Abstract
Background & Aims
Retrospective studies suggest that subjects with chronic hepatitis C and advanced fibrosis who achieve a sustained virological response (SVR) have a lower risk of hepatic decompensation and hepatocellular carcinoma (HCC). In this prospective analysis, we compared the rate of death from any cause or liver transplantation, and of liver-related morbidity and mortality, after antiviral therapy among patients who achieved SVR, virologic nonresponders (NR) and those with initial viral clearance but subsequent breakthrough or relapse (BT/R) in the HALT-C Trial.
Methods
Laboratory and/or clinical outcome data were available for 140 of the 180 SVR patients. Nonresponders (n=309) or BT/R (N=77) were evaluated every 3 months for 3.5 years and then every 6 months thereafter. Outcomes included death, liver-related death, liver transplantation, decompensated liver disease, and HCC.
Results
Median follow-up for SVR, BT/R, and NR patients was 86, 85, and 79 months, respectively. At 7.5 years, the adjusted cumulative rate of death/liver transplantation and of liver-related morbidity/mortality in the SVR group (2.2% and 2.7%, respectively) was significantly lower than that in NR (21.3% and 27.2%, p<0.001 for both) but not the BT/R (4.4% and 8.7%). The adjusted hazard ratio [HR] for time to death/liver transplantation (HR=0.17, 95% CI: 0.06–0.46), or development of liver-related morbidity/mortality (HR=0.15, 95% CI: 0.06–0.38) or HCC (HR=0.19, 95% CI: 0.04–0.80) was significant for SVR compared to NR. Laboratory tests related to liver-disease severity improved following SVR.
Conclusions
Patients with advanced chronic hepatitis C who achieved SVR had a marked reduction in death/liver transplantation, and in liver-related morbidity/mortality, although they remain at risk for HCC.
Keywords: hepatitis C treatment, peginterferon, survival analysis, hepatocellular carcinoma
BACKGROUND AND AIMS
Chronic hepatitis C virus (HCV) infection is a common cause of cirrhosis, hepatocellular carcinoma (HCC), and liver transplantation. Follow-up studies of patients who achieved a sustained virological response (SVR) after antiviral therapy have demonstrated that the majority of patients continue to have undetectable serum HCV RNA, improvement in liver fibrosis, including reversal of cirrhosis, and a reduction in the incidence of decompensated liver disease and HCC as compared with subjects who did not achieve an SVR1–3. These studies notwithstanding, the beneficial effect of achieving an SVR on the outcome of patients with advanced chronic hepatitis C remains incompletely defined because prior studies were retrospective4–7 and included a small number of patients with cirrhosis2 and a relatively limited period of follow-up8. Additionally, few data are available on patients in the United States, because most of these studies were conducted in Japan or Europe4–8. Furthermore, the beneficial effect of interferon and ribavirin treatment on the outcomes of patients with advanced hepatitis C who achieved viral clearance during treatment and who relapsed after discontinuation of treatment has not been established clearly6.
The Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial was a multicenter study involving more than 1,000 patients in the United States with advanced chronic hepatitis C and nonresponse to previous treatment with interferon-based therapy9. During the lead-in phase of the HALT-C Trial, 1,145 patients were treated with a combination of pegylated interferon and ribavirin; 180 achieved SVR. Patients who did not achieve SVR entered the randomized phase of the HALT-C Trial and were followed prospectively for the development of fibrosis progression, decompensated liver disease, HCC, and death. The aim of the current study was to evaluate the effect of achieving SVR on overall mortality and on liver-related morbidity and mortality in this large, prospectively followed cohort of patients from the United States with advanced chronic hepatitis C.
PATIENTS AND METHODS
HALT-C Trial design
The design and primary results of the HALT-C Trial have been reported 9–10. Briefly, patients with chronic hepatitis C meeting the following criteria were entered into the lead-in phase of the HALT-C Trial: advanced hepatic fibrosis (Ishak fibrosis score ≥3) according to a liver biopsy performed within 12 months prior to enrollment; lack of SVR to previous treatment for at least 24 weeks with standard interferon with or without ribavirin; and no history of hepatic decompensation or HCC. All patients in the lead-in phase of HALT-C Trial were prescribed combination therapy with peginterferon alfa-2a 180 ug weekly and weight-based ribavirin 1000–1200 mg daily for 24 weeks.
Patients with detectable serum HCV RNA at treatment Week 20 were classified as nonresponders (NR), and combination therapy was discontinued at Week 24. These patients were randomized to either the maintenance-therapy group (90 ug of peginterferon alfa-2a weekly, without ribavirin) or to no treatment (control group) for the next 3.5 years.
Patients with undetectable serum HCV RNA at Week 20 were considered responders, continued combination therapy for a total duration of 48 weeks, and were monitored to Week 72 (24 weeks posttreatment) to determine if they achieved SVR. If, in a Week-20 responder, HCV RNA became detectable again after Week 20, either during treatment (breakthrough) or after cessation of treatment (relapse), the patient was offered the opportunity to enter the randomized phase of the trial (the breakthrough/relapse [BT/R] cohort).
After randomization, patients were seen every 3 months for 3.5 years and then every 6 months thereafter for an interval medical history, physical examination, and laboratory testing to assess for clinical outcomes and adverse events. Local laboratory tests included complete blood count (CBC), hepatic panel (which included serum albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, and total bilirubin), creatinine (Cr), prothrombin time/international normalized ratio (INR), and alpha fetoprotein (AFP). Quantitative HCV RNA was measured in a central laboratory at each visit during the first 3.5 years. Abdominal ultrasound was performed 6 months after randomization and then every 12 months to screen for HCC.
Design of the current study
According to the initial HALT-C Trial protocol, patients who achieved SVR ceased participation in the Trial after Week 72, although many of the patients continued to be followed outside the HALT-C Trial by the investigators at their respective sites. In 2008, the HALT-C Trial protocol was amended to allow HALT-C Trial clinical centers to contact patients who had achieved SVR and invite them to participate in the current study. The HALT-C Trial protocol amendment was approved by the institutional review boards of all the HALT-C Trial sites. Patients provided informed consent for participation in the initial HALT-C Trial as well as the amended protocol.
The amended protocol allowed for a single study visit consisting of a standard interview regarding the occurrence of hepatic decompensation or a diagnosis of HCC, and a physical examination to identify clinical signs of hepatic decompensation. Blood was drawn to test for CBC, hepatic panel, Cr, INR, AFP, and HCV RNA, and an abdominal ultrasound was performed. Patients who had a history consistent with decompensated liver disease, HCC, or liver transplantation were asked to sign a “release of information” form to allow HALT-C Trial investigators to review medical records related to the event. Patients who agreed to participate but were unable to attend an in-person clinic visit answered a structured telephone interview designed to elicit evidence of decompensated liver disease, HCC, or liver transplantation and were asked to mail a signed release of medical records to the clinical site to allow findings from a recent physical examination, blood tests and abdominal imaging (not performed at a HALT-C clinical site) to be reviewed.
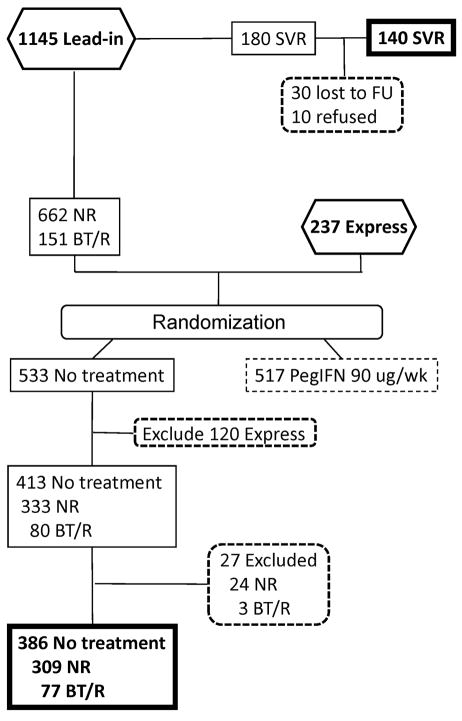
In order to assess the relative impact of achieving SVR on morbidity and mortality, we selected two comparison groups from the 1050 patients randomized into the HALT-C Trial (Figure 1). When selecting these comparison groups, we excluded 237 patients enrolled through the Express pathway because detailed information on whether these patients were nonresponders or breakthrough/relapsers to peginterferon and ribavirin given outside of the HALT-C Trial was not available. We also excluded 517 patients randomized to peginterferon maintenance in order to eliminate a potential effect of maintenance peginterferon on clinical outcomes, although we found no effect of maintenance peginterferon on clinical outcomes in our prior analysis9. Of the remaining 333 nonresponders who were randomized to the no-treatment (control) arm, 24 were excluded for the following reasons: 17 were not followed after randomization, 6 were treated with interferon outside of the HALT-C Trial, and 1 had negative HCV RNA test results during the randomized phase of the HALT-C Trial. Thus, the first comparison group consisted of 309 nonresponder patients randomized to the control arm of HALT-C Trial who were viremic and did not receive subsequent interferon. The second comparison group consisted of patients who had a breakthrough or relapse and who were randomized to the control arm of the HALT-C Trial. Of the 80 breakthrough/relapse patients randomized to the control (untreated) arm, 3 were excluded for the following reasons: 2 were not followed after randomization, and 1 patient was treated with peginterferon and ribavirin outside of the HALT-C Trial. Thus, a total of 77 patients with breakthrough or relapse (BT/R) who were randomized to the control arm and who remained viremic after randomization were included in this analysis.
Figure 1.
Flow-diagram of HALT-C subjects included in the current analysis. Of the 1145 patients entering the lead-in phase and receiving treatment with peginterferon alfa-2a 180 ug/week and ribavirin 1–1.2 grams/day, 180 achieved an SVR. Of these, 140 participated in the current study (SVR group). 813 patients from the HALT-C lead-in phase and 237 patients who were non-responders or relapsers to peginterferon/ribavirin given outside of the HALT-C Trial (Express) were randomized to either no treatment (control arm) or to peginterferon alfa-2a 90ug/week for 3.5 years, and then followed without treatment. For the current analysis, we excluded all patients randomized to receive peginterferon 90 ug/week in order to eliminate a possible effect of long-term interferon on outcomes. Among the 533 patients randomized to no treatment, 120 patients from the Express arm were excluded because data on whether the patients were NR or BT/R was not available. Of the remaining 333 nonresponders in the control arm, 24 were eliminated because they were not followed after randomization (17), received interferon treatment after randomization (6) or had undetectable HCV RNA during follow-up (1). Three of the 80 patients with BT/R were eliminated because they were not followed after randomization (2) or received treatment with peginterferon (1).
The beginning date for this analysis was the day when patients began peginterferon and ribavirin treatment during the lead-in phase of the HALT-C Trial (August, 2000 to January, 2003). The end date for determination of clinical outcomes for the SVR patients was the day of their amended protocol study visit or telephone contact (September, 2008 to March, 2009). The date for assessment of blood tests for SVR patients was the day of the amended protocol study visit or the most recent lab tests from the patient’s medical records (for patients participating by telephone). For the NR and BT/R groups, clinical outcome data were included for the first 7.5 years of participation in the HALT-C Trial. The blood test results from the Month 72 visit for the NR and the BT/R groups were used for analysis of changes in blood tests because a larger number of patients had reached that time point (239 of 386) as compared with the number available at the Month 84 visit (144 of 386). The median follow-up time for SVR patients was 86 months, BT/R 85 months, and NR 79 months. Follow-up rates through month 72 were 60% (185/309) in the NR group and 70% (54/77) in the BT/R group. The survival status of all patients was evaluated by searching the on-line US Social Security Death Index (http://ssdi.rootsweb.ancestry.com), which is generated from the US Social Security Administration’s Death Master File. The SSDI search of patients in the SVR group was undertaken in March, 2009; for patients in the BT/R and NR groups the SSDI search was performed in October, 2009.
Assessment of clinical outcomes
Clinical outcomes were predefined in the HALT-C Trial protocol and included death due to any cause, liver-related death, HCC, and hepatic decompensation (ascites, hepatic encephalopathy, variceal hemorrhage, or spontaneous bacterial peritonitis); we also collected data on liver transplantation. Two definitions of HCC were adopted in the HALT-C Trial, “definite” HCC and “presumed” HCC. Definite HCC was defined by histologic confirmation or a new, ≥2-cm mass lesion on imaging with AFP levels increasing to >1,000 ng/mL. Presumed HCC was defined as a new mass lesion on ultrasound in the absence of histology and AFP <1,000 ng/mL in conjunction with one of the following characteristics: a) two liver imaging studies showing a mass lesion with characteristics of HCC, b) progressively enlarging lesion on ultrasound and leading to death, or c) one additional imaging study showing a mass lesion with characteristics of HCC that either increased in size over time or was accompanied by increasing AFP levels11. An outcome committee, whose members consisted of a rotating panel of three clinical site investigators blinded to study participant and clinical site, reviewed and adjudicated the validity of each clinical outcome.
For the current analysis, we assessed overall mortality (i.e., death from any cause) or liver transplantation, and liver-related morbidity and mortality. Death from any cause or liver transplantation was defined as: any patient who died (of any cause) or had undergone liver transplantation. The four categories of liver-related morbidity and mortality were: 1) Any liver-related clinical outcome: all patients in whom decompensated liver disease (ascites, variceal bleeding, hepatic encephalopathy or spontaneous bacterial peritonitis) or HCC (presumed or definite) developed, or who had undergone liver transplantation, or died related to liver disease. For the calculation of the cumulative incidence of any liver-related outcomes, patients were censored at the time when the first outcome developed. 2) Decompensated liver disease: all patients whose first clinical outcome was decompensated liver disease. 3) HCC: all patients in whom, at any time during the study, definite or presumed HCC developed. 4): Liver-related death or liver transplantation: any patient who died as the result of a liver-related cause, based on the opinion of the clinical site principal investigator, or who had undergone liver transplantation.
Statistical analyses
Statistical analyses were performed at the Data Coordinating Center (New England Research Institute, Watertown, Massachusetts) with SAS software, release 9.1 (SAS Institute, Cary, NC). Chi-square and ANOVA were used to determine categorical and continuous variables that were significantly different between the SVR group and the two comparison groups (NR and BT/R). Cox proportional-hazards regression models were used to evaluate the association between risk factors and each of the five outcomes. Data were censored at the patient’s last follow-up visit or at 7.5 years after a patient began peginterferon and ribavirin treatment, whichever occurred first. Variables with a p-value <0.05 on univariate analysis and variables reported previously to be associated with outcomes were entered into multivariate analyses for each of the five clinical outcomes. One multivariate model was created for each of the five outcomes, and the adjusted cumulative incidence rates for each of the five outcomes were calculated by adjusting for risk factors that were significant on multivariate analyses. Adjusted cumulative rates were compared at the means of the covariates for each group.
Routine blood tests used to assess disease severity in patients with chronic hepatitis C were compared at three time points: 1) baseline (entry into lead-in phase of the HALT-C Trial), 2) approximately 18 months after baseline (Week 72 visit for SVR patients; Month 18 visit for BT/R and NR patients), and 3) approximately 72–84 months after baseline (amended protocol study visit for SVR patients; Month 72 visit for BT/R and NR patients). Paired t-tests were used to compare means of baseline and follow-up laboratory tests between different time points in each group.
RESULTS
Demographic and Clinical Data
Data were obtained on 140 (78%) of the 180 HALT-C Trial patients who achieved SVR. Thirty patients could not be located, and 10 declined to participate. The 40 patients who did not participate did not differ from the 140 who did at baseline or at Week 72 in demographic characteristics, baseline Ishak fibrosis score, or routine blood tests. Specifically, at Week 72 no difference was found between the SVR nonparticipants (n=40) and participants (n=140) for key predictors of clinical outcome such as age (49.8±8.02 vs. 50.0±6.12 for nonparticipants and participants, respectively; p=0.87), albumin (4.3±0.4 vs. 4.2±0.4 g/dL; p=0.26), platelet count (191±56 vs. 191±59 × 1000 per mm3; p=0.97), AFP (3.3±1.5 vs. 3.3±1.7 ng/mL; p=0.88) or alkaline phosphatase (72±20 vs. 78±20 IU/mL; p=0.27). Three of the 140 SVR patients had died and copies of death certificates for two of the three were obtained. Of the 137 surviving participants, 70 were seen in clinic, while 67 were evaluated by telephone interviews supplemented by examination of external medical records. None of the 30 patients with SVR who could not be located were listed as deceased in the on-line US Social Security Death Index.
Baseline demographic as well as clinical and laboratory data on the SVR group and the two comparison groups (BT/R and NR) are shown in Table 1. The three groups differed significantly in race/ethnicity, presence of cirrhosis, hepatitis C genotype, and laboratory values associated with advanced liver disease. SVR patients were more likely to be Caucasian, infected with HCV genotypes other than 1, to have fibrosis (rather than cirrhosis) on baseline biopsy and less likely to have laboratory values associated with advanced liver disease (e.g., low blood counts and albumin, or high INR and AFP) compared with BT/R and NR patients.
Table 1.
Baseline demographic, clinical and laboratory data for patients in the SVR, BT/R, and NR groups.
| Variable | SVR Group (n=140) | BT/R Group (n=77) | NR Group (n=309) | p-value* | |||
|---|---|---|---|---|---|---|---|
| N or mean | % or SD | N or mean | % or SD | N or mean | % or SD | ||
| Age (years) | 48.6 | 6.1 | 48.6 | 5.7 | 49.6 | 7.6 | 0.23 |
| Gender | |||||||
| Male | 107 | 76.4% | 57 | 74.0% | 215 | 69.6% | 0.30 |
| Female | 33 | 23.6% | 20 | 26.0% | 94 | 30.4% | |
| Race/ethnicity | |||||||
| White | 112 | 80.0% | 62 | 80.5% | 209 | 67.6% | 0.001 |
| Black | 8 | 5.7% | 3 | 3.9% | 61 | 19.7% | |
| Hispanic | 15 | 10.7% | 10 | 13.0% | 28 | 9.1% | |
| Others | 5 | 3.6% | 2 | 2.6% | 11 | 3.6% | |
| Stratum | |||||||
| Fibrosis | 111 | 79.3% | 53 | 68.8% | 177 | 57.3% | <0.0001 |
| Cirrhosis | 29 | 20.7% | 24 | 31.2% | 132 | 42.7% | |
| Ishak fibrosis score | 3.6 | 1.2 | 3.9 | 1.2 | 4.2 | 1.3 | <0.0001 |
| Steatosis score | 1.2 | 0.88 | 1.1 | 0.96 | 1.4 | 0.84 | 0.02 |
| Genotype 1 | |||||||
| Yes | 101 | 72.1% | 65 | 85.5% | 291 | 94.5% | <0.0001 |
| No | 39 | 27.9% | 11 | 14.5% | 17 | 5.5% | |
| Genotype 3 | |||||||
| Yes | 14 | 10.0% | 5 | 6.6% | 8 | 2.6% | 0.004 |
| No | 126 | 90.0% | 71 | 93.4% | 300 | 97.4% | |
| Body Mass Index (BMI) | 29.3 | 5.5 | 29.4 | 5.3 | 30.2 | 5.7 | 0.16 |
| WBC ×1000/mm3 | 6.17 | 2.1 | 5.98 | 1.98 | 5.62 | 1.73 | 0.01 |
| Hemoglobin, g/dL | 15.5 | 1.35 | 15.1 | 1.43 | 14.9 | 1.38 | 0.001 |
| Platelet count ×1000/mm3 | 180.2 | 59.2 | 180.7 | 55.8 | 164.2 | 70.5 | 0.02 |
| Creatinine, mg/dL | 0.85 | 0.14 | 0.85 | 0.16 | 0.84 | 0.17 | 0.62 |
| AST, U/L | 89.1 | 54.1 | 93.6 | 76.5 | 89.2 | 57.9 | 0.83 |
| ALT, U/L | 137.5 | 83.5 | 132.2 | 112.9 | 112.0 | 79.6 | 0.008 |
| Alk Phos, U/L | 88.3 | 36.8 | 87.8 | 31.7 | 102.3 | 47.8 | 0.001 |
| T. Bilirubin, mg/dL | 0.74 | 0.34 | 0.79 | 0.40 | 0.78 | 0.36 | 0.45 |
| Albumin, g/dL | 4.04 | 0.37 | 3.94 | 0.34 | 3.84 | 0.39 | <0.0001 |
| INR | 1.02 | 0.09 | 1.02 | 0.09 | 1.04 | 0.11 | 0.04 |
| AFP, ng/ml | 7.9 | 14.2 | 7.70 | 10.6 | 18.2 | 27.5 | <0.0001 |
p-value for comparison of the 3 groups.
Durability of SVR
Ninety-one SVR patients had follow-up HCV RNA testing performed an average of 78.6 ± 15.9 months (range: 22.1 to 99.6 months) after achieving SVR, and 90 of the 91 (99%) had undetectable HCV RNA in serum. The patient with reappearance of HCV RNA was presumed to have a relapse since there were no risk factors for reinfection and genotype 1b was detected at enrollment and at HCV reappearance 15 months following discontinuation of combination treatment. This patient had persistently detectable HCV RNA but no evidence of hepatic decompensation or HCC when last seen 108 months after enrollment in the lead-in phase of the HALT-C Trial.
Clinical Outcomes of SVR Patients
Five patients who achieved SVR (3.6%) had six liver-related clinical outcomes (Table 2). One patient (patient A) had a 3-cm lesion detected on ultrasound performed for his amended study clinic visit, 7.3 years after his baseline visit and 5.8 years after achieving SVR. At entry into the HALT-C Trial, he had a liver biopsy with an Ishak fibrosis score of 4 and his platelet count was 112,000/mm3. The resected specimen revealed a well-differentiated HCC; cirrhosis was present in the nontumor liver. Another patient (patient B) who had an Ishak fibrosis score of 3 on his baseline liver biopsy was found to have a 15-cm lesion on MRI performed to evaluate an elevated AFP during a routine follow-up visit 5.8 years after his baseline visit and 4.4 years after achieving SVR. Biopsy of the lesion confirmed the presence of HCC and cirrhosis in the adjacent liver. This patient died of progressive HCC four months later. A third patient (patient G) was found to have a 1.3-cm liver mass on MRI and underwent transarterial chemoembolization (TACE) twice, followed by liver transplantation, but no tumor was found in the liver explant. This patient did not meet the HALT-C Trial criteria for presumed or definite HCC. Two patients with SVR experienced variceal hemorrhage (patients E and F).
Table 2.
Description of clinical outcomes in patients achieving SVR.
| Patient # | Baseline Histology | Outcome | Time to Outcome (years) | Age at enroll ment | Gender | Comment |
|---|---|---|---|---|---|---|
| A | Fibrosis | HCC | 7.29 | 55 | Male | 3.0 cm mass detected by US at SVR study visit. Cirrhosis and HCC present in resected liver. |
| B | Fibrosis | HCC | 5.80 | 50 | Male | 15 cm mass detected by MRI after elevated AFP noted during routine clinic visit. Cirrhosis and HCC present in liver biopsy. Died of HCC. |
| Death (Liver related) | 6.14 | |||||
| C | Fibrosis | Death (Non-liver related or Unknown) | 5.59 | 63 | Female | Death of unknown cause. Family member reported the death occurred following spinal surgery. |
| D | Fibrosis | Death (Non-liver related) | 3.03 | 49 | Male | Death due to alcohol toxicity. |
| E | Fibrosis | Variceal hemorrhage | 4.13 | 47 | Male | Co-morbidities: obesity and diabetes. |
| F | Fibrosis | Variceal hemorrhage | 3.27 | 46 | Male | Co-morbidities: return to heavy alcohol use |
| G | Cirrhosis | Liver transplant | 7.30 | 47 | Male | 1.3 cm mass in liver on MRI. Treated with TACE twice. Liver transplant 4 months after 2nd TACE did not show HCC. |
The race/ethnicity of the 7 patients was non-Hispanic white.
Two additional SVR patients died, one from alcohol toxicity (patient D) and the other from an unconfirmed cause, although a family member reported that the death had occurred following spinal surgery (patient C). These two deaths were not considered to be liver-related.
Models to Predict Clinical Outcomes
The numbers of patients with death from any cause/liver transplantation and with liver-related outcomes in the SVR, BT/R and NR groups are presented in Table 3. SVR patients had fewer deaths any cause/liver transplantation (4 or 2.9%) and liver-related outcomes (6 outcomes in 5 (3.6%) patients) compared to BT/R (4 or 5.2%) death any cause/transplant; 15 liver–related outcomes in 8 (10.4%) patients and NR (64 or 20.7%) death any cause/transplant; 148 liver–related outcomes in 78 (25.2%) patients. Because the three patient groups differed in baseline severity of liver disease (e.g., Ishak fibrosis score, platelet count, albumin level; Table 1), we performed a Cox proportional hazard regression analysis (Table 4) adjusting for histological stratum (fibrosis or cirrhosis), age, race, platelet count, AST/ALT ratio, albumin, alkaline phosphatase, AFP, and treatment response (SVR, BT/R and NR). These variables were selected because they have been associated with liver disease severity or clinical outcomes in prior HALT-C Trial analyses11–12. Separate multivariate models were developed to assess risk factors associated with the five outcomes analyzed in this study.
Table 3.
Number of events and patients with death from any cause, liver-related death, liver transplantation, decompensated liver disease or HCC in the SVR, BT/R and NR groups.
| Clinical Outcomes* n, (%) | SVR Group (n=140) | BT/R Group (n=77) | NR Group (N=309) |
|---|---|---|---|
| Death (any cause) or liver transplantation | 4 (2.9%) | 4 (5.2%) | 64 (20.7%) |
| Death (any cause) | 3 (2.1%) | 2 (2.6%) | 37 (12.0%) |
| Liver transplantation | 1 (0.7%) | 2 (2.6%) | 34 (11.0%) |
| Decompensated liver disease (total) | 2 (1.4%) | 5 (6.5%) | 43 (13.9%) |
| Variceal hemorrhage | 2 (1.4%) | 2 (2.6%) | 12 (3.9%) |
| Ascites | 0 | 3 (3.9%) | 31 (10.0%) |
| Spontaneous bacterial peritonitis | 0 | 0 | 3 (1.0%) |
| Hepatic encephalopathy | 0 | 1 (1.3%) | 19 (6.1%) |
| HCC (definite or presumed) | 2 (1.4%) | 5 (6.5%) | 28 (9.1%) |
| Liver related death or liver transplantation | 2 (1.4%) | 4 (5.2%) | 49 (15.9%) |
| Liver related death | 1 (0.7%) | 2 (2.6%) | 21 (6.8%) |
| Death from any cause or liver transplantation: | 4 outcomes (4 patients) | 4 outcomes (4 patients) | 71 outcomes) (64 patients) |
| Liver-related outcomes: | |||
| Total no. of outcomes (Total no. of patients*) | 6 outcomes (5 patients) | 15 outcomes (8 patients) | 148 outcomes (78 patients) |
Patients can be counted more than once
Table 4.
Cox proportional hazard analysis for death from any cause or liver transplantation (Model 1), and for liver-related morbidity and mortality (Models 2–5).
| Model 1 Death (any cause) or Liver Transplantation | Model 2 Any liver-related outcome | Model 3 Decompensated liver disease | Model 4 HCC | Model 5 Liver-related death or Liver Transplantation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N=526 (72 events) | N=526 (91 events) | N=526 (50 events) | N=526 (35 events) | N=526 (55 events) | ||||||
| Variables | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI |
| Age (per 1 year) | 1.05 | 1.01–1.10* | ||||||||
| Baseline Platelets (per 10,000/mm3) | 0.88 | 0.83–0.92* | 0.85 | 0.81–0.89* | 0.86 | 0.80–0.92* | 0.85 | 0.79–0.92* | 0.86 | 0.81–0.92* |
| Baseline albumin (per 0.1 g/dL) | 0.89 | 0.83–0.94* | 0.91 | 0.87–0.96* | 0.90 | 0.83–0.96* | 0.88 | 0.83–0.95* | ||
| Baseline alk phos (per 10 U/L) | 1.08 | 1.02–1.13* | ||||||||
| Baseline AFP (per 10 ng/ml) | 1.06 | 1.01–1.12* | ||||||||
| Treatment Response | ||||||||||
| BT/R vs. NR | 0.29 | 0.10–0.79* | 0.46 | 0.22–0.96* | 0.57 | 0.22–1.46 | 0.96 | 0.36–2.54 | 0.43 | 0.15–1.21 |
| SVR vs. NR | 0.17 | 0.06–0.46* | 0.15 | 0.06–0.38* | 0.13 | 0.03–0.53* | 0.19 | 0.04–0.80* | 0.12 | 0.03–0.48* |
The following variables were not significant in any model: race, stratum (fibrosis vs. cirrhosis), and AST/ALT ratio
A low baseline platelet count was significantly associated with all five outcomes while a low baseline albumin was a significant risk factor for all outcomes except HCC (Model 4). Age and baseline alkaline phosphatase were also significant risk factors for the development of HCC (Model 4). Achieving an SVR, when compared with nonresponders, was associated with a significantly lower hazard ratio for each of the five clinical outcomes. Patients with BT/R had a significantly lower hazard ratio for death from any cause/liver transplantation (HR=0.29; 95% CI: 0.10–0.79) and for any liver-related outcome (HR=0.46; 95%CI: 0.22–0.96) when compared with NR. Fibrosis stage, race, and baseline AST/ALT ratio were not statistically significant risk factors in any multivariate model.
Adjusted Rates of Clinical Outcomes in SVR, BT/R and NR Patients
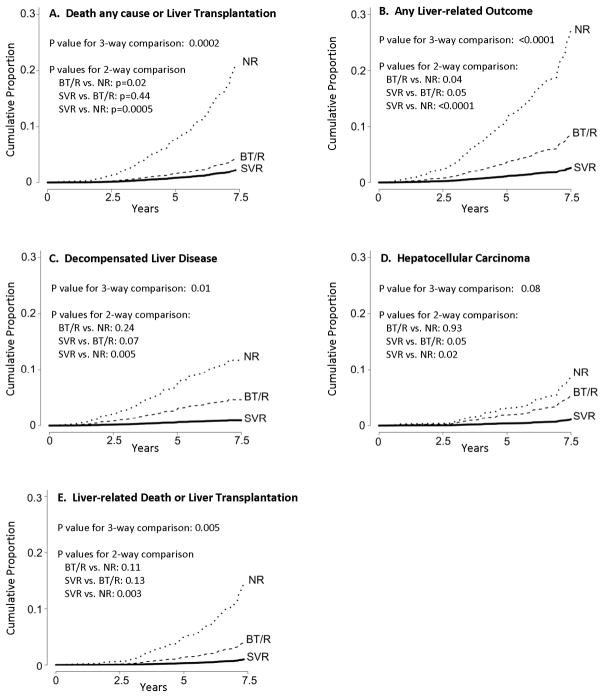
The cumulative rates of death from any cause/liver transplantation, and of liver-related morbidity and mortality, adjusted for the significant risk factors identified in the Cox models, are shown in Figure 2 and Supplemental Table 1. At year 7.5 from enrollment, the adjusted cumulative incidence of outcomes for the SVR, BT/R, and NR patients was, respectively, 2.2%, 4.4% and 21.3% for death from any cause or liver transplantation (p=0.0002); 2.7%, 8.7%, and 27.2% for any liver-related outcome (p<0.0001); 0.9%, 4.7%, and 11.7% for decompensated liver disease (p=0.012); 1.1%, 5.5%, and 8.8% for HCC (p=0.077); 0.99%, 4.1%, and 14.7% for liver-related death or liver transplantation (p=0.005). For each of the five outcomes, the adjusted cumulative proportion of patients with outcomes was lowest for the SVR group, intermediate for the BT/R group, and highest for the NR group of patients. Although the SVR patients had fewer outcomes than the BT/R patients, the adjusted cumulative incidence was not significantly different between the SVR and the BT/R groups for any of the five outcomes (SVR vs. BT/R: p=0.44 for death or liver transplantation, p=0.05 for any liver-related outcome, p=0.07 for decompensated liver disease, p=0.05 for HCC, and p=0.13 for liver-related death or liver transplantation). The adjusted cumulative proportion with death or liver transplantation (p=0.02) or any liver-related outcome (p=0.04) was significantly lower for the BT/R group when compared with the NR patients, but the difference between these two groups was not statistically significant when decompensated liver disease (p=0.24), HCC (p=0.93), liver-related death or liver transplantation (p=0.11) were analyzed individually.
Figure 2.
Cumulative proportion of patients with death from any cause or liver transplantation, or with liver-related clinical outcomes, during 7.5 years for patients with SVR, BT/R and NR, adjusted for variables that were significant in the Cox-proportional hazard analysis. (a) death from any cause or liver transplantation, (b) any liver related outcome, (c) decompensated liver disease, (d) HCC and (e) liver-related death or liver transplantation.
Because there was no effect of long-term peginterferon treatment on the rate of clinical outcomes9, the Cox proportional hazard analysis and the adjusted cumulative survival analysis were repeated after including 400 patients who were randomized to the peginterferon alfa-2a 90ug/week arm of the HALT-C Trial and who were followed after randomization. Including these patients increased the NR group to 638 and the BT/R group to 148. All hazard ratios and cumulative outcome analyses were essentially unchanged, except that statistical significance for SVR vs. NR was stronger, the HR and adjusted survival analyses for SVR vs. BT/R was significant for any liver-related outcome (p<0.05), and the HR and adjusted survival analyses for BT/R vs. NR was significant for liver-related death or liver transplantation (p<0.05) (data not shown).
Changes in Laboratory Test Results
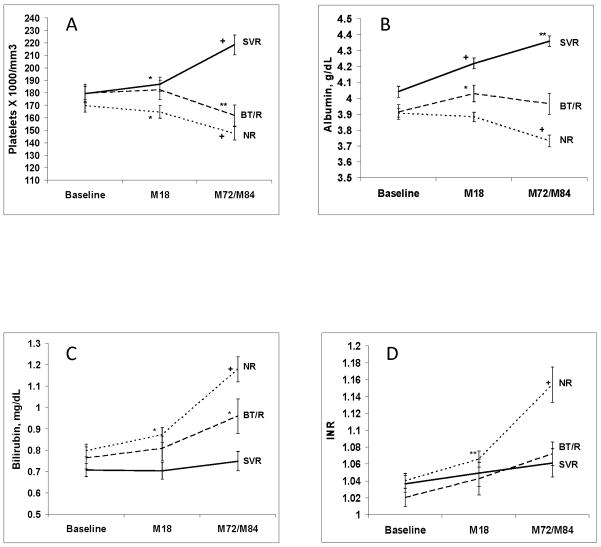
Figure 3 shows changes in selected blood tests over time among patients who had blood tests performed at each of the three time points. Among the SVR patients, platelet count and albumin (shown in Figure 3) as well as AST, ALT and AFP (data not shown) significantly improved from baseline to the most recent values. A significant improvement in platelet count and albumin was also observed between Week 72 (Month 18) (when SVR was attained) and the time of the amended study visit. In contrast, BT/R patients and NR patients had a significant worsening of platelet count and bilirubin between baseline and Month 72 visits, and NR patients also had deterioration in albumin and INR during the same time period.
Figure 3.
Mean (± standard error of the mean) value of laboratory tests at baseline, Month 18, and Month 72/84 for patients with SVR, BT/R and NR. (a) platelet count (× 1,000/mm3) (n=100 (SVR), 54 (BT/R) and 181 (NR)), (b) serum albumin (g/dL) (n=102 (SVR), 54 (BT/R) and 184 (NR)), (c) serum total bilirubin (mg/dL) (n=102 (SVR), 54 (BT/R) and 184 (NR)), and (d) INR (n=85 (SVR), 54 (BT/R) and 179 (NR)). Statistical comparisons within each group are between two adjacent time points (i.e., baseline vs. M18 and M18 vs. M72/84). *p<0.05; **p<0.001; +p<0.0001.
DISCUSSION
We report here the results of a prospective, long-term follow-up study to evaluate the effect of achieving SVR with pegylated interferon and ribavirin treatment on death from any cause or liver transplantation, and on liver-related morbidity and mortality, in a large cohort of patients in the United States with chronic hepatitis C and bridging fibrosis or cirrhosis. Patients who achieved SVR were compared with two groups of patients who were enrolled into the HALT-C Trial at the same time: 1) patients failed to respond to peginterferon and ribavirin (NR) and 2) patients with virologic clearance at Week 20 but subsequent virologic breakthrough during combination antiviral therapy or relapse after completion of therapy (BT/R). In this cohort of patients with advanced chronic hepatitis C, we found that those who achieved SVR after peginterferon and ribavirin had a significantly reduced risk of death from any cause/liver transplantation, and of liver-related morbidity and mortality, when compared with NR patients. Importantly, achieving SVR significantly reduced the risk of developing each component of liver-related morbidity and mortality (i.e., hepatic decompensation, HCC, and liver-related death or liver transplantation) when compared with NR patients.
A strength of the present study was the long duration of prospective follow-up. Patients were identified at entry into the HALT-C Trial and were followed for a median of 79 (NR) to 86 (SVR) months after starting their final course of peginterferon/ribavirin. Our findings on the effect of SVR on liver-related clinical outcomes are similar to those of retrospective, and often smaller, studies from Japan5, 7, 13–15 and Europe6, the results of which supported an approximately 70% to 90% reduction in the risk of liver-related clinical outcomes over a follow-up period of 2 to 6 years in patients achieving an SVR. An interesting observation in our study was the relative rapidity of the effect of achieving an SVR on hepatic decompensation; within one year, rates of decompensation among patients with an SVR and those with NR began to diverge. Both SVR patients in whom HCC developed had no discernable cause for ongoing liver damage. These data underscore the continued risk of HCC in patients with advanced chronic hepatitis C even in those who achieved SVR, as has been noted previously 2, 4, 7–8, 13. Because both SVR patients in whom HCC developed were diagnosed more than 4 years (4.4 and 5.9) after achieving SVR, HCC surveillance should continue for more than 5 years after SVR, and probably for life.
Based on Cox proportional hazard analyses, we found that baseline platelet count was associated independently with all five outcomes, while albumin level was associated independently with four outcomes (not with HCC). Age and alkaline phosphatase were associated with the risk of HCC but not with any other outcome. This observation could suggest that the development of HCC follows a different pathway than, and is temporally independent of, the development of other complications of liver disease. In prior studies, age and gender have been associated with risk of HCC, and we have reported previously that alkaline phosphatase is associated with the risk of HCC in the HALT-C Trial cohort 11.
An interesting and heretofore unreported finding was the intermediate risk of clinical outcomes in the BT/R group, between the risk of that for the NR and the SVR groups. In particular, the adjusted risk of death from any cause/liver transplantation or of any liver-related outcome was significantly lower in the BT/R group than in the NR group. The risks of decompensated liver disease, HCC, and liver-related death or liver transplantation were also lower in the BT/R group than in the NR group, although these differences did not reach statistical significance. These findings suggest that complete viral suppression is associated with a reduced risk of clinical outcomes and that the benefits may outlast the period in which HCV RNA is undetectable16.
Laboratory tests commonly associated with liver disease severity, such as albumin and platelet count, improved in patients achieving an SVR but worsened in patients not achieving SVR. Of particular interest, platelet count and albumin continued to improve between Week 72 and the final visit approximately 5.5 years later in these patients with advanced fibrosis who achieved SVR. In the only prior report of laboratory tests among SVR patients followed for 5 years, George et al.2 were unable to demonstrate improvement in laboratory tests. Therefore, improvement in liver-related blood tests after achieving an SVR in patients with advanced fibrosis is an original finding. One possible explanation for the difference between the prior report and ours is that the majority of patients followed by George and colleagues2 had mild liver fibrosis, with minimal changes in albumin and platelets that would not be expected to improve during follow-up monitoring. Overall, our data demonstrating improvement in liver-related blood tests, when combined with prior studies demonstrating reduction in liver fibrosis1–3, suggest that liver function continues to recover in the years following an SVR in patients with advanced fibrosis/cirrhosis.
This study has several limitations. Seventeen percent of patients who achieved SVR were lost to follow-up and an additional six percent declined to participate. Potentially, decompensated liver disease or HCC may have developed in these patients; therefore, our results may be an underestimate of the rate of clinical outcomes in patients who achieved SVR. We were able to determine, however, that none of the 30 patients who were lost to follow-up died according to a search of the US Social Security Death Index performed at the end of amended study. Another potential limitation was the fact that the SVR patients were not monitored as closely as the BT/R and NR patients and that not all SVR patients were evaluated in person. Nevertheless, medical records with physical examination, blood tests, and/or liver imaging of the patients who were interviewed by phone were reviewed and added reliability to the ascertainment of the occurrence of decompensated liver disease or HCC as of the time of their last follow-up assessment.
In summary, we found that patients with advanced chronic hepatitis C who achieved SVR had significantly lower rates of death from any cause or liver transplantation, and of liver-related morbidity and mortality, compared to patients who failed to eliminate HCV with treatment (NR). Still, patients who achieved SVR remained at risk of HCC for at least 6 years after achieving SVR. Our study also showed that patients who had temporary, but complete viral suppression (BT/R) were less likely to die or undergo liver transplantation, or to experience liver-related complications than NR patients, indicating that the duration of clinical benefit may outlast the period of actual viral suppression. Importantly, laboratory tests associated with liver-disease severity (e.g., platelet count, albumin) continued to improve after patients achieved SVR. Overall, our data indicate that patients with chronic hepatitis C and advanced hepatic fibrosis who achieve SVR have a marked reduction in the risk for death or liver transplantation, or of liver-related complications, and continued improvement in laboratory markers of liver function in the 5–6 years following successful viral eradication.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute, the National Center for Minority Health and Health Disparities and by General Clinical Research Center and Clinical and Translational Science Center grants from the National Center for Research Resources, National Institutes of Health (grant numbers are listed below). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc., through a Cooperative Research and Development Agreement (CRADA) with the National Institutes of Health.
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Gyongyi Szabo, MD, Barbara F. Banner, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Gloria Borders, RN, Michelle Kelley, RN, ANP
Saint Louis University School of Medicine, St Louis, MO: (Contract N01-DK-9-2324) Bruce Bacon, MD, Brent Neuschwander-Tetri, MD, Elizabeth M. Brunt, MD, Debra King, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR-01066; Grant 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center) Raymond T. Chung, MD, Andrea E. Reid, MD, Atul K. Bhan, MD, Wallis A. Molchen, Cara C. Gooch
University of Colorado Denver, School of Medicine, Aurora, CO: (Contract N01-DK-9-2327, Grant M01RR-00051, Grant 1 UL1 RR 025780-01), Thomas Trouillot, MD, Marcelo Kugelmas, MD, S. Russell Nash, MD, Carol McKinley, RN
University of California - Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR-00827) John C. Hoefs, MD, John R. Craig, MD, M. Mazen Jamal, MD, MPH, Muhammad Sheikh, MD, Choon Park, RN
University of Texas Southwestern Medical Center, Dallas, TX: (Contract N01-DK-9-2321, Grant M01RR-00633, Grant 1 UL1 RR024982-01, North and Central Texas Clinical and Translational Science Initiative) Thomas E. Rogers, MD, Peter F. Malet, MD, Janel Shelton, Nicole Crowder, LVN, Rivka Elbein, RN, BSN, Nancy Liston, MPH
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Sugantha Govindarajan, MD, Carol B. Jones, RN, Susan L. Milstein, RN
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042, Grant 1 UL1 RR024986, Michigan Center for Clinical and Health Research) Robert J. Fontana, MD, Joel K. Greenson, MD, Pamela A. Richtmyer, LPN, CCRC, R. Tess Bonham, BS
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Richard K. Sterling, MD, MSc, Melissa J. Contos, MD, A. Scott Mills, MD, Charlotte Hofmann, RN, Paula Smith, RN
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD: T. Jake Liang, MD, David Kleiner, MD, PhD, Yoon Park, RN, Elenita Rivera, RN, Vanessa Haynes-Williams, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: James E. Everhart, MD, Leonard B. Seeff, MD, Patricia R. Robuck, PhD, Jay H. Hoofnagle, MD, Elizabeth C. Wright, PhD
University of Washington, Seattle, WA: (Contract N01-DK-9-2318) David R. Gretch, MD, PhD, Minjun Chung Apodaca, BS, ASCP, Rohit Shankar, BC, ASCP, Natalia Antonov, M. Ed.
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Anne M. Stoddard, ScD, Teresa M. Curto, MSW, MPH, Margaret C. Bell, MS, MPH
Armed Forces Institute of Pathology, Washington, DC: Zachary D. Goodman, MD, PhD, Fanny Monge, Michelle Parks
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
Abbreviations
- AFP
alpha-fetoprotein
- ALT
alanine aminotransferase
- ANOVA
analysis of variance
- AST
aspartate aminotransferase
- BT/R
breakthrough or relapse
- CBC
complete blood count
- CI
confidence ratio
- Cr
creatinine
- HALT-C
hepatitis C antiviral long-term treatment against cirrhosis
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- INR
international normalized ratio
- NR
nonresponder
- RNA
ribonucleic acid
- SSDI
social security death index
- SVR
sustained virological response
Footnotes
This is publication #51 of the HALT-C Trial.
The HALT-C Trial was registered with clinicaltrials.gov (#NCT00006164).
Participation in this study (participants listed alphabetically):
Study concept and design: Marc G. Ghany, Karen L. Lindsay, Anna S.F. Lok, Timothy R. Morgan, Kristin K. Snow
Acquisition of data: Herbert L. Bonkovsky, Jennifer L. De Santo, Adrian M. Di Bisceglie, Jules L. Dienstag, Marc G. Ghany, William M. Lee, Karen L. Lindsay, Anna S.F. Lok, Timothy R. Morgan, Chihiro Morishima, Mitchell L. Shiffman, Kristin K. Snow,
Analysis and interpretation of data: Marc G. Ghany, Hae-Young Kim, Karen L. Lindsay, Anna S.F. Lok, Timothy R. Morgan, Kristin K. Snow
Drafting of the manuscript: Marc G. Ghany, Hae-Young Kim, Karen L. Lindsay, Anna S.F. Lok, Timothy R. Morgan, Kristin K. Snow
Critical revision of manuscript: Herbert L. Bonkovsky, Jennifer L. De Santo, Adrian M. Di Bisceglie, Jules L. Dienstag, William M. Lee, Chihiro Morishima, Mitchell L. Shiffman
The following members of the writing group contributed equally to this manuscript (listed alphabetically): Marc Ghany, Hae-Young Kim, Karen Lindsay, Anna Lok, Timothy Morgan, and Kristin Snow.
Financial Disclosures
The authors disclose the following: T.R. Morgan is on the speaker’s bureau and receives research support from Hoffmann-La Roche, Inc.; K. L. Lindsay was a consultant and received research support from Hoffmann-La Roche, Inc. during this study and is now an employee of Tibotec, Inc. (a subsidiary of Johnson and Johnson), Yardley, NJ.; A.S. Lok is a consultant for Hoffmann-La Roche, Inc.; M. L. Shiffman is a consultant for Hoffmann-La Roche, Inc.; W.M. Lee receives research support from Hoffmann-La Roche, Inc.; A.M. Di Bisceglie is a consultant and receives research support from Hoffmann-La Roche, Inc.; and H.L. Bonkovsky receives research support from Hoffmann-La Roche, Inc. Authors with no financial relationships related to this project are: M.G. Ghany, H.-Y. Kim, K.K. Snow, J.L. De Santo, J. L. Dienstag, and C. Morishima.
Contributor Information
Timothy R. Morgan, Email: timothy.morgan@va.gov.
Marc G. Ghany, Email: MarcG@bdg10.niddk.nih.gov.
Hae-Young Kim, Email: hkim@neriscience.com.
Kristin K. Snow, Email: ksnow@neriscience.com.
Mitchell L. Shiffman, Email: mlshiffman@gmail.com.
Jennifer L. De Santo, Email: Jennifer.DeSanto@ucdenver.edu.
William M. Lee, Email: William.Lee@UTSouthwestern.edu.
Adrian M. Di Bisceglie, Email: dibiscam@slu.edu.
Herbert L. Bonkovsky, Email: Herbert.bonkovsky@carolinashealthcare.org.
Jules L. Dienstag, Email: jdienstag@partners.org.
Chihiro Morishima, Email: chihiro@u.washington.edu.
Karen L. Lindsay, Email: klindsay@usc.edu.
Anna S.F. Lok, Email: aslok@umich.edu.
References
- 1.Camma C, Di Bona D, Schepis F, et al. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology. 2004;39:333–42. doi: 10.1002/hep.20073. [DOI] [PubMed] [Google Scholar]
- 2.George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009;49:729–38. doi: 10.1002/hep.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poynard T, McHutchison J, Manns M, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–13. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 4.Bruno S, Stroffolini T, Colombo M, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–87. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 5.Imazeki F, Yokosuka O, Fukai K, Saisho H. Favorable prognosis of chronic hepatitis C after interferon therapy by long-term cohort study. Hepatology. 2003;38:493–502. doi: 10.1053/jhep.2003.50329. [DOI] [PubMed] [Google Scholar]
- 6.Pradat P, Tillmann HL, Sauleda S, et al. Long-term follow-up of the hepatitis C HENCORE cohort: response to therapy and occurrence of liver-related complications. J Viral Hepat. 2007;14:556–63. doi: 10.1111/j.1365-2893.2006.00829.x. [DOI] [PubMed] [Google Scholar]
- 7.Shiratori Y, Ito Y, Yokosuka O, et al. Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann Intern Med. 2005;142:105–14. doi: 10.7326/0003-4819-142-2-200501180-00009. [DOI] [PubMed] [Google Scholar]
- 8.Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677–84. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 9.Di Bisceglie AM, Shiffman ML, Everson GT, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. The New England Journal of Medicine. 2008;359:2429–41. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee WM, Dienstag JL, Lindsay KL, et al. Evolution of the HALT-C Trial: pegylated interferon as maintenance therapy for chronic hepatitis C in previous interferon nonresponders. Control Clin Trials. 2004;25:472–92. doi: 10.1016/j.cct.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–48. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lok AS, Ghany MG, Goodman ZD, et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology. 2005;42:282–92. doi: 10.1002/hep.20772. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda K, Saitoh S, Arase Y, et al. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: A long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124–30. doi: 10.1002/hep.510290439. [DOI] [PubMed] [Google Scholar]
- 14.Kasahara A, Hayashi N, Mochizuki K, et al. Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Osaka Liver Disease Study Group. Hepatology. 1998;27:1394–402. doi: 10.1002/hep.510270529. [DOI] [PubMed] [Google Scholar]
- 15.Makiyama A, Itoh Y, Kasahara A, et al. Characteristics of patients with chronic hepatitis C who develop hepatocellular carcinoma after a sustained response to interferon therapy. Cancer. 2004;101:1616–22. doi: 10.1002/cncr.20537. [DOI] [PubMed] [Google Scholar]
- 16.Shiffman ML, Morishima C, Dienstag JL, et al. Effect of HCV RNA Suppression During Peginterferon Alfa-2a Maintenance Therapy on Clinical Outcomes in the Halt-C Trial. Gastroenterology. 2009;137:1986–94. doi: 10.1053/j.gastro.2009.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.