Abstract
Processing of the amyloid-β (Aβ) precursor protein (APP) has been extensively studied since it leads to production of Aβ peptides. Toxic forms of Aβ aggregates are considered the cause of Alzheimer’s disease (AD). On the other end, BRI2 is implicated in APP processing and Aβ production. We have investigated the precise mechanism by which BRI2 modulates APP cleavages and have found that BRI2 forms a mature BRI2 polypeptide that is transported to the plasma membrane and endosomes where it interacts with mature APP. Notably, immature forms of APP and BRI2 fail to interact. Mature BRI2 inhibits APP processing by α-, β- and γ-secretases on the plasma membrane and in endocytic compartments. Thus, BRI2 is a specific inhibitor that reduces secretases’ access to APP in the intracellular compartments where APP is normally processed.
Keywords: APP, BRI2, Alzheimer’s disease, Familial British dementia, Familial Danish dementia, Secretases
1. Introduction
Alzheimer’s disease (AD) is the most common cause of dementia and is characterized by neurofibrillary tangles and amyloid plaque brain lesions (Selkoe and Kopan, 2003; Selkoe and Podlisny, 2002; Small and Duff, 2008). Amyloid plaques are extracellular deposits of fibrillary amyloid-β (Aβ) observed in the hippocampus, entorhinal cortex, blood vessels (cerebral amyloid angiopathy or CAA), and other areas. Aβ arises from proteolytic processing of APP a ubiquitous type I transmembrane protein (Selkoe and Kopan, 2003; Sisodia and St George-Hyslop, 2002). Cleavage of APP by the β-secretase releases the APP ectodomain (sAPPβ) and a membrane-bound 99 amino acid-long fragment (C99) (Cole and Vassar, 2007). C99 is cleaved, with somewhat lax site specificity, by γ-secretase into two peptides; the amyloidogenic Aβ peptide, consisting of two major species of 40 and 42 amino acids (Aβ40 and Aβ42, respectively) (Wolfe, 2007) and an intracellular product termed the APP Intracellular Domain (AID or AICD) that regulates cell death (Passer et al., 2000), gene transcription (Cao and Sudhof, 2001) and calcium homeostasis (Leissring et al., 2002). Although Aβ40 represents the major product, Aβ42 is more prone to form pathogenic oligomers (Haass and Selkoe, 2007; Shankar et al., 2008) and is the primary species deposited in amyloid plaques (Hardy, 2006). Alternatively, α-secretase cleaves APP within the Aβ sequence to produce soluble APPα (sAPPα) and the membrane-bound C83 fragment (Marambaud and Robakis, 2005; Wilquet and De Strooper, 2004). About 6%, of all AD cases are caused by autosomal dominant mutations in either APP itself or in Presenilins (PSEN1 and PSEN2) (St George-Hyslop and Petit, 2005), which are key components of a multi-molecular complex with γ-secretase activity (De Strooper, 2003). These studies have provided strong genetic evidence that alteration in APP cleavage by the γ-secretase plays a pathogenic role in AD.
The BRI2 gene encodes a type II membrane protein 266 amino acids long. The precursor immature form of BRI2 (imBRI2) undergoes cleavage in the secretory pathway at the carboxyl-terminus between arginine 243 and glutamic acid 244 (Choi et al., 2004; Kim et al., 1999) to generate a soluble 23 amino acid peptide and a membrane-bound mature BRI2 product (mBRI2, Fig. 1A). Of note, mutations in this region of BRI2, which result in secretion of amyloidogenic peptides, cause two distinct autosomal dominant neurodegenerative disorders (FDD and FBD) (Vidal et al., 1999, 2000). BRI2 is a member of a gene family that comprises also BRI1, which is primarily expressed in chondrocytes (Deleersnijder et al., 1996), and BRI3 (Deleersnijder et al., 1996; Vidal et al., 2001). Similar to BRI2, BRI3 is also cleaved by furin or furin-like convertases and the C-terminal peptide is secreted (Wickham et al., 2005).
Fig. 1.
BRI2 does not alter APP trafficking and only mBRI2 is transported to cell membranes. (A) Schematic drawing of wild type BRI2. Immature BRI2 (imBRI2) is cleaved by a furin-like convertase to mature BRI2 (mBRI2). The cleavage releases a C-terminal 23 amino acid peptide (Bri2–23) from imBRI2. Cytoplasmic, luminal, and BRICHOS (B) domains are indicated. (B) HeLa cells were transfected with Flag-BRI2 or APP as indicated. Cell surface proteins were biotinylated and precipitated with streptavidin beads. Immunoblot analysis of total lysate (T), unbound (U), and bound (B) proteins with 22C11, anti-Flag and αAPPct antibodies. α-Tubulin was not found in the bound fraction demonstrating that intracellular proteins were not biotinylated. (C) Iodixanol gradient fractionation shows that imAPP is concentrated in ER fractions (where the ER-resident protein calnexin fractionates) while mAPP is found in the Golgi complex fraction (marked by the Golgi complex marker GM130) and early endosomal fractions (where EEA1 is enriched). BRI2 expression does not alter APP localization (second panel from the top).
We and others have previously shown that BRI2 inhibits Aβ production and amyloidosis both in cell lines and in animal models of AD. The evidence that reducing BRI2 expression by RNAi in cell lines or gene knock out in mice increases APP processing stresses the physiological relevance of this BRI2 function. Of interest, while BRI3 has a similar function, BRI1 does not (Matsuda, in press). Here, we demonstrate that BRI2 functions as a regulator of APP processing and inhibits Aβ production on the plasma membrane and in endosomes. Indeed, maturation of imBRI2 into mBRI2 and transport of mBRI2 along the secretory pathway are required to generate a functional APP processing inhibitor. These important insights into the biological regulatory mechanisms of APP processing also suggest that targeting BRI2 mimics to the plasma membrane and endosomes is a sound approach to AD therapy.
2. Material and methods
2.1. Cell culture, transfection, plasmids and antibodies
Cell lines, transfection methods, human APP and Flag-human BRI2 were described (Matsuda et al., 2008). To culture mouse dermal fibroblasts, skin was removed from mouse tails, soaked in 70% ethanol, washed in PBS, diced into small pieces and incubated at 37 °C overnight in CO2 incubator in DMEM containing 20% FBS, supplemented with penicillin/streptomycin and 1.6 mg/ml collagenase II. On the next day, clumps were removed by passing through a nylon mesh, and the material was centrifuged at 1000 rpm for 5 min to collect the cells. The collected cells were maintained in DMEM containing 20% FBS and penicillin/streptomycin.
All mutations were created by PCR and confirmed by sequencing. Flag-BRI2-myc has a myc-tag insertion (GEQKLISEEDL) just before the stop codon of Flag-BRI2. Flag-BRI2FR was created by replacing K242R243 to A242A243. To create Flag-BRI2Δ23, the nucleotides coding the last 23 amino acids are deleted from Flag-BRI2.
The following antibodies and antibody beads were used: anti-APP (22C11, Chemicon MAB348); anti-sAPPα (IBL 11088); anti-sAPPβ (IBL 18957); anti-APP C-terminal fragments (CTF) (αAPPct Invitrogen/Zymed 36-6900); anti-Aβ (6E10, Covance 9300-02 and 4G8, Covance 9200-02); anti-calnexin (Stressgen SPA-865), anti-α-tubulin (Sigma DM1A); anti-EEA1 (Sigma E3906); anti-GM130 (Sigma G7295), anti-myc (9B11, Cell Signaling 2276). Antibodies coupled beads used are: anti-Flag M2 beads (Sigma A2220); anti-HA beads (Sigma A2095); anti-myc beads (Sigma A7470). Rabbit polyclonal and goat secondary antibodies conjugated with horseradish peroxidase are from Southern Biotechnology. BRI2 antibody (recognizing amino acids 7–21 of human BRI2) was a generous gift from Dr. Haruhiko Akiyama (Akiyama et al., 2004).
2.2. Iodixanol fractionation
Iodixanol fractionation was performed as previously described (Lee et al., 2000) with slight modifications. HeLa cells were transfected with human APP together with an empty vector or human BRI2. One day after transfection, the cells were washed, scraped in 1 ml of H buffer (20 mM Hepes/NaOH pH 7.4, 1 mM EDTA, 1 mM EGTA, 0.25 M sucrose) and homogenized in a Dounce homogenizer until fewer than 10% of cells remain intact. The homogenate was centrifuged at 1000 × g for 10 min. The post-nuclear supernatant (PNS) was adjusted to 2 ml of 25% iodixanol with 50% iodixanol (5 vol. of 60% iodixanol (Sigma) diluted with one volume of dilution buffer (120 mM Hepes/NaOH pH 7.4, 6 mM EDTA, 6 mM EGTA, 0.25 M sucrose) and 25% iodixanol (1 vol. of 50% iodixanol diluted with 1 vol. of H buffer). 1 ml each of 20%, 18.5%, 17%, 15.5%, 14%, 12.5%, 11%, 9.5%, 8%, 6.5% of iodixanol (50% iodixanol diluted with H buffer) were successively placed on the sample, and centrifuged at 90k × g for 20 h in SW 41Ti (Beckman). Fractions of 0.5 ml each were collected from the top and equal volume of the fractions was used for the immunoblot.
2.3. Immunoprecipitation and metabolic labeling
HeLa cells were transfected with the indicated plasmids and lysed in Hepes-Triton buffer (20 mM Hepes/NaOH pH 7.4, 1 mM EDTA, 150 mM NaCl, 0.5% TritonX-100), supplemented with protease inhibitors. Mouse dermal fibroblasts were also lysed in the Hepes-Triton buffer. The lysates were cleared by centrifuging at 20k × g for 10 min. The cleared lysates were mixed with following antibody beads combination: Flag IP, anti-Flag beads; APP IP, anti-APPct and protein A beads; myc IP, anti-myc beads; endogenous BRI2 IP, anti-BRI2 antibody and protein A beads. Precipitation with rabbit polyclonal antibody and protein A beads (Pierce) was used as a control of αAPPct or BRI2 precipitation. Anti-HA beads were used for a control of anti-Flag or anti-myc beads. After being washed four times with the same buffer, the precipitated beads were boiled in 2× SDS buffer and analyzed by Western blot.
In pulse-chase experiments, the transfected HeLa cells were metabolically labeled for 30 min with 35S cysteine + methionine (ICN) and chased for indicated times. The cell lysates were immunoprecipitated with anti-Flag beads in the RIPA buffer (20 mM Hepes/NaOH pH 7.4, 1 mM EDTA, 150 mM NaCl, 1% Triton-X 100, 0.5% sodium deoxycholate, 0.1% SDS) and subjected to SDS-PAGE and autoradiography as described (Matsuda et al., 2005).
2.4. Biotinylation, streptavidin precipitation, and internalization
Biotinylation and internalization experiments were performed according to Ehlers (2000) with modifications. One day after transfection, HeLa cells were washed three times with cold PBS plus Ca2+ and Mg2+ (PBS-CM) and labeled for 30 min on ice in 0.5 mg/ml sulfo-NHS-SS-biotin (Pierce) dissolved in PBS-CM. Free biotinylation reagent was removed by washing three times with PBS-CM containing 0.1% BSA. The cells were lysed in the RIPA buffer. The lysates were cleared by centrifuging at 20k × g for 10 min, and were mixed with streptavidin agarose beads (Sigma S1638). After collecting unbound lysate, the beads were washed four times with the RIPA buffer, and were boiled in 2× SDS buffer. Comparable volume of the samples was subjected to Western blot. The streptavidin precipitants correspond to four times of total lysates or unbound proteins.
For internalization experiments (Fig. 3A and B and Fig. 4), the cells were biotinylated and washed in PBS-CM containing 0.1% BSA as above. Then the cells were incubated in warm media for indicated periods in CO2 incubator to allow internalization and processing of APP. Cold media was used for time “0”. Placing the cells on ice terminated the reaction. To remove biotin from the cell surface (Fig. 3B), cells were incubated glutathione buffer (50 mM glutathione, 75 mM NaOH, 75 mM NaCl, 1 mM EDTA, 0.2% BSA) on ice for 15 min, twice after the termination of the incubation. The cells were washed four times with PBS-CM, and lysed in the RIPA buffer. The biotinylated proteins were collected and processed as above.
Fig. 3.
mBRI2 and mAPP interact on the plasma membrane and in endocytic vesicles. (A) HeLa cells transfected with APP or APP plus Flag-BRI2 were surface biotinylated. The total lysates (T) were immunoprecipitated with anti-Flag, and eluted with the Flag peptide. The eluate (E) was further precipitated with streptavidin beads, and unbound (U) and bound (B) fractions were collected, and probed for APP, APP CTFs, and BRI2. Material loaded in the B fraction is eight times more than the E and the U fraction. (B) The experiments were performed as in A except that after labeling with biotin, cells were incubated at 37 °C for the indicated times and the biotin remaining on the surface was removed with glutathione after the incubation and before the cells were lysed. The efficacy of biotin removal is confirmed by the absence of mAPP in the B fraction at time 0. (C) Mouse dermal fibroblasts were prepared from the tail skin of the mice that have the indicated genotypes. The total lysates (T) were prepared and immunoprecipitated with an anti-BRI2 antibody. APP, APP CTFs, BRI2, and calnexin in the total lysates and precipitants were detected in western blots with 22C11, αAPPct, anti-BRI2, and anti-calnexin. Calnexin is used as a loading control.
Fig. 4.
BRI2 inhibits APP processing on the plasma membrane and in endocytic vesicles. (A) HeLa cells transfected with APP or APP plus Flag-BRI2 were biotinylated, incubated at 37 °C for the indicated times, lysed and precipitated with streptavidin beads. The precipitants were probed with either αAPPct, which recognize both APP CTFs C83 and C99. (B) Quantification of C99 and C83 levels of the experiment shown in A. The peak point of C83 was defined as 100 s. (C) Cells were transfected, incubated at 37 °C for indicated times. The cell lysates and culture supernatants were precipitated with streptavidin beads as in A. The lysate precipitants were probed for APP, APP CTFs (C99 and C83), C99 (with 6E10 that only recognizes C99), and BRI2. The media precipitants were probed for sAPPα and sAPPβ. (D) Quantification of sAPPα and sAPPβ. The maximum point of each sAPPα and sAPPβ were set to 100.
2.5. Mouse handling
Mice were handled according to the Ethical Guidelines for Treatment of Laboratory Animals of Albert Einstein College of Medicine. The procedures were described and approved in animal protocol number 20040707.
2.6. Image scanning and analysis
Immunoblot images were scanned with Epson perfection 3200 Photo scanner and were analyzed with either NIH Image software or ImageJ 1.4.2.
2.7. Fluorescence imaging of cells
HEK293 cells were grown in 8-well Labtek coverglass chambers (Nunc) and imaged in phenol red-free RPMI supplemented with 10 mM Hepes and 10% fetal bovine serum. For immunofluorescence, cells were fixed for 15 min in 3.7% formaldehyde in 1× PBS, permeabilized by 0.1% triton-X 100 in 1× PBS, blocked for 30 min in 10% fetal bovine serum (block), stained with primary antibody for 1 h in block, washed, stained with secondary antibody for 1 h in block, and then washed and imaged in 1× PBS. Composite figures were prepared using Photoshop CS2 and Illustrator CS software (Adobe).
3. Results and discussion
3.1. Maturation of BRI2 and APP is necessary for their interaction
Recent studies in vitro (Fotinopoulou et al., 2005; Matsuda et al., 2005) and in vivo (Kim et al., 2008; Matsuda et al., 2008) have suggested that wild type BRI2 can suppress Aβ accumulation and prompted us to investigate whether maturation and transport of BRI2 regulate APP processing. Intracellular transport and localization of APP are critical components of Aβ production (Fuentealba et al., 2007; Vetrivel et al., 2007). In fact, α-secretase cleaves mAPP en route to or on the plasma membrane. Consistently, mAPP and C83 localize to the cell surface, as determined in biotinylation assays (Fig. 1B). β-Secretase predominantly cleaves mAPP in early endosomes (Ehehalt et al., 2003; Kaether et al., 2006) while C99 and C83 are processed by the γ-secretase in endocytic compartments (Ehehalt et al., 2003). Notably, BRI2 expression neither altered the imAPP/mAPP ratio nor changed the levels of plasma membrane mAPP (Fig. 1B), indicating that BRI2 does not alter APP maturation and trafficking. Analysis of APP distribution in cellular compartments by iodixanol gradient fractionation confirms this conclusion (Fig. 1C).
Next, we asked whether the maturation and trafficking of BRI2 and APP have any bearing on the formation of BRI2–APP complexes. To this end we transfected HeLa cells with APP and a Flag-BRI2-myc construct (tagged with a Flag epitope at the NH2-terminus and a myc epitope at the COOH-terminus, Fig. 2A) so that the myc epitope will be cleaved off by convertases in the GC. Cell lysates were precipitated with either αFlag, which isolated both mBRI2 and imBRI2 (Fig. 2B), or anti-myc antibody, which precipitated only imBRI2 (Fig. 2C). Significantly, mAPP and C99, but not imAPP and C83, were co-purified when BRI2 was isolated with anti-Flag (Fig. 2B) but not anti-myc (Fig. 2C). Conversely, mBRI2, but not imBRI2, co-precipitated with APP (Fig. 2B and C). Taken together, our data demonstrate that mBRI2 and mAPP interact while imBRI2 and imAPP do not. Thus, BRI2–APP complexes form after the maturation processes of BRI2 and APP are completed at or downstream of the trans-Golgi.
Fig. 2.
Maturation of BRI2 and APP is necessary for their interaction. (A) Schematic representation of Flag-BRI2-myc, which has a Flag tag at the N-terminus and a myc tag at the C-terminus. (B) HeLa cells transfected with APP and Flag-BRI2-myc were immunoprecipitated with anti-HA, anti-Flag, rabbit control (RP) or anti-APPct antibodies. Mouse monoclonal anti-HA and rabbit polyclonal (RP) were used as negative controls for mouse monoclonal αFlag and αAPPct in immunoprecipitation experiments. (C). HeLa cells were transfected as in (B). The total lysates were immunoprecipitated with anti-HA, anti-myc, RP and αAPPct. APP and Flag-BRI2-myc were detected with 22C11 and anti-Flag immunoblots, respectively.
3.2. Mature BRI2 and mature APP interact in “secretase compartments”
In light of our findings, we asked whether mBRI2 and mAPP interact at the cell surface and in the endocytic organelles, where mAPP is cleaved by secretases. HeLa cells co-transfected with BRI2 and APP constructs were surface biotinylated, and the lysates were immunoprecipitated with anti-Flag. BRI2–APP complexes were eluted with the Flag peptide (fraction E). The eluted material was further precipitated with streptavidin (SA) beads. After precipitation and centrifugation, proteins bound to SA (pellet fraction B) and unbound proteins (supernatant fraction U), were recovered. Analysis of the total lysate (T) and fractions E, U and B confirmed that mAPP and C99, but not imAPP and C83, interacted with BRI2 (fraction E) and that mBRI2, but not imBRI2, was present on the cell surface (fraction B, Fig. 3A). mAPP–BRI2 complexes were detected in both the intracellular compartments (fraction U) as well as on the cell surface (fraction B). Notably, C99–BRI2 complexes are detected in intracellular compartments (fraction U), but not on the plasma membrane (fraction B, Fig. 3A). In a complimentary experiment to analyze internalized proteins, biotinylated cells were incubated at 37 °C for either 0, 20 or 60 min and then treated with glutathione to remove biotin from cell membrane proteins. Hence, only internalized proteins are biotinylated. BRI2 bound to biotinylated mAPP after the cells were incubated at 37 °C (fraction B, 20 and 60 min time points, Fig. 3B). Thus, internalized mAPP is complexed to mBRI2 in endocytic compartments. Notably, mBRI2–C99 complexes are only detected intracellularly (overexposed B fraction, 60 min incubation, Fig. 3B). This biotinylated C99 probably originates from cell membrane mAPP that was internalized and processed by β-secretase. Analysis of primary mouse dermal fibroblasts (MDFs) showed that endogenous mouse Bri2 specifically interacts with mAPP and C99, but not imAPP or C83 (Fig. 3C). Since mAPP is found in post-GC compartments, plasma membrane and endocytic vesicles, while C99 is generated mainly in endosomes, our data indicate that under basal conditions BRI2 and mAPP/C99 interact at the cell surface and endocytic compartments.
3.3. BRI2 inhibits APP processing at the plasma membrane and in endocytic compartments
To directly study whether mBRI2 interferes with mAPP processing at the plasma membrane and in endosomes, we labeled plasma membrane proteins with biotin and incubated biotinylated cells at 37 °C to permit internalization and processing of APP. Biotinylated proteins were isolated from both cell lysates and culture supernatant and analyzed by immunoblot with either αAPPct (cell lysates) or anti-sAPPα and anti-sAPPβ (culture supernatant) antibodies. In control transfected cells (Fig. 4A and B), biotinylated C83 and C99 peaked after 30 min of incubation and biotinylated sAPPα and sAPPβ accumulated steadily during the time-course of the experiments (Fig. 4C and D). These changes in levels of the different protein species reflect processing of biotinylated mAPP by either α-secretase or β-secretase. Biotinylated C83 and C99 decreased after 30 min of incubation, consistent with processing by β-secretase. In BRI2 transfected cells, biotinylated C83, sAPPα and sAPPβ levels were dramatically reduced at all time points, indicating inhibition of α- and β-cleavage of mAPP occurs at the plasma membrane and, possibly, after internalization (Fig. 4A–D). The evidence that the levels of biotinylated mAPP decay less rapidly in BRI2 transfected cells support these conclusions (Fig. 4C). Biotinylated C99 levels were initially reduced, consistent with inhibition of β-processing. Strikingly however, C99 steadily accumulated during the 120 min chase (Fig. 4A and B). The stabilization of C99 indicates BRI2 inhibits β-processing of C99. Notably, C83 is not stabilized by BRI2 (Fig. 4A). This is consistent with the fact that BRI2 does not interact with C83 and therefore cannot inhibit γ-cleavage of C83.
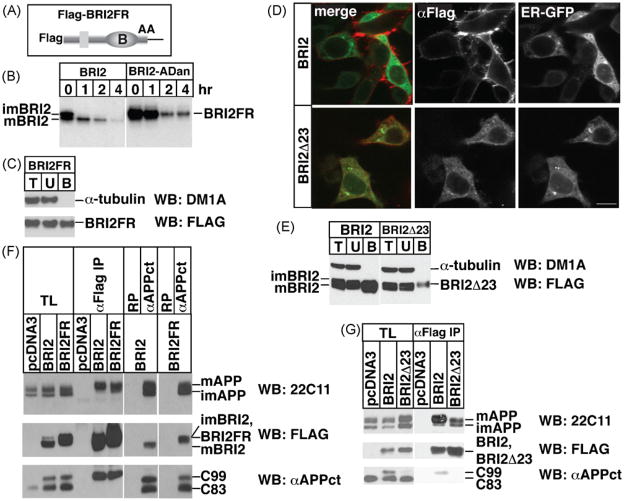
To further refine where BRI2 interacts with APP, we investigated whether compartmentally restricted mutants of BRI2 affected C99 processing. We employed two BRI2 mutants. BRI2FR is a convertase-resistant BRI2 mutant (Fig. 5A). Pulse chase of metabolically labeled transfected cells shows that BRI2FR consists of a single polypeptide that co-migrates with imBRI2, attesting that the mutations have abolished cleavage by convertases (Fig. 5B). Yet, biotinylation of transfected cells reveals that BRI2FR readily traffics to the plasma membrane (Fig. 5C) demonstrating that convertase-cleavage is not required for export of BRI2 to the cell surface. The second mutant, BRI2ΔC23, lacks the COOH-terminal peptide clipped off by convertase and, therefore, corresponds to mBRI2. While immunofluorescence of wild type BRI2 robustly stains the cell surface, BRI2ΔC23 accumulates intracellularly in a pattern that overlaps with the endoplasmic reticulum marker ER-GFP (Fig. 5D). Little, if any, BRI2ΔC23 staining is observed at the plasma membrane. Consistent with the immunofluorescence data, cell surface biotinylation labels a large pool of BRI2, but poorly labels BRI2ΔC23 (Fig. 5E). Together, these data indicate BRI2ΔC23 is inefficiently transported to the cell surface (Fig. 5E). In transfected cells, inhibition of C99 processing by γ-secretase is evident by the accumulation of C99 (Fig. 5F and G). Our data support the hypothesis that the mBRI2–mAPP/C99 complexes form on the plasma membrane and in endocytic vesicles, and that processing of mAPP and C99 complexed to mBRI2 is inhibited. BRI2FR efficiently interacts with mAPP, as well as C99, and inhibits C99 processing by γ-secretase (Fig. 5F) while ER-retained BRI2ΔC23 binds APP poorly and fails to inhibit APP processing (Fig. 5G). The findings that BRI2ΔC23 is retained in the ER and does not inhibit APP processing help explain why BRI2ΔC23 fails to reduce formation of pathogenic amyloid plaques in the brain of the AD mouse model (Kim et al., 2008).
Fig. 5.
Efficient export of BRI2 out of ER is required to inhibit APP processing. (A) Schematic representation of Flag-BRI2FR (K242 and R243 are replaced by alanines). (B) Pulse-chase experiments of HeLa cells transfected with either Flag-BRI2 or Flag-BRI2FR. (C) HeLa cells were transfected with Flag-BRI2FR, surface biotinylated, lysed and the lysates were precipitated with streptavidin, as in Fig. 1B. (D) BRI2 localizes to the cell surface and displays no overlap with the ER marker, ER-GFP. In contrast, BRI2D23 accumulates intracellularly and exhibits colocalization with ER-GFP. HEK cells were transfected with Flag-BRI2 or Flag-BRI2Δ23 together with ER-GFP, and stained with anti-Flag. Scale bar = 10 μm. (E) BRI2Δ23 is poorly presented on the cell surface. HeLa cells were transfected with Flag-BRI2 or Flag-BRI2Δ23. After surface biotinylation and lysis, cell lysates were precipitated with streptavidin beads. T, U and B fractions were probed for α-tubulin and BRI2 or BRI2Δ23 by immunoblot with DM1A and anti-Flag, respectively. α-Tubulin was not biotinylated. Eight times more sample was loaded in the B fractions as compared to T and U fractions. (F) HeLa cells were transfected with Flag-BRI2 or Flag-BRI2FR together with APP. The cell lysates were immunoprecipitated with the indicated antibodies. Total lysates (T) and precipitants were probed for APP, BRI2, and APP CTFs with 22C11, anti-Flag, and αAPPct, respectively. (G) HeLa cells were transfected with pcDNA3, Flag-BRI2, or Flag-BRI2Δ23, together with APP. The total cell lysates (T) and anti-Flag immunoprecipitates (anti-Flag IP) were probed for APP (22C11), BRI2 (anti-Flag), and APP CTF (αAPPct) by immunoblots.
In summary, we demonstrated that maturation and transport of BRI2 along the secretory pathway is essential for generating a competent inhibitor of APP processing. mBRI2 localized to the plasma membrane and in endocytic vesicles where it forms heteromeric mBRI2/mAPP and mBRI2/C99 complexes. Complex formation enables mBRI2 to function as an inhibitor of mAPP cleavage by both α- and β-secretases and protects C99 from γ-processing. Notably, an artificial ER-retained mutant, BRI2Δ23 poorly interacts with mAPP and does not inhibit APP processing. Our data, together with previous findings showing an anti-amyloidogenic effect of the BRI2–23 peptide, suggest that maturation of BRI2 generates two BRI2 metabolites with distinct anti-amyloidogenic activities: mBRI2, which interferes with Aβ42 production, and BRI2–23, which prevents aggregation of Aβ42 into toxic oligomers (Fig. 6). These results provide important new insights into the mechanisms regulating APP metabolism and Aβ production in the central nervous system and establish BRI2 as a potentially important molecular target for an anti-amyloid therapy in AD.
Fig. 6.
A model depicting the anti-amyloidogenic functions of BRI2. N-glycosylated ( ) imAPP localizes in the endoplasmic reticulum (ER) and cis-Golgi. N- and O-glycosylated (
) imAPP localizes in the endoplasmic reticulum (ER) and cis-Golgi. N- and O-glycosylated ( ) mAPP resides in compartments following trans-Golgi and on the plasma membrane (Tomita et al., 1998). Immature BRI2 is processed by a convertase in the secretory pathway at the carboxyl-terminus between R243 and E244 (Kim et al., 1999; Choi et al., 2004) to generate the soluble Bri2–23 peptide and a membrane-bound mBRI2. APP and BRI2 interact only after both molecules have matured. While mBRI2 inhibits processing of APP by all secretases (reducing therefore every APP metabolite including Aβ), Bri2–23 can antagonize formation of Aβ oligomers. The absence of BRI2, like in Bri2 KO mice, results in increased levels of APP metabolites (upper left panel). Molecules and molecules regions are not drawn to scale.
) mAPP resides in compartments following trans-Golgi and on the plasma membrane (Tomita et al., 1998). Immature BRI2 is processed by a convertase in the secretory pathway at the carboxyl-terminus between R243 and E244 (Kim et al., 1999; Choi et al., 2004) to generate the soluble Bri2–23 peptide and a membrane-bound mBRI2. APP and BRI2 interact only after both molecules have matured. While mBRI2 inhibits processing of APP by all secretases (reducing therefore every APP metabolite including Aβ), Bri2–23 can antagonize formation of Aβ oligomers. The absence of BRI2, like in Bri2 KO mice, results in increased levels of APP metabolites (upper left panel). Molecules and molecules regions are not drawn to scale.
Acknowledgments
Support for this research was provided by: the NIH grants NIA RO1 AG22024 (L.D.), NIA RO1 AG21588 (L.D.), NIA R21 AG027139 (L.D.), American Health Assistance Foundation (AHAF) A2003-076 (L.D.) and Alzheimer’s Association IIRG-05-14511 (L.D.). E.L.S. is an Ellison Medical Foundation New Scholar in Aging and is supported by NIA R21 1R21AG032544-01. We thank Samuel Hocine for technical assistance in producing the GFP-BRI2 and GFP-BRI2-ADan constructs.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.neurobiolaging. 2009.08.005.
Footnotes
Disclosure statement: Dr. D’Adamio is a co-founder of Remegenix. Dr. D’Adamio and Dr. Shuji Matsuda are inventors of a patent on BRI2 owned by the AECOM.
References
- Akiyama H, Kondo H, Arai T, Ikeda K, Kato M, Iseki E, Schwab C, McGeer PL. Expression of BRI, the normal precursor of the amyloid protein of familial British dementia, in human brain. Acta Neuropathol (Berl) 2004;107:53–58. doi: 10.1007/s00401-003-0783-1. [DOI] [PubMed] [Google Scholar]
- Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- Choi SI, Vidal R, Frangione B, Levy E. Axonal transport of British and Danish amyloid peptides via secretory vesicles. FASEB J. 2004;18:373–375. doi: 10.1096/fj.03-0730fje. [DOI] [PubMed] [Google Scholar]
- Cole SL, Vassar R. The Alzheimer’s disease beta-secretase enzyme, BACE1. Mol Neurodegener. 2007;2:22. doi: 10.1186/1750-1326-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma–secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- Deleersnijder W, Hong G, Cortvrindt R, Poirier C, Tylzanowski P, Pittois K, Van Marck E, Merregaert J. Isolation of markers for chondro-osteogenic differentiation using cDNA library subtraction. Molecular cloning and characterization of a gene belonging to a novel multigene family of integral membrane proteins. J Biol Chem. 1996;271:19475–19482. doi: 10.1074/jbc.271.32.19475. [DOI] [PubMed] [Google Scholar]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Fotinopoulou A, Tsachaki M, Vlavaki M, Poulopoulos A, Rostagno A, Frangione B, Ghiso J, Efthimiopoulos S. BRI2 interacts with amyloid precursor protein (APP) and regulates amyloid beta (Abeta) production. J Biol Chem. 2005;280:30768–30772. doi: 10.1074/jbc.C500231200. [DOI] [PubMed] [Google Scholar]
- Fuentealba RA, Barria MI, Lee J, Cam J, Araya C, Escudero CA, Inestrosa NC, Bronfman FC, Bu G, Marzolo MP. ApoER2 expression increases Abeta production while decreasing Amyloid Precursor Protein (APP) endocytosis: possible role in the partitioning of APP into lipid rafts and in the regulation of gamma-secretase activity. Mol Neurodegener. 2007;2:14. doi: 10.1186/1750-1326-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hardy J. Alzheimer’s disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis. 2006;9:151–153. doi: 10.3233/jad-2006-9s317. [DOI] [PubMed] [Google Scholar]
- Kaether C, Schmitt S, Willem M, Haass C. Amyloid precursor protein and Notch intracellular domains are generated after transport of their precursors to the cell surface. Traffic. 2006;7:408–415. doi: 10.1111/j.1600-0854.2006.00396.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Miller VM, Levites Y, West KJ, Zwizinski CW, Moore BD, Troendle FJ, Bann M, Verbeeck C, Price RW, Smithson L, Sonoda L, Wagg K, Rangachari V, Zou F, Younkin SG, Graff-Radford N, Dickson D, Rosenberry T, Golde TE. BRI2 (ITM2b) inhibits Abeta deposition in vivo. J Neurosci. 2008;28:6030–6036. doi: 10.1523/JNEUROSCI.0891-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Wang R, Gordon DJ, Bass J, Steiner DF, Lynn DG, Thinakaran G, Meredith SC, Sisodia SS. Furin mediates enhanced production of fibrillogenic ABri peptides in familial British dementia. Nat Neurosci. 1999;2:984–988. doi: 10.1038/14783. [DOI] [PubMed] [Google Scholar]
- Lee DS, Tomita S, Kirino Y, Suzuki T. Regulation of X11L-dependent amyloid precursor protein metabolism by XB51, a novel X11L-binding protein. J Biol Chem. 2000;275:23134–23138. doi: 10.1074/jbc.C000302200. [DOI] [PubMed] [Google Scholar]
- Leissring MA, Murphy MP, Mead TR, Akbari Y, Sugarman MC, Jannatipour M, Anliker B, Muller U, Saftig P, De Strooper B, Wolfe MS, Golde TE, LaFerla FM. A physiologic signaling role for the gamma-secretase-derived intracellular fragment of APP. Proc Natl Acad Sci USA. 2002;99:4697–4702. doi: 10.1073/pnas.072033799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Robakis NK. Genetic and molecular aspects of Alzheimer’s disease shed light on new mechanisms of transcriptional regulation. Genes Brain Behav. 2005;4:134–146. doi: 10.1111/j.1601-183X.2005.00086.x. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Giliberto L, Matsuda Y, Davies P, McGowan E, Pickford F, Ghiso J, Frangione B, D’Adamio L. The familial dementia BRI2 gene binds the Alzheimer gene amyloid-beta precursor protein and inhibits amyloid-beta production. J Biol Chem. 2005;280:28912–28916. doi: 10.1074/jbc.C500217200. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Giliberto L, Matsuda Y, McGowan EM, D’Adamio L. BRI2 inhibits amyloid beta-peptide precursor protein processing by interfering with the docking of secretases to the substrate. J Neurosci. 2008;28:8668–8676. doi: 10.1523/JNEUROSCI.2094-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passer B, Pellegrini L, Russo C, Siegel RM, Lenardo MJ, Schettini G, Bachmann M, Tabaton M, D’Adamio L. Generation of an apoptotic intracellular peptide by gamma-secretase cleavage of Alzheimer’s amyloid beta protein precursor. J Alzheimers Dis. 2000;2:289–301. doi: 10.3233/jad-2000-23-408. [DOI] [PubMed] [Google Scholar]
- Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Podlisny MB. Deciphering the genetic basis of Alzheimer’s disease. Annu Rev Genomics Hum Genet. 2002;3:67–99. doi: 10.1146/annurev.genom.3.022502.103022. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia SS, St George-Hyslop PH. Gamma-secretase, Notch, Abeta and Alzheimer’s disease: where do the presenilins fit in? Nat Rev Neurosci. 2002;3:281–290. doi: 10.1038/nrn785. [DOI] [PubMed] [Google Scholar]
- Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 2008;60:534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George-Hyslop PH, Petit A. Molecular biology and genetics of Alzheimer’s disease. C R Biol. 2005;328:119–130. doi: 10.1016/j.crvi.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Tomita S, Kirino Y, Suzuki T. Cleavage of Alzheimer’s amyloid precursor protein (APP) by secretases occurs after O-glycosylation of APP in the protein secretory pathway. Identification of intracellular compartments in which APP cleavage occurs without using toxic agents that interfere with protein metabolism. J Biol Chem. 1998;273:6277–6284. doi: 10.1074/jbc.273.11.6277. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Gong P, Bowen JW, Cheng H, Chen Y, Carter M, Nguyen PD, Placanica L, Wieland FT, Li YM, Kounnas MZ, Thinakaran G. Dual roles of the transmembrane protein p23/TMP21 in the modulation of amyloid precursor protein metabolism. Mol Neurodegener. 2007;2:4. doi: 10.1186/1750-1326-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal R, Calero M, Revesz T, Plant G, Ghiso J, Frangione B. Sequence, genomic structure and tissue expression of human BRI3, a member of the BRI gene family. Gene. 2001;266:95–102. doi: 10.1016/s0378-1119(01)00374-2. [DOI] [PubMed] [Google Scholar]
- Vidal R, Frangione B, Rostagno A, Mead S, Revesz T, Plant G, Ghiso J. A stop-codon mutation in the BRI gene associated with familial British dementia. Nature. 1999;399:776–781. doi: 10.1038/21637. [DOI] [PubMed] [Google Scholar]
- Vidal R, Revesz T, Rostagno A, Kim E, Holton JL, Bek T, Bojsen-Moller M, Braendgaard H, Plant G, Ghiso J, Frangione B. A decamer duplication in the 3° region of the BRI gene originates an amyloid peptide that is associated with dementia in a Danish kindred. Proc Natl Acad Sci USA. 2000;97:4920–4925. doi: 10.1073/pnas.080076097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham L, Benjannet S, Marcinkiewicz E, Chretien M, Seidah NG. Beta-amyloid protein converting enzyme 1 and brain-specific type II membrane protein BRI3: binding partners processed by furin. J Neurochem. 2005;92:93–102. doi: 10.1111/j.1471-4159.2004.02840.x. [DOI] [PubMed] [Google Scholar]
- Wilquet V, De Strooper B. Amyloid-beta precursor protein processing in neurodegeneration. Curr Opin Neurobiol. 2004;14:582–588. doi: 10.1016/j.conb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Wolfe MS. When loss is gain: reduced presenilin proteolytic function leads to increased Abeta42/Abeta40. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007;8:136–140. doi: 10.1038/sj.embor.7400896. [DOI] [PMC free article] [PubMed] [Google Scholar]