Abstract
Atrial fibrillation (AF) is a common cardiac arrhythmia with potentially life-threatening complications. Drug therapies for treatment of AF that seek long-term maintenance of normal sinus rhythm remain elusive due in large part to proarrhythmic ventricular actions. Kv1.5, which underlies the atrial specific IKur current, is a major focus of research efforts seeking new therapeutic strategies and targets. Recent work has shown a novel effect of antiarrhythmic drugs where compounds that block Kv1.5 channel current can also alter ion channel trafficking. This work further suggests that the pleiotropic effects of antiarrhythmic drugs may be separable. Although this highlights the therapeutic potential for selective manipulation of ion channel surface density, it also reveals an uncertainty regarding specificity of modulating trafficking pathways without risk of off-target effects. Future studies may show that specific alteration of Kv1.5 trafficking can overcome the proarrhythmic limitations of current pharmacotherapy and provide an effective method for long-term cardioversion in AF.
Keywords: Cardiovascular, Atrial fibrillation, Cardioversion, Kv1.5, Trafficking, Channels
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia affecting an estimated 2.2 million adults in the in the United States.1 AF is caused by the rapid and irregular activation of the atria by electrical sources outside the normal sinus node, and can be classified as paroxysmal, persistent, or long-standing persistent.2 The occurrence of atrial fibrillation increases dramatically with age, affecting less than 1% of individuals under age 50 to approximately 10% of individuals over age 80.1, 3 Importantly, over the past two decades the age standardized death rate (per 100,000 in the US) has increased from 27.6 to 69.8.4 Therefore, AF presents a significant increasing health risk with an untold burden for healthcare costs.
The combination of inefficient atrial contraction and irregular ventricular rate can lead to serious complications. AF related deaths are due primarily to the increased risk of stroke and heart failure. AF is associated with a nearly 5-fold increase in the risk of embolic stroke with nearly one fourth of all strokes in patients over 80 attributable to AF.5, 6 This increased rate of stroke is due to the rapid, uncoordinated atrial rhythm that leads to inefficient contraction of the atria, resulting in the pooling of blood in the atria and promotion of thrombo-emboli formation. The presence or absence of several other risk factors significantly contributes to the risk of stroke, such as recent cardiac failure, hypertension, age, diabetes, or a history of stroke or transient ischemic attack (TIA).7, 8 In addition to an increased risk of stroke, atrial thrombo-emboli can propagate to regions other than the brain, such as the kidneys, mesenteric circulation, or the heart itself where they may induce myocardial infarction. AF also participates reciprocally with several comorbid conditions including congestive heart failure, thyrotoxic heart disease, and hypertension.9
Current therapy for AF is aimed at rate control or rhythm control.10 In rate control, the goal is to maintain the ventricular rate within a physiological range by slowing atrioventricular conduction. In rhythm control, treatment aims to restore normal sinus rhythm through pharmacological cardioversion or through electrical cardioversion or catheter ablation. Catheter ablation is considered a 2nd-line treatment for AF with published success rates of 22-85%. Higher success rates are often seen for patients with paroxysmal AF.2, 11-13 While approximately half of these patients remain asymptomatic, nearly 30% require a second procedure and 10-25% require additional pharmacological therapy in order to maintain normal sinus rhythm post ablation therapy.14, 15 However, the long-term effectiveness of this technique remains to be fully determined.16, 9
Pharmacological cardioversion makes use of antiarrhythmic drugs targeting cardiovascular ion channels to achieve normal sinus rhythm control in the treatment of AF.17-20 Pharmacological cardioversion has an advantage over catheter ablation in that it is not invasive, however it has been reported to be less effective.21 If class I or III antiarrhythmic agents are administered within the first 24 hours of onset of AF, the reported success rate is 47-84%; however, this drops sharply for AF that persists longer than 48 hours in that the antiarrhythmic therapy can only achieve cardioversion in 15-30% of patients.22
A common negative side effect for antiarrhythmic drug therapy is proarrhythmia in the ventricles due to non-selective ion channel block and/or overlapping expression of ion channels in both the atria and ventricles. More recently, there has been a shift in both academia and industry to target atrial specific currents such in order to terminate AF and maintain normal sinus rhythm while avoiding proarrhythmic risk in the ventricles. One of the main targets in this research effort is the voltage-gated potassium channel Kv1.5 that underlies the I current. Importantly, IKur is selectively reported only in the human atria 17, 20, 23, 24 where it contributes to repolarization and participates in the control of action potential duration. In the human atria, Kv1.5 is a predominant channel mediating repolarization and alterations in its expression level have been demonstrated in pathophysiological states such as persistent and paroxysmal AF and chronic pulmonary arterial hypertension.17, 25 More specifically, there is a marked reduction in Kv1.5 channel protein expression in these pathophysiological states.26,27, 28perhaps as an endogenous compensatory mechanism. Given the atrial specific expression of Kv1.5 and its known alterations in cardiovascular disease, it is no surprise that the development of Kv1.5-specific blockers has been a target of both academic and industrial research efforts for the treatment of AF.29-31 While several compounds have been developed, these antiarrhythmic drugs have been limited by a lack of channel or tissue selectivity, or by poor bioavailability. Therefore, there remains an unmet need for the development of safe new compounds with both atrial selectivity and clinical efficacy for the long-term treatment of AF.
New therapeutic strategies that focus on the regulation of ion channel surface density are emerging from basic research at the bench (Figures 1 and 2).32, 33 Traditional antiarrhythmic drugs target the ion permeability of channels; however, as highlighted above this approach has not yet yielded a satisfactory outcome. There are two ways to decrease IKur, through a direct effect on the conduction properties (classically pore block) of channel subunits or through alterations in surface density of the protein. The steady-state cell surface density of proteins is determined by the balance between the anterograde and retrograde trafficking pathways. Anterograde trafficking ensues only after proper synthesis and processing in the endoplasmic reticulum and golgi, including quality control mechanisms, glycosylation, and post-translation modification (Figure 1).34 Retrograde movement initiates with endocytosis after which internalized proteins can follow multiple routes to different intracellular fates (Figure 1).35 One well-recognized fate is the targeting of internalized proteins to lysosomes or proteasomes followed by degradation (Figure 1). Alternatively, trafficking through recycling endosomes allows proteins to return to the plasma membrane and protects them from degradation (Figure 1).36 Sorting at early endosomes to Rab-GTPase specific compartments is now established as an important event determining the intracellular fate of internalized proteins.37-39 Another important component of the endocytic machinery regulating protein surface levels is the coordinated movement of molecular motors. In general, protein trafficking is highly coordinated between long-range events, involving the microtubule-based kinesin and dynein motors, and short-range events using unconventional myosin motors.40-43 There is a significant and growing body of literature about ion channel trafficking from synthesis to sorting to degradation in multiple tissues and cells systems that has been reviewed previously.44-48 Our discussion will center mainly on recent work focused on the acute modulation of ion channel density at the plasma membrane in the heart where relatively little is known about protein trafficking.
Figure 1.

Potential therapeutic intervention points in the trafficking of membrane proteins. Each arrow represents a regulatory step in the trafficking of membrane proteins that could serve as a potential therapeutic target for modulating steady-state cells surface levels of ion channels. The left half of this figure represents an area where much work has been done in the hERG field for the treatment of LQTS and other arrhythmias. The right half represents and exciting developing field for the regulation of Kv1.5 membrane levels in the treatment of atrial fibrillation. Endoplasmic Reticulum (ER); Recycling Endosome (RE); Late Endosome (LE).
Figure 2.
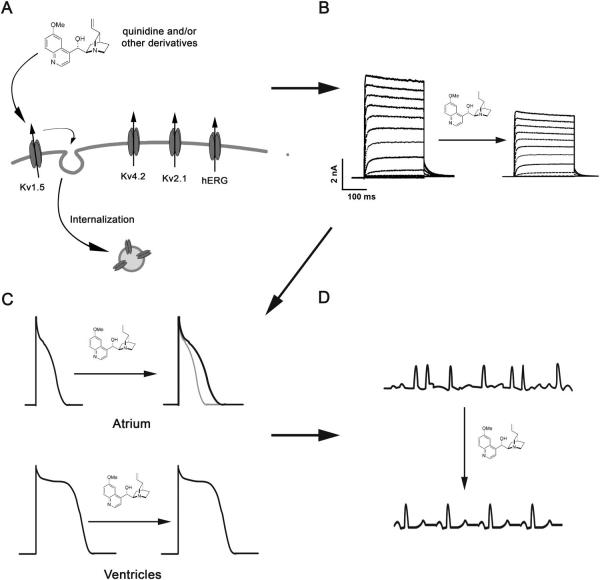
Antiarrhythmic drug-induced internalization of atrial specific Kv1.5 as a novel therapeutic target for AF. (A) Drug-induced internalization is specific to the atrial potassium channel Kv1.5. (B) Kv1.5 specific internalization results in a decrease in IKur density. (C) Decreased IKur may result in an increase in atrial, not ventricular, action potential duration. (D) Increased atrial action potential duration may terminate atrial fibrillation and restore normal sinus node rhythm control.
While the precise mechanisms regulating plasma membrane localization and targeting of Kv1.5 in atrial myocytes have not been fully elucidated, several key components and steps are now known. The formation of functional Kv1.5 begins in the endoplasmic reticulum where tertiary folding is coupled to formation of the quaternary structure through tetramerization of the T1 domain in the amino terminus of this channel.49 The first transmembrane segment (S1) of Kv1.5 has also been implicated in the co-assembly of homo- and heterotetrameric K+ channels.50 In addition to protein folding and assembly, subunit composition and post-translational modification can play a crucial role in determining the plasma membrane levels of functional Kv1.5.51-60 At the plasma membrane, localization to specific membrane microdomains and association with scaffolding proteins into macromolecular signaling complexes may contribute to the stability and biological function of Kv1.5.61-67 Despite association with scaffolding proteins, Kv1.5 has been shown to undergo dynamic trafficking at the plasma membrane through constitutive internalization and recycling.68 Internalization of Kv1.5 occurs via a dynein-mediated, microtubule dependent pathway.68, 69 Following internalization, sorting of Kv1.5 into specific, rab-dependent endocytic compartments determines the intracellular fate of the channel. Specifically, association of Kv1.5 with rab4- or rab11-containing endocytic vesicles is associated with recycling of the channel back to the plasma membrane, whereas association with rab7-containing vesicles denotes channel degradation.68-70 In addition, ubiquitin modification of Kv1.5 has been described in agreement with data implicating the proteasome in channel degradation.32, 52, 71-73 As the molecular machinery and endogenous regulatory mechanisms of Kv1.5 surface density begin to emerge, one new therapeutic horizon is the extrinsic manipulation of Kv1.5 surface levels.
The concept of drugs modulating ion conduction and/or surface density of channels it not new. For example, the antiarrhythmic agents ketoconazole and fluoxetine have been shown to reduce hERG density by at least 50% following 48 hours of treatment,74-77 whereas pentamidine and probucol reduce cell-surface hERG without affecting ion conduction.77-80 Research into the therapeutic potential of antiarrhythmic drugs that alter channel trafficking has primarily focused on hERG. Alterations in hERG mediated IKr current, whether drug-induced or a result of the over 200 naturally occurring mutations of this channel, may induce or contribute to the development of long QT syndrome. In particular, long QT syndrome type II which results from retention of misfolded hERG in the quality control pathways of the ER.81, 82 Nearly 70% of these mutant channels can be rescued to the plasma membrane by antiarrhythmic drugs such as E4031.81, 83 These drugs likely act to stabilize misfolded protein through facilitation of quality control machinery in the ER such as Hsc70 and Hsp90 that have been shown to exist in a macromolecular complex with hERG and facilitate its maturation and export from the ER.82, 84 Thus far research has focused primarily on hERG folding and maturation with little investigation into the molecular mechanisms regulating anterograde trafficking from the ER to the plasma membrane. None-the-less, these studies give credence to the idea that antiarrhythmic drugs may be developed to manipulate specific ion channel trafficking pathways as a novel therapeutic approach for treating cardiac arrhythmias.
Recently, we have reported a previously unrecognized mechanism of antiarrhythmic drug action in the acute modulation of Kv1.5 channel trafficking.32 Using quinidine, an antiarrhythmic agent which has both class Ia actions85, 86 and class III actions in mammalian atrium and ventricle, we demonstrated that channel blockers can both inhibit ion conduction and regulate the stability of the channel protein within the membrane. In this study, quinidine resulted in a dose- and time-dependent internalization of Kv1.5, concomitant with channel block. Interestingly, this quinidine-induced internalization of Kv1.5 was found to be subunit-dependent and stereospecific32 which highlights the possibility for the development of atrial selective agents that specifically modulate surface density.
Drug-induced internalization may occur for channel subunits selectively expressed in the atria.32 Quinidine exhibits promiscuous block of cardiovascular ion channels, including several members of the Kv channel family. This lack of specificity raised the question as to whether the drug-induced internalization was specific to Kv1.5 or a general mechanism contributing to the block of current from multiple ion channels. To address this specificity, we examined the effect of quinidine on two other prominent cardiovascular potassium channel subunits expressed endogenously in the human atrium and ventricle, Kv4.2 and Kv2.1. Although ion permeability of both channels is blocked by quinidine (IC50 of 10 μmol/L and 20 μmol/L quinidine, respectively),87, 88 neither Kv4.2 nor Kv2.1 internalized in response to any drug concentration tested over the time course studied. This subunit specificity may permit the development of drugs that avoid or reduce undesired proarrhythmic ventricular side effects.
The potential to separate drug-induced channel internalization from pore block stimulates further interest in the design of drugs to modulate trafficking pathways. Within the Kv1.5 channel protein there is partial, but not complete, overlap in the binding sites required for quinidine-induced internalization and pore block (Figure 3).32 This was reveled through alanine-scanning mutagenesis of four amino acid residues within the highly conserved drug binding site of Kv channels.89, 90 As with pore block, quinidine-induced internalization was abolished with the T480A and I508A mutations; however, unlike block, Kv1.5-L510A and V512A underwent quinidine-induced internalization indistinguishable from wild-type.32, 89, 90 Interestingly, Kv1.5-P532L, a naturally occurring mutation (outside of the conserved drug binding site) in atrial fibrillation patients that results in a rightward shift in the dose response curve for quinidine block of channel current91 caused an equivalent (~10-fold shift in the EC50 value) decrease in the sensitivity to quinidine-induced channel internalization.32 Together, the fact that these mutations alter the open channel block and/or internalization of Kv1.5 demonstrate that quinidine is inducing its effects through direct binding to the channel and not through off-target effects such as mediators of channel trafficking. Importantly, the subunit dependence and incomplete overlap in amino acid requirements indicate that individual components of the Kv1.5 channel structure are critical for the quinidine-induced internalization. However, additional studies of alanine-scanning mutants within the putative conserved drug-binding site are necessary to compare effects on surface density to those reported for pore block (Figure 3). Nevertheless, these data highlight the possibility for development of new agents that specifically enhance Kv1.5 channel internalization as an alternative and potentially beneficial new therapeutic strategy.
Figure 3.
Strategies to isolate drug-induced internalization from pore block. Cartoon representation of the potential drug binding site of the Kv1.5 channel pore. (1) Ion channel mutagenesis can be used to fully characterize the incomplete overlap in the drug binding site for pore block and channel internalization. (2) Multiple moieties within the quinidine molecule can be studied in structure activity analysis to identify the pharmacophore for drug-induced internalization.
In order to develop new compounds that selectively modulate Kv1.5 surface density, the pharmacophore(s) responsible for pore block and internalization would need to be isolated. The observation that quinine, a diastereomer of quinidine, blocks Kv1.5 current in a dose-dependent fashion without enhancing internalization, suggests that a highly stereospecific binding event is required to induce channel internalization, rather than some non-stereospecific interaction with the cell membrane. Quinidine embodies structural features that enable it to induce endocytosis of Kv1.5. It should be possible to identify individual components of the quinidine structure that are critical for this stereospecific internalization/effect. In addition, these studies should be expanded to modern, more clinically relevant antiarrhythmic drugs. These structural features can be identified through the definition of structure-activity relationships (SAR), and then exploited in the design of novel chemical compounds with greater potency and selectivity for inducing endocytosis vs. direct block of the channel (Figure 3).
Another important therapeutic aspect of quinidine-induced internalization of Kv1.5 is that it is acute and reversible, further suggesting that it may be effective for acute cardioversion. In our study we found that the quinidine-induced internalization was rapid, reaching a plateau within ten minutes. Combined with the fact that this drug-mediated trafficking effect occurred at resting membrane potential in our immunocytochemical assays, these data indicate that the rate-limiting factor for this effect is likely equilibration of drug across the membrane. Importantly, the quinidine-induced internalized Kv1.5 recycled back to the plasma membrane at a rate indistinguishable from constitutive channel recycling. These data further show that the quinidine-mediated trafficking effect results in an acute increase in channel endocytosis, specifically, without altering the endogenous recycling of Kv1.5. If a drug were designed to specifically modulate Kv1.5 channel trafficking it could, in theory, be used to cause a rapid internalization of channel with recovery after drug withdrawal. Therefore, acute modulation of ion channel surface levels may offer the selective advantage for a rapid, reversible decrease in IKur with a subsequent increase in action potential duration that may terminate acute onset of AF without altering the overall pool of Kv1.5 channel. In contrast, we found that chronic treatment with quinidine diverted Kv1.5 channel to the proteasomal degradation pathway. It is unclear if this chronic decrease in surface expression is advantageous or an unwanted side-effect of the long-term drug treatment. For instance, the time course of recovery from this repression may precipitate drug-withdrawal side effects. Furthermore, long-term suppression of channel expression may contribute to remodeling of heart tissue in which a decrease in Kv1.5 channel protein levels has already been documented. The alternative is that this chronic suppression of Kv1.5 channel protein levels may overcome the current limitations of acute cardioversion which fails to terminate breakthrough AF or adequately subdue the arrhythmogenic substrate and may result in the benefit of maintained rhythm control.
An important finding that goes beyond the therapeutic potential of this approach is that the drug-induced trafficking effect is calcium-dependent and raises significant issues for drug safety screening.32 The calcium-dependent trafficking component was responsible for a 3-fold shift in the dose response for quinidine and is therefore critically important when considering the in vivo effects of this drug and likely many others. It has been well established that non-antiarrhythmic drugs can have proarrhythmic effects through off target inhibition of cardiovascular ion channel currents. This has been reported for psychiatric drugs, antihistamines, antimicrobials and other compounds, many of which can induce QT prolongation with subsequent risk of torsade de pointes.92 As a result, pharmaceutical companies and regulatory agencies mandate that cardiac ion channel testing be part of the drug safety profiling of all new compounds. Current drug safety profiling is almost entirely focused on a drug’s capacity to block ion conduction. This is commonly tested through electrophysiological measurement of current in the presence or absence of drug, using a calcium chelating agent to isolate the drug-channel interaction. Therefore antiarrhythmic, and non-antiarrhythmic, compounds that alter ion channel trafficking may show greater efficacy and potency in vivo than can be predicted by current drug safety profiling. This may result in a dramatic underestimation of the desired clinical effect and/or undesired side effects of a drug.
The results discussed here highlight the potential for development of new agents that selectively modulate ion conduction and/or the stability of channel protein in the membrane. In addition, manipulation of the trafficking of functional membrane channel may provide an avenue for developing new therapies with atrial selectivity and clinical efficacy and safety for the treatment of AF and potentially other cardiovascular arrhythmias.
Acknowledgments
We thank Dr. Fred Morady, (McKay Professor of Cardiovascular Disease, Professor of Medicine, University of Michigan) for careful reading of and insight towards this manuscript.
Sources of Funding: NIH HL0270973, JRM; Systems and Integrative Biology Training Grant NIH T32 GM008322, SMS
Abbreviations
- AF
Atrial fibrillation
- TIA
Transient ischemic attack
- SAR
structure-activity relationships
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. Jama. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Archives of internal medicine. 1995;155:469–473. [PubMed] [Google Scholar]
- 4.Wattigney WA, Mensah GA, Croft JB. Increased atrial fibrillation mortality: United States, 1980-1998. American journal of epidemiology. 2002;155:819–826. doi: 10.1093/aje/155.9.819. [DOI] [PubMed] [Google Scholar]
- 5.Brand FN, Abbott RD, Kannel WB, Wolf PA. Characteristics and prognosis of lone atrial fibrillation. 30-year follow-up in the Framingham Study. Jama. 1985;254:3449–3453. [PubMed] [Google Scholar]
- 6.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke; a journal of cerebral circulation. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 7.Casella L, Abelmann WH, Ellis LB. Patients with Mitral Stenosis and Systemic Emboli; Hemodynamic and Clinical Observations. Archives of internal medicine. 1964;114:773–781. doi: 10.1001/archinte.1964.03860120085008. [DOI] [PubMed] [Google Scholar]
- 8.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. Jama. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 9.Crandall MA, Bradley DJ, Packer DL, Asirvatham SJ. Contemporary management of atrial fibrillation: update on anticoagulation and invasive management strategies. Mayo Clinic proceedings. 2009;84:643–662. doi: 10.1016/S0025-6196(11)60754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. The New England journal of medicine. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 11.Karch MR, Zrenner B, Deisenhofer I, et al. Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation: a randomized comparison between 2 current ablation strategies. Circulation. 2005;111:2875–2880. doi: 10.1161/CIRCULATIONAHA.104.491530. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson B, Chen X, Pehrson S, et al. Recurrence of pulmonary vein conduction and atrial fibrillation after pulmonary vein isolation for atrial fibrillation: a randomized trial of the ostial versus the extraostial ablation strategy. American heart journal. 2006;152:537, e531–538. doi: 10.1016/j.ahj.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 13.Oral H, Veerareddy S, Good E, et al. Prevalence of asymptomatic recurrences of atrial fibrillation after successful radiofrequency catheter ablation. Journal of cardiovascular electrophysiology. 2004;15:920–924. doi: 10.1046/j.1540-8167.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- 14.Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 15.d’Avila A, Ruskin JN. Nonpharmacologic strategies: the evolving story of ablation and hybrid therapy. The American journal of cardiology. 2008;102:20H–24H. doi: 10.1016/j.amjcard.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 16.Packer DL, Asirvatham S, Munger TM. Progress in nonpharmacologic therapy of atrial fibrillation. Journal of cardiovascular electrophysiology. 2003;14:S296–309. doi: 10.1046/j.1540-8167.2003.90403.x. [DOI] [PubMed] [Google Scholar]
- 17.Amos GJ, Wettwer E, Metzger F, et al. Differences between outward currents of human atrial and subepicardial ventricular myocytes. The Journal of physiology. 1996;491(Pt 1):31–50. doi: 10.1113/jphysiol.1996.sp021194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camm AJ. Safety considerations in the pharmacological management of atrial fibrillation. International journal of cardiology. 2008;127:299–306. doi: 10.1016/j.ijcard.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Camm AJ, Savelieva I. Advances in antiarrhythmic drug treatment of atrial fibrillation: where do we stand now? Heart Rhythm. 2004;1:244–246. doi: 10.1016/j.hrthm.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Mays DJ, Foose JM, Philipson LH, Tamkun MM. Localization of the Kv1.5 K+ channel protein in explanted cardiac tissue. The Journal of clinical investigation. 1995;96:282–292. doi: 10.1172/JCI118032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SS, Knight BP. Electrical and pharmacologic cardioversion for atrial fibrillation. The Medical clinics of North America. 2008;92:101–120. xi. doi: 10.1016/j.mcna.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--excutive summary. Rev Port Cardiol. 2007;26:383–446. [PubMed] [Google Scholar]
- 23.Brendel J, Peukert S. Blockers of the Kv1.5 channel for the treatment of atrial arrhythmias. Current medicinal chemistry. 2003;1:273–287. doi: 10.2174/1568016033477441. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993;73:1061–1076. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- 25.Ravens U. Potassium channels in atrial fibrillation: targets for atrial and pathology-specific therapy? Heart Rhythm. 2008;5:758–759. doi: 10.1016/j.hrthm.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Brundel BJ, Van Gelder IC, Henning RH, et al. Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K+ channels. Journal of the American College of Cardiology. 2001;37:926–932. doi: 10.1016/s0735-1097(00)01195-5. [DOI] [PubMed] [Google Scholar]
- 27.Michelakis ED, Weir EK. The pathobiology of pulmonary hypertension. Smooth muscle cells and ion channels. Clinics in chest medicine. 2001;22:419–432. doi: 10.1016/s0272-5231(05)70281-1. [DOI] [PubMed] [Google Scholar]
- 28.Sweeney M, Yuan JX. Hypoxic pulmonary vasoconstriction: role of voltage-gated potassium channels. Respiratory research. 2000;1:40–48. doi: 10.1186/rr11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford JW, Milnes JT. New drugs targeting the cardiac ultra-rapid delayed-rectifier current (I Kur): rationale, pharmacology and evidence for potential therapeutic value. Journal of cardiovascular pharmacology. 2008;52:105–120. doi: 10.1097/FJC.0b013e3181719b0c. [DOI] [PubMed] [Google Scholar]
- 30.Nattel S, Carlsson L. Innovative approaches to anti-arrhythmic drug therapy. Nat Rev Drug Discov. 2006;5:1034–1049. doi: 10.1038/nrd2112. [DOI] [PubMed] [Google Scholar]
- 31.Waldo AL. A perspective on antiarrhythmic drug therapy to treat atrial fibrillation: there remains an unmet need. American heart journal. 2006;151:771–778. doi: 10.1016/j.ahj.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Schumacher SM, McEwen DP, Zhang L, et al. Antiarrhythmic drug-induced internalization of the atrial-specific k+ channel kv1.5. Circ Res. 2009;104:1390–1398. doi: 10.1161/CIRCRESAHA.108.192773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Heyden MA, Smits ME, Vos MA. Drugs and trafficking of ion channels: a new pro-arrhythmic threat on the horizon? British journal of pharmacology. 2008;153:406–409. doi: 10.1038/sj.bjp.0707618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nature reviews. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 35.Olson TM, Alekseev AE, Liu XK, et al. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Human molecular genetics. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 36.London B, Guo W, Pan X, et al. Targeted replacement of KV1.5 in the mouse leads to loss of the 4-aminopyridine-sensitive component of I(K,slow) and resistance to drug-induced qt prolongation. Circ Res. 2001;88:940–946. doi: 10.1161/hh0901.090929. [DOI] [PubMed] [Google Scholar]
- 37.Marks DL, Pagano RE. Endocytosis and sorting of glycosphingolipids in sphingolipid storage disease. Trends Cell Biol. 2002;12:605–613. doi: 10.1016/s0962-8924(02)02399-1. [DOI] [PubMed] [Google Scholar]
- 38.Maxfield FR, McGraw TE. Endocytic recycling. Nat.Rev.Mol.Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 39.Prekeris R. Rabs, Rips, FIPs, and endocytic membrane traffic. ScientificWorldJournal. 2003;3:870–880. doi: 10.1100/tsw.2003.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown SS. Cooperation between microtubule- and actin-based motor proteins. Annu.Rev.Cell Dev.Biol. 1999;15:63–80. doi: 10.1146/annurev.cellbio.15.1.63. [DOI] [PubMed] [Google Scholar]
- 41.Dantzig JA, Liu TY, Goldman YE. Functional studies of individual myosin molecules. Ann.N.Y.Acad.Sci. 2006;1080:1–18. 1–18. doi: 10.1196/annals.1380.002. [DOI] [PubMed] [Google Scholar]
- 42.Langford GM. Myosin-V, a versatile motor for short-range vesicle transport. Traffic. 2002;3:859–865. doi: 10.1034/j.1600-0854.2002.31202.x. [DOI] [PubMed] [Google Scholar]
- 43.Seabra MC, Coudrier E. Rab GTPases and myosin motors in organelle motility. Traffic. 2004;5:393–399. doi: 10.1111/j.1398-9219.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- 44.Steele DF, Eldstrom J, Fedida D. Mechanisms of cardiac potassium channel trafficking. The Journal of physiology. 2007;582:17–26. doi: 10.1113/jphysiol.2007.130245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steele DF, Zadeh AD, Loewen ME, Fedida D. Localization and trafficking of cardiac voltage-gated potassium channels. Biochemical Society transactions. 2007;35:1069–1073. doi: 10.1042/BST0351069. [DOI] [PubMed] [Google Scholar]
- 46.Deutsch C. The birth of a channel. Neuron. 2003;40:265–276. doi: 10.1016/s0896-6273(03)00506-3. [DOI] [PubMed] [Google Scholar]
- 47.Deutsch C. Potassium channel ontogeny. Annual review of physiology. 2002;64:19–46. doi: 10.1146/annurev.physiol.64.081501.155934. [DOI] [PubMed] [Google Scholar]
- 48.Staudacher I, Schweizer PA, Katus HA, Thomas D. hERG: protein trafficking and potential for therapy and drug side effects. Current opinion in drug discovery & development. 13:23–30. [PubMed] [Google Scholar]
- 49.Robinson JM, Deutsch C. Coupled tertiary folding and oligomerization of the T1 domain of Kv channels. Neuron. 2005;45:223–232. doi: 10.1016/j.neuron.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 50.Babila T, Moscucci A, Wang H, Weaver FE, Koren G. Assembly of mammalian voltage-gated potassium channels: evidence for an important role of the first transmembrane segment. Neuron. 1994;12:615–626. doi: 10.1016/0896-6273(94)90217-8. [DOI] [PubMed] [Google Scholar]
- 51.Jindal HK, Folco EJ, Liu GX, Koren G. Posttranslational modification of voltage-dependent potassium channel Kv1.5: COOH-terminal palmitoylation modulates its biological properties. Am J Physiol Heart Circ Physiol. 2008;294:H2012–2021. doi: 10.1152/ajpheart.01374.2007. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Foster K, Li Q, Martens JR. S-acylation regulates Kv1.5 channel surface expression. Am J Physiol Cell Physiol. 2007;293:C152–161. doi: 10.1152/ajpcell.00480.2006. [DOI] [PubMed] [Google Scholar]
- 53.England SK, Uebele VN, Kodali J, Bennett PB, Tamkun MM. A novel K+ channel beta-subunit (hKv beta 1.3) is produced via alternative mRNA splicing. The Journal of biological chemistry. 1995;270:28531–28534. doi: 10.1074/jbc.270.48.28531. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez T, Navarro-Polanco R, Arias C, et al. Assembly with the Kvbeta1.3 subunit modulates drug block of hKv1.5 channels. Molecular pharmacology. 2002;62:1456–1463. doi: 10.1124/mol.62.6.1456. [DOI] [PubMed] [Google Scholar]
- 55.Kwak YG, Hu N, Wei J, et al. Protein kinase A phosphorylation alters Kvbeta1.3 subunit-mediated inactivation of the Kv1.5 potassium channel. The Journal of biological chemistry. 1999;274:13928–13932. doi: 10.1074/jbc.274.20.13928. [DOI] [PubMed] [Google Scholar]
- 56.Kwak YG, Navarro-Polanco RA, Grobaski T, Gallagher DJ, Tamkun MM. Phosphorylation is required for alteration of kv1.5 K(+) channel function by the Kvbeta1.3 subunit. The Journal of biological chemistry. 1999;274:25355–25361. doi: 10.1074/jbc.274.36.25355. [DOI] [PubMed] [Google Scholar]
- 57.Uebele VN, England SK, Chaudhary A, Tamkun MM, Snyders DJ. Functional differences in Kv1.5 currents expressed in mammalian cell lines are due to the presence of endogenous Kv beta 2.1 subunits. The Journal of biological chemistry. 1996;271:2406–2412. doi: 10.1074/jbc.271.5.2406. [DOI] [PubMed] [Google Scholar]
- 58.Uebele VN, England SK, Gallagher DJ, et al. Distinct domains of the voltage-gated K+ channel Kv beta 1.3 beta-subunit affect voltage-dependent gating. The American journal of physiology. 1998;274:C1485–1495. doi: 10.1152/ajpcell.1998.274.6.C1485. [DOI] [PubMed] [Google Scholar]
- 59.Mason HS, Latten MJ, Godoy LD, Horowitz B, Kenyon JL. Modulation of Kv1.5 currents by protein kinase A, tyrosine kinase, and protein tyrosine phosphatase requires an intact cytoskeleton. Molecular pharmacology. 2002;61:285–293. doi: 10.1124/mol.61.2.285. [DOI] [PubMed] [Google Scholar]
- 60.Williams CP, Hu N, Shen W, Mashburn AB, Murray KT. Modulation of the human Kv1.5 channel by protein kinase C activation: role of the Kvbeta1.2 subunit. The Journal of pharmacology and experimental therapeutics. 2002;302:545–550. doi: 10.1124/jpet.102.033357. [DOI] [PubMed] [Google Scholar]
- 61.Abi-Char J, El-Haou S, Balse E, et al. The anchoring protein SAP97 retains Kv1.5 channels in the plasma membrane of cardiac myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H1851–1861. doi: 10.1152/ajpheart.01045.2007. [DOI] [PubMed] [Google Scholar]
- 62.Abi-Char J, Maguy A, Coulombe A, et al. Membrane cholesterol modulates Kv1.5 potassium channel distribution and function in rat cardiomyocytes. The Journal of physiology. 2007;582:1205–1217. doi: 10.1113/jphysiol.2007.134809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eldstrom J, Choi WS, Steele DF, Fedida D. SAP97 increases Kv1.5 currents through an indirect N-terminal mechanism. FEBS letters. 2003;547:205–211. doi: 10.1016/s0014-5793(03)00668-9. [DOI] [PubMed] [Google Scholar]
- 64.Folco EJ, Liu GX, Koren G. Caveolin-3 and SAP97 form a scaffolding protein complex that regulates the voltage-gated potassium channel Kv1.5. Am J Physiol Heart Circ Physiol. 2004;287:H681–690. doi: 10.1152/ajpheart.00152.2004. [DOI] [PubMed] [Google Scholar]
- 65.Martens JR, Sakamoto N, Sullivan SA, Grobaski TD, Tamkun MM. Isoform-specific localization of voltage-gated K+ channels to distinct lipid raft populations. Targeting of Kv1.5 to caveolae. The Journal of biological chemistry. 2001;276:8409–8414. doi: 10.1074/jbc.M009948200. [DOI] [PubMed] [Google Scholar]
- 66.Maruoka ND, Steele DF, Au BP, et al. alpha-actinin-2 couples to cardiac Kv1.5 channels, regulating current density and channel localization in HEK cells. FEBS letters. 2000;473:188–194. doi: 10.1016/s0014-5793(00)01521-0. [DOI] [PubMed] [Google Scholar]
- 67.Mathur R, Choi WS, Eldstrom J, et al. A specific N-terminal residue in Kv1.5 is required for upregulation of the channel by SAP97. Biochemical and biophysical research communications. 2006;342:1–8. doi: 10.1016/j.bbrc.2006.01.110. [DOI] [PubMed] [Google Scholar]
- 68.McEwen DP, Schumacher SM, Li Q, et al. Rab-GTPase-dependent endocytic recycling of Kv1.5 in atrial myocytes. The Journal of biological chemistry. 2007;282:29612–29620. doi: 10.1074/jbc.M704402200. [DOI] [PubMed] [Google Scholar]
- 69.Choi WS, Khurana A, Mathur R, et al. Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circ Res. 2005;97:363–371. doi: 10.1161/01.RES.0000179535.06458.f8. [DOI] [PubMed] [Google Scholar]
- 70.Balse E, El-Haou S, Dillanian G, et al. Cholesterol modulates the recruitment of Kv1.5 channels from Rab11-associated recycling endosome in native atrial myocytes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14681–14686. doi: 10.1073/pnas.0902809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benson MD, Li QJ, Kieckhafer K, et al. SUMO modification regulates inactivation of the voltage-gated potassium channel Kv1.5. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1805–1810. doi: 10.1073/pnas.0606702104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boehmer C, Laufer J, Jeyaraj S, et al. Modulation of the voltage-gated potassium channel Kv1.5 by the SGK1 protein kinase involves inhibition of channel ubiquitination. Cell Physiol Biochem. 2008;22:591–600. doi: 10.1159/000185543. [DOI] [PubMed] [Google Scholar]
- 73.Kato M, Ogura K, Miake J, et al. Evidence for proteasomal degradation of Kv1.5 channel protein. Biochemical and biophysical research communications. 2005;337:343–348. doi: 10.1016/j.bbrc.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 74.Dennis A, Wang L, Wan X, Ficker E. hERG channel trafficking: novel targets in drug-induced long QT syndrome. Biochemical Society transactions. 2007;35:1060–1063. doi: 10.1042/BST0351060. [DOI] [PubMed] [Google Scholar]
- 75.Rajamani S, Eckhardt LL, Valdivia CR, et al. Drug-induced long QT syndrome: hERG K+ channel block and disruption of protein trafficking by fluoxetine and norfluoxetine. British journal of pharmacology. 2006;149:481–489. doi: 10.1038/sj.bjp.0706892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takemasa H, Nagatomo T, Abe H, et al. Coexistence of hERG current block and disruption of protein trafficking in ketoconazole-induced long QT syndrome. British journal of pharmacology. 2008;153:439–447. doi: 10.1038/sj.bjp.0707537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yeung KS, Meanwell NA. Inhibition of hERG channel trafficking: an under-explored mechanism for drug-induced QT prolongation. ChemMedChem. 2008;3:1501–1502. doi: 10.1002/cmdc.200800170. [DOI] [PubMed] [Google Scholar]
- 78.Cordes JS, Sun Z, Lloyd DB, et al. Pentamidine reduces hERG expression to prolong the QT interval. British journal of pharmacology. 2005;145:15–23. doi: 10.1038/sj.bjp.0706140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo J, Massaeli H, Li W, et al. Identification of IKr and its trafficking disruption induced by probucol in cultured neonatal rat cardiomyocytes. The Journal of pharmacology and experimental therapeutics. 2007;321:911–920. doi: 10.1124/jpet.107.120931. [DOI] [PubMed] [Google Scholar]
- 80.Kuryshev YA, Ficker E, Wang L, et al. Pentamidine-induced long QT syndrome and block of hERG trafficking. The Journal of pharmacology and experimental therapeutics. 2005;312:316–323. doi: 10.1124/jpet.104.073692. [DOI] [PubMed] [Google Scholar]
- 81.Ficker E, Dennis A, Kuryshev Y, Wible BA, Brown AM. HERG channel trafficking. Novartis Foundation symposium. 2005;266:57–69. discussion 70-54, 95-59. [PubMed] [Google Scholar]
- 82.Walker VE, Atanasiu R, Lam H, Shrier A. Co-chaperone FKBP38 promotes HERG trafficking. The Journal of biological chemistry. 2007;282:23509–23516. doi: 10.1074/jbc.M701006200. [DOI] [PubMed] [Google Scholar]
- 83.Ficker E, Obejero-Paz CA, Zhao S, Brown AM. The binding site for channel blockers that rescue misprocessed human long QT syndrome type 2 ether-a-gogo-related gene (HERG) mutations. The Journal of biological chemistry. 2002;277:4989–4998. doi: 10.1074/jbc.M107345200. [DOI] [PubMed] [Google Scholar]
- 84.Ficker E, Dennis AT, Wang L, Brown AM. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ Res. 2003;92:e87–100. doi: 10.1161/01.RES.0000079028.31393.15. [DOI] [PubMed] [Google Scholar]
- 85.Colatsky TJ. Mechanisms of action of lidocaine and quinidine on action potential duration in rabbit cardiac Purkinje fibers. An effect on steady state sodium currents? Circ Res. 1982;50:17–27. doi: 10.1161/01.res.50.1.17. [DOI] [PubMed] [Google Scholar]
- 86.Slawsky MT, Castle NA. K+ channel blocking actions of flecainide compared with those of propafenone and quinidine in adult rat ventricular myocytes. The Journal of pharmacology and experimental therapeutics. 1994;269:66–74. [PubMed] [Google Scholar]
- 87.Caballero R, Pourrier M, Schram G, et al. Effects of flecainide and quinidine on Kv4.2 currents: voltage dependence and role of S6 valines. British journal of pharmacology. 2003;138:1475–1484. doi: 10.1038/sj.bjp.0705199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang T, Snyders DJ, Roden DM. Inhibition of cardiac potassium currents by the vesnarinone analog OPC-18790: comparison with quinidine and dofetilide. The Journal of pharmacology and experimental therapeutics. 1997;280:1170–1175. [PubMed] [Google Scholar]
- 89.Decher N, Kumar P, Gonzalez T, Pirard B, Sanguinetti MC. Binding site of a novel Kv1.5 blocker: a “foot in the door” against atrial fibrillation. Molecular pharmacology. 2006;70:1204–1211. doi: 10.1124/mol.106.026203. [DOI] [PubMed] [Google Scholar]
- 90.Decher N, Pirard B, Bundis F, et al. Molecular basis for Kv1.5 channel block: conservation of drug binding sites among voltage-gated K+ channels. The Journal of biological chemistry. 2004;279:394–400. doi: 10.1074/jbc.M307411200. [DOI] [PubMed] [Google Scholar]
- 91.Drolet B, Simard C, Mizoue L, Roden DM. Human cardiac potassium channel DNA polymorphism modulates access to drug-binding site and causes drug resistance. The Journal of clinical investigation. 2005;115:2209–2213. doi: 10.1172/JCI23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Keller GA, Ponte ML, Di Girolamo G. Other Drugs Acting on Nervous System Associated with QT-Interval Prolongation. Current drug safety. 2009 doi: 10.2174/157488610789869256. [DOI] [PubMed] [Google Scholar]