Abstract
The efficacy of a stabilized chemical analog of double-stranded ribonucleic acid (RNA), PIKA, as prophylaxis against infection with 5 different influenza A virus subtypes, including the 2009 swine-origin pandemic H1N1 virus, was evaluated in mice. Intranasal treatment with PIKA resulted in significant reduction of viral replication in the respiratory tract. The inhibitory effect was mediated by rapid infiltration of immune cells into the lungs, and production of inflammatory cytokines. While TLR3 is important for the optimal production of these inflammatory cytokines, inhibition of viral replication was still observed in TLR3−/− mice. In addition, a significant synergistic effect in inhibiting H5N1 virus replication was observed when PIKA was co-administered with oseltamivir. The broad-spectrum protection provided by PIKA makes it an attractive option for prophylaxis from infection with influenza A viruses.
Keywords: TLR3, influenza, immunoprophylaxis
Introduction
Influenza is an acute respiratory disease associated with significant morbidity and mortality worldwide, particularly among the elderly and young children (Izurieta et al., 2000; Neuzil et al., 2000; Nicholson, Wood, and Zambon, 2003). The newly emerged swine-origin H1N1 virus has caused the first influenza pandemic of this century (Centers for Disease Control and Prevention, 2009b). The enzootic of highly pathogenic H5N1 influenza A viruses in poultry in Asia with sporadic transmission to humans also raises concerns of a possible pandemic (Beigel et al., 2005; Lipatov et al., 2004). Vaccination is the most effective tool for controlling an influenza pandemic, but it is estimated that the global suppliers of vaccines can only produce ~2.5 billion doses within the first 12 months following receipt of a new vaccine strain and will take 4 years to meet the global demand (International Federation of Pharmaceutical Manufacturers & Associations, 24 February 2009). While anti-influenza drugs, such as neuraminidase inhibitors (oseltamivir and zanamivir) or ion-channel blockers (adamantanes) have been used for treatment and prophylaxis (Hayden et al., 1999; Treanor et al., 2000), drug resistance occurs and the appearance of drug resistant viruses before or during a pandemic would severely affect the efficacy of treatment strategies (de Jong et al., 2005; Hayden, 2006a; Hayden, 2006b; Hurt et al., 2007; Kiso et al., 2004; Le et al., 2005; McKimm-Breschkin et al., 2007). Therefore, additional anti-viral strategies are needed.
In contrast to the adaptive immune system, the innate immune system provides broad-spectrum defense that can be activated immediately or within hours after infection. It is believed that these anti-viral responses play a critical role in limiting the replication and dissemination of pathogens, providing time for adaptive immune effectors to develop. For example, different innate cell types, such as neutrophils (Fujisawa, 2008) and macrophages (Herold et al., 2008) contribute to the control of influenza A virus replication in vivo and plasmacytoid dendritic cells (pDCs) produce large amounts of type 1 interferon after exposure to influenza (Jego et al., 2003; Thitithanyanont et al., 2007).
We were interested in targeting innate immune mechanisms because they may be less strain-specific than adaptive immune mechanisms. We have previously demonstrated that the administration of a stabilized chemical analog of double stranded (ds) RNA (PIKA) was able to inhibit the replication of 3 mouse-adapted laboratory strains (H1N1 and H3N1) of influenza A virus in mice (Lau, Tang, and Ooi, 2009). In the current study, we extended these observations to influenza A viruses with pandemic potential and variable virulence, including three clinical isolates from humans, an H5N1 virus, an H9N2 virus and a swine-origin H1N1 virus from the 2009 pandemic. Using a number of assays, we also showed that a heightened antiviral state was achieved in the respiratory tract shortly after administration of the drug; several chemokines were produced and different cell types infiltrated into the lungs. This study demonstrates the feasibility of harnessing innate immunity to provide broad-spectrum protection against multiple influenza A virus subtypes, an attractive approach as a first line of defense in the event of an influenza pandemic. We also evaluated a combination of PIKA and oseltamivir for the prevention of H5N1 virus infection in mice.
Materials and Methods
Mice
Six to 8-week-old female BALB/c mice (Taconic Farms, Inc., Germantown, NY) were used in all mouse experiments. The B6;129S1-Tlr3tm1Flv/J (stock number 005217) and B6129SF2/J (stock number 101045) were purchased from The Jackson Laboratory (Bar Harbor, ME). The animal protocols were approved by the National Institutes of Health Animal Care and Use Committee and the experiments were conducted at the NIH.
Viruses
Viruses used in this study were kindly provided by Dr Robert G. Webster, St. Jude Children's Research Hospital, Memphis, TN, Dr Alexander Klimov, Influenza Division, Centers for Disease Control and Prevention, Atlanta, GA and Dr David Swayne, Southeast Poultry Research Laboratory, USDA, Athens, GA. Virus stocks were propagated in the allantoic cavity of 9-day old embryonated specific-pathogen-free hen's eggs (Charles River Laboratories, Wilmington, MA) at 37°C. Clarified allantoic fluids were aliquoted and stored at −80°C.
Administration of PIKA and oseltamivir phosphate
PIKA was obtained from NewBiomed PIKA Pte Ltd. (Singapore). The endotoxin level of the lot of PIKA used in this study was determined by an independent accredited laboratory and was < 1 endotoxin unit / mg of PIKA. Mice were lightly anesthetized with 4% isoflurane and inoculated intranasally with 100 μg of PIKA in 50 μL of sterile PBS. Control mice received 50 μL of sterile PBS. Oseltamivir phosphate (Tamiflu, 75 mg, Roche, New Jersey) was obtained from a local pharmacy. The contents of 75 mg capsules were emptied from the shell and dissolved in water. Mice were given oseltamivir by oral gavage twice daily at 10 mg/kg/day in a volume of 200 μL for 3 days. Control mice received sterile water on the same schedule.
Viral titration assay
Lungs and nasal turbinates were harvested, weighed, and homogenized in L-15 medium to prepare a 10% w/v tissue homogenate. The materials were clarified by low-speed centrifugation and viral titers of the samples were determined using MDCK cells as previously described and expressed as log10 TCID50/g of tissue (Gillim-Ross et al., 2008).
Cytokine analysis using Bio-plex and ELISA
The concentration of various cytokines or chemokines in clarified lung homogenates (10% w/v in L15 media) harvested at specified time points were measured by the Bio-plex Protein Array system (Bio-rad, Hercules CA). The assay was performed according to the manufacturer's instructions and the results were analyzed using the Bio-Plex manager software. One hundred microliters of lung homogenate were used to measure IFN-β using a mouse IFN-β ELISA kit (PBL Biomedical Laboratories, Piscataway,NJ).
Preparation of single cell suspensions from lungs
Single cell suspensions were prepared from lungs as previously described (Lau et al., 2006). In brief, the lungs were harvested, finely minced and digested in RPMI-1640 medium containing 2 mg/mL of collagenase A (Roche Diagnostics GmbH, Mannheim, Germany) for 30 min at 37°C. The collagenase A-treated tissues were then homogenized by pressing against sieves (40 nm, BD Falcon) with plastic plungers. Red blood cells were removed from the samples by hypotonic shock with 0.15 M ammonium chloride in 17 mM Tri-HCl for 5 min and were resuspended in PBS with 1% fetal calf serum (FCS) for flow cytometry analysis.
FACS analysis
Monoclonal antibodies (MAbs) against mouse CD3 (PerCP), CD4 (PE or ALPC), CD8 (PE or ALPC), CD11b (PerCP), CD11c (ALPC), Ly-6G (PE), Ly-6G/C (PE), CD14 (FITC), CD19 (PE), B220 (PerCP) were purchased from BD PharMingen (San Diego, CA). Cell suspensions were treated with Mouse Fc Block™ (BD PharMingen, CA) on ice for 15 mins before staining with various combinations of MAb for 30 mins on ice. Cells were washed twice with PBS with 1% FCS before analysis on a FACSCalibur (BD Biosciences). A total of 80,000 events were acquired for each lung sample. The data were analyzed by FlowJo (Tree Star, Inc. Ashland, OR).
NF-κB reporter gene assay
The mouse RIG-I and MDA-5 expressing plasmids were from InvivoGen (San Diego, CA) and human embryonic kidney (HEK) 293 cells were transfected with the plasmids and the assay was performed as previously described (Lau, Tang, and Ooi, 2009).
ELISA
ELISAs were performed with serum samples as described previously (Deliyannis et al., 1998) using BPL-inactivated A/Puerto Rico/8/34 as the coating antigen. The optical density of each well was measured at 405 nm using the SpectraMax M5 microplate reader (Molecular Devices. Sunnyvale, CA).
Statistical analysis
The significance of differences between different groups was assessed by the Mann-Whitney test using Prism 5 (GraphPad Software, Inc. San Deigo, CA). In selected experiments, where the sample size of each group was less than 5, the unpaired t test was used. Reported p values < 0.05 are considered significantly different.
Results
Inhibition of viral replication in the respiratory tract of mice after PIKA administration
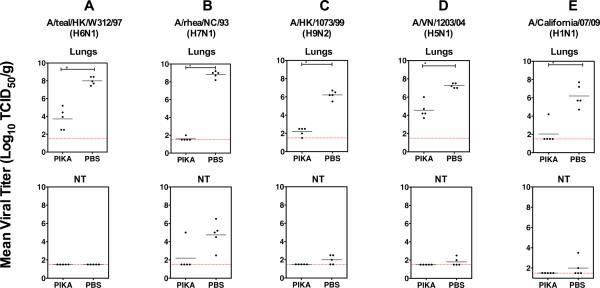
We previously showed that after intranasal (i.n.) administration of PIKA, pulmonary viral titers of three different laboratory strains of influenza virus (A/Puerto Rico/8/34 (H1N1), A/WS/33 (H1N1) and a reassortant of A/Memphis/1/71 (H3N1)) were substantially reduced compared to a PBS control group (Lau, Tang, and Ooi, 2009). In this study, we proceeded to evaluate the efficacy of this strategy against 5 wild-type (wt) influenza A viruses with pandemic potential. Groups of five lightly anesthetized 8 to 10-week-old female BALB/c mice were given 100 μg of PIKA or PBS, in a volume of 50 μL i.n. 6 hrs prior to infection with 4 avian influenza viruses, A/teal/HK/W312/97 (H6N1), A/rhea/NC/93 (H7N1), A/HK/1073/99 (H9N2) and A/Vietnam/1203/2004 (H5N1) and an isolate from the 2009 H1N1 pandemic A/California/07/2009 (H1N1). Apart from the H5N1 virus, for which the challenge dose was set at 15 TCID50 because it is exceptionally virulent in mice, other viruses were administered at a dose of 50 TCID50. Mice continued to receive daily treatment with 100 μg of PIKA i.n. for two days before they were sacrificed on day 3 post-infection (p.i.) and virus titers in nasal turbinates (NT) and lungs were determined. As shown in Fig. 1A–E, the viruses replicated to high titers in the lungs of mice treated with PBS. Lung virus titers were statistically lower in mice that were treated daily with PIKA than in the PBS-control group (p <0.05, Mann-Whitney test). The efficacy of PIKA against the H6N1 virus could only be assessed in the lower respiratory tract because the virus did not replicate to detectable levels in the NT of mice. For the other viruses, 5/5 (H7N1), 3/5 (H9N2), 2/5 (H5N1) and 2/5 (H1N1) of the PBS-treated mice had virus detected in the NT. Except for the H7N1 experiment in which one mouse in the PIKA-treated group had virus detected in the NT, none of the PIKA-treated mice in other challenge groups had virus detected in the NT, demonstrating the effectiveness of the treatment in inhibiting viral replication in the upper respiratory tract. In summary, the results suggest that PIKA prophylaxis can effectively inhibit the replication of several subtypes of wt influenza A viruses including the newly emerged H1N1 pandemic virus, leading a substantial reduction of viral titers in the respiratory tract.
Figure 1. Administration of PIKA inhibited replication of influenza viruses in the respiratory tract of mice.
Groups of five mice each were given 100 μg of PIKA in PBS intranasally 6 h before challenge with the indicated viruses. The mice continued to receive PIKA treatment once a day for two additional days. Lungs and NT were harvested on day 3 post-infection. Virus titers in the lungs and NT were determined in MDCK cells. The lower limit of detection was 101.5 TCID50 per gram of tissue. The horizontal bars represent the geometric mean of the group. The percent reduction in mean viral titer relative to the control group treated with PBS is shown above each column of data. The `*' symbol indicates that the difference between the groups was statistically significant (p<0.05).
Administration of PIKA protects mice from lethality following challenge with A/Puerto Rico/8/34
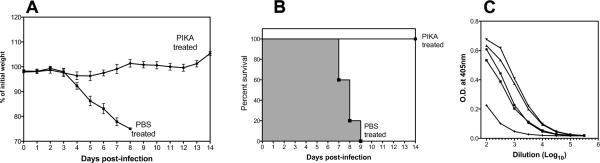
To determine the significance of the reduction in pulmonary virus titer observed in our previous study (Lau, Tang, and Ooi, 2009) and in Fig. 1, groups of 5 mice were treated with PIKA as previously described, were challenged with 4LD50 of A/Puerto Rico/8/34 and monitored for 2 weeks. As shown in Fig. 2A–B, mice that received 3 doses of PIKA were completely protected from lethal challenge with no significant weight loss and 100% survival, whereas all of the mice that received PBS treatment demonstrated significant weight loss and succumbed to infection. In addition, virus-specific antibodies were detected in the sera of the PIKA-treated mice on day 14 post-infection (Fig. 2C).
Figure 2. Administration of PIKA protected mice from lethal challenge and initiated influenza-specific antibody responses.
(A–B) Groups of five mice each were given PIKA or PBS as previously described and challenged with 4 LD50 of A/Puerto Rico/8/34 intranasally. The mice continued to receive PIKA treatment once a day for two additional days. Weight loss and survival rate was monitored for 2 weeks. (C) Serum samples were collected from PIKA-treated mice on day 14 post-infection and influenza-specific antibody titers were determined by ELISA.
Co-administration of PIKA with Oseltamivir provides synergistic protection against challenge with VN04 (H5N1) in mice
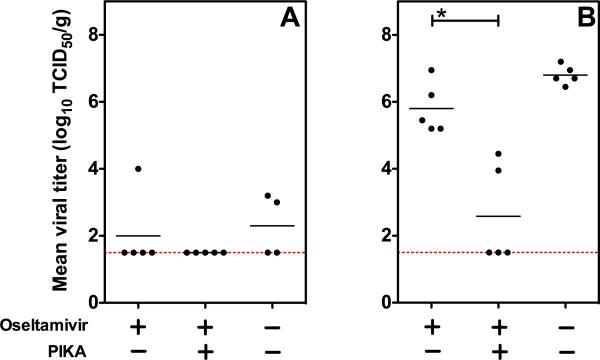
Although oseltamivir phosphate (Tamiflu) has been used widely in treating infections with highly pathogenic H5N1 influenza A viruses in humans, many patients still succumb to infection (Beigel et al., 2005) and therefore more effective treatment is needed. We proceeded to determine the benefit of co-administering PIKA with oseltamivir for protection of mice against H5N1 virus infection in mice. Groups of 5 mice were given 100 μg of PIKA i.n. and 10 mg/kg/day of oseltamivir by oral gavage 6 hours before challenge with 15 TCID50 of VN04 (H5N1, ~6 MLD50) wt virus i.n. The mice continued to receive daily treatment with PIKA and 2 doses of oseltamivir treatment daily. The dose of oseltamivir used was selected based on a study by Yen et al. that demonstrated that a 5-day regimen of 10 mg/kg/day protected 50% of mice infected with 5MLD50 of VN04 (H5N1) wt virus (Yen et al., 2005). Three days after infection, the level of viral replication in various organs was determined. This experimental design allowed us to determine the virologic benefit of the combined therapy over oseltamivir treatment alone. We had also intended to evaluate whether the combination of PIKA and oseltamivir would offer a survival benefit, but mice that received a 5-day course of oseltamivir showed 60–100% mortality on day 6 compared with 0–20% mortality in a group that received water alone by gavage on the same schedule, regardless of whether they were infected with an influenza virus. We infer that the filler material present in commercial oseltamivir might contribute to the increased death rate observed in mice because the dose of the drug is similar to that reported by Yen et al. (Yen et al., 2005).
As shown in Fig. 3A, challenging mice with 15 TCID50 of VN04 (H5N1) wt virus did not lead to significant viral replication in the NT on day 3 post-infection, even in mock-treated mice and therefore, the difference in the level of protection between the combined or oseltamivir alone regimen did not reach statistical significance. However, none of the mice that received the combined treatment had detectable levels of virus, while some mice that received oseltamivir alone or mock-treated had virus detected in the NT (1/5 and 2/4 respectively). In the lungs, there was a significant reduction in pulmonary viral titers in mice that received the combined treatment compared to the groups that received oseltamivir alone or mock-treatment (Fig. 3b, p < 0.05 Mann-Whitney test). Three out of five mice that received the combined treatment had no detectable level of virus in their lungs on day 3 post-infection and the average viral titer was lower than the group that received oseltamivir alone (Fig. 3) or PIKA alone (Fig. 1 D). These results suggest that targeting different anti-viral mechanisms is a feasible approach in achieving a synergistic virologic effect in combating H5N1 virus infection.
Figure 3. Synergistic anti-viral effect achieved against H5N1 infection in mice by co-administering PIKA with oseltamivir.
(A–B) Groups of five mice were given either oseltamivir alone by oral gavage or together with 100 μg of PIKA i.n. 6 hours before challenge with 15 TCID50 of VN04 wt. Mice continued to receive treatment for 2 additional days. NT and lungs were harvested for viral titration on day 3 post-infection. Virus titers in the NT (A) and lungs (B) were determined in MDCK cells. The lower limit of detection was 101.5 TCID50 per gram of tissue. The horizontal bars represent the geometric mean of the group. The `*' symbol indicates that the difference between the groups was statistically significant (p<0.05).
Administration of PIKA led to changes in the cellular composition and chemokine production in the lungs of mice
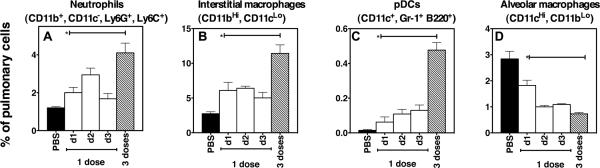
To establish the mechanism(s) responsible for the reduction in viral titers achieved by PIKA, mice that were given 1 or 3 doses of PIKA were sacrificed at various time points to examine the lungs for changes in cellularity. Single cell suspensions were made as previously described (Lau et al., 2006) and different cell populations were identified using cellular markers conjugated with fluorochromes. Using this approach, we were able to identify ~70% of the cell population in the lungs (data not shown). As shown in Fig. 4A, there was a 2-fold increase in neutrophils (CD11b+ CD11c−, Ly6G+, Ly6C+) within 24 hours of a single dose of PIKA, reaching a peak on day 2 post-administration. The number of neutrophils in the lungs of mice after 3 doses of PIKA was significantly greater than in mice that received a single dose of the drug (p< 0.05, unpaired t test). There was an infiltration of interstitial macrophages (CD11bHi, CD11cLo) in the lungs, that peaked on day 1 post-inoculation and was maintained at an elevated level for the next 72 hours (Fig. 4B). On the other hand, the infiltration of pDCs (CD11c+, Gr-1+, B220+(Nakano, Yanagita, and Gunn, 2001) into the lungs was more gradual, continuing to increase for 72 hours after a single dose of PIKA (Fig. 4C). Administration of 3 consecutive doses of PIKA led to a significant increase in both interstitial macrophages and pDCs (p< 0.05, unpaired t test). However, there was a reduction in the number of alveolar macrophages (CD11CHi, CD11bLo) within 24 hours of PIKA treatment that was maintained for 72 hours (Fig. 4D). There were no significant changes in the numbers of natural killer cells, NKT cells, B lymphocytes, CD4+ and CD8+ T lymphocytes in the lungs (data not shown).
Figure 4. Administration of PIKA changed the cellular composition of the lungs.
Groups of three mice were given 100 μg of PIKA i.n. in PBS and sacrificed 1, 2 or 3 days later. An additional group received 100 μg of PIKA i.n. once a day for 3 consecutive days and mice were sacrificed 24 hours after the last inoculation. Single-cell suspensions were prepared and the cells were stained with the indicated cellular makers. Eighty thousand events were acquired for each sample. The bars and error bars represent the mean and standard errors of the groups. The `*' symbol indicates that the difference between the groups was statistically significant (p<0.05).
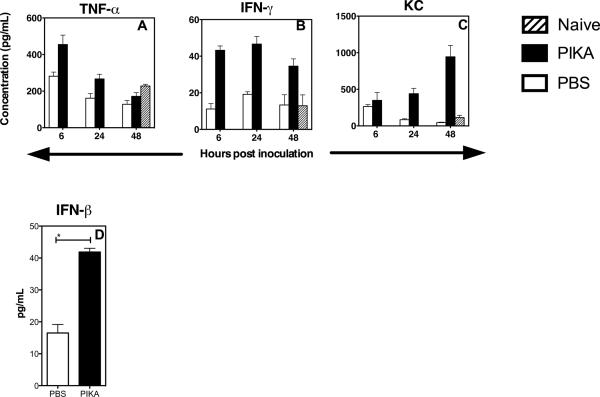
Since cytokines and chemokines are responsible for cellular migration, we examined the levels of different cytokines and chemokines in the lungs at different time points after a single dose of PIKA. The lungs were homogenized and clarified homogenates were analyzed in duplicate with the Bio-plex Protein Array system to determine the protein levels of cytokines and chemokines. As shown in Fig. 5A, an early response of TNF-α was seen, with an increase 6 hours after administration of PIKA and return to baseline by 48 hours. The production of other cytokines such as IFN-γ, Rantes, MCP-1 and MIP-1b, increased 6 hours following administration of PIKA and was sustained for 48 hours (Fig. 5B and data not shown). An increase in production of KC was observed at 24 and 48 hours following PIKA administration (Fig. 5C). In addition, there was also an increase in the production of IFN-β in lungs at 24 hours after administration of PIKA (Fig. 5D). In summary, these data suggest that intranasal administration of PIKA leads to the activation of innate immunity in the respiratory tract, leading to infiltration of the lungs by immune cells and the production of cytokines and chemokines that are associated with inhibition of viral replication in the respiratory tract of mice.
Figure 5. Administration of PIKA induced production of cytokines in the lungs.
Groups of three mice each were given 100 μg of PIKA i.n. in PBS and were sacrificed at the indicated time. The lungs were stored at −80°C till all samples were collected and homogenized in 1 mL of RPMI-1640 media. (A–C) To measure the level of different cytokines/ chemokines in the samples, 50 μL of the clarified samples were tested in duplicate using the Bio-plex Protein Array system. (D) For IFN-β, 100 μL of the clarified samples were tested using an ELISA kit. The bars and error bars represent the mean and standard error of the groups.
TLR3 is dispensable in mediating the anti-viral response to PIKA in mice
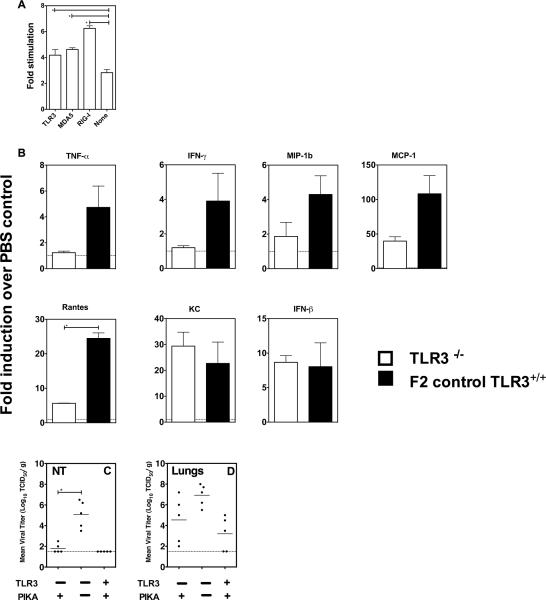
Using a plasmid-expression system, we previously showed that PIKA interacts with TLR3 (Lau, Tang, and Ooi, 2009). Using the same expression system, we showed that, in addition to TLR3, PIKA can also be recognized by melanoma differentiation-associated gene 5 (MDA-5) and retinoic acid inducible gene I (RIG-I) (Fig. 6A). Since TLR3 has been identified as a receptor for dsRNA with abundant expression in the lungs (Alexopoulou et al., 2001), mice with a targeted defect in the TLR3 gene were used to determine the role of TLR3 in mediating the anti-viral effect of PIKA. TLR3−/− and TLR3+/+ mice were given 100 μg of PIKA or PBS intranasally and the concentrations of various cytokines in lung homogenates were determined. As shown in Fig. 6B, compared with TLR3+/+mice, the TLR3−/− mice showed a general trend of diminished cytokine production. The level of reduction varied between cytokines, with no increase of TNF-α and IFN-γ detected in TLR3−/− mice after PIKA stimulation. For other cytokines such as MIP-1b, Rantes and MCP-1, there was an increase in production in TLR3−/− mice in response to PIKA treatment, however, the fold-increase was lower than those observed with TLR3+/+ mice, with the difference for Rantes reaching statistical significance (p < 0.05). The production of KC and IFN-β was comparable between the two strains of mice. The results suggest that TLR3 is important for optimal cytokine production in response to PIKA. To determine the impact of the diminished cytokine responses on the anti-viral effect of PIKA, TLR3−/− mice were treated with PIKA or PBS and were challenged with 50 TCID50 of the H7N1 virus. This virus was chosen because it was able to replicate to high titer in both the upper and lower respiratory tract of mice (Fig. 1B), allowing us to determine the importance of TLR3 in both areas. As shown in Fig. 6C, the virus replicated to high titer in the NT in the TLR3−/− mice treated with PBS and the titer was significantly higher than in mice treated with PIKA (p< 0.05, Mann-Whitney test). The virus titers in the lungs of the TLR3−/− mice were variable (Fig. 6D), and the mean titer in the two groups of mice was not statistically different. The results suggest that the production of cytokines induced by PIKA in the absence of TLR3 signaling, was still sufficient to inhibit viral replication in vivo.
Figure 6. TLR3 is dispensable in mediating the anti-viral responses of PIKA in mice.
(A) Human embryonic kidney (HEK 293 cells) were transfected with an NF-κB-luciferase reporter gene and a β-galactosidase-expressing plasmid with or without co-transfection of the indicated TLR3, MDA-5 or RIG-I receptor-expressing plasmids. Twenty–four hours after transfection, the cells were stimulated with 50 ng of PIKA in plain medium for 6 hours before the cells were lysed and luciferase activity in the lysates was determined. The data were normalized to β-galactosidase activity and expressed as fold-increase relative to expression in cells that were transfected with respective receptor-expressing plasmids without PIKA stimulation. The bar and error bars represent the mean and standard error of 8 replicates and are representative of two independent experiments. (B) Groups of three TLR3−/− or TLR3+/+ mice received PIKA or PBS intranasally and were sacrificed 24 hours later. Lung homogenates were prepared and the concentration of various cytokines was determined as previously described. Data are expressed as the fold-increase in PIKA-treated mice over PBS-treated mice. Groups of five TLR3−/− or TLR3+/+ mice received PIKA and were challenged with the H7N1 virus as previously described. Virus titers in the NT (C) and lungs (D) were determined in MDCK cells. The horizontal bars represent the geometric mean of the group. The `*' symbol indicates that the difference between the groups was statistically significant (p<0.05).
Discussion
We have demonstrated the efficacy of PIKA against different influenza A virus subtypes with pandemic potential that have caused infections in humans, including the newly emerged 2009 H1N1 pandemic virus (Butt et al., 2005; Centers for Disease Control and Prevention, 2009b; de Jong et al., 2005; Peiris et al., 1999; Subbarao et al., 1998). Although it may seem obvious that a drug that induces an interferon-induced antiviral state would prevent infection by influenza viruses, a demonstration of the efficacy of this approach against viruses of varied virulence is important and the virologic benefit of combining PIKA prophylaxis with oseltamivir is novel. We have also explored the mechanism underlying the antiviral effects of PIKA.
While immunoprophylaxis with poly IC has been described using mouse-adapted influenza strains (Saravolac et al., 2001; Wong et al., 1995; Wong et al., 1999), the mechanism responsible for the anti-viral effects has not been studied in detail. Our data show that a number of immune effectors were activated following intranasal administration of PIKA. The initial wave of cytokine production occurred 6 hours following administration of PIKA included cytokines TNF-α and IFN-γ, that have anti-viral properties (Seo and Webster, 2002). Inflammatory cytokines, such as Rantes (CCL5) (Dorner et al., 2002) and Monocyte Chemotactic Protein-1 (MCP-1) were produced in response to PIKA and led to the recruitment of effector cells such as neutrophils, macrophages and pDCs into the lungs within 24 hours of administration of a single dose of PIKA. Fujisawa et al. have demonstrated the important role of polymorphonuclear leukocytes (PMN) in the early response to influenza virus infection (Fujisawa et al., 1987) and Hashimoto et al. demonstrated that neutrophils and macrophages were capable of phagocytosing influenza-induced apoptotic cells in vivo (Hashimoto et al., 2007), thereby disrupting the infectious cycle of the virus. Several investigators have used monoclonal antibodies to deplete neutrophils and/or macrophages in vivo and found an increase in viral titers associated with severe clinical disease (Tate, Brooks, and Reading, 2008; Tumpey et al., 2005). pDCs have the ability to produce large amounts of type 1 IFNs after exposure to influenza virus (Jego et al., 2003; Thitithanyanont et al., 2007) and the release of type 1 IFNs can lead to the activation of IFN-stimulated genes with anti-viral functions, such as Mx proteins, Protein Kinase R and 2'5' oligoadenylate synthetase (reviewed in (Samuel, 2001). Since it is more difficult for pathogens to simultaneously develop resistance to two or more pathways than to a single pathway, the induction of antiviral effects by multiple pathways reduces the likelihood of viruses becoming resistant to PIKA. In addition, we demonstrated that the synergism achieved by co-administering PIKA with oseltamivir was sufficient to inhibit viral replication of the H5N1 virus (Fig. 1D and Fig. 3). Because the strategy of activating generic anti-viral mechanisms by targeting the innate immune system is effective against a number of viruses, including herpes simplex virus type-2 (Bernstein et al., 2001; Harrison, Miller, and Bernstein, 1994), cytomegalovirus (Chen et al., 1988), parainfluenza (Stokes et al., 1998), West Nile virus (Morrey et al., 2004) and influenza virus (Wong et al., 2007; Wong et al., 2005), we believe PIKA may have the potential to inhibit the growth of other viral pathogens.
While the anti-viral properties of dsRNA compounds, such as poly ICLC, have been well documented, they are often associated with side effects. In a study by Wong et al, mice showed ~11% weight loss and hypothermia after receiving 2 doses of poly ICLC (~20 μg per dose, Wong et al., 1999). In contrast, PIKA displays anti-viral properties in mice without causing significant weight loss. Although TLR3 has been identified as a receptor for dsRNA and cells from TLR 3−/− mice showed reduced responses to poly I:C in vitro (Alexopoulou et al., 2001; De Miranda et al., 2009), our experiment in TLR3−/− mice suggested that other receptors might also be involved in mediating the anti-viral activity of PIKA. Arimura et al. and Kato et al. who demonstrated that the interferon-inducing activity and recognition by RIG-I and MDA-5 of Poly I:C are dependent on the length of the Poly I:C molecules (Arimura, 1975; Kato et al., 2008). PIKA contains dsRNA greater than 100 base pairs in size (Peter Brazier, unpublished data), its receptor usage in vivo might be different from those used by poly I:C due to different size distribution. In addition, while Gitlin et al. reported that MDA-5, rather than TLR3, played an important role in mediating the type 1 interferon response to poly I:C in vivo (Gitlin et al., 2006), others showed that both MDA-5 and TLR3 are required for optimal biological activity of poly I:C (Kumar et al., 2008; Trumpfheller et al., 2008). The contradictory results could be because poly I:C was administered by different routes in these studies. We were unable to determine the relative importance of RIG-I and MDA-5 on the anti-viral effect of PIKA in vivo and are currently investigating how PIKA can access MDA-5 and RIG-I, which are cytosolic sensors.
In an influenza challenge experiment using TLR3−/− and wild-type mice, Le Goffic et al. demonstrated that TLR3 activation in the context of an influenza virus infection resulted in an increase in cytokine production and the number of inflammatory cells in the lungs, resulting in immunopathology and reduced survival (Le Goffic et al., 2006). The sequence of events in our experiments was different: mice were pretreated with PIKA that induced TLR3 activation and cytokine production. When the mice were subsequently infected with influenza virus, there was reduced viral replication, less inflammation and improved survival. In our lethal challenge experiment, the mice pretreated with PIKA showed no significant weight loss after infection, an observation that is markedly different from Le Goffic's study. We speculate that timing of TLR3 activation relative to influenza virus infection determines the outcome.
Wong et al. reported recently that administration of dsRNA condensed with poly-L-lysine and carboxymethylcellulose improved the survival of mice challenged with a lethal dose of A/chicken/Henan/5/2004 (a clade 2 H5N1 virus) (Wong et al., 2009). In addition, Tuvim et al. showed that induction of local pulmonary inflammation led to protection against influenza infection in mice (Tuvim et al., 2009). Our findings further extend these observations by demonstrating the breadth of protection that PIKA can provide against a range of influenza viruses with pandemic potential. Furthermore, the transient inhibition of viral replication mediated by PIKA allows mice to be primed and develop influenza-specific antibody responses that can provide long-term protection from re-infection. A number of studies have shown that intranasal administration of exogenous type I interferon protects mice, ferrets and guinea pigs from influenza virus infection (Kugel et al., 2009; Tumpey et al., 2007; Van Hoeven et al., 2009). Activation of the innate immune system using synthetic compounds to induce endogenous type I interferon production might be more cost-effective than exogenous type I interferon administration.
Since it remains uncertain which influenza strain(s) will eventually emerge as a pandemic virus, drugs that target the innate immune system and provide broad-spectrum anti-viral activity have the potential to be used as a first-line of defense. Our findings are promising and provide support for further evaluation of PIKA for prophylaxis. Areas for further study include an assessment of the risk of immunologic tolerance resulting from stimulation of the innate immune system (Trinchieri and Sher, 2007), the risk of secondary bacterial infection that is a common complication of influenza virus infection (Centers for Disease Control and Prevention, 2009a; Morens, Taubenberger, and Fauci, 2008; Sun and Metzger, 2008) and studies to establish the feasibility of using PIKA prophylaxis in humans including the timing and duration of treatment.
Supplementary Material
Single cell suspensions were prepared from lungs and stained with various cocktails of antibodies. Eighty thousands events were acquired for each sample.
Acknowledgments
We thank Jadon Jackson and Amber McCall for their excellent technical assistance. This research was supported by funds from the Intramural Research Program of the NIAID, NIH and Future Systems Directorate, Ministry of Defence, The Republic of Singapore.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Arimura H. Correlation between molecular size and interferon- inducing activity of poly I:C. Acta Virol. 1975;19(6):457–66. [PubMed] [Google Scholar]
- Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, Nicoll A, Touch S, Yuen KY. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353(13):1374–85. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- Bernstein DI, Harrison CJ, Tomai MA, Miller RL. Daily or weekly therapy with resiquimod (R-848) reduces genital recurrences in herpes simplex virus-infected guinea pigs during and after treatment. J Infect Dis. 2001;183(6):844–9. doi: 10.1086/319262. [DOI] [PubMed] [Google Scholar]
- Butt KM, Smith GJ, Chen H, Zhang LJ, Leung YH, Xu KM, Lim W, Webster RG, Yuen KY, Peiris JS, Guan Y. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J Clin Microbiol. 2005;43(11):5760–7. doi: 10.1128/JCM.43.11.5760-5767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) - United States, May-August 2009. MMWR Morb Mortal Wkly Rep. 2009a;58(38):1071–4. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Swine influenza A (H1N1) infection in two children--Southern California, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009b;58(15):400–2. [PubMed] [Google Scholar]
- Chen M, Griffith BP, Lucia HL, Hsiung GD. Efficacy of S26308 against guinea pig cytomegalovirus infection. Antimicrob Agents Chemother. 1988;32(5):678–83. doi: 10.1128/aac.32.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong MD, Tran TT, Truong HK, Vo MH, Smith GJ, Nguyen VC, Bach VC, Phan TQ, Do QH, Guan Y, Peiris JS, Tran TH, Farrar J. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005;353(25):2667–72. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- De Miranda J, Yaddanapudi K, Hornig M, Lipkin WI. Astrocytes recognize intracellular polyinosinic-polycytidylic acid via MDA-5. FASEB J. 2009;23(4):1064–71. doi: 10.1096/fj.08-121434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliyannis G, Jackson DC, Dyer W, Bates J, Coulter A, Harling-McNabb L, Brown LE. Immunopotentiation of humoral and cellular responses to inactivated influenza vaccines by two different adjuvants with potential for human use. Vaccine. 1998;16(20):2058–68. doi: 10.1016/s0264-410x(98)00080-2. [DOI] [PubMed] [Google Scholar]
- Dorner BG, Scheffold A, Rolph MS, Huser MB, Kaufmann SH, Radbruch A, Flesch IE, Kroczek RA. MIP-1alpha, MIP-1beta, RANTES, and ATAC/lymphotactin function together with IFN-gamma as type 1 cytokines. Proc Natl Acad Sci U S A. 2002;99(9):6181–6. doi: 10.1073/pnas.092141999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H. Neutrophils play an essential role in cooperation with antibody in both protection against and recovery from pulmonary infection with influenza virus in mice. J Virol. 2008;82(6):2772–83. doi: 10.1128/JVI.01210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H, Tsuru S, Taniguchi M, Zinnaka Y, Nomoto K. Protective mechanisms against pulmonary infection with influenza virus. I. Relative contribution of polymorphonuclear leukocytes and of alveolar macrophages to protection during the early phase of intranasal infection. J Gen Virol. 1987;68(Pt 2):425–32. doi: 10.1099/0022-1317-68-2-425. [DOI] [PubMed] [Google Scholar]
- Gillim-Ross L, Santos C, Chen Z, Aspelund A, Yang CF, Ye D, Jin H, Kemble G, Subbarao K. Avian influenza h6 viruses productively infect and cause illness in mice and ferrets. J Virol. 2008;82(21):10854–63. doi: 10.1128/JVI.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A. 2006;103(22):8459–64. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CJ, Miller RL, Bernstein DI. Posttherapy suppression of genital herpes simplex virus (HSV) recurrences and enhancement of HSV-specific T-cell memory by imiquimod in guinea pigs. Antimicrob Agents Chemother. 1994;38(9):2059–64. doi: 10.1128/aac.38.9.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Moki T, Takizawa T, Shiratsuchi A, Nakanishi Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178(4):2448–57. doi: 10.4049/jimmunol.178.4.2448. [DOI] [PubMed] [Google Scholar]
- Hayden FG. Antiviral resistance in influenza viruses--implications for management and pandemic response. N Engl J Med. 2006a;354(8):785–8. doi: 10.1056/NEJMp068030. [DOI] [PubMed] [Google Scholar]
- Hayden FG. Antivirals for influenza: historical perspectives and lessons learned. Antiviral Res. 2006b;71(2–3):372–8. doi: 10.1016/j.antiviral.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Hayden FG, Treanor JJ, Fritz RS, Lobo M, Betts RF, Miller M, Kinnersley N, Mills RG, Ward P, Straus SE. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282(13):1240–6. doi: 10.1001/jama.282.13.1240. [DOI] [PubMed] [Google Scholar]
- Herold S, Steinmueller M, von Wulffen W, Cakarova L, Pinto R, Pleschka S, Mack M, Kuziel WA, Corazza N, Brunner T, Seeger W, Lohmeyer J. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205(13):3065–77. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt AC, Selleck P, Komadina N, Shaw R, Brown L, Barr IG. Susceptibility of highly pathogenic A(H5N1) avian influenza viruses to the neuraminidase inhibitors and adamantanes. Antiviral Res. 2007;73(3):228–31. doi: 10.1016/j.antiviral.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, Black S, Shinefield H, Fukuda K. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342(4):232–9. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–34. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205(7):1601–10. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiso M, Mitamura K, Sakai-Tagawa Y, Shiraishi K, Kawakami C, Kimura K, Hayden FG, Sugaya N, Kawaoka Y. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet. 2004;364(9436):759–65. doi: 10.1016/S0140-6736(04)16934-1. [DOI] [PubMed] [Google Scholar]
- Kugel D, Kochs G, Obojes K, Roth J, Kobinger GP, Kobasa D, Haller O, Staeheli P, von Messling V. Intranasal administration of alpha interferon reduces seasonal influenza A virus morbidity in ferrets. J Virol. 2009;83(8):3843–51. doi: 10.1128/JVI.02453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Koyama S, Ishii KJ, Kawai T, Akira S. Cutting edge: cooperation of IPS-1- and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J Immunol. 2008;180(2):683–7. doi: 10.4049/jimmunol.180.2.683. [DOI] [PubMed] [Google Scholar]
- Lau YF, Deliyannis G, Zeng W, Mansell A, Jackson DC, Brown LE. Lipid-containing mimetics of natural triggers of innate immunity as CTL-inducing influenza vaccines. Int Immunol. 2006;18(12):1801–13. doi: 10.1093/intimm/dxl114. [DOI] [PubMed] [Google Scholar]
- Lau YF, Tang LH, Ooi EE. A TLR3 ligand that exhibits potent inhibition of influenza virus replication and has strong adjuvant activity has the potential for dual applications in an influenza pandemic. Vaccine. 2009;27(9):1354–64. doi: 10.1016/j.vaccine.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goffic R, Balloy V, Lagranderie M, Alexopoulou L, Escriou N, Flavell R, Chignard M, Si-Tahar M. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2(6):e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le QM, Kiso M, Someya K, Sakai YT, Nguyen TH, Nguyen KH, Pham ND, Ngyen HH, Yamada S, Muramoto Y, Horimoto T, Takada A, Goto H, Suzuki T, Suzuki Y, Kawaoka Y. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437(7062):1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- Lipatov AS, Govorkova EA, Webby RJ, Ozaki H, Peiris M, Guan Y, Poon L, Webster RG. Influenza: emergence and control. J Virol. 2004;78(17):8951–9. doi: 10.1128/JVI.78.17.8951-8959.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKimm-Breschkin JL, Selleck PW, Usman TB, Johnson MA. Reduced sensitivity of influenza A (H5N1) to oseltamivir. Emerg Infect Dis. 2007;13(9):1354–7. doi: 10.3201/eid1309.07-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198(7):962–70. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrey JD, Day CW, Julander JG, Blatt LM, Smee DF, Sidwell RW. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antivir Chem Chemother. 2004;15(2):101–9. doi: 10.1177/095632020401500202. [DOI] [PubMed] [Google Scholar]
- Nakano H, Yanagita M, Gunn MD. CD11c(+)B220(+)Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194(8):1171–8. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Jr., Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342(4):225–31. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362(9397):1733–45. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris M, Yuen KY, Leung CW, Chan KH, Ip PL, Lai RW, Orr WK, Shortridge KF. Human infection with influenza H9N2. Lancet. 1999;354(9182):916–7. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14(4):778–809. doi: 10.1128/CMR.14.4.778-809.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravolac EG, Sabuda D, Crist C, Blasetti K, Schnell G, Yang H, Kende M, Levy HB, Wong JP. Immunoprophylactic strategies against respiratory influenza virus infection. Vaccine. 2001;19(17–19):2227–32. doi: 10.1016/s0264-410x(00)00450-3. [DOI] [PubMed] [Google Scholar]
- Seo SH, Webster RG. Tumor necrosis factor alpha exerts powerful anti-influenza virus effects in lung epithelial cells. J Virol. 2002;76(3):1071–6. doi: 10.1128/JVI.76.3.1071-1076.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes JR, Sorkness RL, Kaplan MR, Castleman WL, Tomai MA, Miller RL, Lemanske RF., Jr. Attenuation of virus-induced airway dysfunction in rats treated with imiquimod. Eur Respir J. 1998;11(2):324–9. doi: 10.1183/09031936.98.11020324. [DOI] [PubMed] [Google Scholar]
- Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279(5349):393–6. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med. 2008;14(5):558–64. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- Tate MD, Brooks AG, Reading PC. The role of neutrophils in the upper and lower respiratory tract during influenza virus infection of mice. Respir Res. 2008;9:57. doi: 10.1186/1465-9921-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thitithanyanont A, Engering A, Ekchariyawat P, Wiboonut S, Limsalakpetch A, Yongvanitchit K, Kum-Arb U, Kanchongkittiphon W, Utaisincharoen P, Sirisinha S, Puthavathana P, Fukuda MM, Pichyangkul S. High susceptibility of human dendritic cells to avian influenza H5N1 virus infection and protection by IFN-alpha and TLR ligands. J Immunol. 2007;179(8):5220–7. doi: 10.4049/jimmunol.179.8.5220. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, Singh S, Kinnersley N, Ward P, Mills RG. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283(8):1016–24. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7(3):179–90. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- Trumpfheller C, Caskey M, Nchinda G, Longhi MP, Mizenina O, Huang Y, Schlesinger SJ, Colonna M, Steinman RM. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci U S A. 2008;105(7):2574–9. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, Schultz-Cherry S, Solorzano A, Van Rooijen N, Katz JM, Basler CF. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79(23):14933–44. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpey TM, Szretter KJ, Van Hoeven N, Katz JM, Kochs G, Haller O, Garcia-Sastre A, Staeheli P. The Mx1 gene protects mice against the pandemic 1918 and highly lethal human H5N1 influenza viruses. J Virol. 2007;81(19):10818–21. doi: 10.1128/JVI.01116-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvim MJ, Evans SE, Clement CG, Dickey BF, Gilbert BE. Augmented lung inflammation protects against influenza A pneumonia. PLoS One. 2009;4(1):e4176. doi: 10.1371/journal.pone.0004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoeven N, Belser JA, Szretter KJ, Zeng H, Staeheli P, Swayne DE, Katz JM, Tumpey TM. Pathogenesis of 1918 pandemic and H5N1 influenza virus infections in a guinea pig model: antiviral potential of exogenous alpha interferon to reduce virus shedding. J Virol. 2009;83(7):2851–61. doi: 10.1128/JVI.02174-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JP, Christopher ME, Salazar AM, Dale RM, Sun LQ, Wang M. Nucleic acid-based antiviral drugs against seasonal and avian influenza viruses. Vaccine. 2007;25(16):3175–8. doi: 10.1016/j.vaccine.2007.01.051. [DOI] [PubMed] [Google Scholar]
- Wong JP, Christopher ME, Viswanathan S, Karpoff N, Dai X, Das D, Sun LQ, Wang M, Salazar AM. Activation of toll-like receptor signaling pathway for protection against influenza virus infection. Vaccine. 2009;27(25–26):3481–3. doi: 10.1016/j.vaccine.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JP, Nagata LP, Christopher ME, Salazar AM, Dale RM. Prophylaxis of acute respiratory virus infections using nucleic acid-based drugs. Vaccine. 2005;23(17–18):2266–8. doi: 10.1016/j.vaccine.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Wong JP, Saravolac EG, Sabuda D, Levy HB, Kende M. Prophylactic and therapeutic efficacies of poly(IC.LC) against respiratory influenza A virus infection in mice. Antimicrob Agents Chemother. 1995;39(11):2574–6. doi: 10.1128/aac.39.11.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JP, Yang H, Nagata L, Kende M, Levy H, Schnell G, Blasetti K. Liposome-mediated immunotherapy against respiratory influenza virus infection using double-stranded RNA poly ICLC. Vaccine. 1999;17(13–14):1788–95. doi: 10.1016/s0264-410x(98)00439-3. [DOI] [PubMed] [Google Scholar]
- Yen HL, Monto AS, Webster RG, Govorkova EA. Virulence may determine the necessary duration and dosage of oseltamivir treatment for highly pathogenic A/Vietnam/1203/04 influenza virus in mice. J Infect Dis. 2005;192(4):665–72. doi: 10.1086/432008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Single cell suspensions were prepared from lungs and stained with various cocktails of antibodies. Eighty thousands events were acquired for each sample.