycle in women with irritable bowel syndrome. American Journal of Gastroenterology. 2003;98:420–430. doi: 10.1111/j.1572-0241.2003.07233.x. [DOI] [PubMed] [Google Scholar]
- 39.Heitkemper MM, Jarrett M, Cain KC, Shaver J, Walker E, Lewis L. Daily gastrointestinal symptoms in women with and without a diagnosis of IBS. Digestive Diseases and Sciences. 1995;40:1511–1519. doi: 10.1007/BF02285200. [DOI] [PubMed] [Google Scholar]
- 40.Houghton LA, Lea R, Jackson N, Whorwell PJ. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50:471–474. doi: 10.1136/gut.50.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson NA, Houghton LA, Whorwell PJ, Currer B. Does the menstrual cycle affect anorectal physiology? Digestive Diseases and Sciences. 1994;39:2607–2611. doi: 10.1007/BF02087697. [DOI] [PubMed] [Google Scholar]
- 42.Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93:1867–72. doi: 10.1111/j.1572-0241.1998.540_i.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee SY, Kim JH, Sung IK, Park HS, Jin CJ, Choe WH, Kwon SY, Lee CH, Choi KW. Irritable bowel syndrome is more common in women regardless of the menstrual phase: a Rome II-based survey. J Korean Med Sci. 2007;22:851–4. doi: 10.3346/jkms.2007.22.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simmons L, Heitkemper M, Shaver J. Gastrointestinal function during the menstrual cycle. Health Care Women Int. 1988;9:201–9. doi: 10.1080/07399338809515818. [DOI] [PubMed] [Google Scholar]
- 45.Whitehead WE, Cheskin LJ, Heller BR, Robinson JC, Crowell MD, Benjamin C, Schuster MM. Evidence for exacerbation of irritable bowel syndrome during menses. Gastroenterology. 1990;98:1485–1489. doi: 10.1016/0016-5085(90)91079-l. [DOI] [PubMed] [Google Scholar]
- 46.Triadafilopoulos G, Finlayson M, Grellet C. Bowel dysfunction in postmenopausal women. Women & Health. 1998;27:55–66. doi: 10.1300/J013v27n04_04. [DOI] [PubMed] [Google Scholar]
- 47.Barakzai MD, Gregory J, Fraser D. The effect of culture on symptom reporting: Hispanics and irritable bowel syndrome. J Am Acad Nurse Pract. 2007;19:261–7. doi: 10.1111/j.1745-7599.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 48.Ruigomez A, Garcia Rodriguez LA, Johansson S, Wallander MA. Is hormone replacement therapy associated with an increased risk of irritable bowel syndrome? Maturitas. 2003;44:133–40. doi: 10.1016/s0378-5122(02)00321-3. [DOI] [PubMed] [Google Scholar]
- 49.Palomba S, Orio F, Jr., Manguso F, Russo T, Falbo A, Lombardi G, Doldo P, Zullo F. Leuprolide acetate treatment with and without coadministration of tibolone in premenopausal women with menstrual cycle-related irritable bowel syndrome. Fertil Steril. 2005;83:1012–20. doi: 10.1016/j.fertnstert.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Gonenne J, Esfandyari T, Camilleri M, Burton DD, Stephens DA, Baxter KL, Zinsmeister AR, Bharucha AE. Effect of female sex hormone supplementation and withdrawal on gastrointestinal and colonic transit in postmenopausal women. Neurogastroenterol Motil. 2006;18:911–8. doi: 10.1111/j.1365-2982.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 51.Kim YJ, Ban DJ. Prevalence of irritable bowel syndrome, influence of lifestyle factors and bowel habits in Korean college students. Int J Nurs Stud. 2005;42:247–54. doi: 10.1016/j.ijnurstu.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 52.Sandler RS, Stewart WF, Liberman JN, Ricci JA, Zorich NL. Abdominal pain, bloating, and diarrhea in the United States: Prevalence and impact. Digestive Diseases and Sciences. 2000;45:1166–1171. doi: 10.1023/a:1005554103531. [DOI] [PubMed] [Google Scholar]
- 53.Tan YM, Goh KL, Muhidayah R, Ooi CL, Salem O. Prevalence of irritable bowel syndrome in young adult Malaysians: a survey among medical students. J Gastroenterol Hepatol. 2003;18:1412–6. doi: 10.1046/j.1440-1746.2003.03212.x. [DOI] [PubMed] [Google Scholar]
- 54.Celebi S, Acik Y, Deveci SE, Bahcecioglu IH, Ayar A, Demir A, Durukan P. Epidemiological features of irritable bowel syndrome in a Turkish urban society. J Gastroenterol Hepatol. 2004;19:738–43. doi: 10.1111/j.1440-1746.2004.03367.x. [DOI] [PubMed] [Google Scholar]
- 55.Cain KC, Jarrett ME, Burr RL, Rosen S, Hertig VL, Heitkemper MM. Gender differences in gastrointestinal, psychological, and somatic symptoms in irritable bowel syndrome. Dig Dis Sci. 2009;54:1542–9. doi: 10.1007/s10620-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perveen I, Hasan M, Masud MA, Bhuiyan MM, Rahman MM. Irritable bowel syndrome in a Bangladeshi urban community: prevalence and health care seeking pattern. Saudi J Gastroenterol. 2009;15:239–43. doi: 10.4103/1319-3767.56099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Houghton LA, Brown H, Atkinson W, Morris J, Fell C, Whorwell PJ, Lockhart S, Keevil B. 5-hydroxytryptamine signalling in irritable bowel syndrome with diarrhoea: effects of gender and menstrual status. Aliment Pharmacol Ther. 2009;30:919–29. doi: 10.1111/j.1365-2036.2009.04121.x. [DOI] [PubMed] [Google Scholar]
- 58.Shen L, Kong H, Hou X. Prevalence of irritable bowel syndrome and its relationship with psychological stress status in Chinese university students. J Gastroenterol Hepatol. 2009;24:1885–90. doi: 10.1111/j.1440-1746.2009.05943.x. [DOI] [PubMed] [Google Scholar]
- 59.Schmulson M, Adeyemo M, Gutierrez-Reyes G, Charua-Guindic L, Farfan-Labonne B, Ostrosky-Solis F, Diaz-Anzaldua A, Medina L, Chang L. Differences in gastrointestinal symptoms according to gender in Rome II positive IBS and dyspepsia in a Latin American population. Am J Gastroenterol. 105:925–32. doi: 10.1038/ajg.2010.58. [DOI] [PubMed] [Google Scholar]
- 60.Ringel Y, Williams RE, Kalilani L, Cook SF. Prevalence, characteristics, and impact of bloating symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:68–72. doi: 10.1016/j.cgh.2008.07.008. quiz 3. [DOI] [PubMed] [Google Scholar]
- 61.Landis JR, Koch GG. A review of statistical methods in the analysis of data arising from observer reliability studies, Parts I and II. Statistica Neerlandica. 1975;29:101–23. 151–61. [Google Scholar]
- 62.Hinds JP, Stoney B, Wald A. Does gender or the menstrual cycle affect colonic transit? American Journal of Gastroenterology. 1989;84:123–126. [PubMed] [Google Scholar]
- 63.Altman G, Cain KC, Motzer S, Jarrett M, Burr R, Heitkemper M. Increased symptoms in female IBS patients with dysmenorrhea and PMS. Gastroenterol Nurs. 2006;29:4–11. doi: 10.1097/00001610-200601000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Hungin AP, Chang L, Locke GR, Dennis EH, Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther. 2005;21:1365–75. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 65.Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. Irritable bowel syndrome: A 10-yr natural history of symptoms and factors that influence consultation behavior. Am J Gastroenterol. 2008 doi: 10.1111/j.1572-0241.2007.01740.x. [DOI] [PubMed] [Google Scholar]
- 66.Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: Postal survey of patients. British Medical Journal. 1997;314:779–782. doi: 10.1136/bmj.314.7083.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andrews EB, Eaton SC, Hollis KA, Hopkins JS, Ameen V, Hamm LR, Cook SF, Tennis P, Mangel AW. Prevalence and demographics of irritable bowel syndrome: results from a large web-based survey. Aliment Pharmacol Ther. 2005;22:935–42. doi: 10.1111/j.1365-2036.2005.02671.x. [DOI] [PubMed] [Google Scholar]
- 68.Thompson WG, Irvine EJ, Pare P, Ferrazzi S, Rance L. Functional gastrointestinal disorders in Canada: first population-based survey using Rome II criteria with suggestions for improving the questionnaire. Dig Dis Sci. 2002;47:225–35. doi: 10.1023/a:1013208713670. [DOI] [PubMed] [Google Scholar]
- 69.Sperber AD, Friger M, Shvartzman P, Abu-Rabia M, Abu-Rabia R, Abu-Rashid M, Albedour K, Alkranawi O, Eisenberg A, Kazanoviz A, Mazingar L, Fich A. Rates of functional bowel disorders among Israeli Bedouins in rural areas compared with those who moved to permanent towns. Clin Gastroenterol Hepatol. 2005;3:342–8. doi: 10.1016/s1542-3565(04)00553-1. [DOI] [PubMed] [Google Scholar]
- 70.Coffin B, Dapoigny M, Cloarec D, Comet D, Dyard F. Relationship between severity of symptoms and quality of life in 858 patients with irritable bowel syndrome. Gastroenterologie Clinique et Biologique. 2004;28:11–15. doi: 10.1016/s0399-8320(04)94834-8. [DOI] [PubMed] [Google Scholar]
- 71.Van der Horst VG, Holstege G. Sensory and motor components of reproductive behavior: pathways and plasticity. Behavioural Brain Research. 1998;92:157–167. doi: 10.1016/s0166-4328(97)00188-5. [DOI] [PubMed] [Google Scholar]
- 72.van der Horst HE, van Dulmen AM, Schellevis FG, van Eijk JT, Fennis JF, Bleijenberg G. Do patients with irritable bowel syndrome in primary care really differ from outpatients with irritable bowel syndrome? Gut. 1997;41:669–674. doi: 10.1136/gut.41.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simren M, Abrahamsson H, Svedlund J, Bjornsson ES. Quality of life in patients with irritable bowel syndrome seen in referral centers versus primary care: the impact of gender and predominant bowel pattern. Scandinavian Journal of Gastroenterology. 2001;36:545–552. doi: 10.1080/003655201750153476. [DOI] [PubMed] [Google Scholar]
- 74.Gwee KA, Wee S, Wong ML, Png DJ. The prevalence, symptom characteristics, and impact of irritable bowel syndrome in an asian urban community. Am J Gastroenterol. 2004;99:924–31. doi: 10.1111/j.1572-0241.2004.04161.x. [DOI] [PubMed] [Google Scholar]
- 75.Xiong LS, Chen MH, Chen HX, Xu AG, Wang WA, Hu PJ. A population-based epidemiologic study of irritable bowel syndrome in South China: stratified randomized study by cluster sampling. Aliment Pharmacol Ther. 2004;19:1217–24. doi: 10.1111/j.1365-2036.2004.01939.x. [DOI] [PubMed] [Google Scholar]
- 76.Lu CL, Chen CY, Lang HC, Luo JC, Wang SS, Chang FY, Lee SD. Current patterns of irritable bowel syndrome in Taiwan: The Rome II questionnaire on a Chinese population. Alimentary Pharmacology and Therapeutics. 2003;17:217–224. doi: 10.1046/j.1365-2036.2003.01711.x. [DOI] [PubMed] [Google Scholar]
- 77.Drossman DA, Weinland SR. Commentary: sociocultural factors in medicine and gastrointestinal research. Eur J Gastroenterol Hepatol. 2008;20:593–5. doi: 10.1097/MEG.0b013e3282f53a37. [DOI] [PubMed] [Google Scholar]
- 78.Jain AP, Gupta OP, Jajoo UN, Sidhwa HK. Clinical profile of irritable bowel syndrome at a rural based teaching hospital in central India. Journal of the Association of Physicians of India. 1991;39:385–386. [PubMed] [Google Scholar]
- 79.Pimparkar BD. Irritable colon syndrome. J Indian Med Assoc. 1970;54:95–103. [PubMed] [Google Scholar]
- 80.Ragnarsson G, Hallbook O, Bodemar G. Abdominal symptoms are not related to anorectal function in the irritable bowel syndrome. Scandinavian Journal of Gastroenterology. 1999;34:250–258. doi: 10.1080/00365529950173645. [DOI] [PubMed] [Google Scholar]
- 81.Chang L, Naliboff BD, Labus JS, Schmulson M, Lee OY, Olivas TI, Stains J, Mayer EA. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2006;291:R277–R284. doi: 10.1152/ajpregu.00729.2005. [DOI] [PubMed] [Google Scholar]
- 82.Jun DW, Park HY, Lee OY, Lee HL, Yoon BC, Choi HS, Hahm JS, Lee MH, Lee DH, Kee CS. A population-based study on bowel habits in a Korean community: prevalence of functional constipation and self-reported constipation. Dig Dis Sci. 2006;51:1471–7. doi: 10.1007/s10620-006-9087-3. [DOI] [PubMed] [Google Scholar]
- 83.Katsinelos P, Lazaraki G, Kountouras J, Paroutoglou G, Oikonomidou I, Mimidis K, Koutras C, Gelas G, Tziomalos K, Zavos C, Pilpilidis I, Chatzimavroudis G. Prevalence, bowel habit subtypes and medical care-seeking behaviour of patients with irritable bowel syndrome in Northern Greece. Eur J Gastroenterol Hepatol. 2009;21:183–9. doi: 10.1097/MEG.0b013e328312eb97. [DOI] [PubMed] [Google Scholar]
- 84.Pare P, Ferrazzi S, Thompson WG, Irvine EJ, Rance L. An epidemiological survey of constipation in Canada: definitions, rates, demographics, and predictors of health care seeking. American Journal of Gastroenterology. 2001;96:3130–3137. doi: 10.1111/j.1572-0241.2001.05259.x. [DOI] [PubMed] [Google Scholar]
- 85.Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40–47. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 86.Meier R, Beglinger C, Dederding JP, Meyer-Wyss B, Fumagali M, Rowedder A, Turberg Y, Brignoli R. Influence of age, gender, hormonal status and smoking habits on colonic transit time. Neurogastroenterology and Motility. 1995;7:235–238. doi: 10.1111/j.1365-2982.1995.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 87.Lampe JW, Fredstrom SB, Slavin JC, Potter JD. Sex differences in colonic function: a randomised trial. Gut. 1993;34:531–536. doi: 10.1136/gut.34.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shih YC, Barghout VE, Sandler RS, Jhingran P, Sasane M, Cook S, Gibbons DC, Halpern M. Resource utilization associated with irritable bowel syndrome in the United States 1987-1997. Dig Dis Sci. 2002;47:1705–15. doi: 10.1023/a:1016471923384. [DOI] [PubMed] [Google Scholar]
- 89.Jonsson B, Gardsell P, Johnell O, Redlund-Johnell I, Sernbo I. Remembering fractures: fracture registration and proband recall in southern Sweden. J Epidemiol Community Health. 1994;48:489–90. doi: 10.1136/jech.48.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Naliboff BD, Mayer EA. Brain imaging in IBS: Drawing the line between cognitive and non-cognitive processes. Gastroenterology. 2006 doi: 10.1053/j.gastro.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 91.Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 92.Lee OY, Mayer EA, Schmulson M, Chang L, Naliboff B. Gender-related differences in IBS symptoms. Am J Gastroenterol. 2001;96:2184–93. doi: 10.1111/j.1572-0241.2001.03961.x. [DOI] [PubMed] [Google Scholar]
- 93.Levy RL, Olden KW, Naliboff BD, Bradley LA, Francisconi C, Drossman DA, Creed F. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology. 2006;130:1447–58. doi: 10.1053/j.gastro.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 94.Drossman DA, Talley NJ, Leserman J, Olden KW, Barreiro MA. Sexual and physical abuse and gastrointestinal illness. Review and recommendations. Ann Intern Med. 1995;123:782–94. doi: 10.7326/0003-4819-123-10-199511150-00007. [DOI] [PubMed] [Google Scholar]
- 95.Drossman DA, Li Z, Leserman J, Toomey TC, Hu YJ. Health status by gastrointestinal diagnosis and abuse history. Gastroenterology. 1996;110:999–1007. doi: 10.1053/gast.1996.v110.pm8613034. [DOI] [PubMed] [Google Scholar]
- 96.Drossman DA, Leserman J, Li Z, Keefe F, Hu YJ, Toomey TC. Effects of coping on health outcome among women with gastrointestinal disorders. Psychosomatic Medicine. 2000;62:309–317. doi: 10.1097/00006842-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 97.Koloski NA, Talley NJ, Boyce PM. Predictors of health care seeking for irritable bowel syndrome and nonulcer dyspepsia: a critical review of the literature on symptom and psychosocial factors. Am J Gastroenterol. 2001;96:1340–9. doi: 10.1111/j.1572-0241.2001.03789.x. [DOI] [PubMed] [Google Scholar]
- 98.Sperber AD, Shvartzman P, Friger M, Fich A. A comparative reappraisal of the Rome II and Rome III diagnostic criteria: are we getting closer to the ‘true’ prevalence of irritable bowel syndrome? Eur J Gastroenterol Hepatol. 2007;19:441–7. doi: 10.1097/MEG.0b013e32801140e2. [DOI] [PubMed] [Google Scholar]
- 99.Saito YA, Locke GR, Talley NJ, Zinsmeister AR, Fett SL, Melton LJ., 3rd A comparison of the Rome and Manning criteria for case identification in epidemiological investigations of irritable bowel syndrome. American Journal of Gastroenterology. 2000;95:2816–2824. doi: 10.1111/j.1572-0241.2000.03192.x. [DOI] [PubMed] [Google Scholar]
- 100.Heitkemper M, Chang L. Do fluctuations in ovarian hormones affect gastrointestinal symptoms in women with irritable bowel syndrome? Gender Medicine. 2009 doi: 10.1016/j.genm.2009.03.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laessle RG, Tuschl RJ, Schweiger U, Pirke KM. Mood changes and physical complaints during the normal menstrual cycle in healthy young women. Psychoneuroendocrinology. 1990;15:131–8. doi: 10.1016/0306-4530(90)90021-z. [DOI] [PubMed] [Google Scholar]
- 102.Li TJ, Yu BP, Dong WG, Luo HS, Xu L, Li MQ. Ovarian hormone modulates 5-hydroxytryptamine 3 receptors mRNA expression in rat colon with restraint stress-induced bowel dysfunction. World J Gastroenterol. 2004;10:2723–6. doi: 10.3748/wjg.v10.i18.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Houghton LA, Perry H, Morris J, Whorwell PJ, Keevil B. Plasma 5-Hydroxytryptamine concentration varies with gender and menstrual status in irritable bowel syndrome patients with diarrhoea (IBS-D) but not healthy volunteers. Gastroenterology. 2008;134:A-681. [Google Scholar]
- 104.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 105.Vasiadi M, Kempuraj D, Boucher W, Kalogeromitros D, Theoharides TC. Progesterone inhibits mast cell secretion. Int J Immunopathol Pharmacol. 2006;19:787–94. doi: 10.1177/039463200601900408. [DOI] [PubMed] [Google Scholar]
- 106.Koren G, Maltepe C, Navioz Y, Wolpin J. Recall bias of the symptoms of nausea and vomiting of pregnancy. Am J Obstet Gynecol. 2004;190:485–8. doi: 10.1016/j.ajog.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 107.Sherman JJ, LeResche L. Does experimental pain response vary across the menstrual cycle? A methodological review. Am.J Physiol Regul.Integr.Comp Physiol. 2006;291:R245–R256. doi: 10.1152/ajpregu.00920.2005. [DOI] [PubMed] [Google Scholar]
- 108.Lagiou P, Tamimi R, Mucci LA, Trichopoulos D, Adami HO, Hsieh CC. Nausea and vomiting in pregnancy in relation to prolactin, estrogens, and progesterone: a prospective study. Obstet Gynecol. 2003;101:639–44. doi: 10.1016/s0029-7844(02)02730-8. [DOI] [PubMed] [Google Scholar]
- 109.Fujii Y. [Postoperative nausea and vomiting and their sex differences]. Masui. 2009;58:59–66. [PubMed] [Google Scholar]
- 110.Brandes JM. First-trimester nausea and vomiting as related to outcome of pregnancy. Obstet Gynecol. 1967;30:427–31. [PubMed] [Google Scholar]
- 111.Klebanoff MA, Koslowe PA, Kaslow R, Rhoads GG. Epidemiology of vomiting in early pregnancy. Obstet Gynecol. 1985;66:612–6. [PubMed] [Google Scholar]