Abstract
Objectives
Therapy with broad-spectrum antibiotics is a common practice for premature infants. This treatment can reduce the biodiversity of the fecal microbiota and may be a factor in the cause of necrotizing enterocolitis (NEC). In contrast, probiotic treatment of premature infants reduces the incidence of NEC. We hypothesized that one mechanism for these observations is the influence of bacteria on postnatal development of the mucosal immune system.
Methods
Expression of immune molecules and microbial sensors was investigated in the postnatal mouse gastrointestinal tract by real-time PCR. Subsequently, two-week-old specific pathogen free (SPF) and microbial reduced (antibiotic treated, MR) mice were compared for immune molecule and microbial sensor expression, mesenteric lymph node (MLN) T-cell numbers and activation, intestinal barrier function/permeability, systemic lymphocyte numbers, and T-cell phenotype commitment.
Results
Toll-like receptor (TLR) 2, 4, and 5 expression was highest in 2-week-old SPF mice, and this expression was decreased in MR mice. There was no difference in intestinal tight-junctional function, as evaluated by FITC-dextran uptake, but MR mice had increased bacteria translocation across the intestinal epithelial barrier. MR mice had significantly fewer splenic B-cells and MLN CD4+ T-cells, but there were normal numbers of splenic T-cells. These systemic T-cells from MR mice produced more IL-4, and less interferon-γ and IL-17, indicative of a maintenance of the fetal, T-helper cell type 2 phenotype.
Conclusions
This study shows that intestinal commensal microbiota have an influence on early postnatal immune development. Determining specific bacteria and/or bacterial ligands critical for this development could provide insight into the mechanisms by which broad-spectrum antibiotics and/or probiotic therapy influences the development of the mucosal immune system and mucosal-related diseases.
Keywords: Toll-like receptors, NEC, probiotics, commensal microbiota, antibiotics
Introduction
Following birth, the sterile newborn intestine is colonized by environmental microorganisms that expand exponentially to numbers that exceed the total number of host mammalian cells 1. The current paradigm holds that a state of mutualism is essential for overall health, growth, and development 2. Acquiring intestinal microbiota is an active process where host and microbial factors mediate the intestinal bacterial composition 3.
The systemic and mucosal immune functions of newborns are markedly different than adults 4. The fetal immune system has evolved to avoid maternal rejection rather than to process potential pathogens 5. The neonatal immune system undergoes extensive postnatal development 6 and the acquisition of intestinal microbiota is a major determinant of early immune development 7. For example, the T cell population of the fetus favors a naïve or T-helper cell 2 (Th2) phenotype which undergoes a switch to a T-helper 1 (Th1) or T-helper 17 (Th17) phenotype upon exposure to intestinal microbiota to facilitate protection against potential injurious microorganisms 8. The classic Th1, Th2, and Th17 phenotypes are largely defined by their associated cytokine production: Th1 (interferon-γ (IFN-γ)), Th2 (interleukin-4 (IL-4)), and Th17 (IL-17). The first step in this phenotype commitment is believed to be the interaction between commensal bacteria and the mucosal immune system. The microbial sensors known as toll-like receptors (TLRs) sense specific bacterial products, i.e. peptidoglycan (TLR2), lipopolysaccharide (LPS)(TLR4), flagellin (TLR5), with a resultant innate immune response mediated in large part by chemokine production 9.
Extremely premature human infants have a different postnatal experience than term infants. They often receive broad spectrum antimicrobials and experience delayed feedings. Thus, these infants acquire a skewed commensal microbiota which we hypothesize could adversely affect the developing mucosal and systemic immune function. This phenomenon may in part explain in a high incidence of necrotizing enterocolitis (NEC) in this patient population10. Recent reports of probiotic therapy reducing the incidence of NEC in premature infants 11, 12give credence to the notion that acquiring intestinal commensal bacteria in the early postnatal period are of great importance. In addition, recent studies from our laboratory have demonstrated that 2-week old mice lacking TLR2, TLR4, or both TLR2 and 4, as well as microbial reduced mice have increased intestinal injury in a model of NEC (Tatum, PM, et.al, in press, Journal of Pediatric Surgery). These results support the idea that the presence of commensal bacteria and the sensing of those bacteria through TLRs is important in preventing NEC.
Previous work examining the role of commensal microbiota in the development of immune function have utilized adult germfree mice or animals colonized with a defined microbiota (gnotobiotic) 13. These studies have indicated that in the absence of a commensal microbiota, mice have decreased IgA, reduced intraepithelial lymphocytes, reduced Peyer’s patch and mesenteric lymph node (MLN) size and cellularity, decreased responses to T-cell mitogens, and altered susceptibility to pathogenic organisms 14, 15. As the neonatal immune system is not fully developed during normal acquisition of commensal microbiota, these studies on fully developed adults may not accurately reflect the outcome of this crucial interaction. Previous studies in suckling rats have demonstrated that antibiotic induced microbial restriction during early neonatal development results in decreased Paneth cell products 16. Additionally, it has been reported in C57BL/6 mice that exposure to microbial products after vaginal delivery transiently increases chemokine expression by intestinal epithelial cells; a result that is not seen after a sterile cesarean delivery 17. However, the effects of these colonizing microorganisms and their microbial products on the initial establishment of mucosal and systemic immunity have not been evaluated. As some components of the developing postnatal murine immune system are similar to that of premature human infants, it is a useful model for the investigation of the establishment of this ecosystem 18. The aim of this study is to characterize early postnatal mucosal and systemic immune function during the neonatal period when mice are acquiring their intestinal microbiota. We then manipulated exposure to these microbiota during this period by antibiotic treatment of pups (microbial reduced (MR)) to recapitulate the clinical course of premature infants. We hypothesized that newborn mice that have altered acquisition of normal microbiota will demonstrate significant changes in postnatal immune development.
Materials and Methods
Mice
Adult C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME), raised under specific pathogen free (SPF) conditions, and acclimatized to our facility 2 weeks prior to mating. SPF mice were chosen because they are known to be free of bacterial, viral, and parasitic mouse pathogens, as opposed to “conventional” mice, which are not known to be free of pathogens. SPF conditions, as defined by the UAB Animal Resources Program, can be found at http://main.uab.edu/Sites/ComparativePathology/surveillance/. The pups were born and housed under SPF or experimental conditions (as noted) and nursed until time of sacrifice. All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Alabama at Birmingham.
RNA isolation and gene expression
RNA isolation and gene expression by quantitative real-time reverse-transcriptase polymerase chain reaction (RT-PCR) was performed as previously described 38,49. Briefly, small intestine and colon were harvested for analysis of the expression of microbial sensors and inflammatory molecules. RNA was isolated, genomic DNA contamination was removed, and then RNA was transcribed into cDNA. RT-PCR was performed using Applied Biosystems® primer probe sets. Specific gene RNA levels were determined using crossing thresholds read by the RT cycler MX3000P® (Stratagene®, La Jolla, CA). Fluorescence thresholds were averaged generating a gene specific numeral, which then could be normalized to the average expression of the 18S housekeeping gene and further stratified by particular strain and experimental condition. Gene expression was calculated as an average fold change when compared to control strain values, and shown on a log 2 scale as fold changes from the control baseline (=1). The protocol for this data analysis format is provided in the Applied Biosystems manufacturer’s instructions (4371095 Rev A, PE Applied Biosystems).
Epithelial versus lamina propria TLR expression
Small intestine (SI) and colonic epithelium was removed to determine the mucosal location of TLR expression at two weeks of age. Briefly, SI and colon from two week old animals were removed and opened along the anti-mesenteric border. The tissue was then placed in Hank’s balanced salt solution-plus (HBBS+) (HBSS 500 mL, 25mM HEPES, 1% fetal bovine serum (HyClone Laboratories, Logan, UT), 100U penicillin, and 100 µg streptomycin). The tissue was gently inverted several times to remove the luminal contents. To remove the epithelial layer, the tissue was then transferred to HBSS w/o calcium and magnesium + 5mM EDTA and agitated at 100 RPM for 15 minutes at 37°C. The tissue was removed and the supernatant, which contained the epithelium, was saved on ice. The process was repeated and the final volume of supernatant underwent centrifugation at 1,500 RPM for 5 minutes at 4°C. The pellet was re-suspended in Trizol™ for RNA isolation (as above). Adequate removal of epithelium was confirmed by hematoxylin and eosin (H&E) staining of histologic sections of the remaining tissue.
Microbial Reduction
Antibiotics were added to the drinking water based on a modification of Hans, et al 20. Microbially reduced (MR) animals were maintained by the addition of streptomycin sulfate (4.8 mg/mL), ampicillin (1.2mg/mL), metronidazole (1.2mg/mL), and vancomycin (0.6mg/mL) (Sigma, St. Louis, MO) to sterile drinking water ad libitum and changed bi-weekly. Antibiotic treatment of female C57BL/6 mice was initiated 5 days following mating and maintained throughout the experimental period, including the nursing period up until sacrifice of the pups. The choice of these antibiotics was based on an approximation of the early postnatal experience in extremely premature human infants in the NICU.
Analysis of fecal samples by PCR-Denaturing gel electrophoresis (PCR-DGGE)
DNA extraction of fecal samples for PCR-DGGE analysis was performed as described 21. Primers were used for PCR amplification targeting different variable regions of the 16S rDNA. The forward primer sequence was ACTACGTGCCAGCAGCCCGCCGGGCCGCGGCCCGCCCGCCCGCGGGGGCACGGGGGACTACGTGCCAGCAGCC and the reverse sequence was GGACTACCAGGG TATCTAATCC. Analysis of PCR products was performed using a gradient denaturant gel which was scanned using a GS-710 Calibrated Imaging Densitometer® (Bio-Rad Inc., Hercules, CA). Selected bands were excised from the gel and DNA extracted via a crush soak process. The DNA was used for an additional PCR using the above primers joined to M13 vector sequences as previously described 22 and the sequence analyzed using the online Ribosomal Database Project provided by the Center for Microbial Ecology at Michigan State University (http://rdp.cme.msu.edu/).
Flow cytometry
To conduct lymphocyte population analysis spleens and MLNs underwent mechanical disruption, erythrocyte lysis and preparation for flow cytometry as previously described 50. Lymphocytes were labeled for the lymphocyte markers CD4, CD45, CD8, CD3, CD19, CD44, CD45, CD62L, or CD69 (BD Biosciences®, San Diego, CA). Flow cytometry was performed using the Becton Dickson FACs Caliber® (Franklin Lakes, NJ) utilizing the Cell Quest® analysis software (San Diego CA) and analyzed with FlowJo® software (Tree Star, Inc, Ashland, OR).
Bacterial translocation and barrier permeability experiments
Green florescent protein (GFP) producing Escherichia coli (E. coli) (pET 21d/eTS GFP #4) were a gift from Dr. Casey Weaver (UAB) and cultured as described 23. GFP E. coli was inoculated into liquid Luria–Bertani broth (LB) with 100 mg/L ampicillin (Sigma-Aldrich, St. Louis, MO) and incubated at 37°C overnight with agitation. A final dilution of 5 ×1010 CFU/mL was prepared in sterile media, as determined by optical density at 450 nm. (OD450 /3 × 109 =CFU/mL)
Two-week old SPF and MR pups and their dams were orally inoculated with 1 × 108 CFU/mL of GFP E. coli on day 1, 2, 3, and 4. On day 1, antibiotic therapy was discontinued in the MR group prior to bacterial challenge. On day 6, the pups were sacrificed and individual mesenteric lymph nodes (MLNs), spleen, and liver were harvested using sterile technique. Cecal contents were obtained after the organ harvest. The organs and cecal contents were used to inoculate LB agar plates containing ampicillin and incubated overnight at 37°C. GFP producing colonies were confirmed by fluorescence. The presence of ≥ 1 GFP E. coli colony was defined as being positive for bacterial translocation for that organ.
Barrier permeability was assessed by analyzing serum concentrations of fluorescein isothiocyanate (FITC)-dextran (MW 4,000, Sigma-Aldrich, St. Louis, MO) following oral gavage as previously described 24. Briefly, mice were orally gavaged 4 hours prior to sacrifice using an 80mg/ml stock of FITC-dextran, for a total dose of 60mg/100g of body weight. Total blood volume was collected at sacrifice via cardiac puncture. Blood samples were coagulated in the dark at 4°C for one hour prior to centrifugation and serum harvest. Samples were analyzed using the Synergy™ HT Multi-Mode Microplate Reader (Bio-Tek Technologies, Winooski, Vermont) and standardized to FITC loaded control serum, FITC was detectable between 25-0.195ng/ml.
Splenocyte culture and cytokine analysis by ELISA
Spleens were harvested from SPF and MR neonates following sacrifice. A single cell suspension was filtered through a 70 µM Nitex® membrane (Tetko, Elmsford, NY). Erythrocytes were lysed using ammonium chloride and the splenocytes were washed twice in HBSS+. The cells were resuspended in R10 medium (RPMI 1640, 10% FBS, 1% GlutaMAX-I™ (Invitrogen™, Carlsbad, CA,) and 50 µg/mL gentamicin sulfate) and inoculated at 2×105 cells/well onto 96 well culture plates, coated with hamster αCD3 (clone 145-2C11) or hamster IgG control (clone UC8-4B3) at a concentration of 1 µg/mL (BD Biosciences, San Diego, CA). Cells were incubated at 37° C in a humidified atmosphere containing 5%CO2/95% air for 96 hours. Supernatants were analyzed for expression of IFN-γ, IL17, IL10, IL4, and IL2 with BioLegend® cytokine ELISA reagents and read at 450nm with a VERSA-Max® microplate reader (San Diego, CA) and analyzed using Softmax Pro® software (Molecular Devices, Sunnyvale, CA).
Statistical analysis
Sigma Stat 2.03 (SPSS Inc., Chicago, IL 60606 USA) software was used for data analysis. Student’s t-test and one-way ANOVA were used for continuous variables and Fisher’s exact test was used for categorical variables. Non-parametric analysis was performed with a Mann-Whitney test. Gene expression differences were analyzed by using Student’s test to compare ΔΔCT and standard deviation between the experimental and control animals. A p value < 0.05 was regarded as significant.
Results
Bacterial sensing molecules during early development
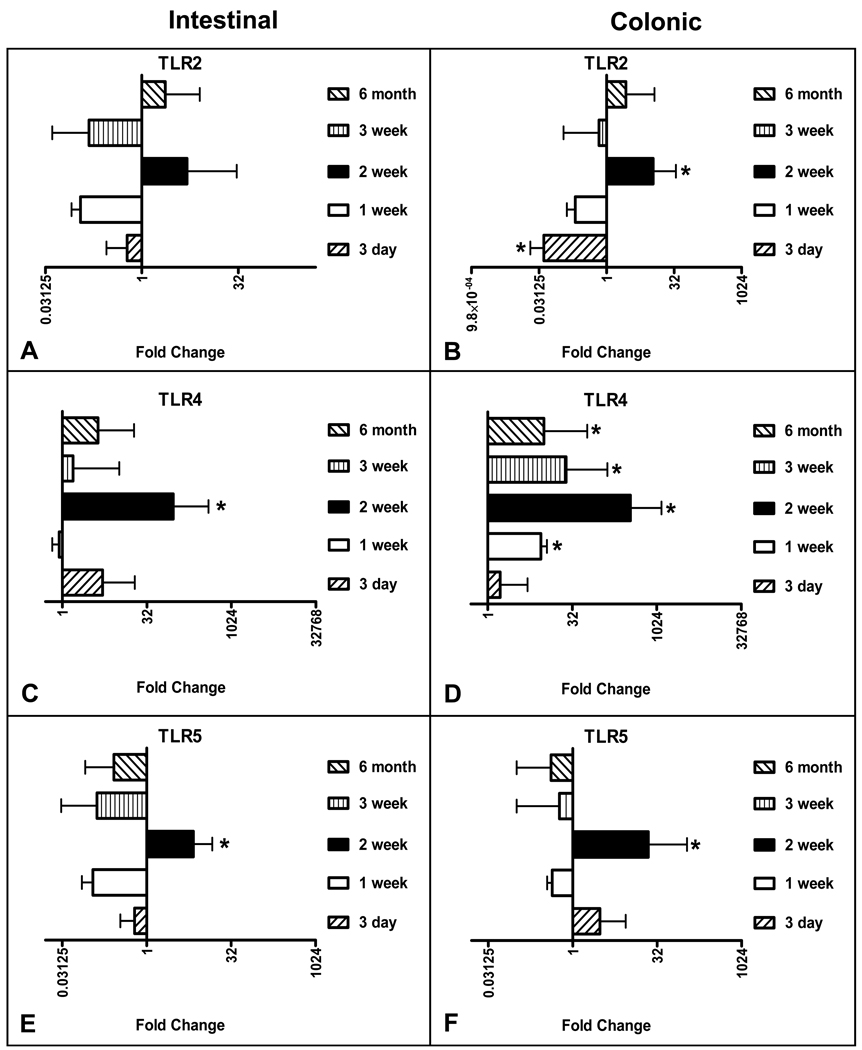
Previous work has shown that immature intestinal cells express a basal level of the LPS signaling molecule TLR4 25. Additionally, several investigators have demonstrated that the expression and responsiveness of TLR2 and TLR4 is regulated by exposure to bacterial product ligand 26–28. To determine the early postnatal response to commensal microbial colonization with regards to TLR expression, cohorts of SPF C57BL/6 mice were sacrificed at multiple developmental time points. RNA was harvested from the SI and colon after which gene expression was determined using RT-PCR analysis and values were normalized to E20.5 levels. This was done based on the assumption that prenatal organs at this stage would be fully developed, but are sterile due to a lack exposure to a commensal microbiota. Relative the E20.5 expression, TLR2, TLR4 and TLR5 were significantly elevated in the two week old mouse colon (Figure 1, B, D, and F). In addition, SI expression of TLR4 and TLR5 were also significantly greater in the two week old cohorts (Figure 1, C and E). While not achieving statistically significance, the same trend was observed with regards to SI TLR2 expression (Figure 1, A). These expression peaks were attenuated in the three week old animals and remained diminished into adulthood. Interestingly the increased level of expression at two weeks coincides with the period of microbial expansion in the postnatal murine gastrointestinal tract 29, 30. In addition, the relative organ specific expression of TLRs was consistent with increased microbial colonization in the colon compared to the SI. Thus, the induction of TLR expression at two weeks is greatest in the colon, the organ with the greatest number of commensal microbiota.
Figure 1.
Quantitative RT-PCR analysis of TLR gene expressin in SPF mice at various postnatal ages. (A) Small intestinal TLR2, (B) colonic TLR2, (C) small intestinal TLR4, (D) colonic TLR4, (E) small intestinal TLR5, (F) colonic TLR5. Data is expressed on a log2 scale and normalized to 18S rRNA expression, with fold change calculated by comparison with the level of expression in E20.5 mice. (E20.5 n=7, Day 3 n=8, Week 1 n=10, Week 2 n=13, Week 3 n=9, Week 6 n=7). The bar graph to the right represents increased expression and to the left decreased expression.
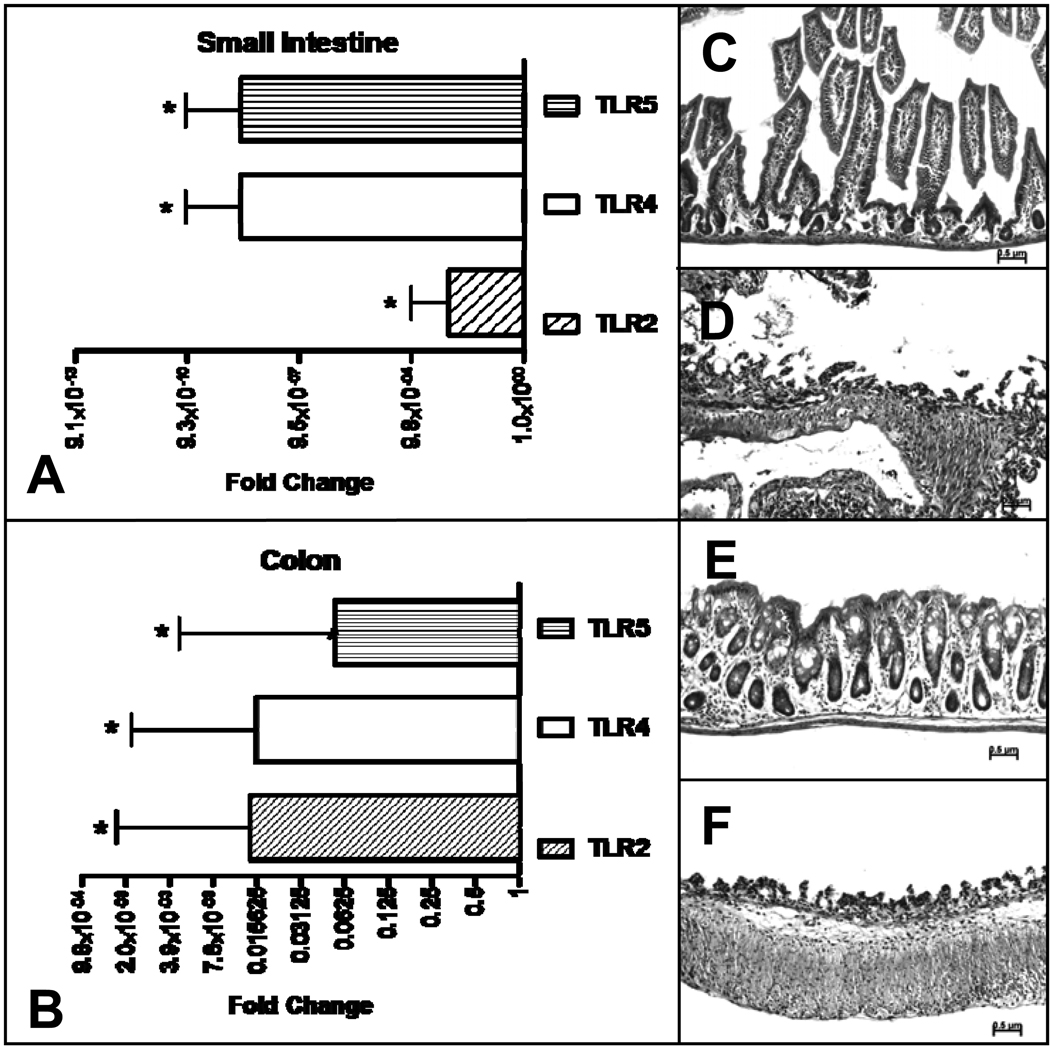
Epithelial versus lamina propria TLR expression at two week postnatal development
To determine the mucosal compartment of the increased TLR expression, separate cohorts of two week old pups raised in the SPF environment were studied. After isolation of the epithelium, the relative expression of epithelial derived TLR2, TLR4, and TLR5 were compared to that of the total SI or colon. Relative to that of the total SI or colon, the epithelial expression of TLR2, TLR4, and TLR5 was significantly less, indicating that the majority of the increased expression was a result of induction of TLRs in the cells of the lamina propria (Figure 2, A,B). The removal of the epithelium by EDTA treatment was confirmed in each sample by histological examination (Figure 2, C,D,E,F).
Figure 2.
Quantitative RT-PCR analysis of TLR gene expression in SPF mice, comparing epithelial expression to total small intestinal or colonic expression. (A) Small intestinal TLR 2, 4, and 5 expression, (B) colonic TLR 2, 4, and 5 expression, (C) H & E of small intestinal section without and (D) with EDTA digestion. (E) and (F) are similar sections of colon without and with EDTA digestion. RT-PCR data is expressed in log2 scale and normalized to 18S rRNA expression with fold change calculated comparing epithelial expression to total intestinal or colonic expression, n=6. The bar graph to the right represents increased expression and to the left decreased expression.
Commensal intestinal microbial reduction using antibiotics
The developmental experiments above identified the two week period as a potential check point of microbial-host interaction. Therefore we set out to reproduce a simulated microbial reduced environment during this period by using antibiotics added to recently bred dam drinking water. This replicated the broad-spectrum antibiotic treatment that is observed in the early postnatal period of premature human neonates.
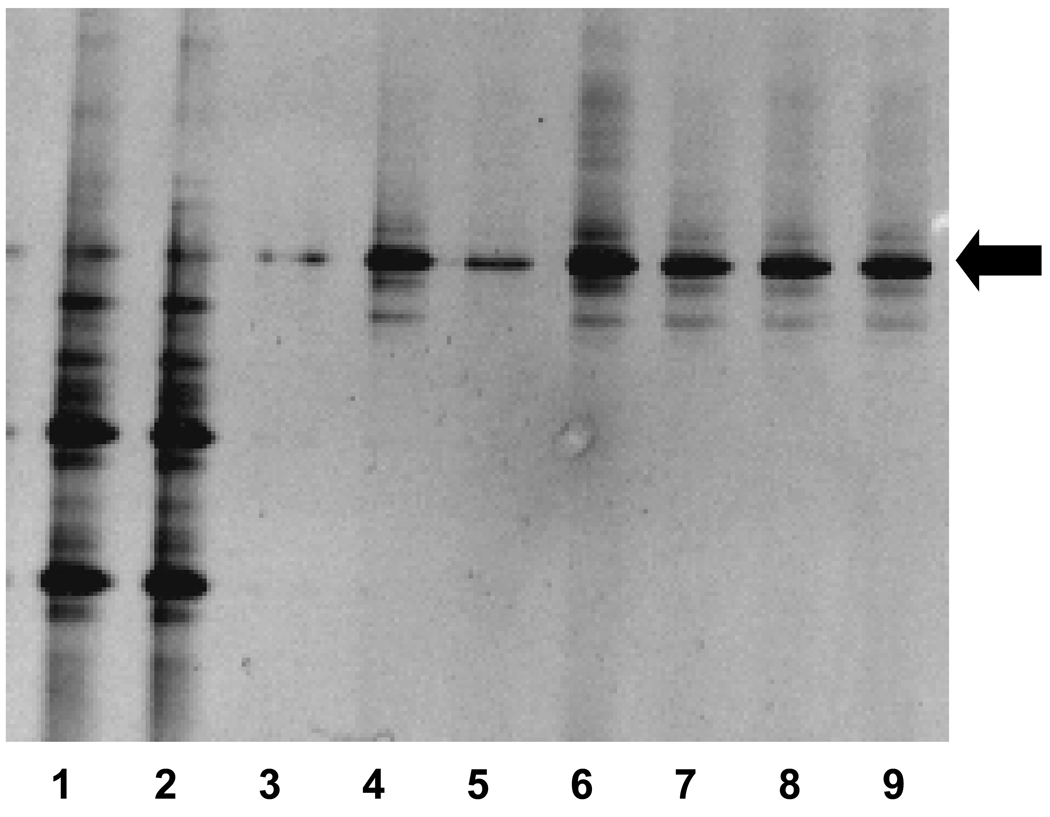
To determine the alteration in maternal microbiota induced by the broad-spectrum antibiotic treatment, feces from the MR and SPF 2-week old mice were analyzed for the presence and diversity of bacteria using amplification of 16s rRNA. Following PCR with conserved bacterial 16s rRNA primers, a DGGE analysis revealed a relatively diverse microbial population in the SPF feces (Figure 3, Lanes 1,2) compared to that of MR mice (Figure 3, Lanes 3–9). A consistent band representing single bacterial species was observed in the MR mice (Figure 3, arrow). This band was also noted in the SPF mice. Sequence analysis revealed this band corresponded with Pseudomonas aeruginosa with 93% sequence similarity as found in the Ribosomal Database Project. Thus, the mice reared by dams receiving antimicrobial therapy had a marked reduction in microbial diversity with a selection for a potentially pathogenic bacterium.
Figure 3.
PCR-DGGE analysis of feces from 2-week-old SPF and MR mice. Lanes 1,2=SPF, lanes 3–9 MR. The black arrow indicates persistent banding pattern observed in the MR mice.
Microbial reduction results in alteration of bacterial sensor and immune function molecule expression
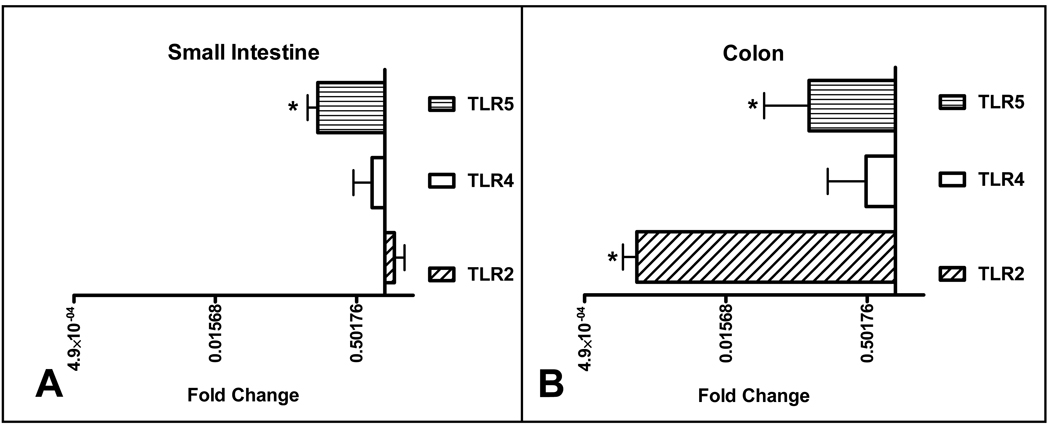
Since there was an increased expression of TLR2, TLR4, and TLR5 in 2-week old mice compared to the other cohorts, we hypothesized that MR mice would have less expression of these microbial sensing molecules. The SI expression of TLR5 in the MR mice was significantly less than the SPF controls, with no difference in TLR2 and TLR4 expression (Figure 4, A). In contrast, there was marked reduction in TLR2 and TLR5 in the MR colons with a trend toward less TLR4 expression. (Figure 4, B). These results support the notion that commensal microbiota are critical for the induced TLR expression observed at 2 weeks postpartum.
Figure 4.
Quantative RT-PCR analysis of TLR gene expression in MR mice compared to SPF. RT-PCR data is expressed in log2 scale and normalized to 18S rRNA expression with fold change calculated comparing MR to SPF. (MR n=10, SPF n=10). The bar graph to the right represents increased expression and to the left decreased expression.
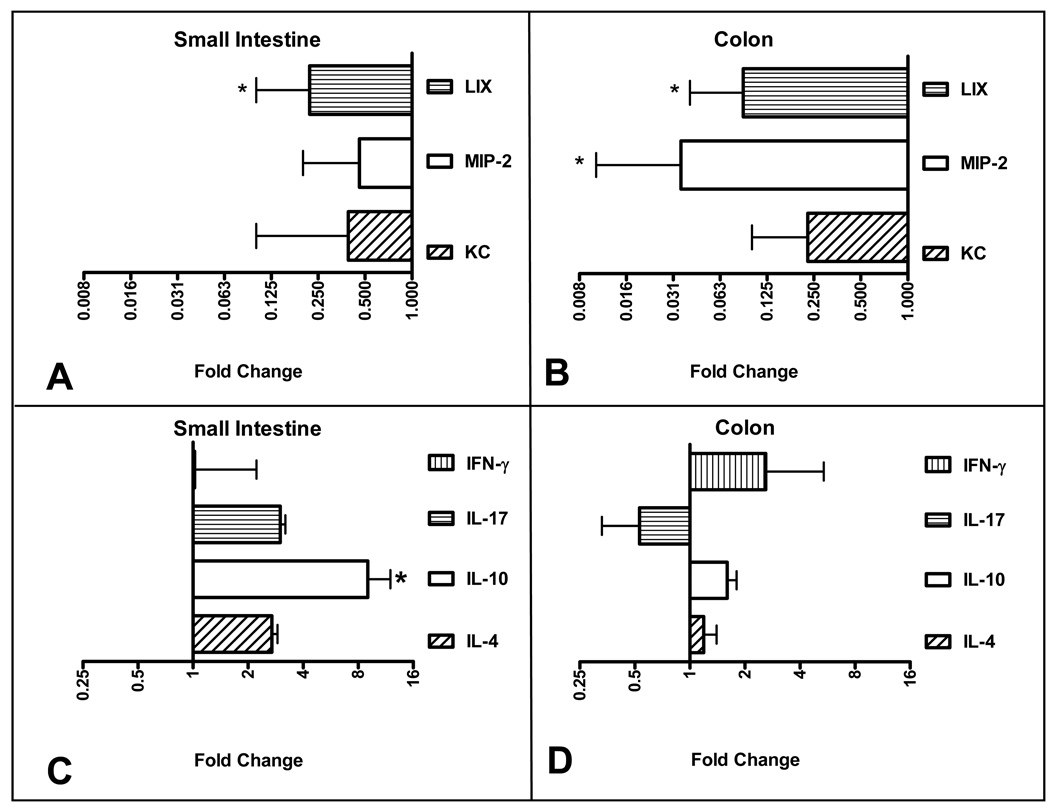
Bacterial signaling through TLRs results in an initial innate immune response, including the production of neutrophil-attracting chemokines 27. Therefore, we speculated that the MR 2-week old mice would have a diminished chemokines bearing the glutamic acid-leucine-arginine and cysteine-amino acid-cysteine (ELR+ CXC) motif. These chemokines are the murine equivalent of human IL-8. As is seen in Figure 5, there was decreased expression of three ELR+ CXC chemokines in the MR mice compared to SPF controls. Specifically, there was significantly less SI and colonic LPS-inducible CXC chemokine (LIX, CXCL5) expression (Figure 5, A,B). In addition, there was significantly less colonic macrophage inflammatory protein-2 (MIP-2, CXCL2) (Figure 5, B). While there was a trend toward decreased expression of SI and colonic keratinocyte-derived chemokine (KC, CXCL1), this reduction did not achieve statistical significance. As was previously seen with regards to intestinal versus colonic differences, we observed a greater relative reduction in ELR+ CXC expression in the colon which has orders of magnitude more microbiota.
Figure 5.
Quantative RT-PCR analysis of cytokine and chemokine expression in MR mice compared to SPF. (A) Small intestinal chemokine expression, (B) colonic chemokine expression, (C) small intestinal cytokine expression, (D) colonic cytokine expression. RT-PCR data is expressed in log2 scale and normalized to 18S rRNA expression with fold change calculated comparing MR to SPF. (MR n=10, SPF n=10). The bar graph to the right represents increased expression and to the left decreased expression.
We next set out to determine the role of microbiota in T-cell phenotype associated adaptive immune development. In general, there was no difference in mucosal IL-4, IL-17, or IFN-γ expression between the MR and SPR 2 week old animals (Figure 5, C,D). There was higher IL-10 expression in the MR SI but no difference in colonic expression. In summary, taken together, these data demonstrate that the presence of intestinal and colonic commensal bacteria induce their own signaling molecules, trigger an innate mucosal immune response, but do not seem to affect the early postnatal adaptive mucosal response.
The role of microbiota in MLN T-cell activation
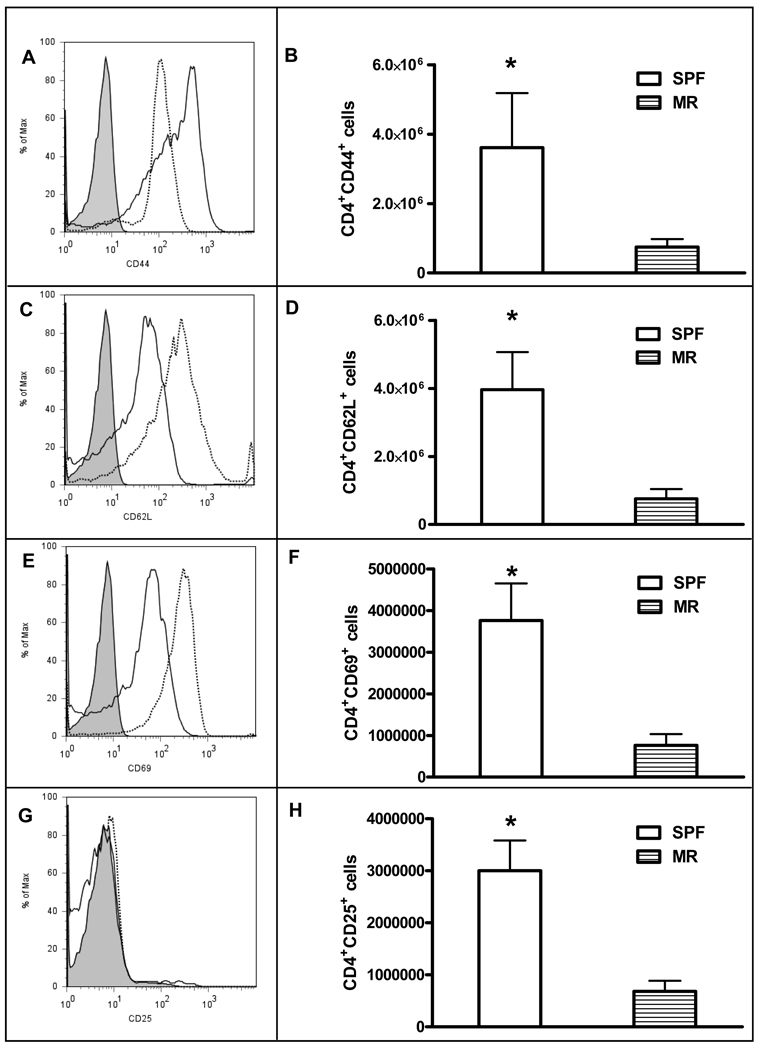
The MLNs from MR and SPF 2 week-old animals were analyzed for evidence of T-cell activation. Overall, we saw no statistically significant difference in the percentage of T-cells expressing the activation markers CD44, CD62L, CD69 or CD25 (data not shown). There was a trend towards less CD44 expression (Figure 6, A) and greater CD62L and CD69 (Figure 6, C, E); while there was similar CD25 expression (Figure 6, G) in the SPF mice compared the MR mice. However, the mean fluorescence intensity between the groups for each activation marker was not significantly different.
Figure 6.
Flow cytometry analysis of MLN derived T-cell activation markers comparing MR to SPF mice. (A)(C)(E)(G) represent histograms of T-cell surface activation markers CD44, CD62L, CD69, and CD25, respectively. Dotted line=SPF, solid line=MR, gray filled= unlabeled SPF cells. (B)(D)(F)(H) represent absolute numbers of CD4+ cells that are CD44, CD62L, CD69, and CD25 positive. White bars=SPF, stipped bars=MR. Results are 3 separate experiments where the MLNs from 3–5 mice were pooled for analysis. *p<0.05.
Next we analyzed the number of T-cells expressing the selected activation markers. While the percentage of activated CD4+ cells was not different between the SPF and MR cohorts, the mean absolute number of CD4+ cells per MLN was significantly decreased in the MR mice (8.6 ± 2.4 × 106 (SPF) versus 1.7 ± 0.42 × 106 (MR), p=0.0012). This alteration in total number of cells resulted in a significantly fewer CD44+, CD62L+, CD69+, and CD25+ T-cells in the MR mice compared to the SPF group (Figure 6, B, D, F, and H). Taken together, these results suggest that in the early postnatal period, the presence of commensal bacteria induces the expansion of activated T-cells but does not alter the intensity of surface expression of markers of activation.
Alterations in microbiota effect intestinal barrier function
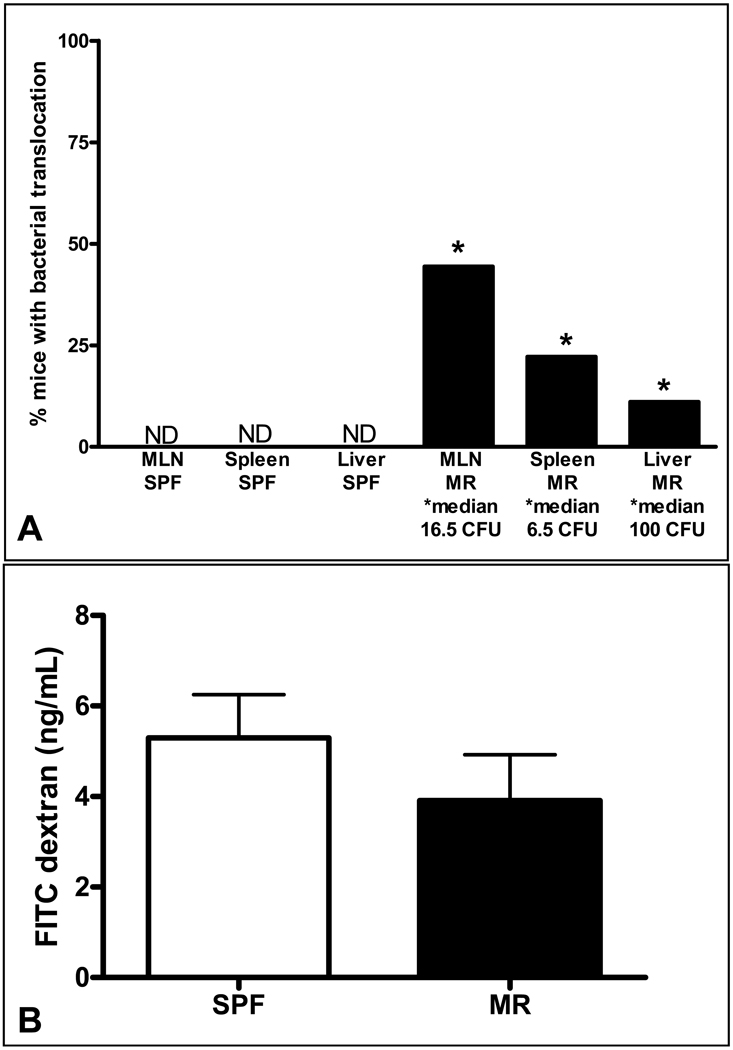
While the mechanisms by which broad-spectrum antibiotics and probiotics alter the incidence of NEC in premature infants are unknown, one hypothesis is that these bacteria protect the host from intestinal bacterial translocation. To test the hypothesis that mice with an altered commensal microbiota secondary to early antibiotic exposure would have a relative loss of barrier function, cohorts of 2-week old MR and SPF pups were orally inoculated with GFP labeled E. coli. Six days after inoculation, pups were sacrificed under sterile conditions, and cecal contents and peripheral lymphoid organs were cultured and analyzed for the presence of GFP E. coli. Twenty-four hours following initial culture, GFP producing colonies were detected in the cecal contents from all innoculated neonates, indicating adequate colonization. Colonies were detected in the MLN, spleen, and liver of MR animals, while organs from SPF neonates were demonstrably free of GFP labeled organisms (Figure 7, A).
Figure 7.
Bacterial translocation as a function of intestinal barrier permeability (A). The columns represent the percentage of animals with positive GFP E. coli colonies cultured from mesenteric lymph nodes (MLN), spleen, and liver from SPF (first three columns) and MR (last three columns) neonates. The median number of colony forming units (CFU)/whole organ culture is expressed below the X-axis. ND-none detected, * p< 0.05, (MR n=12, SPF n=11). Epithelial barrier function assay (B). The columns represent serum FITC-dextran concentrations four hours after oral gavage. The concentration is expressed as mean and standard deviation. (MR n=9, SPF=8).
To test whether bacterial translocation was a result of increased intestinal permeability, MR and SPF mice were orally gavaged with FITC-dextran and sera harvested after four hours. The mean serum FITC-dextran concentration of the MR and SPF mice was not significantly different (Figure 7, B), arguing that paracellular barrier permeability and epithelial tight junctions were intact.
Role of microbiota in the development of systemic T and B cell populations
The preceding experiments demonstrated that the acquisition of commensal bacteria in the first two weeks of life is a major determinant of postnatal innate mucosal immune development. We next wanted to determine if MR pups would have altered numbers and proportions of peripheral lymphocytes. Splenocytes were labeled with fluorescently tagged lymphocyte markers and analyzed using flow cytometry. Overall, there were significantly more lymphocytes in the spleen in SPF mice compared to the MR group, similar to what was observed in the MLN experiments. There was no difference in the absolute number or percentage CD45+CD3+ lymphocytes, indicating that early in postnatal life, microbiota have little effect on systemic T-cell numbers (Table 1). In contrast, there were significantly more CD45+CD19+ lymphocytes, thus showing that commensal bacteria influence early postnatal B-cell numbers.
Table 1.
Spleen lymphocyte populations analyzed by flow cytometry
| CD45+CD3+ | CD45+CD19+ | Total Number | |
|---|---|---|---|
| SPF neonates | 3.06 ± 1.82(4.6) | 18.4 ± 6.17(27.4) | 67.2 ± 29.6 |
| MR neonates | 2.02 ± 0.82(6.4) | 8.53 ± 1.68*(27.2) | 31.4 ± 5.30* |
Values are expressed as 106 cells, number in parentheses is the percentage of total number of splenocytes.
p<0.05
SPF=specific pathogen free MR=microbial reduced
Role of microbiota in the development of systemic T cell phenotype
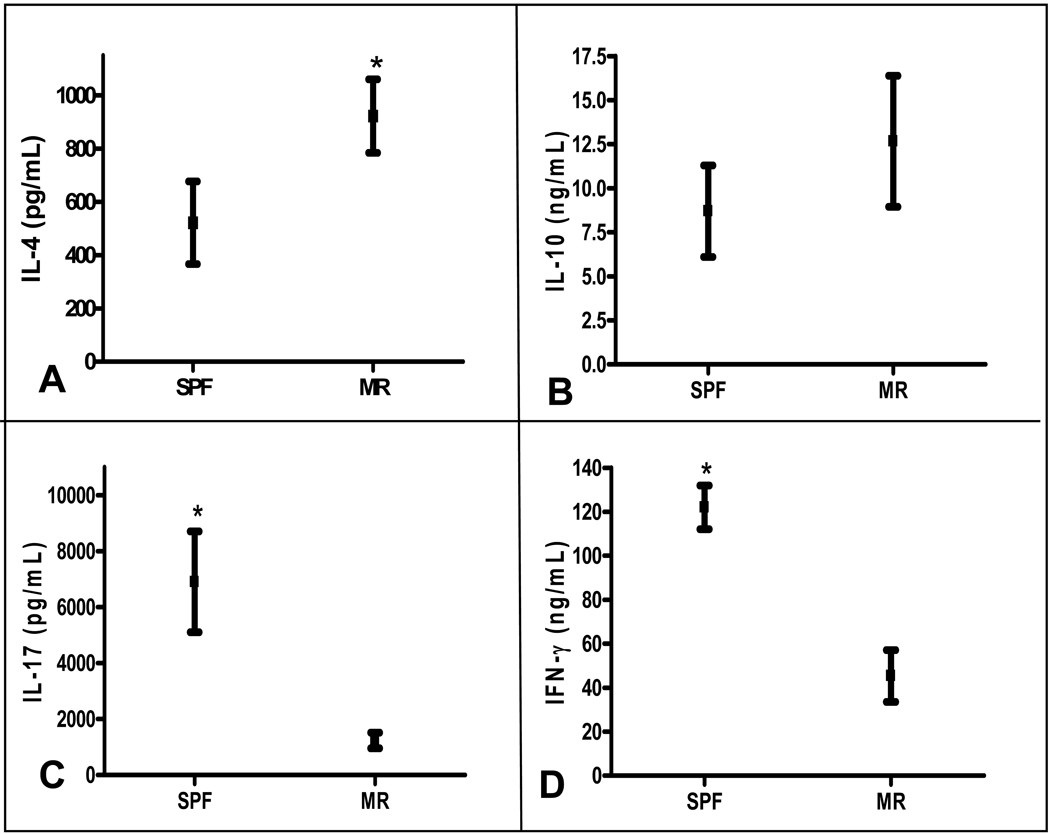
While the overall percentage of T-cells was similar in the SPF and MR groups, we next set out to test the hypothesis that acquisition of commensal intestinal microbiota influences systemic T-helper cell phenotypic commitment. Following a 96 hour culture, the supernatants from splenocyte cultures obtained from 2 week old SPF and MR mice were analyzed for cytokine production. When compared to MR conditions, there was a significantly greater concentration of IFN-γ and IL-17 in the SPF splenocyte cultures (Figure 8 C, D). In contrast, the MR cultures had significantly greater amount of IL-4 and a trend toward more IL-10 production (Figure 8, A, B). These results demonstrate that in the absence of intestinal microbiota, newborns remain committed to a systemic Th2 phenotype and fail to switch to the Th1 and Th17 pathways, which are essential for defending against microbial infections.
Figure 8.
Cytokine ELISA results from MR and SPF splenocyte cultures after αCD3 activation. (A) IL4, (B) IL10, (C) IL17, and (D) IFN-γ. *p<0.05 (MR n=10, SPF n=10).
Conclusions
The results of these experiments demonstrate that early acquisition of commensal microbiota significantly influences neonatal mucosal and systemic immune development and function. From a mechanistic prospective, these experiments suggest that during early postnatal mucosal immune development, the colonization of the intestine with microbiota results in a self-induction process of bacterial sensors with subsequent activation of innate mucosal immune processes. This activation process is temporal and rapidly attenuated as the host ages. The presence of commensal bacteria also promotes intestinal barrier function against potential pathogens and stimulates the number of activated T-cells in the MLN. In concert, the acquisition of commensal intestinal bacteria induces expansion of systemic B-cells and thus also shaped early humoral immunity. Lastly, this colonization process induces the switch from the fetal Th2 state that promotes intrauterine fetal-maternal coexistence to a Th1/Th17 state that prevents invasion of potential pathogens.
Our results agree with other work demonstrating that intestinal TLR2 and TLR4 are expressed in the sterile fetal environment 31. Constitutive expression of baseline TLRs would be necessary if increased expression is inducible. The increased expression of TLRs at 2 weeks postnatal development reflects a relative increase not an absolute value. The downregulation of TLRs occurs in the same timeframe as TLR tolerance has been reported to occur, indicating that both functional and physical mechanisms are induced by exogenous stimuli to control intestinal responses to TLR ligands 32.
These results also tease out the specific roles of commensal microbiota and breast milk in the induction of TLR expression 33. TLR expression in human adult and fetal epithelial cells was different when treated with breast milk supplement medium 34. In our study, both the SPF and MR pups received breast milk, thus the difference in microbial sensor expression was specifically related to the presence or absence of commensal microbiota.
Other investigators have demonstrated that germ-free animals lacking a commensal microbiota are at risk for invasive bacteria 14, 35, 36. Our findings demonstrate that this barrier function is established early the postnatal period. Another difference in our results is that while MR mice were in a microbial reduced environment, the dam was not from a gnotobiotic environment and as such was able to confer passive immunity by both transplacental or breast milk pathways. Even though the MR pups were nursed by immunocompetent mothers, the lack of normal commensal microbiota created an environment allowing bacterial translocation. Interestingly, the FITC-dextran experiments revealed similar serum levels in the MR and SPF pups, indicating no differences in the paracellular mucosal permeability in the early postnatal period. Thus, the increased bacterial translocation is not a result of a “leaky” intestinal epithelial barrier, but due to a more global impairment of immune function. As chemokines are critical for the appropriate trafficking of innate and adaptive immune cells to the intestine, the decreased expression of these molecules may explain the absence of appropriate immune mechanisms to clear the administered GFP-E.coli
However, some commensal microbial products have been shown to alter mucosal epithelial integrity and barrier function. An in vitro study of human intestinal epithelial cells has demonstrated that the tight junction, ZO-1, is increased in the presence of TLR2 ligands 37. In addition, our laboratory has shown that adult mice lacking TLR2 have greater injury in an ischemia/reperfusion model of NEC 38 and that 2 week old MR mice or mice lacking TLR2 or TLR4 also have increased ischemia/reperfusion injury (Tatum, PM, et.al, in press, Journal of Pediatric Surgery). Thus, intestinal sensing of commensal microbiota is also essential to impart protection against acute intestinal injury.
Of additional interest is that the MR mice have reduced bacteria but are not germ-free. The PCR-DGGE results show broad spectrum antibiotics in this model reduce not only the number of bacteria but more importantly the bacterial diversity. It is unlikely that any one commensal bacterium is the major determinant of postnatal development but rather it is the interplay between several species. Our findings are in agreement with previous studies looking at the introduction of defined bacterial microbiota into gnotobiotic mice, which show that a diverse population of bacteria is necessary 39, 40. Of interest was the predominance of Pseudomonas in the MR mice. This finding has also been reported in a case-control study in very low birth weight infants reporting an association with prolonged use of antibiotics with pseudomonas and NEC 41. Thus, our findings seem to recapitulate the early postnatal experience of premature infants in the NICU receiving broad spectrum antibiotics.
Our findings of activation of innate immunity in response to microbiota have been previously shown in adult-germ free mice 15. A unique finding of our study is the relative increase in IL-10 gene expression in the MR at 2-week-old mice. As IL-10 is considered a regulatory cytokine, it is unclear why mice with a reduced microbiota would have greater expression of the gene. It is possible that this increase reflects the earlier response to food antigens and the development of tolerance in an environment with markedly less bacteria 42.
The finding of increased B cells in SPF animals is consistent with the observation in humans that Peyer’s patches and other mucosa-associated lymphoid tissue do not contain secondary follicles with germinal centers and B-2 B-cells at birth. These structures develop several weeks later, reflecting that this activation needs exogenous stimuli 43. However, this early postnatal increase in B cells could also reflect expansion of B-1 B progenitor cells 44. In addition, antibiotic therapy early in life has been shown to impair humoral immunity and response to oral antigens, thus demonstrating the role for microbiota to direct postnatal immune development 45.
The temporal and functional nature of Th2 and Th1/Th17 commitment as it relates to health and disease and the need for microbiota is well documented 46. Our study again focuses on the timely commitment during the early postnatal period. Several studies have shown that progesterone induces the production of Th2 cytokines, presumably to prevent allograft (fetus) rejection 47. It appears that evolution has selected for a switch from a Th2 to Th1/17 preponderance early in development, as a means of protection against potential pathogens. Our study shows that neonatal animals that fail to acquire commensal microbiota remain in a Th2 state and are at risk for pathogenic infections.
Lastly our study offers a potential mechanism for how the commensal microbiota is involved in the susceptibility to NEC. The alteration and/or delay in establishing a normal commensal microbiota has been theorized as a possible mechanism for NEC 48. Our data shows that the interplay of microbiota and immune function, during the early postnatal period is critical and that the treatment of premature infants with probiotic medication may help to mimic the establishment of a normal commensal microbiota and therefore help to establish a normal mucosal and systemic immune system.
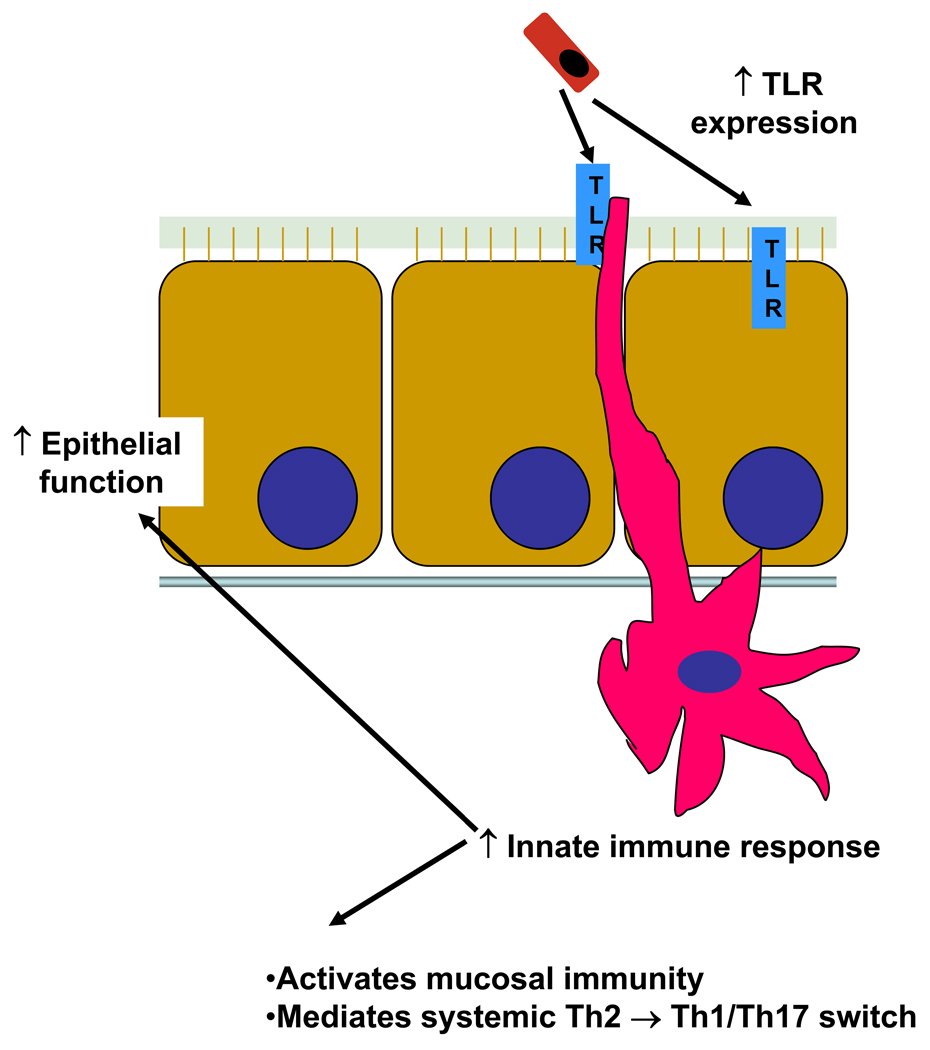
In conclusion, we have demonstrated that the acquisition of a commensal microbiota is necessary for the early postnatal development of immune function. In this model, newly arriving commensal bacteria are sensed by cells, both in the epithelium and in the lamina prorpia that express a basal level of TLRs. These bacteria induce addition expression of host TLR and an accompanying innate response. This early immune response is necessary for activation of the mucosal immune function, prevention of bacterial translocation, and as a check point for the switch from the in utero Th2 predominant phenotype to a mature Th1/Th17 T-cell commitment. (Figure 9). Determining the specific bacterium and/or bacterial products that are essential for this process could provide insight into the mechanism of both disease and protection in neonatal sepsis and NEC.
Figure 9.
Proposed mechanism of commensal bacteria acquisition and early postnatal mucosal and immune development.
Acknowledgments
We would like to thank Dr. Vance McCracken (Southern Illinois University at Edwardsville), Jamie McNaught, and the Cell and Molecular Pathology Core of the Digestive Disease Developmental Center at UAB for assistance with the PCR-DGGE experiments and histology, Peggie McKie-Bell for animal husbandry assistance, and Dr. Suzanne M. Michalek for the use of her flow cytometer.
This work was supported in part by the NIH grants R01 DK059911, P01 DK071176, T32A107051, K08 HD46506, American Asthma Foundation grant 06-0167, Juvenile Diabetes Research Foundation Grant 36-2008-930, and the University of Alabama at Birmingham Digestive Diseases Research Development Center Grant #P30 DK064400. Aspects of this project were conducted in biomedical research space that was constructed with funds supported in part by the NIH grant C06RR020136.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bäckhed F, Ley R, Sonnenburg J, Peterson D, Gordon J. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Hooper L, Midtvedt T, Gordon J. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 3.Ley R, Bäckhed F, Turnbaugh P, Lozupone C, Knight R, Gordon J. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 5.Trowsdale J, Betz A. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7(3):241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder HJ, Zhang L, Philips Slow, programmed maturation of the immunoglobulin HCDR3 repertoire during the third trimester of fetal life. Blood. 2001;98(9):2745–2751. doi: 10.1182/blood.v98.9.2745. [DOI] [PubMed] [Google Scholar]
- 7.Salzman N, Underwood M, Bevins C. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol. 2007;19(2):70–83. doi: 10.1016/j.smim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Morein B, Blomqvist G, Hu K. Immune responsiveness in the neonatal period. J Comp Pathol. 2007;137 Suppl 1:S27–S31. doi: 10.1016/j.jcpa.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Parker L, Whyte M, Dower S, Sabroe I. The expression and roles of Toll-like receptors in the biology of the human neutrophil. J Leukoc Biol. 2005;77(6):886–892. doi: 10.1189/jlb.1104636. [DOI] [PubMed] [Google Scholar]
- 10.Lemons J, Bauer C, Oh W, Korones S, Papile L, Stoll B, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001;107(1):E1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 11.Barclay A, Stenson B, Simpson J, Weaver L, Wilson D. Probiotics for necrotizing enterocolitis: a systematic review. J Pediatr Gastroenterol Nutr. 2007;45(5):569–576. doi: 10.1097/MPG.0b013e3181344694. [DOI] [PubMed] [Google Scholar]
- 12.Alfaleh K, Bassler D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2008;(1):CD005496. doi: 10.1002/14651858.CD005496.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Williams A, Probert C, Stepankova R, Tlaskalova-Hogenova H, Phillips A, Bland P. Effects of microflora on the neonatal development of gut mucosal T cells and myeloid cells in the mouse. Immunology. 2006;119(4):470–478. doi: 10.1111/j.1365-2567.2006.02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macpherson A, Uhr T. Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Ann N Y Acad Sci. 2004;1029:36–43. doi: 10.1196/annals.1309.005. [DOI] [PubMed] [Google Scholar]
- 15.Macpherson A, Hapfelmeier S, McCoy K. The armed truce between the intestinal microflora and host mucosal immunity. Semin Immunol. 2007;19(2):57–58. doi: 10.1016/j.smim.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Schumann A, Nutten S, Donnicola D, Comelli E, Mansourian R, Cherbut C, et al. Neonatal antibiotic treatment alters gastrointestinal tract developmental gene expression and intestinal barrier transcriptome. Physiol Genomics. 2005;23(2):235–245. doi: 10.1152/physiolgenomics.00057.2005. [DOI] [PubMed] [Google Scholar]
- 17.Masumoto J, Yang K, Varambally S, Hasegawa M, Tomlins S, Qiu S, et al. Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J Exp Med. 2006;203(1):203–213. doi: 10.1084/jem.20051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCracken V, Lorenz R. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol. 2001;3(1):1–11. doi: 10.1046/j.1462-5822.2001.00090.x. [DOI] [PubMed] [Google Scholar]
- 19.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 20.Hans W, Schölmerich J, Gross V, Falk W. The role of the resident intestinal flora in acute and chronic dextran sulfate sodium-induced colitis in mice. Eur J Gastroenterol Hepatol. 2000;12(3):267–273. doi: 10.1097/00042737-200012030-00002. [DOI] [PubMed] [Google Scholar]
- 21.Simpson J, Martineau B, Jones W, Ballam J, Mackie R. Characterization of fecal bacterial populations in canines: effects of age, breed and dietary fiber. Microb Ecol. 2002;44(2):186–197. doi: 10.1007/s00248-002-0001-z. [DOI] [PubMed] [Google Scholar]
- 22.O'Sullivan L, Webster G, Fry J, Parkes R, Weightman A. Modified linker-PCR primers facilitate complete sequencing of DGGE DNA fragments. J Microbiol Methods. 2008;75(3):579–581. doi: 10.1016/j.mimet.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Schultz C, Edrington T, Schroeder S, Hallford D, Genovese K, Callaway T, et al. Effect of the thyroid on faecal shedding of E.coli O157:H7 and Escherichia coli in naturally infected yearling beef cattle. J Appl Microbiol. 2005;99(5):1176–1180. doi: 10.1111/j.1365-2672.2005.02705.x. [DOI] [PubMed] [Google Scholar]
- 24.Cario E, Gerken G, Podolsky D. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132(4):1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 25.Savidge T, Newman P, Pan W, Weng M, Shi H, McCormick B, et al. Lipopolysaccharide-induced human enterocyte tolerance to cytokine-mediated interleukin-8 production may occur independently of TLR-4/MD-2 signaling. Pediatr Res. 2006;59(1):89–95. doi: 10.1203/01.pdr.0000195101.74184.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan J, Frey R, Malik A. TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J Clin Invest. 2003;112(8):1234–1243. doi: 10.1172/JCI18696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan J, Malik A. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med. 2003;9(3):315–321. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Gao X, Fan J, Liu Q, Anwar K, Frey R, et al. LPS activation of Toll-like receptor 4 signals CD11b/CD18 expression in neutrophils. Am J Physiol Lung Cell Mol Physiol. 2005;288(4):L655–L662. doi: 10.1152/ajplung.00327.2004. [DOI] [PubMed] [Google Scholar]
- 29.Makhoul I, Sujov P, Ardekian L, Kassis I, Smolkin T, Abu-Elnaa'j I, et al. Factors influencing oral colonization in premature infants. Isr Med Assoc J. 2002;4(2):98–102. [PubMed] [Google Scholar]
- 30.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91(441):48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 31.Fusunyan R, Nanthakumar N, Baldeon M, Walker W. Evidence for an innate immune response in the immature human intestine: toll-like receptors on fetal enterocytes. Pediatr Res. 2001;49(4):589–593. doi: 10.1203/00006450-200104000-00023. [DOI] [PubMed] [Google Scholar]
- 32.Lotz M, Gütle D, Walther S, Ménard S, Bogdan C, Hornef M. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203(4):973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newburg D, Walker W. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007;61(1):2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 34.LeBouder E, Rey-Nores J, Raby A, Affolter M, Vidal K, Thornton C, et al. Modulation of neonatal microbial recognition: TLR-mediated innate immune responses are specifically and differentially modulated by human milk. J Immunol. 2006;176(6):3742–3752. doi: 10.4049/jimmunol.176.6.3742. [DOI] [PubMed] [Google Scholar]
- 35.Berg R, Garlington A. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23(2):403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lecuit M, Sonnenburg J, Cossart P, Gordon J. Functional genomic studies of the intestinal response to a foodborne enteropathogen in a humanized gnotobiotic mouse model. J Biol Chem. 2007;282(20):15065–15072. doi: 10.1074/jbc.M610926200. [DOI] [PubMed] [Google Scholar]
- 37.Cario E, Gerken G, Podolsky D. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127(1):224–238. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Aprahamian C, Lorenz R, Harmon C, Dimmit R. Toll-like receptor 2 is protective of ischemia-reperfusion-mediated small-bowel injury in a murine model. Pediatr Crit Care Med. 2008;9(1):105–109. doi: 10.1097/01.PCC.0000288717.44702.C0. [DOI] [PubMed] [Google Scholar]
- 39.Martin F, Wang Y, Sprenger N, Yap I, Lundstedt T, Lek P, et al. Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Mol Syst Biol. 2008;4:157. doi: 10.1038/msb4100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Che C, Pang X, Hua X, Zhang B, Shen J, Zhu J, et al. Effects of human fecal flora on intestinal morphology and mucosal immunity in human flora-associated piglet. Scand J Immunol. 2009;69(3):223–233. doi: 10.1111/j.1365-3083.2008.02211.x. [DOI] [PubMed] [Google Scholar]
- 41.Leigh L, Stoll B, Rahman M, McGowan JJ. Pseudomonas aeruginosa infection in very low birth weight infants: a case-control study. Pediatr Infect Dis J. 1995;14(5):367–371. doi: 10.1097/00006454-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Allam J, Peng W, Appel T, Wenghoefer M, Niederhagen B, Bieber T, et al. Toll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cells. J Allergy Clin Immunol. 2008;121(2):368–374. doi: 10.1016/j.jaci.2007.09.045. e361. [DOI] [PubMed] [Google Scholar]
- 43.Bauer E, Williams B, Smidt H, Verstegen M, Mosenthin R. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr Issues Intest Microbiol. 2006;7(2):35–51. [PubMed] [Google Scholar]
- 44.Herzenberg L, Tung J. B cell lineages: documented at last! Nat Immunol. 2006;7(3):225–226. doi: 10.1038/ni0306-225. [DOI] [PubMed] [Google Scholar]
- 45.Nutten S, Schumann A, Donnicola D, Mercenier A, Rami S, Garcia-Rodenas C. Antibiotic administration early in life impairs specific humoral responses to an oral antigen and increases intestinal mast cell numbers and mediator concentrations. Clin Vaccine Immunol. 2007;14(2):190–197. doi: 10.1128/CVI.00055-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forchielli M, Walker W. The role of gut-associated lymphoid tissues and mucosal defence. Br J Nutr. 2005;93 Suppl 1:S41–S48. doi: 10.1079/bjn20041356. [DOI] [PubMed] [Google Scholar]
- 47.Piccinni M, Scaletti C, Mavilia C, Lazzeri E, Romagnani P, Natali I, et al. Production of IL-4 and leukemia inhibitory factor by T cells of the cumulus oophorus: a favorable microenvironment for pre-implantation embryo development. Eur J Immunol. 2001;31(8):2431–2437. doi: 10.1002/1521-4141(200108)31:8<2431::aid-immu2431>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 48.Neu J, Chen M, Beierle E. Intestinal innate immunity: how does it relate to the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14(3):137–144. doi: 10.1053/j.sempedsurg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Staley E, Schoeb T, Lorenz R. Differential susceptibility of p-glycoprotein deficient mice to colitis induction by environmental insults. Inflamm Bowel Dis. 2009;15(5):684–696. doi: 10.1002/ibd.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newberry R, McDonough J, McDonald K, Lorenz R. Postgestational lymphotoxin/lymphotoxin beta receptor interactions are essential for the presence of intestinal B lymphocytes. J Immunol. 2002;168(10):4988–4997. doi: 10.4049/jimmunol.168.10.4988. [DOI] [PubMed] [Google Scholar]