Abstract
Natural killer (NK) cells play a critical role in host defense against viral infections. However chronic HIV-1 infection is associated with an accumulation of dysfunctional NK cells, that poorly control viral replication. The underlying mechanisms for this NK cell mediated dysfunction are not understood. Certain tumors evade NK cell mediated detection by dampening NK cell activity through the downregulation of NKG2D, via the release of soluble NKG2D-ligands, resulting in a potent suppression of NK cell function. Here we show that chronic HIV-1 infection is associated with a specific defect in NKG2D-mediated NK cell activation, due to reduced expression and transcription of NKG2D. Reduced NKG2D expression was associated with elevated levels of the soluble form of the NKG2D-ligand, MIC-A, in patient sera, likely released by HIV+ CD4+ T-cells. Thus, like tumors, HIV-1 may indirectly suppress NK cell recognition of HIV-1-infected CD4+ T-cells by enhancing NKG2D-ligand secretion into the serum resulting in a profound impairment of NK cell function.
Introduction
Natural killer (NK) cells play a critical role in the host‘s first line defense against tumors and viral infections (Trinchieri, 1989). Although these cells only constitute a small fraction of circulating lymphocytes, NK cells are capable of rapidly destroying infected or malignant cells without the need for prior antigen sensitization (Kiessling, Klein, and Wigzell, 1975). NK cells do not express an antigen specific receptor, as do other lymphocytes. Instead, NK cells encode a variety of different activating and inhibitory receptors, belonging to several different receptor families (Lanier, 2005). Thus, NK cell activation is dependent on a delicate balance of stimulatory and inhibitory signals elicited by the multiple NK cell receptor-ligand interactions that take place following NK cell-target cell interaction (Lanier, 2005).
One of the most potent activating NK cell receptors is NKG2D; a homodimeric, C-type, lectin like, type II transmembrane receptor encoded within the NK gene complex on Chromosome 12 (Bauer et al., 1999; Lanier, 2005; Raulet, 2003). In humans, NKG2D is expressed on most NK cells, CD8-T cells and γδT cells(Bauer et al., 1999; Raulet, 2003). The activating signal triggered by the NKG2D-ligand-complex is one of the few dominant activating signals, as it can override inhibitory signals (Diefenbach et al., 2001). A prerequisite of NKG2D-mediated killing is the interaction of NKG2D with one of its numerous ligands. NKG2D ligands are not expressed by healthy tissues but have been demonstrated to be induced upon cell stress due to heat shock, viral infection, or malignant transformation (Bauer et al., 1999; Raulet, 2003). Among these molecules, human NKG2D ligands include MHC class I-related chain (MIC) A and B, and several UL-16 binding proteins (ULBP-1, 2, 3 and 4) (Cao et al., 2008; Groh et al., 1996; Groh et al., 2001).
NKG2D-dependent NK cell cytotoxicity has been shown to be critical in the control and clearance of several viral infections (Biron, Byron, and Sullivan, 1989; Etzioni et al., 2005; Fang, Lanier, and Sigal, 2008). Furthermore, several viruses have evolved to encode NKG2D-ligand homologues in order to subvert the immunological pressure exerted by this strong activating receptor (Arase et al., 2002). NKG2D and its ligands have been strongly implicated in tumor immuno-surveillance (Lanier, 2001; Li et al., 2009; Salih et al., 2003; Smyth et al., 2004; Vetter et al., 2002). While NKG2D is a well conserved molecule, its ligands are highly polymorphic (Cao et al., 2008; Diefenbach et al., 2001; Groh et al., 1996; Lanier, 2005) and have been associated with differential disease outcome in a number of neurological, autoimmune, infectious disease and cancer models (Fdez-Morera et al., 2006; Gambelunghe et al., 2007; Lopez-Arbesu et al., 2007; Shirts et al., 2007). In addition, in a recently published genome wide association study, a single-nucleotide polymorphism in the MICB gene has now been linked to AIDS-non progression (Limou et al., 2009).
Malignant cells have devised a mechanism to evade detection by NKG2D through proteolytic shedding of NKG2D-ligands (NKG2D-L) from the surface of target cells. NKG2DLs are enzymatically cleaved from the surface of tumor cells releasing large concentrations of NKG2DL into the extracellular milieu (Kaiser et al., 2007; Salih, Rammensee, and Steinle, 2002; Waldhauer et al., 2008). NKG2D is rapidly internalized following engagement with ligands, rendering NK cells temporarily refractory to stimulation. Thus, elevated levels of soluble NKG2DLs in the peripheral circulation can interact with NK cells at a distance from the tumor, resulting in NKG2D downregulation and the generation of anergized NK cells (Groh et al., 2002; Jinushi et al., 2008; Salih, Rammensee, and Steinle, 2002). Typically, high levels of secreted NKG2DL are present in the sera of particular cancer patients but not in healthy subjects (Jinushi et al., 2008; Kaiser et al., 2007), and are associated with the inactivation of NK cells via NKG2D, thus indirectly protecting these tumor cells from direct elimination by NK cells that circulate in their vicinity (Jinushi et al., 2008; Kaiser et al., 2007; Waldhauer et al., 2008). Similarly, NK cell function becomes progressively dysfunctional during chronic HIV-1 infection, however the role of NKG2D/NKG2DLs in this dysfunction has not been addressed.
Several classes of proteolytic enzymes have been implicated in NKG2DL shedding including the endoplasmic reticulum protein 5 (ERP5) and the matrix metalloproteinases (MMPs) (Kaiser et al., 2007; Salih, Rammensee, and Steinle, 2002). The MMPs, also designated matrixins, which hydrolyze components of the extracellular matrix, play a central role in many biological processes, and have been implicated in both normal and pathological tissue remodeling, as well as inflammatory responses (Hijova, 2005). Several reports suggest that progressive HIV-1 infection is associated with elevated production and secretion of MMPs, which may contribute to infection-associated immunopathology, dysfunctional T cell responses, and dysregulated myeloid cell trafficking (Dezube, Sullivan, and Koon, 2006; Ramankulov et al., 2008; Webster and Crowe, 2006; Yokoyama et al., 2008). However, changes in MMP expression in HIV infected CD4+ T cells have not yet been assessed, nor has their role in NKG2DL secretion. Here we show new data demonstrating reduced NKG2D expression on NK cells strongly linked to the level of ex vivo NK cell activation. Alterations in NKG2D expression were furthermore associated with aberrant NKG2D mediated recognition of target cells, related to elevated secretion of the NKG2DL, MICA, into patient sera. These data suggest for the first time, that active HIV-1 replication may subvert NK cells indirectly by driving NK cell dysfunction via the cleavage and release of NKG2DL into the peripheral circulation.
Patients, materials and methods
Subjects
A total of 100 subjects were recruited for this study, including 35 healthy HIV negative control subjects, 25 untreated chronically HIV-1-infected subjects with a median viral load of 77,000 copies/ml (range 3,310–379,251 copies/ml) and CD4+ T cell count of 448/mm3 (range 65–1041/mm3), 25 HIV-infected subjects receiving highly active antiretroviral therapy (HAART) with a median viral load of 3,079 copies/ml (range <50–17,300 copies/ml) and CD4 counts of 621/mm3 (range 192–1162/mm3), and 15 subjects that spontaneously controlled HIV-1 replication without antiretroviral therapy (referred to as HIV-1 controllers) with a median viral load of 1,841 copies/ml (range<50–5,360 copies/ml) and CD4+ T cell count of 683 (range 169–1290/mm3). The MGH institutional review board approved the study, and each subject gave written informed consent for participation in the study.
Cell lines
K562 and 221 cell lines were cultured in RPMI 1640 medium supplemented with glutamine, 10% fetal calf serum, penicillin, and streptomycin. Both cell lines are MHC-class I devoid.
Flow cytometry and antibodies
Peripheral blood mononuclear cells (PBMCs) were obtained after Ficoll-Hypaque density gradient centrifugation of whole blood. At the same time, plasma was collected from each patient for the MIC-A ELISA (below). To determine NKG2D surface expression and NK cells responses following stimulation with different target cells (221 cells or K562 cells), PBMCs were resuspended at 2×106/ml cells in RPMI medium supplemented with glutamine, 10% fetal calf serum, penicillin, and streptomycin. Ex vivo NKG2D-expression was analyzed immediately by staining PBMCs with CD3-Pacific Blue, CD8-Alexa 700, CD16- APC-Cy7, CD56-Pe-Cy-7, CD69-PE, NKG2D-APC for 20 minutes. The cells were then washed, resuspended in 1% paraformaldehyde (Sigma, St. Louis, MO), and at least 5×105 cells were acquired on a Becton Dickinson (BD) LSRII flow cytometer. To gate on NK cells, we first gated on CD3-negative lymphocytes. Then CD56 and/or CD16-positive cells were included in the NK cell gate. Thus NK cells included both CD56-negative NK cells that have been shown previously to accumulate in HIV infection (Alter et al., 2005; Mavilio et al., 2005) as well as CD16-negative CD56dim NK cells that may represent a subset of NK cells that have recently lost CD16 following activation (Liu et al., 2009), thereby including the largest fraction of NK cells based on known subsets.
The functional capacity of NKG2D+ NK cells was assessed following stimulation of PBMC with either 221 or K562 target cells, as described previously (Alter et al., 2005). Thus, PBMCs were resuspended at 106cells/ml in media containing CD107a-Pe-Cy5 (BD) and Golgi Stop (3µg/ml), in the presence of either target cell line at an effector:target ratio of 10:1, medium alone served as a negative control. PBMCs were stimulated for 4hrs at 37°C and 5% CO2. PBMCs were stained with CD3-Pacific Blue, CD8-Alexa 700, CD16- APC-Cy7, CD56-Pe-Cy-7, CD69-PE, NKG2D-APC (BD Biosciences, San Jose, CA) for 20 minutes, washed with PBS and resuspended in 1% paraformaldehyde (Sigma, St. Louis, MO). All samples were acquired on a BD LSR II (BD Biosciences, San Jose, CA). Five hundred thousand to 106 events were acquired and analyzed using FlowJo software. NKG2D-downregulation following stimulation was calculated as Δ% of NKG2D expression on responding and non-responding NK cells following stimulation. Thus we calculated: the percent change in NKG2D expression on CD107aneg NK cells – % changes in NKG2D expression on the surface of CD107apos NK cells to determine the capacity of NK cells to downregulate NKG2D following stimulation.
To determine the influence of sMICA levels on NKG2D downregulation on NK cells after K562 stimulation, PBMCs of four HIV-1 negative control individuals were incubated for 2hrs at 37° degrees in 5% CO2 in presence of plasma derived from chronic untreated HIV-1 infected individuals with different sMICA levels. After incubation, PBMCs were stimulated with K562 as described above in the presence of Brefeldin A (0.5µg/ml) and stained after 4hrs with CD3-Pacific Blue, CD8-Alexa 700, CD16- APC-Cy7, CD56-Pe-Cy-7, NKG2D-APC (BD Biosciences, San Jose, CA) for 20 minutes, washed with PBS and resuspended in 1% paraformaldehyde (Sigma, St. Louis, MO). All samples were acquired on a BD LSR II (BD Biosciences, San Jose, CA). 106 events were acquired and analyzed using FlowJo software. The effect of increasing doses of sMICA on NK cell mediated NKG2D downregulation was calculated as the ratio of the NKG2D MFI on [stimulated / unstimulated] NK cells.
Isolation of CD4+-T cells
CD4-T cells were isolated from PBMCs derived from HIV-1 infected patients and uninfected subjects by negative selection using MACS human CD4+ T cell isolation kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) as instructed by the manufacturer.
NK cell isolation
NK cells were purified using the NK cell enrichment cocktail RosetteSep (StemCell Technologies Inc., Vancouver, Canada). NK cells were lysed for RNA purification and subsequent cDNA synthesis, and then used for quantitative RT-PCR.
Quantitative Real Time-Polymerase Chain reaction (q-RT-PCR)
Transcript levels of NKG2D, its ligands, and intracellular enzymes that are able to cleave these proteins, were determined by quantitative real time PCR. Total RNA was isolated from purified NK cells or isolated CD4+ T cells using the RNeasy plus KIT (Quiagen, Hilden, Germany), which includes a genomic DNA elimination step, and reverse transcribed using SuperScriptIII (Invitrogen, Karlsruhe, Germany) according to the manufacturer’s protocol. The resulting cDNA was amplified with NKG2D-, NKG2D-ligand- or proteolytic enzymes-specific primer pairs in duplicate. Cycling conditions for real-time PCR were: 50 degrees for 2 min, 95 degrees for 5 minutes, 95 degrees for 30 sec, 60 degrees for 1min, 95 degrees for 1 min, 55 degrees for 30 sec, and 95 degrees for 30 seconds. A total of 40 cycles were performed. Amplification was monitored using SYBR Green Full velocity mastermix (Stratagene, Cedar Creek, TX) on a Mxpro 3000 and 3005 qPCR cycler. Samples were normalized to GAPDH RNA to account for the variability in the initial concentration of the total RNA and conversion efficiency of the reverse transcription reaction. Primers used were: GAPDH forward ACCCACTCCTCCACCTTTGA, reverse TGGTGGTCCAGGGGTCTTAC; NKG2D forward CTGGGAGATGAGTGAATTTCATA, reverse GACTTCACCAGTTTA-AGTAAATC; MMP2 forward CGCTCTGTCTCCTGGGCT, reverse AGGTATTG-CACTGCCAACTCTT; MMP7 forward AACTCCCGCGTCATAGAAAT, reverse GATACGATCCTGTAGGTGAC; ERP5 forward TGCGGCACGCTGCAGGGCT, reverse TTGACAGTGACCACACCATGGAGCATA; MIC-A forward TTCTGG-CTGGCATCTTCCCT, reverse TCCCATGTCTTATTTCCCA; MIC-B forward TTCTG-GCCGTCGCCTTCCCT, and reverse TCCCAGGTCTCAGCTCCCA.
MICA-ELISA
The commercially available MIC-A Elisa kit from Bamomab (Munich, Germany) was used to quantify soluble NKG2D ligand MICA (sMICA), as directed. Briefly, plates were coated overnight with the capture anti-MICA mAb AMO-1 at 2 µg/ml in PBS, then blocked by addition of 100 µl of 15% BSA for 2 h at 37°C and washed. Standards and samples were added and the plates were incubated for 2 h at 37°C. Plates were washed and the detection mAb BAMO-3 at 5 µg/ml in 7.5% BSA-PBS was added for 2 h at 37°C. Plates were then washed and anti-mouse IgG2a-HRP (1:8000 in 7.5% BSA-PBS) was added for 1 h at 37°C. Plates were then washed and developed using the Tetramethylbenzidine Peroxidase Substrate System (KPL, Gaithersburg, MD). The absorbance was measured at 450 nm.
MMP-inhibitor induced expression of MIC-A/B expression
PBMC were isolated from the peripheral blood of HIV-negative donors and stimulated for 3 days with 0.3ug of a CD3/CD8 bispecific antibody in the presence of 50 units of IL-2 in complete RPMI. This culture results in the enrichment of a population of highly activated CD4+ T cells that were subsequently infected at a multiplicity of infection of 1 for 3 hours, and then placed in culture in the presence or absence of the MMP inhibitor GM6001 (Gallardin, Calbiochem) for 2 days. The mean fluorescence intensity of MIC-A/B-PE (BD Biosciences) was then quantified on the surface of CD3+ T cells on a BD LSR2. A minimum of 3×105 events were acquired and all experiments were analyzed using FlowJo software.
Statistical analysis
Differences between groups were determined using a Mann-Whiney U, non-parametric test, for assessing differences between independent samples. A wilcoxon-matched pairs test was employed to define whether differences existed between matched samples. . A Friedman test was employed to define whether differences existed between repeated measures represented in matched observations. Significant differences were noted to be p<0.05.
Results
Altered NKG2D expression on NK cells during chronic untreated HIV-1 infection
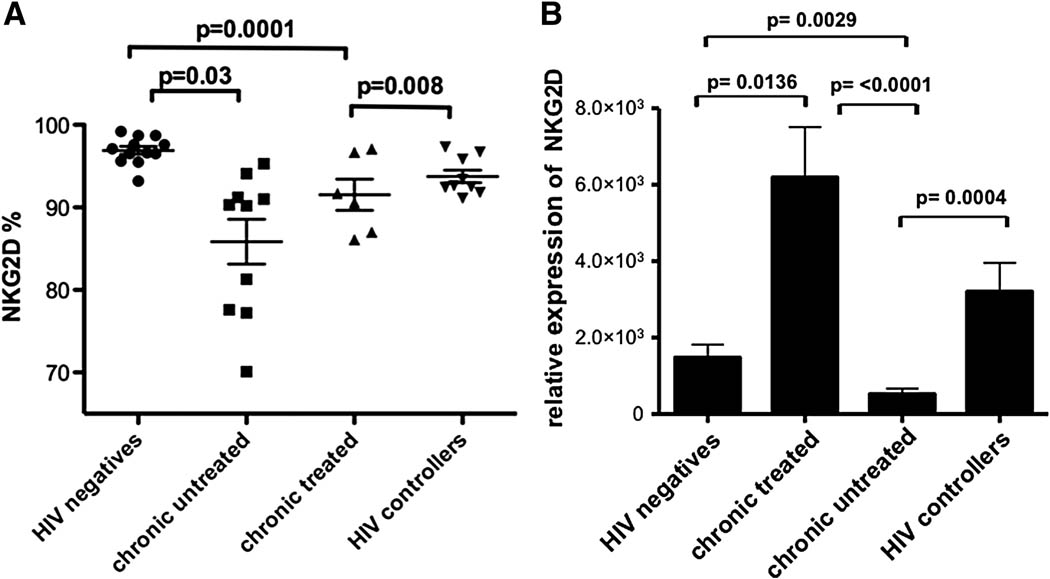
NKG2D is expressed on almost all NK cells in healthy individuals, and is crucial for optimal NK cell mediated clearance of some virally infected cells (Biron, Byron, and Sullivan, 1989; Etzioni et al., 2005). While a small number of studies have examined NKG2D expression on NK cells in chronically infected patient populations (Mavilio et al., 2003), less is known about the expression pattern of this critical activating receptor on NK cell populations in spontaneous controllers and non-controllers on or off anti-retroviral therapy. Here we analyzed changes in surface expression of the receptor NKG2D on NK cells in HIV-1 infected individuals compared to healthy controls. The frequency of NKG2D+ NK cells was reduced in all HIV-infected individuals compared to healthy controls (Figure 1A). However, the percentage of NK cells expressing NKG2D was most noticeably reduced on the surface of NK cells from chronically infected individuals with active viral replication in the absence of antiretroviral therapy compared to uninfected controls or individuals that spontaneously control HIV-1 replication in vivo (p=0.0001 and p=0.008, respectively, Figure 1A). Furthermore, viral suppression in the presence of antiretroviral therapy was associated with a slight but non-significant increase in the frequency of NKG2D+ NK cells compared to untreated subjects; however the NKG2D+ NK cell density remained significantly lower than those observed in uninfected controls (p =0.03, Figure 1A). Furthermore, despite the fact that significant changes were observed in the frequency of NKG2D expressing NK cells in different HIV-infected populations, no relationship was observed between NKG2D expression and clinical parameters including CD4+ T cell count or viral load, nor with an elevation of CD56neg NK cells (data not shown), suggesting that other immunological phenomena may drive reduced expression of NKG2D on the surface of NK cells.
Figure 1. Reduced expression of NKG2D on NK cells in HIV-infection.
NKG2D expression was compared among individuals with chronic untreated (triangles, n=10), chronic treated (squares, n=6), HIV controllers (transparent circles, n=10), and HIV-negative controls (filled circles, n=9) (A). Similarly, NKG2D transcription was compared among the same groups of individuals (B).
To determine whether these changes in surface expression were paralleled by changes in NKG2D transcription, we compared NKG2D transcript levels in bulk purified NK cells isolated from the different clinical subgroups. NKG2D transcriptional levels were significantly reduced in untreated HIV-1 infected subjects compared to uninfected controls (p=0.0029, Fig. 1B). Interestingly, the level of NKG2D transcription was elevated in HIV-1 infected individuals on antiretroviral therapy compared to HIV-1-uninfected controls (p=0.0136), suggesting that viral suppression with antiretroviral therapy results in a compensatory reconstitution of NKG2D transcription, but incomplete NKG2D protein expression on the surface of NK cells (Figure 1A). Similarly, HIV-1 controllers exhibited enhanced transcription of NKG2D compared to chronic untreated individuals (p=0.0004, Figure 1B) associated with a higher frequency of NKG2D expressing NK cells (Figure 1A), indicating durable viral control is associated with normal NKG2D transcription and surface expression. Overall, chronic progressive HIV-1 infection is associated with a loss of NKG2D expression and transcription.
Compromised NKG2D-dependent NK cell responses in chronic untreated HIV-1 infection
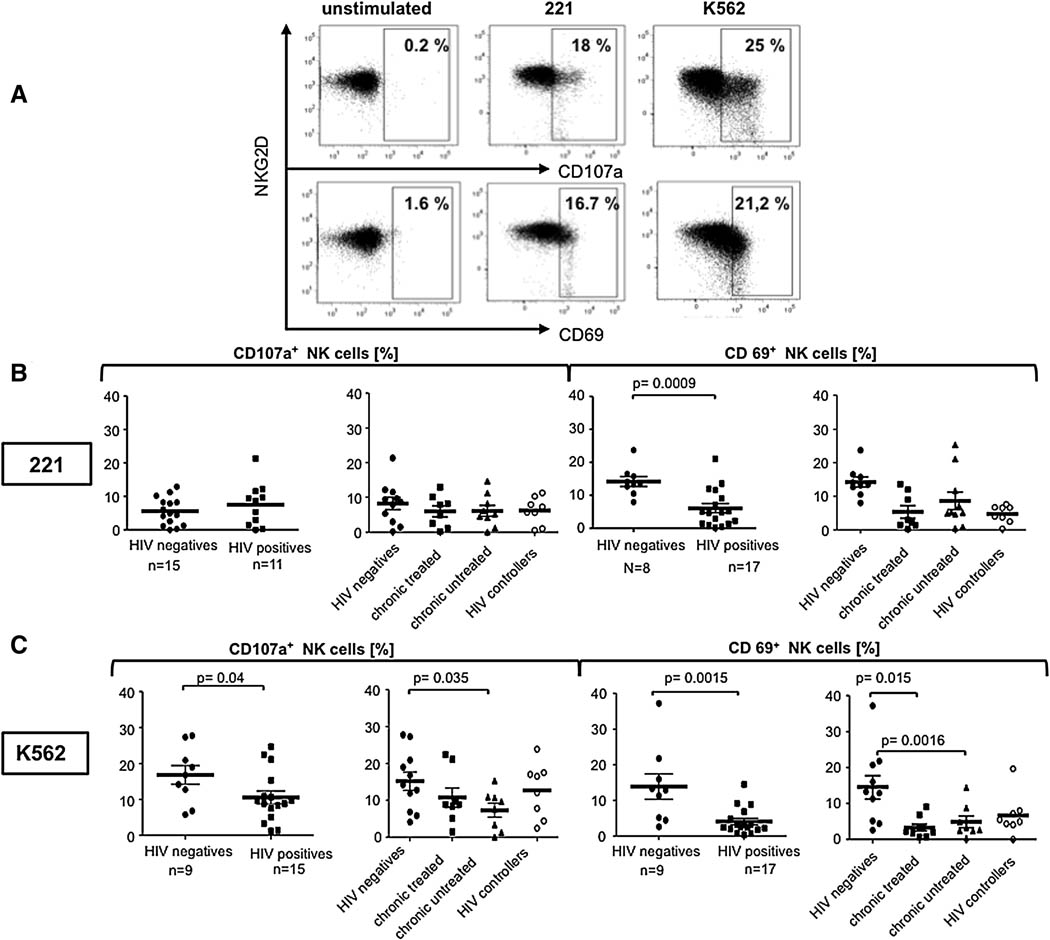
Chronic HIV-1 infection is associated with an accumulation of anergic NK cells (Alter et al., 2005; Mavilio et al., 2005). Given the protective nature of NKG2D in other viral models, we sought to investigate whether specific changes occurred in the quality of NKG2D-dependent NK cell responses following HIV-1 infection. NK cell degranulation (CD107a-upregulation) and activation (CD69-upregulation) following stimulation with MHC class 1 target cells were compared among HIV-1 infected individuals and uninfected controls. Two different MHC class 1 target cell lines were employed for these experiments, 221 cells, a MHC-class 1 devoid B lymphoblastoid cell line that induces natural cytotoxicity receptor (NCR) mediated activation of NK cells, and K562 cells, an MHC deficient human erythromyeloblastoid leukemia cell line, that activates NK cells in an NKG2D-dependent manner (Lozzio and Lozzio, 1977; Shimizu and DeMars, 1989). Following stimulation, NK cell activation (quantified by CD69-upregulation) was significantly reduced in HIV-1 infected individuals in response to both cell lines (Figures 2B and C), however NK cell degranulation was only compromised following stimulation with K562 cells (Figure 2C). These data suggest that while overall NK cell activation is dysregulated in HIV infection, a specific dysfunction exists within the NK cell mediated NKG2D-induced degranulation pathway during HIV-1 infection.
Figure 2. Reduced NKG2D- dependent degranulation and activation of NK cells in chronic untreated HIV-1 infection.
Flow plots (A) show representative raw flow cytometric data from a single individual measuring NK cell degranulation (CD107a expression) (top) and activation (CD69 expression)(bottom) following stimulation with 221 cells (middle) and K562 cells (right). The dot plot represents the overall NK cell degranulation (CD107a) and activation (CD69) of NK cells following stimulation with 221 cells (B) or K562 (C) cells in HIV- infected (squares) or uninfected individuals (circles). The dot plots represent the distribution of NK cell responses among the different patient subpopulations .
To determine whether suppression of viral replication to undetectable levels by antiretroviral therapy can restore the observed defect in the NKG2D-dependent activation of NK cells, we compared NK cell activation and degranulation in response to K562 and 221 cells in both treated and untreated HIV-1-infected individuals compared to spontaneous controllers and HIV-1 negative controls. Little difference was observed in the NK cell response to 221 cells among the clinical groups (Figure 2B), suggesting that the NCR pathway appears to be relatively spared from dysfunction during chronic HIV-1 infection. However, subjects with progressive chronic HIV infection exhibited a more profound defect in NK cell degranulation in response to K562 cells (Figure 2C). In contrast, both spontaneous controllers, who maintain low viral loads, and treated chronics degranulated slightly less efficiently than HIV-negative controls, suggesting that active viral replication at higher levels is required for the induction of dysfunctional NK cell activity in response to K562 cells. Interestingly, NK cells from both the treated and untreated chronic patients were poorly activated in response to this NKG2D-activating cell line (Figure 2C). These data suggest that NKG2D-dependent NK cell degranulation can only be partially reconstituted by highly active antiretroviral therapy.
Dysregulation of NKG2D down-modulation in HIV-1 infected individuals
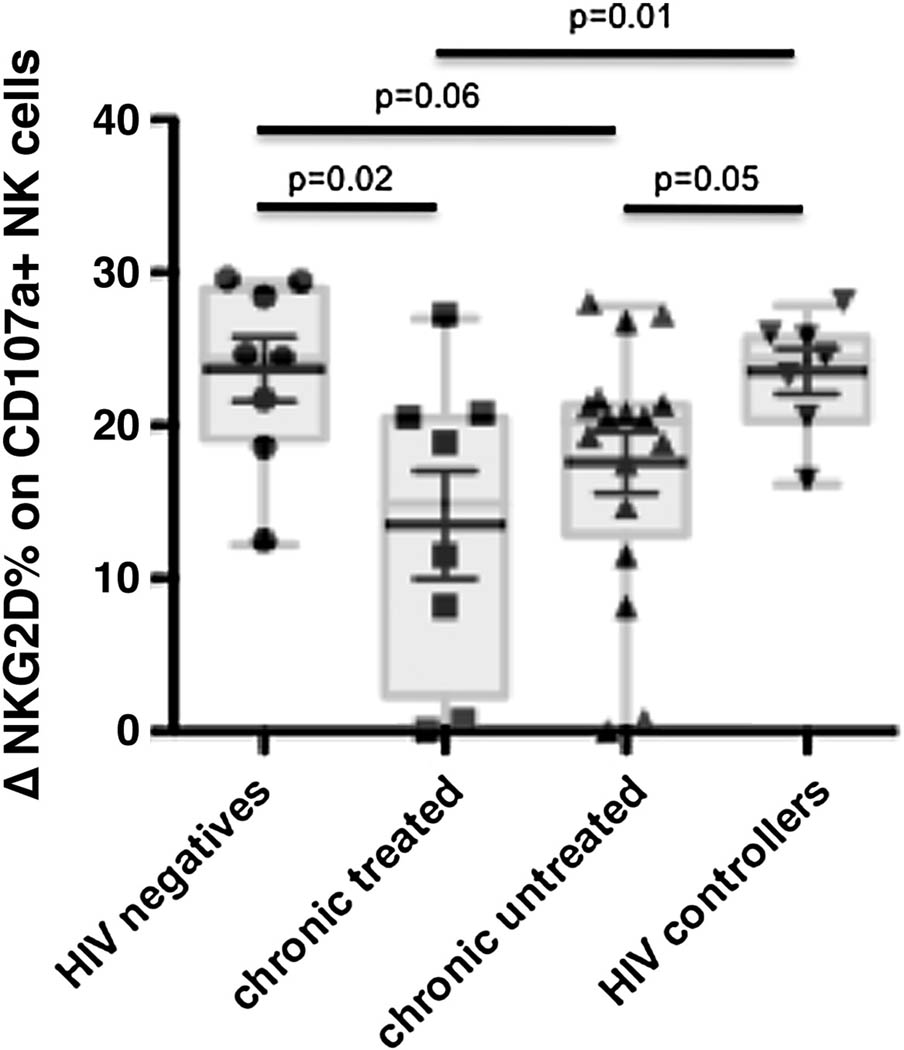
Following NK cell stimulation, many activating receptors are downregulated to avoid activation induced cell death (Huard and Karlsson, 2000; Linsley et al., 1993; Valitutti et al., 1997). Along these lines, NKG2D is downmodulated from the surface of NK cells following stimulation with one of its ligands (Groh et al., 2002; Wiemann et al., 2005). Thus, a functional NK cell downregulates NKG2D following stimulation as a normal homeostatic mechanism to prevent activation induced cell death, and serves as a marker of functional activation via NKG2D. Given the critical nature of NKG2D to prevent cell death, we sought to determine whether HIV infection resulted in any change in the capacity of NK cells to downregulate this activating receptor from the surface of activated NK cells. Thus we compared the mean fluorescence intensity (MFI) change in NKG2D-expression on degranulating versus non-degranulating NK cells after stimulation with K562 cells among the different infected populations. As anticipated, NK cells from HIV-1 negative controls and controllers downregulated NKG2D effectively following stimulation with K562, and therefore displayed a high difference (Δ) in NKG2D expression following stimulation (Figure 3). In contrast, significantly less NKG2D was lost on NK cells derived from treated HIV progressive patients and there was a trend towards a reduced NKG2D-loss in untreated chronically infected patient compared to uninfected controls (p=0.02 and p=0.06, respectively), resulting in lower differences (Δ) in NKG2D expression (Figure 3). However, both treated and untreated chronic patients exhibited reduced NKG2D losses compared to NK cells from HIV controllers (p=0.01 and p=0.05, respectively) suggesting that NK cells in progressive infection lose the capacity to downregulate NKG2D, potentially due to the fact that they express low levels of this molecule to begin as well as additional defects that may prevent homeostatic protection from apoptosis. Thus chronic HIV-1 infection is associated with an overall defect in NKG2D activation and homeostatic regulation.
Figure 3. Chronic HIV infection is associated with impaired downregulation of NKG2D-dependent NK cell activation.
Downregulation of NKG2D on CD107a+ cells was measured in Δ percent and compared among HIV negatives (n=10), HIV-1 infected HAART naïve (n=10), HIV-1 infected individuals receiving HAART (n=6) and HIV controllers (n=10) after stimulation with K562 cells (NKG2D target cell line).
Chronic HIV-1 infection is associated with elevated levels of soluble NKG2D-ligand, MIC-A, in patient sera
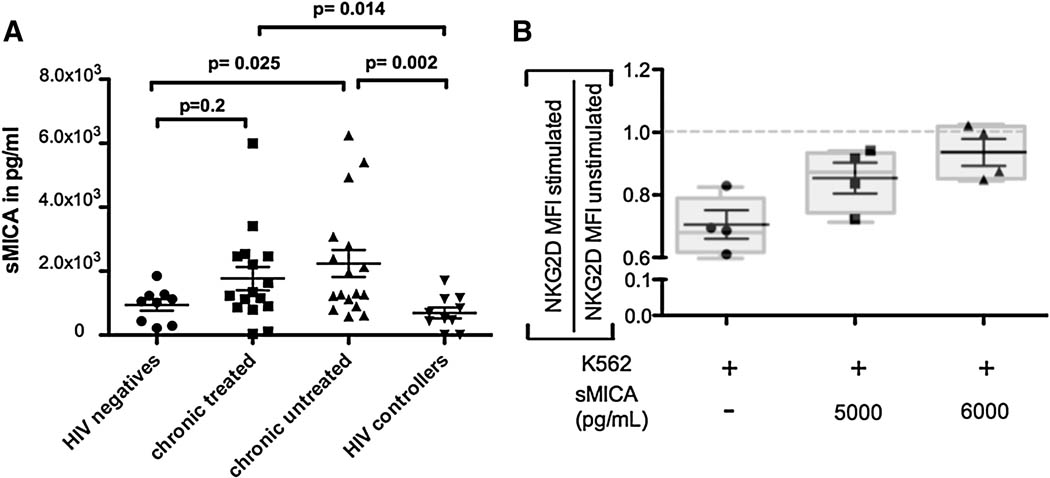
As reported previously, some tumors have evolved mechanisms to evade NKG2D-mediated NK cell elimination, such as the release of the soluble NKG2D-ligand, MHC-class 1 related protein A (Groh et al., 2002; Salih et al., 2003; Waldhauer et al., 2008). Soluble MICA (sMICA) can inactivate NKG2D expressing NK cells at a distance, resulting in a downregulation of NKG2D expression on NK cells thereby preventing NK cells from recognizing and eliminating MICA expressing tumor cells. To determine whether differences in sMICA may account for the dysregulation of the NKG2D-pathway in HIV-1 infected individuals, we compared sMICA in the sera of HIV-1 infected and uninfected individuals, including HIV-1 controllers. sMICA levels were significantly higher in untreated, chronically infected individuals compared to healthy controls and HIV-1 controllers (p=0.02 and p=0.002, respectively Figure 4A). While treated chronics only tended to have higher levels of sMICA in their peripheral circulation compared to HIV-negative controls (p=0.2), they exhibited significantly higher levels of sMICA in serum compared to HIV controllers (p=0.014), suggesting that therapeutic reduction of active viral replication may not completely shut down MICA secretion potentially resulting in persistently compromised NK cell activity. Thus, chronic HIV-1 infection is associated with elevated levels of MICA in patient sera that may represent a novel evasion strategy employed by HIV-1 to subvert detection by NKG2D-expressing NK cells.
Figure 4. A. Elevated secretion of the NKG2D ligand MICA in chronic HIV-1 infection.
(A) Dot plots represent serum levels of soluble NKG2DL MICA among individuals with chronic untreated (triangles, n=17), chronic treated (squares, n=16), HIV controllers (transparent circles, n=10), and HIV-negative controls (filled circles, n=9). (B) Graphs depict the ratio of NKG2D expression on stimulated : unstimulated NK cells in the presence of increasing doses of sMICA in 4 individuals, suggesting that increasing doses of sMICA reduces the capacity of NK cells to downregulate NKG2D. The dotted line represents the average NKG2D MFI in unstimulated NK cells.
Elevated levels of sMICA result in impaired NKG2D downregulation
As mentioned above, in healthy individuals, NKG2D is downregulated from the surface of NK cells following stimulation with one of its ligands (Groh et al., 2002; Wiemann et al., 2005). As a consequence, stimulation with K562 cells will lead to a decrease of the MFI of NKG2D on degranulating cells as a result of NKG2D loss (result: NKG2D MFI low, Δ MFI high) under normal conditions. To determine whether increased levels of sMICA result in aberrant NKG2D responsiveness (Groh et al., 2002), we incubated PBMCs from healthy controls with increasing levels of sMICA and then stimulated them with K562 cells. Our results show a positive correlation between sMICA and NKG2D MFI on degranulating cells after stimulation (Figure 4B), i.e. the higher the sMICA value of the serum the less NKG2D is downregulated from the cell surface after activation, which indicates that sMICA may impair NKG2D-mediated activation of NK cells and therefore impair NK cell effector function.
Elevated transcription of NKG2DL cleaving enzymes in HIV-infected CD4+ T cells
In the setting of Multiple Myeloma (MM), MICA is secreted from the surface of tumor cells by the proteolytic enzyme ERP5 (Jinushi et al., 2008; Kaiser et al., 2007). In addition, MMPs have also been shown to cleave and therefore release of MICA into the peripheral circulation (Salih, Rammensee, and Steinle, 2002; Waldhauer et al., 2008). To determine which class of enzymes may be involved in the cleavage of MICA from the surface of HIV-1 infected CD4+ T cells, we looked at the mRNA transcriptional level of different MMPs (including MMP 1,2,7,9) and ERP5- in HIV-1 infected and uninfected CD4+ T cells derived from infected and non-infected individuals. QPCR analysis revealed significantly higher transcription levels of MMP-7 and a tendency to higher MMP-2 levels in CD4+-T helper cells from infected individuals compared to the control group (Figure 5) potentially contributing to elevated MICA shedding in chronic untreated patients. In contrast, no difference was observed for MMP-1 and MMP-9 (data not shown). Furthermore, no difference was observed in ERP5 transcript levels in purified CD4+ T cells suggesting that this protein is likely not responsible for NKG2DL shedding in HIV-1 infected CD4+ T cells, as it is in MM (Figure 5). These results suggest that MMP-2 and MMP-7 may be involved in increased NKG2DL release into the peripheral circulation.
Figure 5. Upregulation of MMP-2 and MMP-7, but not ERP5 in CD4-T helper cells.
Depicted bars compare transcriptional levels of proteolytic enzymes MMP-2, MMP-7, and ERP-5 between HIV positives (n=8) and the healthy control group (n=8).
HIV infection results in the upregulation of NKG2DLs in CD4+ T cells
To define whether HIV infection is able to induce the expression of NKG2DLs, we first examined whether in vitro infection of CD4+ T cells altered NKG2DL transcription. Thus activated CD4+ T cells were generated from 8 different HIV-negative donors, and were infected in vitro. Following 2 days of coculture RNA was extracted and the level of MICA and MICB expression were quantified by qPCR and the values were normalized to GAPDH. HIV-infected CD4+ T cells expressed significantly higher levels of MICA transcripts compared to matched uninfected CD4+ T cells (p=0.003, Figure 6A). In contrast, no change was observed in MICB transcript levels (data not shown). These data strongly suggest that activation of CD4+ T cells alone is not sufficient to upregulate NKG2D-ligand, but that HIV infection preferentially induces the upregulation of MICA transcripts.
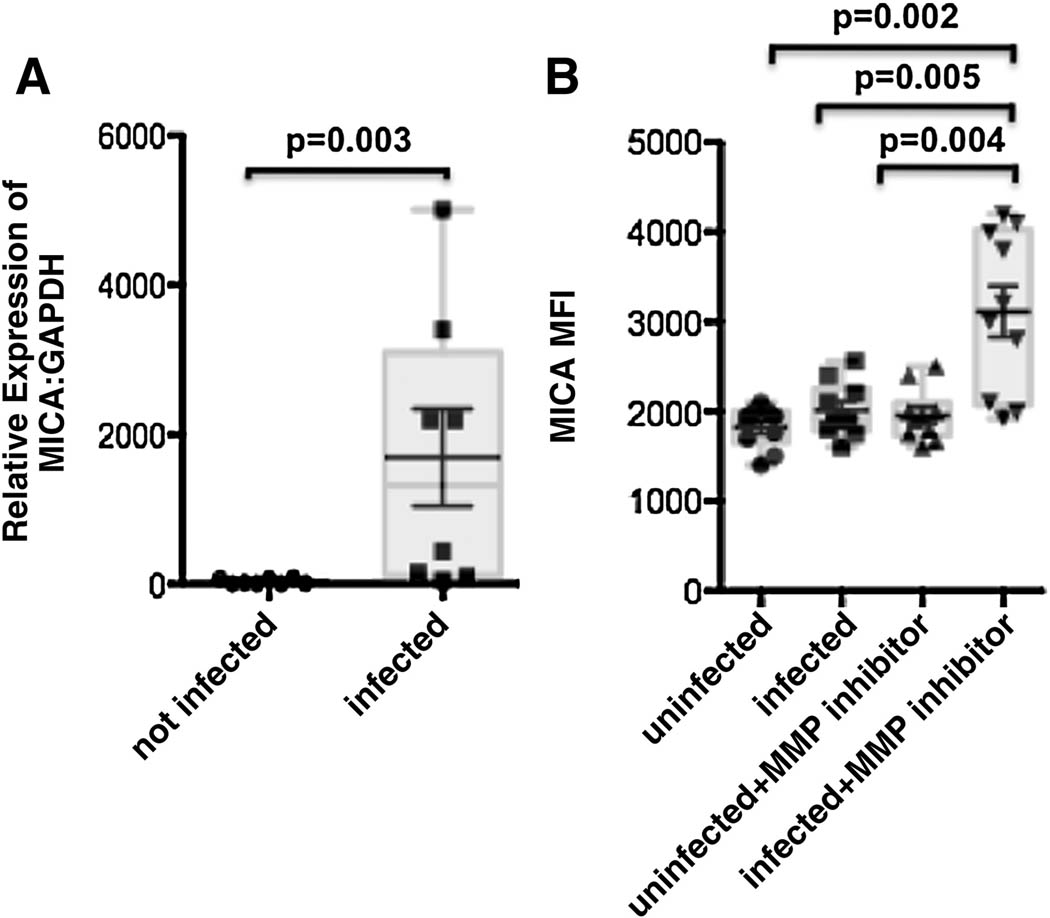
Figure 6. In vitro HIV infection induces NKG2D-ligand, MICA, expression on CD4+ T cells.
(A) The whisker plot depicts the relative expression of MICA transcripts: to the house keeping gene GAPDH transcripts in in vitro uninfected (circles) or infected (squares) CD4+ T cells from 4 HIV-negative donors. (B) The whisker plots represent the mean fluorescence intensity of MICA on the surface of uninfected or infected CD4+ T cells (gated on CD3+ cells) in the presence or absence of the MMP-inhibitor GM6001.
Despite the upregulation of MICA-transcripts, we did not observe any change in the expression of MICA on the surface of HIV-infected CD4+ T cells (Figure 6B). These data suggest that MICA may either not be translated upon transcriptional activation or that MICA proteins are rapidly cleaved from the surface of the cell surface, due to the upregulation of MMPs (Figure 5). Thus to test the second hypothesis, we blocked MMP activity using a general MMP-inhibitor GM6001. In the absence of the MMP-inhibitor, MICA expression did not increase on the surface of infected cells (Figure 6B). However, in the presence of the inhibitor, we observed a significant upregulation of MICA expression, that did not appear on uninfected MMP-inhibitor treated cells, strongly suggesting that MICA expression is induced on infected CD4+ T cells upon HIV infection. However, HIV has evolved a mechanism to counteract this activity by upregulating MMP activity that reduces the susceptibility of infected cells to NKG2D-mediated elimination by rapidly eliminating its ligands.
Discussion
The quality of NK cell-mediated cytotoxicity is critical for the host immune response against infections (Biron, Byron, and Sullivan, 1989; Etzioni et al., 2005; Trinchieri, 1989). However, several features of the NK cell response in HIV-1 infection are aberrant. These include changes in the distribution of NK cell subsets, changes in NK cell receptor expression, and a dramatic loss of NK cell function (Alter et al., 2005; Liu et al., 2009; Mavilio et al., 2005; Zaunders et al., 1995), including changes in NKG2D expression (Mavilio et al., 2003). It has been postulated that persistent viral replication may drive these changes in NK cell phenotype and function, however the underlying mechanisms that accounts for this dysregulation are yet to be defined. Several tumors are able to evade NK cell detection through the release of NKG2DLs that bind NKG2D on the surface of NK cells, thus driving them into a refractory state (Groh et al., 2002). Here we show for the first time that, similar to some tumors, HIV-1 is able to evade NK cell detection by inactivating these cells at a distance via the release of NKG2DLs from the surface of infected cells by MMP-2 and -7. These data elucidate a novel mechanism by which HIV-1 is able to indirectly evade NK cells and can lead to the consequential accumulation of dysfunctional NK cells in chronic infection.
NKG2D plays a critical role in NK cell-mediated control and clearance of a number of different infections (Biron, Byron, and Sullivan, 1989; Etzioni et al., 2005; Fang, Lanier, and Sigal, 2008). We show new data demonstrating that NKG2D expression and transcription are both reduced in NK cells in chronic untreated HIV-1 infection compared to spontaneous controllers. These changes in NKG2D expression were also associated with a reduced capacity of NK cells to respond to NKG2D-target cell lines, K562, but not other target cell lines. These data suggest that NKG2D-dependent degranulation in NK cells may be specifically dysfunctional in HIV-1 infection.
A number of tumors result in a profound downmodulation of NKG2D (Groh et al., 2002; Salih, Rammensee, and Steinle, 2002; Wiemann et al., 2005), in the presence of high levels of the soluble NKG2DL MICA (sMICA). Shedding of MICA by proteolytic enzymes has been clearly associated with disease progression in certain cancer models such as MM and other cancer models {Jinushi, 2008 #6827}. Here we looked at the sMICA level in the plasma of different clinical subgroups and found sMICA to be significantly elevated in chronic HIV-1 infection compared to controllers and negative controls. These data indicate that HIV-1 may have evolved a mechanism to release this stress signal from the surface of infected cells as a means to evade detection by NK cells. Previous work has shown that HIV-1 is able to evade NKG2D-detection, as the Nef protein is able to downregulate some of NKG2DLs from the surface of infected cells (Cerboni et al., 2007). The fact that Nef has evolved this capacity strongly suggests that NKG2D+ NK cells place sufficient pressure on virally infected cells that the virus has devised a means to circumvent this activity. However, the downregulation of NKG2DL still does not account for the dysregulated NK cell activity that is observed over the course of HIV-1 infection. Here we suggest that the additional release of NKG2DLs from the surface of infected CD4+ T cells or other susceptible cells may account for the induction of NK cell dysfunction, rendering NK cells refractory if they come in contact with an infected cell. Thus the persistent release of MICA, while protecting cells from direct NKG2D-mediated recognition, may also result in a progressive accumulation of dysfunctional NK cells over time, in line with the observed accumulation of anergic NK cells in the peripheral blood of chronically infected individuals. Thus, HIV-1 is able to evade NK cells in two different manners, the downregulation of MICA from the surface of infected cells via NEF and the shedding of MICA from the cell surface.
Previous work suggests that both MMPs and ERP5 are associated with MICA shedding (Kaiser et al., 2007; Salih, Rammensee, and Steinle, 2002). Here we did not detect any differences in ERP5 transcription among the CD4+-T cells derived from different clinical groups of HIV-1 infected patients compared to controls (Figure 5). These data suggest that MMPs rather than ERP5 may be involved in MICA shedding in HIV-1-infection. Thus inhibition of these enzymes might represent a promising novel therapeutic approach to consider in the future to promote NK cell mediated elimination of HIV-infected CD4+ T cells. This approach has already been used successfully in a number of clinical trials that employed synthetic inhibitors of MMPs to treat AIDS related malignancies (Brown, 2000; Dezube, Sullivan, and Koon, 2006).
In conclusion, here we show that HIV-1 may indirectly drive NK cell exhaustion via the release of NKG2DLs into the peripheral circulation, mediated by elevated levels of MMPs in HIV-1 infected CD4+ T cells. This novel evasion strategy provides an additional means by which HIV-1 is able to subvert detection by NK cells and also possibly NKG2D+ T cells. New strategies aimed at inhibiting MMP activity or blocking the binding of soluble NKG2DL may provide novel avenues by which to improve NK cell activity, potentially allowing them to gain enhanced control over viral replication in vivo.
Acknowledgements
This work was supported by the Koeln Fortune Program of the Faculty of Medicine, University of Cologne, Germany (AN), and the Bill & Melinda Gates Foundation (MA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, Waring MT, Streeck H, Johnston MN, Staller KD, Zaman MT, Yu XG, Lichterfeld M, Basgoz N, Rosenberg ES, Altfeld M. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005 doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296(5571):1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320(26):1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- Brown PD. Ongoing trials with matrix metalloproteinase inhibitors. Expert Opin Investig Drugs. 2000;9(9):2167–2177. doi: 10.1517/13543784.9.9.2167. [DOI] [PubMed] [Google Scholar]
- Cao W, Xi X, Wang Z, Dong L, Hao Z, Cui L, Ma C, He W. Four novel ULBP splice variants are ligands for human NKG2D. Int Immunol. 2008;20(8):981–991. doi: 10.1093/intimm/dxn057. [DOI] [PubMed] [Google Scholar]
- Cerboni C, Neri F, Casartelli N, Zingoni A, Cosman D, Rossi P, Santoni A, Doria M. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol. 2007;88(Pt 1):242–250. doi: 10.1099/vir.0.82125-0. [DOI] [PubMed] [Google Scholar]
- Dezube BJ, Sullivan R, Koon HB. Emerging targets and novel strategies in the treatment of AIDS-related Kaposi's sarcoma: bidirectional translational science. J Cell Physiol. 2006;209(3):659–662. doi: 10.1002/jcp.20795. [DOI] [PubMed] [Google Scholar]
- Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413(6852):165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzioni A, Eidenschenk C, Katz R, Beck R, Casanova JL, Pollack S. Fatal varicella associated with selective natural killer cell deficiency. J Pediatr. 2005;146(3):423–425. doi: 10.1016/j.jpeds.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Fang M, Lanier LL, Sigal LJ. A role for NKG2D in NK cell-mediated resistance to poxvirus disease. PLoS Pathog. 2008;4(2):e30. doi: 10.1371/journal.ppat.0040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fdez-Morera JL, Tunon A, Rodriguez-Rodero S, Rodrigo L, Martinez-Borra J, Gonzalez S, Lopez-Vazquez A, Lahoz CH, Lopez-Larrea C. Clinical behavior of multiple sclerosis is modulated by the MHC class I-chain-related gene A. Tissue Antigens. 2006;67(5):409–414. doi: 10.1111/j.1399-0039.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- Gambelunghe G, Brozzetti A, Ghaderi M, Candeloro P, Tortoioli C, Falorni A. MICA gene polymorphism in the pathogenesis of type 1 diabetes. Ann N Y Acad Sci. 2007;1110:92–98. doi: 10.1196/annals.1423.011. [DOI] [PubMed] [Google Scholar]
- Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci U S A. 1996;93(22):12445–12450. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2(3):255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419(6908):734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- Hijova E. Matrix metalloproteinases: their biological functions and clinical implications. Bratisl Lek Listy. 2005;106(3):127–132. [PubMed] [Google Scholar]
- Huard B, Karlsson L. KIR expression on self-reactive CD8+ T cells is controlled by T-cell receptor engagement. Nature. 2000;403(6767):325–328. doi: 10.1038/35002105. [DOI] [PubMed] [Google Scholar]
- Jinushi M, Vanneman M, Munshi NC, Tai YT, Prabhala RH, Ritz J, Neuberg D, Anderson KC, Carrasco DR, Dranoff G. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci U S A. 2008;105(4):1285–1290. doi: 10.1073/pnas.0711293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser BK, Yim D, Chow IT, Gonzalez S, Dai Z, Mann HH, Strong RK, Groh V, Spies T. Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature. 2007;447(7143):482–486. doi: 10.1038/nature05768. [DOI] [PubMed] [Google Scholar]
- Kiessling R, Klein E, Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Lanier LL. A renaissance for the tumor immunosurveillance hypothesis. Nat Med. 2001;7(11):1178–1180. doi: 10.1038/nm1101-1178. [DOI] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Li K, Mandai M, Hamanishi J, Matsumura N, Suzuki A, Yagi H, Yamaguchi K, Baba T, Fujii S, Konishi I. Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: high expression of ULBP2 is an indicator of poor prognosis. Cancer Immunol Immunother. 2009;58(5):641–652. doi: 10.1007/s00262-008-0585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limou S, Le Clerc S, Coulonges C, Carpentier W, Dina C, Delaneau O, Labib T, Taing L, Sladek R, Deveau C, Ratsimandresy R, Montes M, Spadoni JL, Lelievre JD, Levy Y, Therwath A, Schachter F, Matsuda F, Gut I, Froguel P. Delfraissy JF, Hercberg S, Zagury JF. Genomewide association study of an AIDS-nonprogression cohort emphasizes the role played by HLA genes (ANRS Genomewide Association Study 02) J Infect Dis. 2009;199(3):419–426. doi: 10.1086/596067. [DOI] [PubMed] [Google Scholar]
- Linsley PS, Bradshaw J, Urnes M, Grosmaire L, Ledbetter JA. CD28 engagement by B7/BB-1 induces transient down-regulation of CD28 synthesis and prolonged unresponsiveness to CD28 signaling. J Immunol. 1993;150(8 Pt 1):3161–3169. [PubMed] [Google Scholar]
- Liu Q, Sun Y, Rihn S, Nolting A, Tsoukas PN, Jost S, Cohen K, Walker B, Alter G. Matrix metalloprotease inhibitors restore impaired NK cell-mediated antibody-dependent cellular cytotoxicity in human immunodeficiency virus type 1 infection. J Virol. 2009;83(17):8705–8712. doi: 10.1128/JVI.02666-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Arbesu R, Ballina-Garcia FJ, Alperi-Lopez M, Lopez-Soto A, Rodriguez-Rodero S, Martinez-Borra J, Lopez-Vazquez A, Fernandez-Morera JL, Riestra-Noriega JL, Queiro-Silva R, Quinones-Lombrana A, Lopez-Larrea C, Gonzalez S. MHC class I chain-related gene B (MICB) is associated with rheumatoid arthritis susceptibility. Rheumatology (Oxford) 2007;46(3):426–430. doi: 10.1093/rheumatology/kel331. [DOI] [PubMed] [Google Scholar]
- Lozzio BB, Lozzio CB. Properties of the K562 cell line derived from a patient with chronic myeloid leukemia. Int J Cancer. 1977;19(1):136. doi: 10.1002/ijc.2910190119. [DOI] [PubMed] [Google Scholar]
- Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, Marcenaro E, Bottino C, Moretta L, Moretta A, Fauci AS. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A. 2003;100(25):15011–15016. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, O'Shea MA, Kinter A, Kovacs C, Moretta A, Fauci AS. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A. 2005;102(8):2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramankulov A, Lein M, Johannsen M, Schrader M, Miller K, Jung K. Plasma matrix metalloproteinase-7 as a metastatic marker and survival predictor in patients with renal cell carcinomas. Cancer Sci. 2008;99(6):1188–1194. doi: 10.1111/j.1349-7006.2008.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3(10):781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, Steinle A. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102(4):1389–1396. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169(8):4098–4102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, DeMars R. Production of human cells expressing individual transferred HLA-A,-B,-C genes using an HLA-A,-B,-C null human cell line. J Immunol. 1989;142(9):3320–3328. [PubMed] [Google Scholar]
- Shirts BH, Kim JJ, Reich S, Dickerson FB, Yolken RH, Devlin B, Nimgaonkar VL. Polymorphisms in MICB are associated with human herpes virus seropositivity and schizophrenia risk. Schizophr Res. 2007;94(1–3):342–353. doi: 10.1016/j.schres.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Swann J, Kelly JM, Cretney E, Yokoyama WM, Diefenbach A, Sayers TJ, Hayakawa Y. NKG2D recognition and perforin effector function mediate effective cytokine immunotherapy of cancer. J Exp Med. 2004;200(10):1325–1335. doi: 10.1084/jem.20041522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Advances in Immunology. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valitutti S, Muller S, Salio M, Lanzavecchia A. Degradation of T cell receptor (TCR)-CD3-zeta complexes after antigenic stimulation. J Exp Med. 1997;185(10):1859–1864. doi: 10.1084/jem.185.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Groh V, thor Straten P, Spies T, Brocker EB, Becker JC. Expression of stress-induced MHC class I related chain molecules on human melanoma. J Invest Dermatol. 2002;118(4):600–605. doi: 10.1046/j.1523-1747.2002.01700.x. [DOI] [PubMed] [Google Scholar]
- Waldhauer I, Goehlsdorf D, Gieseke F, Weinschenk T, Wittenbrink M, Ludwig A, Stevanovic S, Rammensee HG, Steinle A. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008;68(15):6368–6376. doi: 10.1158/0008-5472.CAN-07-6768. [DOI] [PubMed] [Google Scholar]
- Webster NL, Crowe SM. Matrix metalloproteinases, their production by monocytes and macrophages and their potential role in HIV-related diseases. J Leukoc Biol. 2006;80(5):1052–1066. doi: 10.1189/jlb.0306152. [DOI] [PubMed] [Google Scholar]
- Wiemann K, Mittrucker HW, Feger U, Welte SA, Yokoyama WM, Spies T, Rammensee HG, Steinle A. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J Immunol. 2005;175(2):720–729. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Grunebach F, Schmidt SM, Heine A, Hantschel M, Stevanovic S, Rammensee HG, Brossart P. Matrilysin (MMP-7) is a novel broadly expressed tumor antigen recognized by antigen-specific T cells. Clin Cancer Res. 2008;14(17):5503–5511. doi: 10.1158/1078-0432.CCR-07-4041. [DOI] [PubMed] [Google Scholar]
- Zaunders J, Carr A, McNally L, Penny R, Cooper DA. Effects of primary HIV-1 infection on subsets of CD4+ and CD8+ T lymphocytes. AIDS. 1995;9(6):561–566. doi: 10.1097/00002030-199506000-00005. [DOI] [PubMed] [Google Scholar]