Abstract
The often studied stretch reflex is fundamental to the involuntary control of posture and movement. Nevertheless, there remains controversy regarding its functional role. Many studies have demonstrated that stretch reflexes can be modulated in a task appropriate manner. This review focuses on modulation of the long latency stretch reflex, thought to be mediated, at least in part, by supraspinal pathways. For example, this component of the stretch reflex increases in magnitude during interactions with compliant environments, relative to the sensitivity during interactions with rigid environments. This suggests that reflex sensitivity increases to augment limb stability when that stability is not provided by the environment. However, not all results support the stabilizing role of stretch reflexes. Some studies have demonstrated that involuntary responses within the time period corresponding to the long latency reflex can destabilize limb posture. We propose that this debate stems from the fact that multiple perturbation-sensitive pathways can contribute to the long latency stretch reflex and that these pathways have separate functional roles. The presented studies suggest that neural activity occurring within the period normally ascribed to the long latency stretch reflex is highly adaptable to current task demands and possibly should be considered more intelligent than “reflexive.”
Keywords: stretch reflex, long latency, adaptation, impedance, stiffness
Introduction
Observations of stretch reflexes, rapid excitatory responses of a muscle following stretch, were reported as early as 1751 by Robert Whytt (Pearce 1997). Since that time it has been revealed that the stretch reflex is a complex muscle reaction, with multiple excitatory responses occurring at different latencies following a muscle stretch (Hammond 1955). In the human upper limb for example, stretching the biceps brachii produces a ‘short latency’ response in the same muscle beginning approximately 20 ms after the onset of stretch, and a ‘longer latency’ response beginning around 50 ms after stretch onset (Hammond 1955; Marsden et al. 1972). Both short and longer latency responses are generally regarded as involuntary actions since they occur prior to the fastest voluntary reaction, which in the biceps brachii has been shown to begin 90-100 ms following an auditory or proprioceptive ‘go’ signal (Hammond 1956).
Though usually considered to be involuntary, the behavior of both short and long latency stretch reflexes can be modulated in a task dependent manner. This modulation has led to much debate regarding the functional role of these fundamental responses. There is strong evidence from decerebrate animal preparations that the shortest latency stretch reflexes, mediated by the spinal cord, serve to compensate for muscle nonlinearities and regulate muscle stiffness over a wide range of operating conditions (Nichols and Houk 1976; Hoffer and Andreassen 1981). In these studies, stretch reflexes generally augment the intrinsic properties of a muscle to oppose external perturbations of muscle length, thereby increasing stability of the musculoskeletal system. While there also are ample data supporting contributions of the short latency stretch reflex to stiffness regulation in humans, the role of longer latency stretch reflexes is less clear. Longer latency reflexes also have been reported to contribute to limb stiffness and stability, but counter examples have been provided in which these involuntary actions appear to destabilize limb posture (see review by Hasan 2005). We propose that these apparently contradictory results arise largely from the fact that multiple pathways can contribute to perturbation-evoked muscle activity occurring in the period corresponding to the long latency stretch reflex, and the attempt to ascribe a single functional role to these multiple pathways. This review summarizes literature supporting this proposal, and describes conditions under which the role of the long latency stretch reflex is consistent with the regulation of limb stiffness and stability.
Pathways mediating the stretch reflex
Liddell and Sherrington (1924) first described the neural pathway mediating the stretch reflex in the decerebrate cat. They described a pathway with a single synapse in the spinal cord separating the Ia afferent fiber from the homonymous α motoneuron. This monosynaptic pathway is considered to be a major contributor to the short latency component of the stretch reflex observed in human studies (Magladery et al. 1951; Burke et al. 1984).
In contrast to the short latency stretch reflex, there is less certainty regarding the pathways mediating longer latency stretch reflexes. Part of this uncertainty arises from the generic use of the term ‘long latency stretch reflex,” which is used to describe a wide range of perturbation-elicited responses occurring after the shortest latency response. Lee and Tatton (1975) parceled the components of the stretch reflex according to the time at which they occurred after perturbation onset; M1 was used to denote the initial short latency response, while M2 and M3 were used to describe later responses. Other studies have used different terminology to describe these short, medium and long latency components of the stretch reflex. While these distinctions based on latency have proven useful in the study of specific joints and experimental conditions, it is difficult to define a unique set of latencies that can be used to describe results across studies (Jacobs and Horak 2007) since these three potential components of the stretch reflex are not always present (Lenz et al. 1983), and the latency at which they occur varies across subjects, joints and conditions. As such, we will refer simply to short latency and long latency components of the stretch reflex in this review. Below, we consider the various pathways thought to contribute to long latency stretch reflexes, with the understanding that these contributions will often be separated according to latency and complexity, representing a continuum between rapid ‘reflexive’ responses and intelligent voluntary control, the distinction between which can be difficult to discern (Prochazka et al. 2000).
When Hammond (1956) first reported on stretch reflex behavior in the human upper limb, he suggested that the long latency component could be due either to the activation of slow afferent fibers originating in the stretched muscle or to the action of a longer reflex pathway carrying sensory information from Ia afferents to supraspinal structures. In the thirty years following Hammond’s report, data were presented to support each of the two theories, suggesting that both may contribute to the final common pathway within the time period corresponding to the long latency stretch reflex. Slower conducting afferents have been shown to contribute to longer latency reflexes in the lower limb (Corna et al. 1995; Grey et al. 2001). These conclusions were based largely on the effects of tizanidine, an α2 agonist that depresses transmission from group II, but not group I, muscle spindle afferents (Bras et al. 1989; Skoog 1996). Evidence for which afferents contribute to the long latency stretch reflex in the upper limb is less somewhat less clear since contradictory evidence has been provided (see review by Matthews 1991). However, recent evidence suggests that both group Ia and group II afferents likely contribute to this response (Lourenco et al. 2006).
There does appear to be a reasonable consensus that the portion of the long latency reflex attributable to transmission along Ia afferent fibers is at least partially mediated by the cortex (Matthews 1991). The ascending limb of this transcortical reflex has been defined by observations that, in the monkey, fast muscle afferents project to area 3a within the primary sensory cortex (for a review see Jones and Porter 1980) and that neurons within area 3a project directly to the primary motor cortex (Jones et al. 1978; Ghosh et al. 1987; Huerta and Pons 1990). Microstimulation studies in the cat and monkey have also provided evidence that the motor cortex receives sensory input directly from the thalamus (Asanuma et al. 1979; Asanuma et al. 1979). The extent to which each of these pathways is involved in the generation of long-latency stretch reflex responses has not yet been determined. The descending limb of the transcortical reflex loop is formed by pyramidal tract neurons in the primary motor cortex (area 4) that project monosynaptically to spinal motoneurons (Bernard et al. 1953; Landgren et al. 1962). Some of the most convincing physiological evidence for cortical involvement in the long latency stretch reflex pathway comes from recordings of activity within corticomotoneuronal cells in non-human primates (Evarts 1973; Cheney and Fetz 1984). These studies demonstrate that cells descending from the motor cortex to synapse monosynaptically on motoneurons, show enhanced activity following limb perturbations and prior to the reflexive excitation of the stretched muscle (Figure 1).
Figure 1.
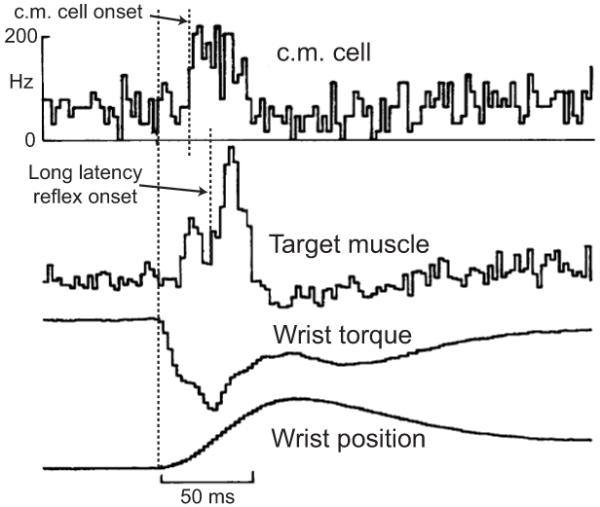
Recordings made from corticomotorneuronal (c.m.) cells demonstrating activity in those cells following a wrist torque perturbation and immediately prior to the expression of the long latency reflex in the stretched muscle. Modified with permission from S Karger AG, Basel (Cheney and Fetz 1984).
Evidence supporting the idea that the long latency stretch reflex is mediated by a transcortical pathway has also been produced in humans. For example, cortical electroencephalographic potentials have been recorded in humans immediately prior to long latency stretch reflex responses elicited by wrist perturbations (MacKinnon et al. 2000), and the amplitude of these potentials has been shown to vary with the velocity of the perturbation in a similar manner to the muscular reflex (Abbruzzese et al. 1985). Transcranial magnetic stimulation (TMS) over motor cortical areas also has been shown to influence the behavior of longer latency stretch reflexes (Palmer and Ashby 1992; Petersen et al. 1998), again suggesting that they are mediated at least in part by the motor cortex. Evidence of motor cortical involvement in the long-latency reflex response holds particular interest in relation to the regulation of posture and movement since it provides a neural basis for context-dependent modulation of our most rapid responses to perturbations or errors.
Brainstem pathways may also contribute to long latency stretch reflexes. The brainstem is known to play an important role in the automatic postural responses that occur following perturbations of whole body posture in standing animals (Lyalka et al. 2005; Honeycutt et al. 2009), or even to the rapid corrective responses following perturbations of an individual limb during standing (Stapley and Drew 2009). It has been suggested that the brainstem plays an important role in the initial response to perturbations of whole body posture, and that the specifics of that role may change according to appropriate priming from the cortex (Jacobs and Horak 2007). Presently, little is known about the role that the brainstem plays in response to perturbations that do not directly compromise whole body posture.
Supraspinal regulation of the stretch reflex
Modulation of short latency stretch reflexes
The traditional view of the short latency stretch reflex as stereotyped and unreceptive to adaptation based on changes in cognition such as intention or learning has been challenged by evidence that its amplitude can be altered according to anticipation of an expected stimulus or voluntary action. While there are many tasks in which the short latency stretch reflex displays limited flexibility, it has been shown that in some tasks the amplitude of both the short latency stretch reflex and the H-reflex can be altered hundreds of milliseconds before a prepared action (Kots 1977). The earliest changes in reflex sensitivity that occur prior to an expected voluntary action appear to be linked to individuals’ perception of the task environment, whereas closer to the initiation of action, reflex amplitudes are tightly coupled to the role of each muscle in the upcoming action (Kots 1977). The idea that we are capable of adjusting our state of preparedness in a manner appropriate for specific anticipated events has been referred to as preparatory ‘set’ (Prochazka 1989). A potential mechanism for regulating preparatory set has also been identified with demonstrations that γ-motoneurons controlling muscle spindle activity are not always coactivated with α-motoneurons driving activation of extrafusal fibers of the same muscle (Taylor and Cody 1974; Goodwin and Luschei 1975; Prochazka et al. 1976; Loeb and Duysens 1979). The idea of ‘fusimotor set’ (Prochazka 1983; Prochazka 1989) suggests that changes in fusimotor activity, independent of extrafusal muscle activation, can be used to regulate the sensitivity of muscle spindles in a manner that is appropriate for a prepared task or expected stimulus. In support of this idea, Ludvig, Cathers and Kearney (2007) recently demonstrated that humans are capable of voluntarily modulating the sensitivity of the short latency stretch reflex rapidly and in the absence of changes in extrafusal muscle activation. In the same experiment they also showed that changes in stretch reflex sensitivity produced concomitant changes in joint stiffness. Evidence for rapid modulation of short latency reflexes in accordance with changing task goals also has recently been presented (Mutha et al. 2008).
Although it is clear that short latency stretch reflexes can be modulated by voluntarily, presumably via supraspinal mechanisms, there is a wide range of tasks for which they have been demonstrated to remain constant as long as the tonic activity to the motoneuron pool remains fixed (Doemges and Rack 1992; MacKinnon et al. 2000; Lewis et al. 2006; Kurtzer et al. 2008; Pruszynski et al. 2008). Such tasks represent an excellent model within which to examine the potential for independent modulation of the long latency stretch reflex.
Modulation of long latency stretch reflexes
The transcortical pathway contributing to the long latency stretch reflex provides another opportunity for modulating the amplitude and duration of this response in a task dependent manner. For example, several investigators have demonstrated that the amplitude of long latency stretch reflexes is dependent upon the mechanical properties of the environment with which our limbs interact (Doemges and Rack 1992; Doemges and Rack 1992; Dietz et al. 1994; Perreault et al. 2008). When perturbations are induced at a single joint, changes in the relative stability of the environment induce concomitant changes in the sensitivity of the long latency stretch reflex response such that reflex amplitudes are larger when individuals interact with compliant, as compared to stiff, mechanical interfaces (Doemges and Rack 1992; Doemges and Rack 1992; Dietz et al. 1994). These changes in reflex gain occur despite the level of tonic activity in the stretched muscles being held constant. These reported changes in the sensitivity of the long-latency stretch reflex without corresponding changes in either tonic levels of muscle activity or the amplitude of the short latency stretch reflex is consistent with the proposal that changes in reflex gain can be induced by supraspinal structures. Furthermore, the reported increases in reflex sensitivity during interactions with more compliant environments strongly suggest that an important role of this reflex is to regulate the stability of the limb when that stability is not provided by the environment.
An alternative role of the long latency stretch reflex is that it generates a pattern of muscle activity appropriate for prepared volitional movements (Hasan 2005). This view stems from observations that the amplitude of the long latency stretch reflex is altered by changes in how a subject is instructed respond to limb perturbations (Hammond 1956). It has been suggested, for example, that changes in the size of the long-latency response prior to movement are the result of prepared voluntary actions being released early by external stimuli (when eliciting reflexes the most likely trigger is the limb perturbation itself, although auditory stimuli are also possible contributors) and superimposed on the long-latency stretch response that would be present in the absence of any motor plan (Crago et al. 1976). Triggered actions can occur as early as 70 ms after a stretch of the biceps brachii when individuals are instructed to respond to a limb perturbation, regardless of whether the muscle of interest is stretched or not (Crago et al. 1976; Koshland and Hasan 2000). The latency of the triggered muscle activity is similar to that of long latency responses observed when subjects do not respond to a limb perturbation. The superposition of triggered actions on transcortical long latency stretch responses results in an increase or decrease in the total response observed, depending on whether the instruction was to oppose or assist the perturbation respectively (Koshland and Hasan 2000). Indeed, it has recently been demonstrated that in an upper limb task in which limb perturbations were applied immediately prior to movements in each of four directions, the muscle activity recorded within a window traditionally considered to reflect long latency stretch reflexes was modulated in a manner consistent with the subsequent voluntary muscle activation in each movement (Pruszynski et al. 2008). Together, these results support the idea that prepared patterns of voluntary muscle activity can be released earlier than usual by limb perturbations, as has been reported for auditory stimuli (Valls-Sole et al. 1999). The relative efficacy of stimuli in different sensory modalities for hastening voluntary actions has not yet been investigated.
Given that muscle activity recorded in the time period corresponding to the long latency stretch reflex may contribute to both the regulation of limb mechanics and the early release of a preplanned motor action, it is conceivable that each of these roles is subserved by a different neural substrate. While cortical and subcortical elements may contribute to long latency reflexes in humans, as described above, the role of the cortex seems to change with task. Specifically, its role in reflex regulation appears to differ between postural or precision tasks and those involving pre-planned ballistic movements, similar to the “resist” instructions used in many reflex studies (e.g. Crago et al. 1976; Lewis et al. 2006). Early evidence for this dual role of the motor cortex came from the work of Evarts and Fromm (1978), who demonstrated that pyramidal tract neurons activated by elbow perturbations are modulated more by postural perturbations delivered during small precision movements, as needed for the fine control of posture, than those delivered immediately prior to ballistic movements (Fig. 2). A lack of cortical involvement in reflex modulation during ballistic tasks also has been noted in human studies (MacKinnon et al. 2000; Lewis et al. 2006) using both TMS and electroencephalograms. These results suggest that neurons within the primary motor cortex regulate the gain of the long latency reflex during tasks in which feedback control is critical while a second supraspinal structure issues feedforward commands to initiate prepared actions, and these actions may be triggered by external stimuli. It seems likely that commands from the two sources may summate as they converge on the final common pathway that is the α-motoneuron, and that the contribution of each pathway to the net response will depend on the specific task being performed.
Figure 2.
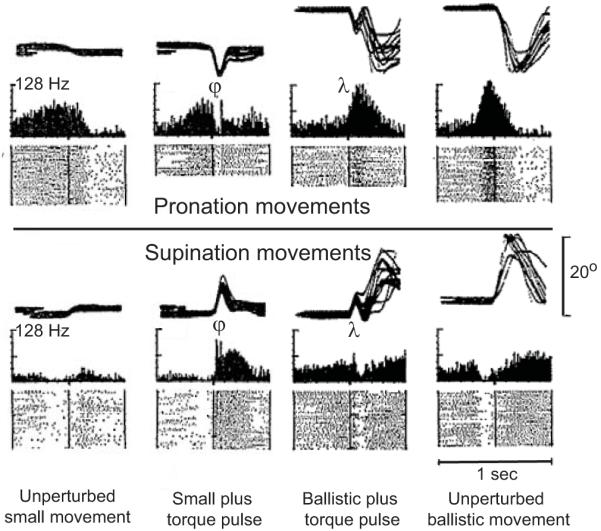
Recordings of pyramidal tract neurons during small corrective pronation/supination movements and larger ballistic movements. Joint position traces, neural histograms and rasters of unit discharge are shown for each condition. Responses are shown with and without the addition of a perturbing torque pulse applied at the onset of the small corrective movements or immediately prior to the ballistic movements. Larger responses (both excitatory and inhibitory) are observable when the perturbation is applied as the monkey makes a small forearm movement (ϕ) compared to responses recorded immediately prior to ballistic movements (λ). Modified with permission from Elsevier B.V., Amsterdam (Evarts and Fromm (1978)).
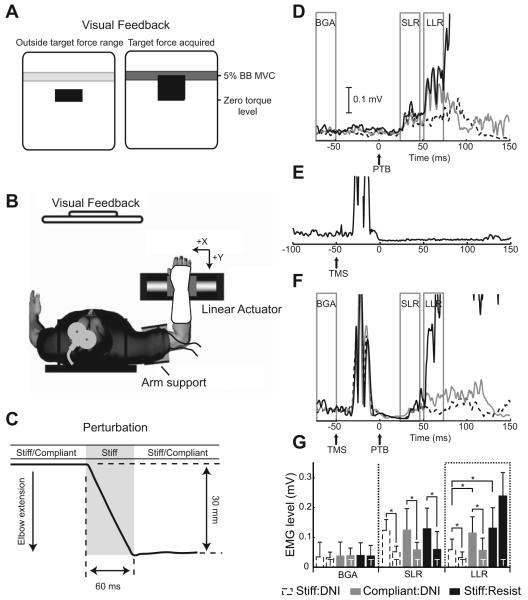
Recent evidence from our laboratory supports the idea that the motor cortex is principally responsible for regulating the gain of the long latency stretch reflex during changes in environmental stability, but is not involved in the release of previously planned motor actions (Shemmell et al. 2009). In this experiment, stretch reflexes were assessed as participants interacted with stiff and compliant haptic environments. Subjects were instructed to maintain a constant level of elbow flexion torque, assisted by visual feedback (Fig. 3A). When the target torque was reached, the elbow was rapidly extended using a linear servo motor (Fig. 3B). Identical perturbations were applied within each mechanical environment (Fig. 3C). When subjects were instructed to not intervene with the perturbation (DNI), long latency reflexes were increased during interactions with the compliant environment (Compliant:DNI) relative to those elicited during interactions with the stiff environment (Stiff:DNI), as shown in Fig. 3D. A similar enhancement of long latency reflex sensitivity was observed when subjects were instructed to resist the imposed displacement as rapidly as possible, while interacting with the stiff environment (Stiff:Resist, Fig. 3D). When the motor cortex was transiently suppressed using TMS (Fig. 3E), the modulation of the long latency reflex observed during the Compliant:DNI task was substantially reduced (Fig. 3F,G). Conversely, motor cortical inhibition did not affect the increased amplitude of the long latency response that occurred during the Stiff:Resist task (Fig. 3F,G). These results support the idea that muscle activity typically attributed to the long latency stretch reflex arises from multiple distinct pathways, one that contributes to the regulation of limb stability and another associated with the early release of preplanned motor actions (Crago et al. 1976; Rothwell et al. 1980). The existence of these distinct pathways, each associated with a distinct functional role, clarifies previous seemingly contradictory results attempting to ascribe a single role to the long latency stretch reflex (Hasan 2005).
Figure 3.
Inhibition of the primary motor cortex reduces modulation of the long latency stretch reflex corresponding to changes in environmental stability but not changes in prepared response. (A) Visual feedback provided to subjects to ensure that a constant level of tonic activity was maintained in the biceps brachii muscle. (B) A linear motor imposed perturbations to extend the elbow joint while simulating either a stiff or compliant haptic environment. (C) Ramp-and-hold perturbations delivered by the linear actuator moved the wrist 30 mm along the –x axis, thus extending the elbow joint and stretching the biceps brachii. The actuator controller remained stiff throughout Stiff:DNI and Stiff:Resist trials and switched rapidly from compliant to stiff during Compliant:DNI trials in order to ensure consistent joint displacements; “Resist” and “DNI” correspond to how the subject was instructed to react to the perturbation in each of the haptic environments. (D) The response of the biceps brachii to stretches imposed at time zero during low-level (5% MVC) activation shows both short (SLR) and long latency reflex (LLR) responses. The amplitude of the LLR is shown to vary with both task and environment. (E) A single trial in which TMS was applied during a contraction of the biceps brachii at 5% MVC. The silent period following the excitatory motor evoked potential lasts longer than 150 ms following the TMS trigger. (F) Data from the same participant as in D shows reductions in the LLR responses obtained within a period of cortical silence in the Stiff:DNI and Compliant:DNI conditions. No reduction in LLR amplitude is evident in the Stiff:Resist condition. (G) Group means (N=8 subjects) are shown for background muscle activity (BGA), SLR and LLR responses in each experimental condition. For more details see Shemmell et al. (2009).
While we have focused mainly on reflex contributions to the maintenance of limb stability in static postures, the motor cortex also has been implicated in the reflex control of limb mechanics during movement (Kimura et al. 2006). In these experiments, subjects were trained to reach through two opposing force fields, one that perturbed the arm medially and the other laterally. Long latency stretch reflexes were shown to adapt in a manner that helped compensate for the forces generated by each field. As in the postural study described above, the observed reflex modulation was abolished by appropriately timed TMS delivered to the motor cortex. These results are consistent with a role of the motor cortex in regulating the reflex contributions needed to compensate for changing mechanical environments.
The above results demonstrate the role of the primary motor cortex in the regulation of long latency stretch reflex gain during interactions with different mechanical environments, but do not identify which neural structure is responsible for the release of previously planned motor actions. Motor actions triggered by proprioceptive inputs are initiated at a similar latency to responses to auditory startle stimuli, which preferentially activate neurons within the brainstem (Colebatch and Porter 1987; Lingenhohl and Friauf 1992; Yeomans et al. 2002). In individuals preparing a motor action, auditory startle stimuli hasten the release of the intended action such that it occurs earlier than the voluntary reaction time, while preserving the intended pattern of muscle activity (Rothwell et al. 2002; Carlsen et al. 2004). It is therefore possible that pre-planned motor actions triggered by limb perturbations are mediated by subcortical structures similar to those involved in the initiation of action following an auditory startle. This idea is supported by evidence that removing the support surface during feline walking triggers startle-like muscle responses which are immediately preceded by activity in neurons of the pontomedullary reticular formation (Stapley and Drew 2009). Startle-like muscle responses are also often observed when humans encounter an unanticipated change in surface height during walking (van der Linden et al. 2007). The integration of signals from a number of proprioceptive modalities in the human brainstem, similar to that observed in the cat, could provide a mechanism for prepared motor actions to be hastened by joint perturbations. Such brainstem mediated mechanisms have long been proposed to play a role in the initial automatic response to perturbations of stance (Deliagina et al. 2008), which shares some similarities to the individual limb perturbations considered here. It is evident, however, that the brainstem does not act alone in the regulation of these automatic postural responses. Preparatory changes in the cortex prior to expected perturbations of body posture appear to play a role in determining postural set (Mochizuki et al. 2008; Petersen et al. 2009), and may also participate in priming the brainstem for context-appropriate rapid reactions to expected disturbances (Jacobs and Horak 2007). Such cortically mediated priming may well contribute to the reflex modulation observed in the resist paradigms described above.
Reflex contributions to limb mechanics
Muscle stiffness
The reflex modulation described above strongly suggests that stretch reflexes can contribute to the regulation of limb stability. This is consistent with the numerous studies describing how stretch reflexes contribute the mechanical properties of a limb. During the maintenance of posture, these mechanical properties often are quantified in terms of stiffness, which is the steady state force generated in response to an imposed static displacement of limb posture. Houk was among the first to propose that stretch reflexes serve to regulate muscle stiffness (Houk 1972). Nichols and Houk (1976) demonstrated this role in the cat soleus muscle, showing that stretch reflexes can compensate for muscle nonlinearities, such as yielding, to keep stiffness relatively constant during stretch and release. Hoffer and Andreassen (1981) extended these results throughout the physiological range of length and tension. They demonstrated that reflexes contribute to the stiffness of the muscle throughout this range and that they serve to keep stiffness nearly constant for muscle forces above approximately 25% of maximum. Both of these studies were conducted in a decerebrate animal preparation, where it is possible to characterize muscle stiffness with and without reflexes intact.
Joint stiffness
Less direct methods are needed to quantify reflex contributions to stiffness in human subjects, although most of these have also concluded that stretch sensitive reflexes contribute substantially to the net stiffness of an intact joint. Perturbation evoked changes in muscle activity, as recorded by electromyograms (EMGs), have long been used to infer stretch reflex contributions to muscle, joint and limb mechanics (Hammond 1956; Jaeger et al. 1982; Lacquaniti and Soechting 1986; Kurtzer et al. 2008). Such studies are useful for quantifying the time course of reflex action and the patterns of activation across multiple muscles. They do not, however, provide quantitative measures of how these reflexes alter muscle and joint stiffness. An alternate approach is to temporarily block or reduce transmission from the afferent pathways mediating the stretch reflex. Often used techniques include ischemia (Allum et al. 1982; Gottlieb et al. 1983; Sinkjaer and Hayashi 1989), vibration (Allum et al. 1982) or electrical stimulation (Sinkjaer 1988; Carter et al. 1990). Such studies, performed on numerous individual joints in the human upper and lower limbs, have suggested that reflexes can contribute between 30-50% of the net torque generated in response to postural perturbations, although precise estimates depend on the specific experimental conditions. Similar conclusions have been reached using more computationally intensive system identification methods (Kearney et al. 1997; Zhang and Rymer 1997; Perreault et al. 2000) that allow reflex contributions to joint stiffness to be quantified without resorting to interventions that may impair normal physiological function.
Multijoint stiffness
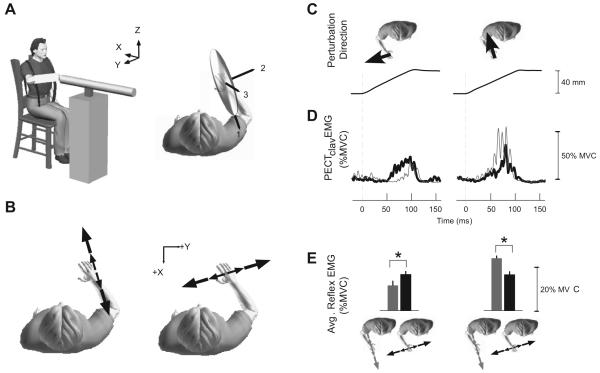
The stiffness of an individual joint or limb is not constant, but can be varied to adapt the mechanical properties of a limb to the specific requirements of a task (Gribble et al. 2003; Selen et al. 2006). Hogan first proposed that the nervous system may explicitly control stiffness and that the redundancy of the human motor system may allow stiffness to be controlled independent from movements or forces required to complete a given task (Hogan 1985). For a multijoint system such as the human arm, measures of endpoint stiffness often are used to quantify limb mechanics at the point of contact with the environment (Mussa-Ivaldi et al. 1985; Gomi and Osu 1998; Perreault et al. 2001; Franklin and Milner 2003). Such measures are directional, indicating that the limb is more resistant to perturbations along certain directions than others. This directionality can be described graphically in terms of an ellipsoid (Mussa-Ivaldi et al. 1985), the long axis of which indicates the orientation of maximal limb stiffness (Fig. 4A).
Figure 4.
Experiments demonstrating how stretch reflexes adapt to compensate for changes in the mechanical properties of the environment with which a subject interacts. (A) Robot used to estimate arm impedance and the corresponding estimate from a single subject. (B) Orientation of the unstable haptic environments used for this same subject. These environments were oriented along the primary and secondary axes of endpoint stiffness, as indicated in A. (C) Ramp-and-hold perturbations used to elicit stretch reflexes as subjects interacted with each of the environments shown in B. (D) reflex EMGs recorded from the clavicular head of the pectoralis (PECTclav). EMGs were elicited by the perturbation directions shown above each set of traces. The light gray traces correspond to reflexes elicited during interactions with the haptic environment aligned to the direction of maximal endpoint stiffness. Black traces correspond to reflexes elicited during interactions with the orthogonal environment (see corresponding directions shown in E). (E) Group results (N=5 subjects) comparing reflex EMGs in each environment. Comparisons were made only at matched levels of background muscle activity. Colors correspond the environments shown at the bottom of the figure. See (Krutky et al. 2010) for more details.
For a fixed posture, control over limb stiffness can be regulated through feedforward changes in voluntary muscle activation, leading to specific patterns of co-contraction (Franklin et al. 2003; Franklin et al. 2004), or via changes in reflex sensitivity. Selective co-contraction has the advantage of changing the intrinsic stiffness of a limb through increases in the number of active crossbridges within each muscle (Rack and Westbury 1974). This provides an immediate opposition to externally imposed disturbances at the expense of increased metabolic cost due to sustained contractions even in the absence of unexpected disturbances. In contrast, heightened reflex sensitivity can increase limb stiffness at a lower metabolic cost since the corresponding increases in muscle activation would occur only following postural perturbations. There may be a constraint on the magnitude of allowable reflex gains due to transmission delays in the stretch reflex pathways and the corresponding influence on limb stability, though the destabilizing influence of these delays may be mitigated by the nonlinearities present in the neuromuscular system, as has been suggested for both stretch reflexes (Stein et al. 1995) and feedback from Golgi tendon organs (Prochazka et al. 1997).
Modulation of reflex contributions to limb stiffness
Evidence for reflex contributions to the active regulation of limb stiffness has been demonstrated using a number of different paradigms. It recently was demonstrated that reflex contributions to joint stiffness can be controlled independently from the intrinsic (non-reflexive) contributions (Ludvig et al. 2007). Reflex sensitivity also can be modulated involuntarily, to compensate for changes in the mechanical properties of the environment with which a limb is interacting. This has been demonstrated by showing that the sensitivity of stretch reflexes increases during interactions with compliant environments relative to that observed during interactions with more rigid environments (Akazawa et al. 1983; Doemges and Rack 1992; Dietz et al. 1994). These results suggest that stretch reflexes may serve to increase joint stiffness and stability during tasks in which that stability is not provided by the environment. These increases occur not only at individual joints, but also throughout the limb (Perreault et al. 2008) providing the possibility that reflexes can alter the directional characteristics of whole limb stiffness in a task appropriate manner.
Many tasks, such as tool use, compromise arm stability along specific directions. Stretch reflexes tuned to those directions could present an efficient mechanism for regulating arm impedance in a task appropriate manner. To be effective, such tuning should adapt not only to the mechanical properties of the environment but rather to those properties in relation to the arm. Evidence for such adaptation was recently provided by examining how stretch reflexes throughout the arm adapt to environments that compromise limb stability along specific directions (Krutky et al. 2010). In these experiments, a 3 degrees-of-freedom robot was used to perturb limb posture and to simulate different haptic environments (Fig. 4A). The tested environments were unstable, having the characteristics of a negative stiffness spring acting along a line (Fig. 4B). These were either aligned or orthogonal to the direction of maximal endpoint stiffness for each subject (Fig. 4A); endpoint stiffness was measured in a separate experiment, using techniques described previously (Trumbower et al. 2009). Stretch reflexes were elicited by applying ramp-and-hold perturbations to the endpoint of the arm (Fig. 4C). These perturbations were oriented along the direction of each unstable haptic environment with which with the subjects interacted. Identical displacement perturbations were applied in each environment. Reflex EMGs were measured in 8 muscles spanning the elbow and shoulder. Reflexes in any specific muscle were compared only at matched levels of background activity within that muscle. Representative results are shown for one muscle, the clavicular head of the pectoralis (Fig. 4D, E). The results demonstrated a preferential increase in reflex sensitivity to perturbations applied specifically along the direction of the destabilizing environment with which the subjects were interacting. Importantly, this preferential increase in reflex sensitivity was observed only when the magnitude of the environmental instability exceeded the endpoint stiffness of the arm along the same direction. These results are consistent with task-specific reflex modulation that is tuned to the mechanical properties of the environment relative to those of the human arm. They demonstrate a highly adaptable involuntary mechanism that may be used to modulate limb impedance along specific directions. However, the precise effect of this reflex modulation on the mechanical properties of the limb has yet to be quantified since most of the techniques used to quantify reflex behavior about a single joint cannot be readily applied to a multijoint system.
Summary
In this review, we have argued that a fundamental role of the human stretch reflex is to regulate the mechanical properties of a limb and to adapt those properties in a task appropriate manner. Furthermore, we emphasize that the regulation limb mechanics is but one important role of the perturbation-evoked muscle activity that can be observed in the time period often attributed to the long latency stretch reflex. Our recent results demonstrate that there are at least two distinct neural pathways that can contribute to the muscle activity recorded in this time period, and that each of these pathways has a distinct functional role.
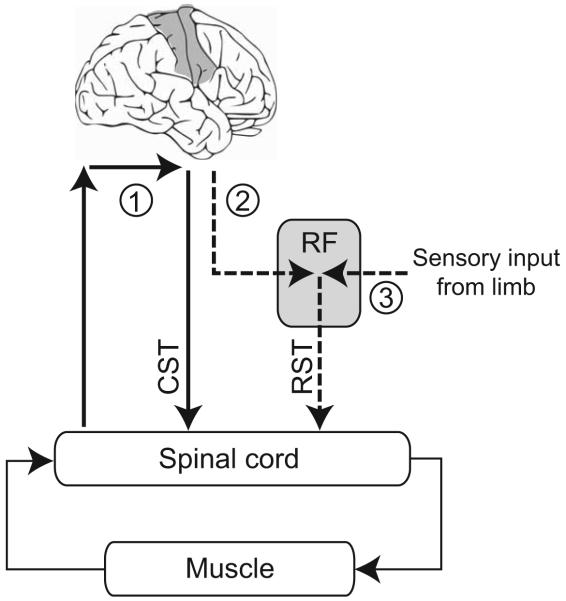
Based on the literature reviewed above, Fig. 5 summarizes our proposal for the pathways that might contribute to the long latency stretch reflex during the tasks that involve the regulation of limb stability or the early release of a pre-planned motor action. We suggest that the long latency stretch reflex pathways contributing to the regulation of limb mechanics are mediated at least in part by the primary motor cortex, exhibiting flexible control over long latency stretch reflexes, as has been proposed for some time. The task specific nature of motor cortical involvement is consistent with earlier primate work, as well as more recent human studies. The pathways contributing to the reflex modulation observed in the presence of a pre-planned movement are less clear, although our data strongly suggest that they involve structures different from those contributing to the regulation of limb mechanics. Many lines of evidence point to a role for the brainstem. Motor actions triggered by proprioceptive inputs are initiated at a similar latency to responses to auditory startle stimuli, which preferentially activate neurons within the brainstem. Furthermore, startling acoustic stimuli can hasten the release of intended actions such that they occurs within the time period often attributed to long-latency stretch reflexes. Brainstem pathways, most notably those involving the reticular formation, have long been implicated in the control of standing posture and also respond to sensory input from the limbs. It is therefore plausible that pre-planned motor actions triggered by perturbations of limb posture are also mediated by subcortical structures similar to those involved in the maintenance of body posture and startle reflexes. As has been suggested for the control of body posture, the priming of an appropriate motor response from the brainstem is likely to involve the motor cortical areas involved in planning.
Figure 5.
A schematic diagram representing the pathways potentially contributing to the stretch reflex response during the regulation of limb mechanics and the release of a prepared motor action. The sensorimotor cortex (1) regulates long latency components of the stretch reflex relevant to the regulation of limb mechanics through transmission along the corticospinal tract (CST), as indicated by the solid, bold lines. The release of prepared motor actions may involve brainstem pathways, including the reticular formation (RF), shown by the dashed, bold lines. The preparation of voluntary is thought to influence the excitability of neurons within the brainstem, most likely through corticobulbar fibers (2). Such prepared actions can be released by startling acoustic stimuli that activate cells within the pontomedullary reticular formation, releasing motor commands transmitted along the reticulospinal tract (RST). We suggest that the release of prepared actions may also be released by sensory input from the limb (3), although the specific modality is yet to be determined. The summation of descending corticospinal and reticulospinal signals may explain apparently contradictory evidence regarding the role of stretch reflex modulation in the regulation of limb mechanics.
The fact that multiple pathways with distinct functional roles can contribute to the rapid motor response to an imposed perturbation suggests that these responses may be more intelligent than reflexive, in concurrence with arguments made previously (Prochazka et al. 2000). This intelligence argues against attempts to ascribe a single functional role to the stretch reflex, especially for responses beyond the shortest latency.
Acknowledgments
This work was supported by NIH grant NS053813.
References
- Abbruzzese G, Berardelli A, Rothwell JC, Day BL, Marsden CD. “Cerebral potentials and electromyographic responses evoked by stretch of wrist muscles in man.”. Experimental Brain Research. 1985;58(3):544–551. doi: 10.1007/BF00235870. [DOI] [PubMed] [Google Scholar]
- Akazawa K, Milner TE, Stein RB. “Modulation of reflex EMG and stiffness in response to stretch of human finger muscle.”. Journal of Neurophysiology. 1983;49(1):16–27. doi: 10.1152/jn.1983.49.1.16. [DOI] [PubMed] [Google Scholar]
- Allum JHJ, Mauritz K-H, Vogele H. “The mechanical effectiveness of short latency reflexes in human triceps surae muscles revealed by ischaemia and vibration.”. Experimental Brain Research. 1982;48:153–156. doi: 10.1007/BF00239584. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Larsen KD, Yumiya H. Integration in the nervous system. H. Asanuma and V. J. Wilson; Tokyo, Igaku-shoin: 1979. Direct sensory pathway to the motor cortex: a basis of cortical reflexes; pp. 223–238. [Google Scholar]
- Asanuma H, Larsen KD, Yumiya H. “Receptive-fields of thalamic neurons projecting to the motor cortex in the cat.”. Brain Research. 1979;172(2):217–228. doi: 10.1016/0006-8993(79)90534-1. [DOI] [PubMed] [Google Scholar]
- Bernard CG, Bohm E, Peterson I. “New investigations on the pyramidal system in macaca-mulatta.”. Experientia. 1953;9(3):111–112. doi: 10.1007/BF02178344. [DOI] [PubMed] [Google Scholar]
- Bras H, Cavallari P, Jankowska E, McCrea D. “COMPARISON OF EFFECTS OF MONOAMINES ON TRANSMISSION IN SPINAL PATHWAYS FROM GROUP-I AND GROUP-II MUSCLE AFFERENTS IN THE CAT.”. Experimental Brain Research. 1989;76(1):27–37. doi: 10.1007/BF00253620. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. “Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex.”. J Neurophysiol. 1984;52(3):435–448. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Carlsen A, Chua R, Inglis J, Sanderson D, Franks I. “Prepared movements are elicited early by startle.”. Journal of motor behavior. 2004;36(3):253–264. doi: 10.3200/JMBR.36.3.253-264. [DOI] [PubMed] [Google Scholar]
- Carter RR, Crago PE, Keith MW. “Stiffness regulation by reflex action in the normal human hand.”. Journal of Neurophysiology. 1990;64(1):105–118. doi: 10.1152/jn.1990.64.1.105. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. “Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey.”. J Physiol. 1984;349:249–272. doi: 10.1113/jphysiol.1984.sp015155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG, Porter R. “‘Long-latency’ responses occurring with startle in the conscious monkey.”. Neurosci Lett. 1987;77(1):43–48. doi: 10.1016/0304-3940(87)90604-5. [DOI] [PubMed] [Google Scholar]
- Corna S, Grasso M, Nardone A, Schieppati M. “Selective depression of medium-latency leg and foot muscle responses to stretch by an alpha(2)-agonist in humans.”. Journal of Physiology-London. 1995;484(3):803–809. doi: 10.1113/jphysiol.1995.sp020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago PE, Houk JC, Hasan Z. “Regulatory actions of human stretch reflex.”. Journal of Neurophysiology. 1976;39(5):925–935. doi: 10.1152/jn.1976.39.5.925. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Beloozerova IN, Zelenin PV, Orlovsky GN. “Spinal and supraspinal postural networks.”. Brain Res Rev. 2008;57(1):212–221. doi: 10.1016/j.brainresrev.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Discher M, Trippel M. “Task-dependent modulation of short- and long-latency electromyographic responses in upper limb muscles.”. Electroencephalogr Clin Neurophysiol. 1994;93(1):49–56. doi: 10.1016/0168-5597(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Doemges F, Rack PMH. “Changes in the stretch reflex of the human first dorsal interosseous muscle during different tasks.”. J Physiol. 1992;447:563–573. doi: 10.1113/jphysiol.1992.sp019018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doemges F, Rack PMH. “Task-dependent changes in the response of human wrist joints to mechanical disturbance.”. J Physiol. 1992;447:575–585. doi: 10.1113/jphysiol.1992.sp019019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV. “Motor Cortex Reflexes Associated with Learned Movement.”. Science. 1973;179(4072):501–503. doi: 10.1126/science.179.4072.501. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Fromm C. Cerebral motor control in man: Long loop mechanisms. Vol. 4. J. E. Desmedt; Basel, Karger: 1978. The pyramidal tract neuron as summing point in a closed-loop control system in the monkey; pp. 56–69. [Google Scholar]
- Franklin DW, Burdet E, Osu R, Kawato M, Milner TE. “Functional significance of stiffness in adaptation of multijoint arm movements to stable and unstable dynamics.”. Exp Brain Res. 2003;151(2):145–157. doi: 10.1007/s00221-003-1443-3. [DOI] [PubMed] [Google Scholar]
- Franklin DW, Milner TE. “Adaptive control of stiffness to stabilize hand position with large loads.”. Exp Brain Res. 2003;152(2):211–220. doi: 10.1007/s00221-003-1540-3. [DOI] [PubMed] [Google Scholar]
- Franklin DW, So U, Kawato M, Milner TE. “Impedance control balances stability with metabolically costly muscle activation.”. J Neurophysiol. 2004;92(5):3097–3105. doi: 10.1152/jn.00364.2004. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Brinkman C, Porter R. “A quantitative study of the distribution of neurons projecting to the precentral motor cortex in the monkey (Macaca-fascicularis).”. Journal of Comparative Neurology. 1987;259(3):424–444. doi: 10.1002/cne.902590309. [DOI] [PubMed] [Google Scholar]
- Gomi H, Osu R. “Task-dependent viscoelasticity of human multijoint arm and its spatial characteristics for interaction with environments.”. J Neurosci. 1998;18(21):8965–8978. doi: 10.1523/JNEUROSCI.18-21-08965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, Luschei ES. “Discharge of spindle afferents from jaw-closing muscles during chewing in alert monkeys.”. Journal of Neurophysiology. 1975;38:560–571. doi: 10.1152/jn.1975.38.3.560. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Agarwal GC, Jaeger RJ. “Response to sudden torques about ankle in man: v effects of peripheral ischemia.”. Journal of Neurophysiology. 1983;50(1):297–312. doi: 10.1152/jn.1983.50.1.297. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Ladouceur M, Andersen JB, Nielsen JB, Sinkjaer T. “Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans.”. Journal of Physiology-London. 2001;534(3):925–933. doi: 10.1111/j.1469-7793.2001.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble PL, Mullin LI, Cothros N, Mattar A. “Role of cocontraction in arm movement accuracy.”. J Neurophysiol. 2003;89(5):2396–2405. doi: 10.1152/jn.01020.2002. [DOI] [PubMed] [Google Scholar]
- Hammond PH. “Involuntary activity in biceps following the sudden application of velocity to the abducted forearm.”. Journal of Physiology-London. 1955;127(3):P23–P25. [PMC free article] [PubMed] [Google Scholar]
- Hammond PH. “The influence of prior instruction to the subject on an apparently involuntary neuro-muscular response.”. J Physiol. 1956;132(1):17–18P. [PubMed] [Google Scholar]
- Hasan Z. “The human motor control system’s response to mechanical perturbation: should it, can it, and does it ensure stability?”. Journal of Motor Behavior. 2005;37(6):484–493. doi: 10.3200/JMBR.37.6.484-493. [DOI] [PubMed] [Google Scholar]
- Hasan Z. “The human motor control system’s response to mechanical perturbation: should it, can it, and does it ensure stability?”. J Mot Behav. 2005;37(6):484–493. doi: 10.3200/JMBR.37.6.484-493. [DOI] [PubMed] [Google Scholar]
- Hoffer JA, Andreassen S. “Regulation of soleus muscle stiffness in premammillary cats: intrinsic and reflex components.”. Journal of Neurophysiology. 1981;45(2):267–285. doi: 10.1152/jn.1981.45.2.267. [DOI] [PubMed] [Google Scholar]
- Hogan N. “The mechanics of multi-joint posture and movement control.”. Biological Cybernetics. 1985;52:315–331. doi: 10.1007/BF00355754. [DOI] [PubMed] [Google Scholar]
- Honeycutt CF, Gottschall JS, Nichols TR. “Electromyographic responses from the hindlimb muscles of the decerebrate cat to horizontal support surface perturbations.”. J Neurophysiol. 2009;101(6):2751–2761. doi: 10.1152/jn.91040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk JC. Biocybernetics IV. J. C. Houk; Jena, Fischer: 1972. The phylogeny of muscular control configurations; pp. 125–144. [Google Scholar]
- Huerta MF, Pons TP. “Primary motor cortex receives input from area 3a in macaques.”. Brain Research. 1990;537(1-2):367–371. doi: 10.1016/0006-8993(90)90388-r. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB. “Cortical control of postural responses.”. J Neural Transm. 2007;114(10):1339–1348. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger R, Gottlieb G, Agarwal G, Tahmoush A. “Afferent contributions to stretch-evoked myoelectric responses.”. Journal of Neurophysiology. 1982;48(2):403–418. doi: 10.1152/jn.1982.48.2.403. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Hendry SHC. “Intra-cortical connectivity of architechtonic fields in somatic sensory, motor and parietal cortex of monkeys.”. Journal of Comparative Neurology. 1978;181(2):291. doi: 10.1002/cne.901810206. &. [DOI] [PubMed] [Google Scholar]
- Jones EG, Porter R. “What is area 3a?”. Brain Research Reviews. 1980;2(1):1–43. doi: 10.1016/0165-0173(80)90002-8. [DOI] [PubMed] [Google Scholar]
- Kearney RE, Stein RB, Parameswaran L. “Identification of intrinsic and reflex contributions to human ankle stiffness dynamics.”. IEEE Transactions on Biomedical Engineering. 1997;44(6):493–504. doi: 10.1109/10.581944. [DOI] [PubMed] [Google Scholar]
- Kimura T, Haggard P, Gomi H. “Transcranial Magnetic Stimulation over Sensorimotor Cortex Disrupts Anticipatory Reflex Gain Modulation for Skilled Action.”. J. Neurosci. 2006;26(36):9272–9281. doi: 10.1523/JNEUROSCI.3886-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland GF, Hasan Z. “Electromyographic responses to a mechanical perturbation applied during impending arm movements in different directions: one-joint and two-joint conditions.”. Exp Brain Res. 2000;132(4):485–499. doi: 10.1007/s002210000356. [DOI] [PubMed] [Google Scholar]
- Kots YM. The organisation of voluntary movement. Plenum; New York: 1977. [Google Scholar]
- Krutky MA, Ravichandran VJ, Trumbower RD, Perreault EJ. “Interactions between limb and environmental mechanics influence stretch reflex sensitivity in the human arm.”. J Neurophysiol. 2010;103(1):429–440. doi: 10.1152/jn.00679.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzer IL, Pruszynski JA, Scott SH. “Long-latency reflexes of the human arm reflect an internal model of limb dynamics.”. Curr Biol. 2008;18(6):449–453. doi: 10.1016/j.cub.2008.02.053. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Soechting JF. “EMG responses to load perturbations of the upper limb: effect of dynamic coupling between shoulder and elbow motion.”. Exp Brain Res. 1986;61(3):482–496. doi: 10.1007/BF00237573. [DOI] [PubMed] [Google Scholar]
- Landgren S, Phillips CG, Porter R. “Minimal synaptic actions of pyramidal impulses on some alpha motoneurons of baboons hand and forearm.”. Journal of Physiology-London. 1962;161(1):91. doi: 10.1113/jphysiol.1962.sp006875. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RG, Tatton WG. “Motor responses to sudden limb displacements in primates with specific CNS lesions and in human patients with motor system disorders.”. Can J Neurol Sci. 1975;2(3):285–293. doi: 10.1017/s0317167100020382. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Tatton WG, Tasker RR. “Electromyographic response to displacement of different forelimb joints in the squirrel monkey.”. J Neurosci. 1983;3(4):783–794. doi: 10.1523/JNEUROSCI.03-04-00783.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GN, Mackinnon CD, Perreault EJ. “The effect of task instruction on the excitability of spinal and supraspinal reflex pathways projecting to the biceps muscle.”. Exp Brain Res. 2006;174(3):413–425. doi: 10.1007/s00221-006-0475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell EGT, Sherrington CS. “Reflexes in response to stretch (myotatic reflexes).”. Proceedings of the Royal Society of London. Series B. 1924;96:212–242. [Google Scholar]
- Lingenhohl K, Friauf E. “Giant neurons in the caudal pontine reticular formation receive short latency acoustic input: an intracellular recording and HRP-study in the rat.”. J Comp Neurol. 1992;325(4):473–492. doi: 10.1002/cne.903250403. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Duysens J. “Activity patterns in individual hindlimb primary and secondary muscle spindle afferents during normal movements in unrestrained cats.”. Journal of Neurophysiology. 1979;54:565–577. doi: 10.1152/jn.1979.42.2.420. [DOI] [PubMed] [Google Scholar]
- Lourenco G, Iglesias C, Cavallari P, Pierrot-Deseilligny E, Marchand-Pauvert V. “Mediation of late excitation from human hand muscles via parallel group II spinal and group I transcortical pathways.”. Journal of Physiology-London. 2006;572(2):585–603. doi: 10.1113/jphysiol.2005.102806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvig D, Cathers I, Kearney RE. “Voluntary modulation of human stretch reflexes.”. Exp Brain Res. 2007;183(2):201–213. doi: 10.1007/s00221-007-1030-0. [DOI] [PubMed] [Google Scholar]
- Lyalka VF, Zelenin PV, Karayannidou A, Orlovsky GN, Grillner S, Deliagina TG. “Impairment and recovery of postural control in rabbits with spinal cord lesions.”. J Neurophysiol. 2005;94(6):3677–3690. doi: 10.1152/jn.00538.2005. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Verrier MC, Tatton WG. “Motor cortical potentials precede long-latency EMG activity evoked by imposed displacements of the human wrist.”. Exp Brain Res. 2000;131(4):477–490. doi: 10.1007/s002219900317. [DOI] [PubMed] [Google Scholar]
- Magladery JW, Porter WE, Park AM, Teasdall RD. “Electrophysiological studies of nerve and reflex activity in normal man. IV. The two-neurone reflex and identification of certain action potentials from spinal roots and cord.”. Bull Johns Hopkins Hosp. 1951;88(6):499–519. [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. “Servo action in human voluntary movement.”. Nature. 1972;238:140–143. doi: 10.1038/238140a0. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. “The human stretch reflex and the motor cortex.”. Trends in Neurosciences. 1991;14(3):87–91. doi: 10.1016/0166-2236(91)90064-2. [DOI] [PubMed] [Google Scholar]
- Mochizuki G, Sibley KM, Esposito JG, Camilleri JM, McIlroy WE. “Cortical responses associated with the preparation and reaction to full-body perturbations to upright stability.”. Clin Neurophysiol. 2008;119(7):1626–1637. doi: 10.1016/j.clinph.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Mussa-Ivaldi FA, Hogan N, Bizzi E. “Neural, mechanical, and geometric factors subserving arm posture in humans.”. The Journal of Neuroscience. 1985;5(10):2732–2743. doi: 10.1523/JNEUROSCI.05-10-02732.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha P, Boulinguez P, Sainburg R. “Visual modulation of proprioceptive reflexes during movement.”. Brain Research. 2008;1246:54–69. doi: 10.1016/j.brainres.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TR, Houk JC. “Improvement in linearity and regulation of stiffness that results from actions of stretch reflex.”. Journal of Neurophysiology. 1976;39:119–142. doi: 10.1152/jn.1976.39.1.119. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. “Evidence that a long latency stretch reflex in humans is transcortical.”. Journal of Physiology. 1992;449:429–440. doi: 10.1113/jphysiol.1992.sp019094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JMS. “Robert Whytt and the stretch reflex.”. Journal of Neurology Neurosurgery and Psychiatry. 1997;62(5):484–484. doi: 10.1136/jnnp.62.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault EJ, Chen K, Trumbower RD, Lewis G. “Interactions with compliant loads alter stretch reflex gains but not intermuscular coordination.”. J Neurophysiol. 2008;99(5):2101–2113. doi: 10.1152/jn.01094.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault EJ, Crago PE, Kirsch RF. “Estimation of intrinsic and reflex contributions to muscle dynamics: a modeling study.”. IEEE Trans Biomed Eng. 2000;47(11):1413–1421. doi: 10.1109/TBME.2000.880092. [DOI] [PubMed] [Google Scholar]
- Perreault EJ, Kirsch RF, Crago PE. “Effects of voluntary force generation on the elastic components of endpoint stiffness.”. Exp Brain Res. 2001;141(3):312–323. doi: 10.1007/s002210100880. [DOI] [PubMed] [Google Scholar]
- Petersen N, Christensen LOD, Morita H, Sinkjaer T, Nielsen J. “Evidence that a transcortical pathway contributes to stretch reflexes in the tibialis anterior muscle in man.”. Journal of Physiology-London. 1998;512(1):267–276. doi: 10.1111/j.1469-7793.1998.267bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TH, Rosenberg K, Petersen NC, Nielsen JB. “Cortical involvement in anticipatory postural reactions in man.”. Exp Brain Res. 2009;193(2):161–171. doi: 10.1007/s00221-008-1603-6. [DOI] [PubMed] [Google Scholar]
- Prochazka A. “The uncoupling of alpha and of static and dynamic fusimotor activity in the cat: fusimotor “set”.”. Proceedings of the International Union of Physiological Sciences. 1983;5:12. [Google Scholar]
- Prochazka A. “Sensorimotor gain control - a basic strategy of motor systems.”. Progress in Neurobiology. 1989;33(4):281–307. doi: 10.1016/0301-0082(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Clarac F, Loeb GE, Rothwell JC, Wolpaw JR. “What do reflex and voluntary mean? Modern views on an ancient debate.”. Exp Brain Res. 2000;130(4):417–432. doi: 10.1007/s002219900250. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gillard D, Bennett DJ. “Implications of positive feedback in the control of movement.”. Journal of Neurophysiology. 1997;77:3237–3251. doi: 10.1152/jn.1997.77.6.3237. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Westerman RA, Ziccone SP. “Discharges of single hindlimb afferents in the freely moving cat.”. Proceedings of the Australian Physiological and Pharmacological society. 1976;6:101. doi: 10.1152/jn.1976.39.5.1090. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Scott SH. “Rapid motor responses are appropriately tuned to the metrics of a visuospatial task.”. J Neurophysiol. 2008;100(1):224–238. doi: 10.1152/jn.90262.2008. [DOI] [PubMed] [Google Scholar]
- Rack PMH, Westbury DR. “The short range stiffness of active mammalian muscle and its effect on mechanical properties.”. Journal of Physiology. 1974;240:331–350. doi: 10.1113/jphysiol.1974.sp010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, MacKinnon CD, Valls-Sole J. “Role of brainstem-spinal projections in voluntary movement.”. Movement Disorders. 2002;17:S27–S29. doi: 10.1002/mds.10054. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Marsden CD. “Influence of voluntary intent on the human long-latency stretch reflex.”. Nature. 1980;286(5772):496–498. doi: 10.1038/286496a0. [DOI] [PubMed] [Google Scholar]
- Selen LP, van Dieen JH, Beek PJ. “Impedance modulation and feedback corrections in tracking targets of variable size and frequency.”. J Neurophysiol. 2006;96(5):2750–2759. doi: 10.1152/jn.00552.2006. [DOI] [PubMed] [Google Scholar]
- Shemmell JBH, An JH, Perreault EJ. “The differential role of motor cortex in stretch reflex modulation induced by changes in environmental mechanics and verbal instruction.”. J Neurosci. 2009;29(42):13255–13263. doi: 10.1523/JNEUROSCI.0892-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T, Hayashi R. “Regulation of wrist stiffness by the stretch reflex.”. J Biomech. 1989;22(11-12):1133–1140. doi: 10.1016/0021-9290(89)90215-7. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Toft E, Andreassen S, Hornemann BC. “Muscle stiffness in human ankle dorsiflexors: intrinsic and reflex components.”. Journal of Neurophysiology. 1988;60(3):1110–1121. doi: 10.1152/jn.1988.60.3.1110. [DOI] [PubMed] [Google Scholar]
- Skoog B. “A comparison of the effects of two antispastic drugs, tizanidine and baclofen, on synaptic transmission from muscle spindle afferents to spinal interneurones in cats.”. Acta Physiologica Scandinavica. 1996;156(1):81–90. doi: 10.1046/j.1365-201X.1996.444159000.x. [DOI] [PubMed] [Google Scholar]
- Stapley PJ, Drew T. “The pontomedullary reticular formation contributes to the compensatory postural responses observed following removal of the support surface in the standing cat.”. J Neurophysiol. 2009;101(3):1334–1350. doi: 10.1152/jn.91013.2008. [DOI] [PubMed] [Google Scholar]
- Stein RB, Hunter IW, Lafontaine SR, Jones LA. “Analysis of Short-latency reflexes in human elbow flexor muscles.”. Journal of Neurophysiology. 1995;73(5):1900–1911. doi: 10.1152/jn.1995.73.5.1900. [DOI] [PubMed] [Google Scholar]
- Taylor A, Cody FWJ. “Jaw muscle spindle activity in the cat during normal movements of eating and drinking.”. Brain Research. 1974;71:523–530. doi: 10.1016/0006-8993(74)90996-2. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Krutky MA, Yang B-S, Perreault EJ. “Use of Self-Selected Postures to Regulate Multi-Joint Stiffness During Unconstrained Tasks.”. PLoS ONE. 2009;4(5):e5411. doi: 10.1371/journal.pone.0005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Sole J, Rothwell JC, Goulart F, Cossu G, Munoz E. “Patterned ballistic movements triggered by a startle in healthy humans.”. J Physiol. 1999;516(Pt 3):931–938. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden MH, Marigold DS, Gabreels FJ, Duysens J. “Muscle reflexes and synergies triggered by an unexpected support surface height during walking.”. J Neurophysiol. 2007;97(5):3639–3650. doi: 10.1152/jn.01272.2006. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Li L, Scott BW, Frankland PW. “Tactile, acoustic and vestibular systems sum to elicit the startle reflex.”. Neuroscience and Biobehavioral Reviews. 2002;26(1):1–11. doi: 10.1016/s0149-7634(01)00057-4. [DOI] [PubMed] [Google Scholar]
- Zhang L-Q, Rymer WZ. “Simultaneous and nonlinear identification of mechanical and reflex properties of human elbow joint muscles.”. IEEE Transactions on Biomedical Engineering. 1997;44(12):1192–1209. doi: 10.1109/10.649991. [DOI] [PubMed] [Google Scholar]