Abstract
Telomeres cap chromosome ends and are critical for genomic stability. Many telomere-associated proteins are important for telomere length maintenance. Recent genome-wide association studies (GWAS) have identified single nucleotide polymorphisms (SNPs) in genes encoding telomere-associated proteins (RTEL1 and TERT-CLPTM1) as markers of cancer risk. We conducted an association study of telomere length and 743 SNPs in 43 telomere biology genes. Telomere length in peripheral blood DNA was determined by Q-PCR in 3,646 participants from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial and Nurses' Health Study. We investigated associations by SNP, gene, and pathway (functional group). We found no associations between telomere length and SNPs in TERT-CLPTM1L or RTEL1. Telomere length was not significantly associated with specific functional groups. Thirteen SNPs from four genes (MEN1, MRE11A, RECQL5, and TNKS) were significantly associated with telomere length. The strongest findings were in MEN1 (Gene-based P=0.006), menin, which associates with the telomerase promoter and may negatively regulate telomerase. This large association study did not find strong associations with telomere length. The combination of limited diversity and evolutionary conservation suggest that these genes may be under selective pressure. More work is needed to explore the role of genetic variants in telomere length regulation.
Keywords: Telomere length, single-nucleotide polymorphism, SNP, telomere biology, epidemiology
Introduction
Telomeres form the ends of chromosomes and help maintain genomic structural integrity (Moon and Jarstfer, 2007). They consist of tandem hexameric (TTAGGG)n nucleotide repeats with a single-stranded overhang and protein complex. The overhang folds back to form a t-loop (Griffith et al., 1999), which prevents the telomere ends from being recognized as break points by the DNA damage repair machinery (Palm and de Lange, 2008). Many proteins bind to or interact with the telomere to maintain telomeric integrity. Shelterin is an ordered associated protein complex that consists of TERF1, TERF2, TINF2, TERF2IP, ACD, and POT1. This complex helps form the t-loop, and protects the telomeres from degradation and inappropriate DNA repair, thereby avoiding end-to-end fusion, atypical recombination, and premature senescence (Palm and de Lange, 2008). The telomerase reverse transcriptase (TERT) and its telomere template-containing RNA component (TERC) are telomere-associated proteins that add telomeric repeats to elongate telomeres (Collins and Mitchell, 2002). Telomerase activity is usually absent in differentiated cells. There are numerous other important telomere-associated proteins and complexes that transiently associates with telomeric DNA, including proteins involved in DNA repair (e.g., ATM and MRE11A) and helicases (e.g., BLM and RECQL) (Aubert and Lansdorp, 2008). A large number of additional proteins interact either directly or indirectly at telomeric ends, and regulate protein-protein and protein-DNA interactions, cellular protein trafficking, and additional telomere-specific functions.
Telomeric repeats range in size from 0.15 to 50 kilobases (kb), and progressively shorten with each cell division (Aubert and Lansdorp, 2008). Telomere length is dependent on many factors including age, replicative history of the cell, chromosome arm, and tissue type (Aubert and Lansdorp, 2008; Wise et al., 2009). There is considerable inter-individual variation in telomere length (Aviv et al., 2009; Nordfjäll et al., 2009), and a genetic influence on length variation. Twin and family studies have estimated the heritability of mean leukocyte telomere length to range from 44% to 84% (Jeanclos et al., 2000; Njajou et al., 2007; Slagboom et al., 1994; Vasa-Nicotera et al., 2004). Quantitative-trait linkage analyses have mapped loci influencing telomere length to chromosomes 12q12.22 and 14q23.2 (Andrew et al., 2006; Vasa-Nicotera et al., 2004). Fine mapping of the 12q12.22 locus identified a polymorphism in the BICD1 gene that was significantly associated with shorter telomere length (Mangino et al., 2008). A genome-wide association study (GWAS) identified two SNPs on chromosome 18q12.2 associated with leukocyte telomere length in the region of the gene VPS34/PIKC3C (Mangino et al., 2009), which has been suggested to be involved in controlling telomere length variation in yeast (Rog et al., 2005). Other recent GWASs have identified associations between leukocyte telomere length and the telomere-associated protein TERC (Codd et al., 2010; Levy et al., 2010) and a gene suggested to be involved with telomere length regulation, OBFC1 (oligonucleotide/oligosaccharide-binding folds containing one) (Levy et al., 2010).
The inverse relationships between telomere length and aging, and its role in age-related and premature aging diseases have been well documented (Aubert and Lansdorp, 2008; Garcia et al., 2007). Telomere attrition has also been associated with inflammatory processes, oxidative stress, and an unhealthy lifestyle (Mirabello et al., 2009; Morlá et al., 2006; von Zglinicki, 2002). There is growing evidence that short telomeres are associated with the initiation and progression of cancer (Blasco MA, 1997; Hackett and Greider, 2002).
Cancer GWAS have shown that SNPs in genes encoding telomere-associated proteins at 5p15.33 (TERT-CLPTM1L locus) and RTEL1 were associated with risk of glioma (Shete et al., 2009; Wrensch et al., 2009), pancreatic (Petersen et al., 2010), and/or lung cancer (Jin et al., 2009; Landi et al., 2009; McKay et al., 2008). In addition, an association study of multiple tumor types suggests that this TERT-CLPTM1L region may contain important markers of overall cancer risk (Rafnar et al., 2009). It is possible that these cancer-associated sequence variants in telomere-associated genes may be associated with shorter telomeres.
Studies of dyskeratosis congenita, an inherited bone marrow failure and cancer predisposition syndrome have also been important in understanding the consequences of telomere dysfunction (Savage and Alter, 2009). Patients with dyskeratosis congenita have extremely short telomeres for their age, very high risk of several cancers, and germline mutations in genes important in the maintenance of telomeres (DKC1, TERC, TERT, NOLA3, TINF2, or NOLA2) (Armanios, 2009; Savage and Alter, 2009). The phenotypic spectrum of telomere biology disorders also includes patients with isolated aplastic anemia (Yamaguchi et al., 2005), acute myelogenous leukemia (Calado et al., 2009), and idiopathic pulmonary fibrosis (Armanios et al., 2007) who may have mutations in TERC or TERT.
The majority of the associated and telomeric complex proteins are highly evolutionarily conserved (de Lange, 2004; Kanoh and Ishikawa, 2003; Li et al., 2000; Mirabello et al., 2008; Nakamura and Cech, 1998; Savage et al., 2005). A recent population genetics study targeting 37 telomere maintenance genes in 53 worldwide populations found that these genes have limited genetic variation (Mirabello et al., 2008). The majority of telomere genes had low diversity, high ancestral allele frequencies, and low population differentiation (Mirabello et al., 2008).
There is little known about how common genetic variation relates to telomere length. We hypothesize that common genetic variation in genes encoding telomere-associated proteins could affect telomere length. We evaluated the association between genetic variation in 43 candidate telomere biology genes and leukocyte telomere length using SNP markers from GWAS of breast and prostate cancers (Hunter et al., 2007; Yeager et al., 2007). These genes encode proteins that are thought to be involved either in telomere length maintenance or with telomere binding proteins necessary for telomere stability and structure. Telomere length data was obtained from prospectively collected blood samples and measured using quantitative-polymerase chain reaction (Q-PCR) by the same laboratory (De Vivo et al., 2009; Mirabello et al., 2009).
Materials and Methods
PLCO Study population
The PLCO Cancer Screening Trial is an ongoing randomized trial with 154,942 persons aged 55 to 74 enrolled between September 1993 and July 2001 from 10 screening centers nationwide (Prorok et al., 2000). Detailed questionnaire data was collected from all subjects at baseline. Participants provided blood and tissue samples for etiologic studies of cancer. Detailed information about the PLCO Trial and eligibility criteria are described elsewhere (Gohagan et al., 2000). Institutional review boards at the U.S. National Cancer Institute and the 10 screening centers approved the PLCO protocol, and all participants provided written informed consent. Participants in this study were male subjects selected for the prospective case-control study of telomere length, prostate cancer risk and life-style variables from Mirabello et al. (Mirabello et al., 2009). In brief, men for this study were selected from the screening arm of the PLCO trial who (a) were of non-Hispanic white race/ethnicity; (b) had a prostate cancer screen (PLCO PSA test) prior to October 1, 2003; (c) completed a baseline questionnaire about cancer risk factors; (d) provided a blood sample between one month and three years prior to prostate cancer diagnosis for cases; (e) age 55-74; and, (f) no history of cancer (other than non-melanoma skin cancer) to study entry. Medical and pathology records related to prostate cancer diagnosis were acquired for men with suspected prostate cancer by screening examination or annual questionnaire, and these data were abstracted by trained medical record specialists. All cases had confirmed aggressive prostate cancer and a Gleason score of ≥ 7. There were a total of 616 prostate cancer cases and 1061 matched male controls. Since there was no difference between the telomere length of cases and controls and no significant association with prostate cancer risk in this study (Mirabello et al., 2009), we combined telomere length data from the cases and controls for the current study.
NHS Study Population
The NHS is a cohort of 121,700 female registered nurses aged 30 to 55 from 11 states in the United States. Detailed questionnaires data was collected at enrollment and biennially thereafter, and blood samples were collected from a subset of 32,826 women. Details of this study are described elsewhere (De Vivo et al., 2009). Participants in the current study were female subjects selected for a case-control study of telomere length and postmenopausal breast cancer risk by De Vivo et al. (De Vivo et al., 2009). In short, eligible cases were (a) postmenopausal woman; (b) had confirmed incident invasive breast cancer diagnosed after blood collection up to June 1, 2004; (d) were of Caucasian ethnicity; and, (c) had no prior diagnosis of cancer. Controls were randomly selected postmenopausal women free of cancer and matched to cases on age, blood collection, and ethnicity. Completion of the questionnaire and submission of the blood sample were considered to imply informed consent. The study protocol was approved by the Committee on Use of Human Subjects of the Brigham and Women's Hospital, Boston, MA. There were 1,122 postmenopausal breast cancer cases and 1,147 controls included in the study. They also found no significant difference between the telomere length of cases and controls and no significant association with breast cancer risk (De Vivo et al., 2009), therefore, we included telomere length data from both cases and controls for the current study.
Telomere length measurement
Details of the assay have been previously described (De Vivo et al., 2009; Mirabello et al., 2009). In brief, genomic DNA was extracted from peripheral blood samples (buffy coats, including all leukocytes) using the QIAmp (Qiagen, Chatsworth, CA) 96-spin blood protocol. The DNA concentration was quantified using a Nanodrop SD-1000 spectrophotometer, and subsequently dried down and re-suspended to ensure accurate and uniform DNA concentrations. Telomere length was measured by a modified version of the quantitative real time polymerase chain reaction (PCR)-based assay (Cawthon, 2002). The ratio of telomere repeat copy number to single-gene (β-globin, 36B4) copy number (T/S) was determined using an Applied Biosystems 7900HT PCR system in a high-throughput 384-well format. Five nanograms of genomic DNA was dried down and then re-suspended in 10 μL of either the telomere or 36B4 PCR reaction mixture. The telomere reaction mixture contained 1X Qiagen Quantitect Sybr Green Master Mix, 2.5 mM of DTT, 270 nM of Tel-1b primer (GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT), and 900 nM of Tel-2b primer (TCCCGACTATCCCTATCCCTATCCCTATCCCTATCCCTA). The PCR reaction ran for 1 cycle at 95°C for 5 minutes, followed by 40 cycles at 95°C for 15 seconds, and 54°C for 2 minutes. The 36B4 reaction mixture consisted of 1X Qiagen Quantitect Sybr Green Master Mix, 300 nM of 36B4U primer (CAGCAAGTGGGAAGGTGTAATCC), and 500 nM of 36B4D primer (CCCATTCTATCATCAACGGGTACAA). The 36B4 PCR reaction ran for 1 cycle at 95°C for 5 minutes, followed by 40 cycles at 95°C for 15 seconds, and 58°C for 1 minute 10 seconds.
All samples of each assay were performed in triplicate. Each 384-well plate included an 8-point standard curve to assess and compensate for interpolated variations in PCR efficiency. Blinded replicate samples were interspersed with the samples to assess inter-plate and intra-plate variability of threshold cycle (Ct) values. The relative average telomere length was calculated as the ratio of telomere repeat copy number to single-gene copy number (T/S) in the study subjects compared with the reference DNA sample; derived by exponentiating the T/S ratio (−dCt) between the average telomere Ct and average 36B4 Ct values. For the PLCO study samples, the coefficients of variation (CV) within triplicates of the telomere and single-gene assay were 1.11% and 0.77%, respectively. For the NHS samples, the CVs of the telomere and single-gene assay were 1.03% and 0.56%, respectively.
Genotyping analysis
We created a subset of genotype data from the GWAS of NHS (Hunter et al., 2007; Cancer Genetic Markers of Susceptibility project: http://cgems.cancer.gov) and PLCO (Yeager et al., 2007; Cancer Genetic Markers of Susceptibility project: http://cgems.cancer.gov) participants with telomere length data (De Vivo et al., 2009; Mirabello et al., 2009) for 43 genes (743 SNPs), including 20kb upstream and 10kb downstream, that have high a priori probabilities of effecting telomere length. We also included an expanded region around TERT-CLPTM1 due to recent findings in this region (Jin et al., 2009; Landi et al., 2009; McKay et al., 2008; Rafnar et al., 2009; Shete et al., 2009), from chromosome 5 position 1,201,710 to 1,445,536, which includes SLC6A19, SLC6A18, TERT, CLPTM1L, and SLC6A3. A detailed description of the genotyping methods and GWAS platforms are given elsewhere (Hunter et al., 2007; Yeager et al., 2007).
SNPs were excluded if they had less than a 90% genotyping rate in either study population or if they failed the Hardy-Weinberg equilibrium test. Individuals were excluded if they had more than 10% missing genotypes.
Data analysis
Linear regression models were used to estimate the association with telomere length for each SNP independently. Models were adjusted for smoking status (categorized as pack-years of smoking: 0, 1-9 pack-years, 10-29 pack-years, ≥30 pack-years), age (categorized as quartiles), case-control status (for both the PLCO and NHS participants), gender, NHS principal components and PLCO principal components (Patterson et al., 2006). For the principal components analysis, pooled case and control samples were analyzed using a set of 14,111 SNPs from the GWAS (Hunter et al., 2007; Yeager et al., 2007) with low pairwise linkage disequilibrium (LD; r2 < 0.01) according to the procedure described in Patterson et al. (2006). Testing for significance using the Tracy-Widom statistics (Patterson et al., 2006) showed four significant principal components for NHS and three for PLCO at the level of P < 0.05. The common allele was used as the referent category for the additive model to evaluate the additive effect of each minor allele. The gene–dose effects for each SNP were estimated by a linear trend test by coding the genotypes based on the number of variant alleles (0, 1 and 2). FDR corrections (Benjamini and Yekutieli, 2001) were conducted by gene (for all SNPs in a gene) for correction of multiple statistical tests.
We conducted gene-level and pathway-level analyses based on Yu et al. (Yu et al., 2009). The gene-level analysis is a global test for the association between the outcome and any subset of SNPs within a given gene or region. The pathway-level analysis is a global test for the association between the outcome and any subset of genes within a given pathway. P-values for these analyses were estimated with 20,000 permutation steps according to the algorithm.(Yu et al., 2009).
Statistical analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC), R language, and PLINK software, version 1.06 (http://pngu.mgh.harvard.edu/purcell/plink/). Haploview version 4.1 (Barrett et al., 2005) was used to determine the degree of LD using SNP data from the HapMap Phase 2 (The International HapMap Consortium, 2003) public database.
Results
Table 1 shows the characteristics of the study subjects, Table 2 shows the genes analyzed by functional pathway, and Supp. Table S1 lists the SNPs in each gene. We analyzed SNP data for 43 gene regions involved in telomere biology, in a dataset consisting of 743 SNPs in 3,646 individuals from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial (Mirabello et al., 2009) and Nurses' Health Study (NHS) (De Vivo et al., 2009). There were 1635 males and 2011 females; their mean age was 60.8 [standard deviation (SD) of 6.5; range 43-74]. The mean relative telomere length (T/S) among the 3,646 subjects was 2.70 (SD 0.47) (Table 1).
Table 1.
Characteristics of study subjects
| PLCO (n = 1635) |
NHS (n = 2011) |
||||
|---|---|---|---|---|---|
| Controls | Cases | Controls | Cases | Overall | |
| n Male | 996 | 639 | 0 | 0 | 1,635 |
| n Female | 0 | 0 | 1,018 | 993 | 2,011 |
| Age groups (n) | |||||
| 43-56 | 255 | 172 | 219 | 214 | 860 |
| 57-61 | 98 | 43 | 378 | 370 | 889 |
| 62-65 | 248 | 153 | 252 | 247 | 900 |
| 66-74 | 395 | 271 | 169 | 162 | 997 |
| Mean Age (SD) | 63.8 (5.0) | 64.1 (4.9) | 58.6 (6.3) | 58.5 (6.4) | 60.8 (6.5) |
| Mean RTL (SD) | 2.58 (0.57) | 2.58 (0.55) | 2.80 (0.36) | 2.79 (0.35) | 2.70 (0.47) |
| Pack-years smoked* (n) | |||||
| 0 | 368 | 280 | 489 | 436 | 1573 |
| 1 - 9 | 123 | 81 | 172 | 171 | 547 |
| 10 - 29 | 220 | 137 | 174 | 195 | 726 |
| ≥30 | 277 | 133 | 166 | 181 | 757 |
|
| |||||
| Total n | 996 | 639 | 1,018 | 993 | 3646 |
n = number of individuals
SD = standard deviation
RTL = relative telomere length
Some individuals were missing smoking status information
PLCO = Prostate, Lung, Colon, Ovarian Cancer Cohort
NHS = Nurses' Health Study
Table 2.
Genes and pathways (i.e., functional group) associated with telomere length
| Pathway | Gene | No. SNPs | No. sig. SNPs† |
Gene P | Pathway P |
|---|---|---|---|---|---|
| Shelterin | ACD | 5 | 0 | 0.307 | 0.52 |
| POT1 | 21 | 0 | 0.222 | ||
| TERF1 | 14 | 0 | 0.523 | ||
| TERF2 | 6 | 0 | 0.126 | ||
| TERF2IP | 4 | 0 | 0.819 | ||
| TINF2 | 5 | 0 | 0.549 | ||
|
| |||||
| DNA repair | ATM | 9 | 0 | 0.189 | 0.384 |
| MRE11A | 12 | 2 | 0.037 | ||
| NBN | 13 | 0 | 0.651 | ||
| RAD50 | 10 | 0 | 0.467 | ||
| RAD51AP1 | 10 | 0 | 0.877 | ||
| RAD51C | 6 | 0 | 0.429 | ||
| RAD51L1 | 142 | 3 | 0.552 | ||
| RAD51L3 | 8 | 1 | 0.299 | ||
| RAD54L | 9 | 1 | 0.17 | ||
| XRCC6 | 3 | 0 | 0.416 | ||
|
| |||||
| Helicase | BLM | 19 | 0 | 0.724 | 0.364 |
| DDX1 | 6 | 0 | 0.92 | ||
| DDX11 | 4 | 0 | 0.751 | ||
| PIF1 | 4 | 0 | 0.407 | ||
| RECQL | 27 | 5 | 0.124 | ||
| RECQL4 | 2 | 0 | 0.356 | ||
| RECQL5 | 4 | 1 | 0.035 | ||
| WRN | 27 | 0 | 0.856 | ||
|
| |||||
| Telomerase associated |
NOLA2 | 3 | 0 | 0.665 | 0.728 |
| NOLA3 | 13 | 1 | 0.367 | ||
| TEP1 | 21 | 1 | 0.506 | ||
| TERC | 1 | 0 | 0.17 | ||
| TERT | 8 | 1 | 0.474 | ||
|
| |||||
| Other | BICD1 | 92 | 3 | 0.321 | 0.058 |
| CLPTM1L | 5 | 2 | 0.074 | ||
| MAD1L1 | 63 | 11 | 0.182 | ||
| MEN1 | 4 | 4 | 0.006 | ||
| MYC | 6 | 0 | 0.319 | ||
| NOLA1 | 3 | 1 | 0.103 | ||
| PARP1 | 14 | 1 | 0.334 | ||
| PARP2 | 13 | 3 | 0.083 | ||
| PINX1 | 32 | 1 | 0.876 | ||
| PRKDC | 13 | 0 | 0.702 | ||
| RTEL1 | 8 | 1 | 0.448 | ||
| SIP1 | 3 | 0 | 0.316 | ||
| TNKS | 33 | 11 | 0.04 | ||
| TNKS2 | 9 | 0 | 0.726 | ||
| TERT | |||||
| Additional * | 29 | 0 | 0.523 | ||
No. = number;
Number of significant SNPs significantly associated with telomere length before correction by gene
All SNPs in an extended region around TERT and CLPTM1L
Association of telomere length with telomere gene variation
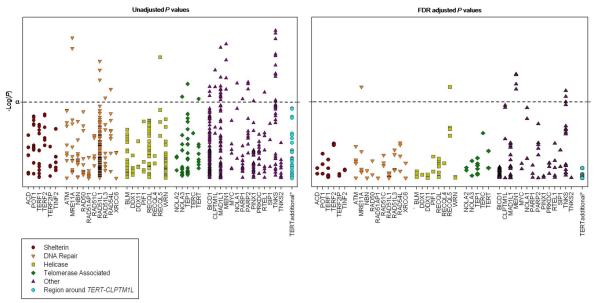
We took three statistical approaches to evaluate genetic variation associated with telomere length, (1) at the individual SNP-level, (2) gene-level, and (3) pathway-level. We used the False Discovery Rate (FDR; Padj) correction to correct for multiple statistical tests at the SNP-level by gene (for all SNPs in a gene). Table 2 shows the most significant SNP in each gene. The SNP approach identified 13 SNPs that were significantly associated with telomere length after correction for multiple tests (Table 3). The direction of the regression coefficient (Beta) represents the effect of each minor allele increasing (+) or decreasing (−) telomere length. All four MEN1 SNPs were inversely associated with telomere length (top SNP, rs670358: Beta −0.046, standard error [SE] 0.017, Padj = 0.014). Two SNPs in MRE11A were positively associated with telomere length (rs12270338 and rs13447720: Beta 0.04, SE 0.012, Padj = 0.025). One SNP in RECQL5 and six SNPs in TNKS were also inversely associated with telomere length (Padj = 0.025-0.049; Table 3). The gene- and pathway-level analyses were global tests used to investigate the association between telomere length and all the SNPs within a gene and all genes within a pathway, respectively. The gene-level analysis identified 4 genes that were significantly associated with telomere length: MRE11A, RECQL5, MEN1, and TNKS (Table 2). MEN1 was the most significant gene (Gene P = 0.006). Genes were grouped by their function in telomere biology for the pathway-level analysis. No single functional pathway showed a significant association with telomere length (Table 2). Within functional pathways, the DNA repair and Other groups appeared to have the most SNPs with significant associations with telomere length, with no strong influence by any one group (Figure 1).
Table 3.
SNPs significantly associated with telomere length after FDR correction
| Gene | SNP ID | Genomic position | Minor Allele |
MAF (%) | Beta† | SE | Ptrend | Padj‡ | |
|---|---|---|---|---|---|---|---|---|---|
| MEN1 | rs669976 | Chr11:64330165 | intronic (IVS8+115) | C | 10.05 | −0.042 | 0.018 | 0.017 | 0.022 |
| MEN1 | rs524386 | Chr11:64341535 | downstream | C | 8.95 | −0.04 | 0.018 | 0.028 | 0.028 |
| MEN1 | rs2957154 | Chr11:64341563 | downstream | G | 25.23 | −0.036 | 0.012 | 0.003 | 0.014 |
| MEN1 | rs670358 | Chr11:64348255 | downstream | A | 10.47 | −0.046 | 0.017 | 0.007 | 0.014 |
| RECQL5 | rs820152 | Chr17:71127683 | upstream | C | 37.74 | −0.029 | 0.010 | 0.006 | 0.025 |
| MRE11A | rs12270338 | Chr11:93787112 | upstream (*5826) | A | 21.61 | 0.036 | 0.012 | 0.004 | 0.025 |
| MRE11A | rs13447720 | Chr11:93804974 | intronic (IVS18−2174) | G | 22.73 | 0.037 | 0.012 | 0.003 | 0.025 |
| TNKS | rs11991621 | Chr8:9443992 | upstream | T | 17.68 | −0.042 | 0.014 | 0.002 | 0.029 |
| TNKS | rs12549064 | Chr8:9479437 | intronic (IVS2+4134) | C | 17.67 | −0.041 | 0.014 | 0.003 | 0.029 |
| TNKS | rs10903314 | Chr8:9504516 | intronic (IVS2−5986) | T | 24.69 | −0.034 | 0.012 | 0.005 | 0.039 |
| TNKS | rs6990300 | Chr8:9585271 | intronic (IVS5+9551) | G | 32.04 | −0.031 | 0.011 | 0.006 | 0.039 |
| TNKS | rs11249943 | Chr8:9645273 | intronic (IVS18-1256) | C | 19.17 | −0.035 | 0.013 | 0.009 | 0.049 |
| TNKS | rs17150478 | Chr8:9678444 | downstream | G | 18.77 | −0.042 | 0.013 | 0.002 | 0.029 |
All analysis were done using linear regression models with telomere length continuous, adjusted for gender, age, smoking status, principal components, and case/control status
MAF = minor allele frequency
SE = standard error
False Discovery Rate (FDR) corrected P-value
Figure 1.
Plot of Ptrend values for each SNP by gene before and after correction for multiple tests. Inset shows the pathway (i.e., functional group) or region designations. Gene names are shown on the x-axis. α = 0.05; dashed line represents an extension of α; * SNPs in the region around TERT and CLPTM1L.
Association of telomere length with SNPs previously associated with cancer
Since recent GWAS have suggested associations between SNPs at the RTEL1 and TERT-CLPTM1L locus and cancer (Jin et al., 2009; Landi et al., 2009; McKay et al., 2008; Petersen et al., 2010; Rafnar et al., 2009; Shete et al., 2009; Wrensch et al., 2009), we evaluated the five SNPs in these regions that were included in our dataset (Table 4). The genotype frequencies of these five SNPS did not differ between the cases and controls in our study population (P > 0.4). None of these SNPs were significantly associated with telomere length in our subjects. We also examined an extended region around the TERT-CLPTM1L locus on 5p13.33 to further investigate this region for any associations with telomere length. No SNPs in this region were significantly associated with telomere length after correction for multiple tests. Eight SNPs in TERT, CLPTM1L or the surrounding region were significantly associated without correction for multiple tests using co-dominant, dominant and/or recessive inheritance models (data not shown). The strongest association was observed for rs31489 in CLPTM1L with a recessive inheritance model (Beta 0.05, SE 0.02, P = 0.005).
Table 4.
SNPs identified in recent association studies and their association with telomere length by inheritance model*
| Co-dominant | Additive** | Dominant | Recessive | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SNP | Genotype | n | % | Beta† | SE | P | P | P | P |
| RTEL1 SNPs | |||||||||
| rs6010620 | GG | 2,129 | 59.2 | 0 | |||||
| GA | 1,268 | 35.3 | −0.001 | 0.016 | 0.927 | ||||
| AA | 197 | 5.5 | 0.014 | 0.033 | 0.676 | 0.69 [G], 0.69 [A] | 0.97 | 0.66 | |
| rs4809324 | TT | 2,865 | 79.7 | 0 | |||||
| TC | 684 | 19.1 | −0.016 | 0.019 | 0.379 | ||||
| CC | 44 | 1.2 | −0.041 | 0.067 | 0.534 | 0.33 [C] | 0.33 | 0.57 | |
| TERT/CLPTM1L SNPs | |||||||||
| rs402710 | CC | 889 | 45.6 | 0 | |||||
| CT | 841 | 43.1 | 0.017 | 0.017 | 0.332 | ||||
| TT | 220 | 11.3 | 0.031 | 0.027 | 0.237 | 0.24 [C] | 0.19 | 0.34 | |
| rs401681 | CC | 1,179 | 32.8 | 0.003 | 0.017 | 0.837 | |||
| CT | 1,698 | 47.2 | 0 | ||||||
| TT | 717 | 19.9 | 0.037 | 0.019 | 0.06 | 0.17 [C] | 0.63 | 0.053 | |
| rs2736100 | GG | 934 | 26.0 | 0.017 | 0.018 | 0.35 | |||
| GT | 1,776 | 49.5 | 0 | ||||||
| TT | 881 | 24.5 | −0.008 | 0.018 | 0.646 | 0.26 [G], 0.26 [T] | 0.25 | 0.41 | |
All analysis were done using linear regression models with telomere length continuous using the most common genotype as the referent, adjusted for gender, age, smoking status, principal components, and case/control status
n = number of individuals
% = percent of individuals
SE = standard error
0 represents the referent genotype
Jin et al., 2009; Landi et al., 2009; McKay et al., 2008; Petersen et al., 2010; Rafnar et al., 2009; Shete et al., 2009; Wrensch et al., 2009
Additive model for the effect of each extra risk allele on telomere length, risk allele is shown in brackets
Discussion
There are many genes involved in telomere length maintenance and stability. We have examined the association between telomere length and genetic variation in 43 telomere biology genes among 3,646 individuals. This large dataset allowed us to investigate genetic variation in candidate genes associated with even small changes in telomere length. No specific functional pathway had a strong effect on telomere length. We noted several significant individual SNP associations after correction for multiple tests; however, we did not find compelling evidence that common variants in these pathways were associated with large differences in telomere length. Four genes (MRE11A, RECQL5, MEN1, and TNKS), and 13 SNPs within these genes had statistically significant effects on telomere length after correction at the gene level, although the effects were all relatively small.
We observed that variation in MRE11A (meiotic recombination 11) was positively associated with telomere length, and the two SNPs identified were in strong LD (r2 = 0.92). MRE11A is part of the telomere MRN (MRE11-RAD50-NBN) complex that senses DNA damage and is involved in modulating t-loop formation (Zhu et al., 2000). This complex positively regulates telomerase-dependent telomere elongation through interactions with ATM and TERF1 (Wu et al., 2007).
One SNP in RECQL5 (RECQ protein-like 5) was found to be inversely associated with telomere length. The RECQ protein family of helicases have critical roles in protecting and stabilizing the genome (Bohr, 2008). Deficiencies in these proteins can lead to genomic instability, premature aging and an increased susceptibility to cancer (Bohr, 2008). Detailed molecular functions of RECQL5 are not clear. RECQL5 has a role in preventing inappropriate homologous recombination (Hu et al., 2007), and RECQL5 deficient mice have an increased susceptibility to cancer.
MEN1 (multiple endocrine neoplasia I) was the most significantly implicated gene, with all SNPs included in this region showing similar inverse associations with telomere length. Surprisingly, there is very little LD across this gene region and these SNPs do not appear to be linked. MEN1 is a strong tumor suppressor gene that encodes menin. Menin associates with the promoter region of TERT, and is thought to negatively regulate TERT (Lin and Elledge, 2003). Through TERT, MEN1 may affect telomere length maintenance, since TERT expression has been shown to stimulate telomerase activity (Lin and Elledge, 2003; Rufer et al., 2001).
Six SNPs in TNKS (tankyrase 1) were similarly negatively associated with telomere length. TNKS is a poly (ADP-ribose) polymerase thought to be a positive regulator of telomere length through its modification of TERF1 (PARsylation), which releases TERF1 repression and allows telomerase to bind and elongate telomeric DNA (Cook et al., 2002; Hsiao and Smith, 2008; Smith and de Lange, 2000). In a small study of genetic variation in nine telomere-associated genes (ACD, POT1, TEP1, TERF1, TERF2, TERF2IP, TERT, TNKS, TNKS2) among 100 breast cancer cases, a SNP in TNKS that significantly associated with telomere length was identified (Varadi et al., 2009). Although this TNKS SNP was not included in our analysis, HapMap (The International HapMap Consortium, 2003) data suggests that this SNP (rs6990097) (Varadi et al., 2009) and one of our significant TNKS SNPs (rs11991621) may be in LD (D′ = 0.92 and r2 = 0.40). In addition, a recent GWAS identified two SNPs in TNKS associated with leukocyte telomere length with P-values of 10−2 to 10−4 (Mangino et al., 2009). One of these SNPs, rs11249943, was also statistically significant in our dataset. Our study is therefore the third to have identified significant associations between genetic variation in TNKS and telomere length.
We included an extended region around TERT-CLPTM1L since recent GWAS (Jin et al., 2009; Landi et al., 2009; McKay et al., 2008; Petersen et al., 2010; Rafnar et al., 2009; Shete et al., 2009) suggested that this region may contain important markers of overall cancer risk. We could not confirm a significant effect of these cancer-associated SNPs (Jin et al., 2009; Landi et al., 2009; McKay et al., 2008; Rafnar et al., 2009; Shete et al., 2009; Wrensch et al., 2009) on telomere length in our study population of 3646 subjects. Rafnar et al. (Rafnar et al., 2009) examined the association between rs401681 and rs2736098 and telomere length in DNA from whole blood, and found that only older subjects (n = 276; born 1925-1935) with the risk allele (rs401681 C allele, and rs2736098 A allele) had significantly shorter telomeres (P = 0.017 and 0.027, respectively) compared with younger women (n = 260; born 1940-1950). They suggested that these variants may gradually shorten telomeres over time with an effect becoming apparent at an older age (Rafnar et al., 2009). The mean age of individuals in our study was 60.8 (age range 43-73), so we did not stratify on age further. The functional significance of these SNP associations with cancer risk requires further investigation.
Variants in intron 1 of BICD1 (most notably rs2630578) were associated with telomere length (Mangino et al., 2008). Our dataset did not include rs2630578 but did include rs1798255, rs2668301, rs10771917 which are in LD with rs2630578. These SNPs in BICD1 were not significantly associated with telomere length after correction for multiple tests in our dataset. Another linkage study found significant linkage of telomere length to a region on chromosome 12 including the DNA helicase DDX11; however, after further analysis of the SNPs in this region, they found no significant effects of DDX11 genotypes on telomere length (Vasa-Nicotera et al., 2004); we observed no significant associations between SNPs in DDX11 and telomere length. Our dataset only included one SNP (rs10936599) in TERC, therefore, we could not determine if the SNP (rs12696304) identified by Codd et al. (2010) in TERC was specifically associated with telomere length. However, these two SNPs appear to be in high LD (D′ = 1.0 and r2 = 0.91), and we could not confirm a significant association in our dataset (P = 0.17). Another recent GWAS identified SNPs in the region of the OBFC1 gene, suggested to be involved with the replication and capping of telomeres, associated with leukocyte telomere length, and they also confirmed an association between TERC and telomere length (Levy et al., 2010). The inconsistencies between many of these studies could be due to differences in genetic background of the study populations, limitations in statistical power, differing telomere lengths in the different cell types analyzed, and/or differences in data interpretation methods. In addition, common SNPs may not have large effects on telomere length. It is also important to point out that our findings are for blood leukocyte DNA and may not necessarily apply to other tissue DNA.
A limitation of our study was that the SNPs available for analysis were limited to those in the specific genotyping platform used for the previous GWAS studies from which our individual data were drawn. This resulted in limited representation of SNPs in some regions; e.g., we only had one SNP in TERC. Strengths of our study include our comprehensive analysis of SNPs in the vast majority of known telomere-associated genes using 3 statistical approaches (at the individual SNP-level, gene-level and pathway-level), the analysis of telomere length in peripheral blood samples by Q-PCR from two studies done by the same laboratory, and the large sample size (3646 individuals).
The relatively limited effect of common germline genetic variants on telomere length could be due to the fact that the majority of these genes are highly evolutionarily conserved (de Lange, 2004; Kanoh and Ishikawa, 2003; Li et al., 2000; Mirabello et al., 2008; Nakamura and Cech, 1998; Savage et al., 2005). Our recent population genetic study of the majority of telomere maintenance genes using a similar approach of extracting genotype data from a GWA platform identified little variation in these genes as a group (Mirabello et al., 2008). MEN1 was not included in that gene-set, but the SNPs in TNKS, RECQL5, and MRE11A all had low population differentiation [FST ≤0.06; genome-wide average for autosomal SNPs is 0.10 ~ 0.15 (Akey et al., 2002; Shriver et al., 2004; Shriver et al., 2005; Weir et al., 2005)] and heterozygosity (0.22-0.37) (Mirabello et al., 2008). There was also evidence of evolutionary selection in TNKS (Mirabello et al., 2008; Savage et al., 2005; The International HapMap Consortium, 2007). A large amount of variation in telomere genes may not be tolerated due to their critical roles in telomere maintenance and chromosomal stability. Rare variants (i.e., mutations) may only contribute to rare telomere biology disorders, such as dyskeratosis congenita. Telomere length and the rate of telomere length change are highly variable among individuals, and have been shown to be effected by many factors including race, BMI, smoking, inflammation, and oxidative stress (Aviv et al., 2009; Mirabello et al., 2009; Nordfjäll et al., 2009). It may be that telomere length is controlled by small contributions from many environmental factors and/or polymorphisms.
In summary, this study of common genetic variation in candidate genes and telomere length identified SNPs in four genes that are associated with telomere length after correction for multiple tests by gene. However, the effects on telomere length were found to be small, and we found no association using global tests by functional pathway. Common SNPs, like those used in GWAS, may not have strong effects on telomere length. The combination of limited nucleotide diversity, high ancestral allele frequencies, and evolutionary conservation suggest that these genes may be under selective pressure. Additional studies of rare variants will be helpful in understanding the role of genetic variation in telomere length regulation.
Supplementary Material
Acknowledgments
We are grateful to the NHS and PLCO participants for their valuable contributions. The work of Drs. Mirabello, Yu, Chatterjee, Hayes and Savage was supported [in part] by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute.
Footnotes
Supporting Information for this preprint is available from the Human Mutation editorial office upon request (humu@wiley.com)
References
- Akey J, Zhang G, Zhang K, Jin L, Shriver M. Interrogating a high-density SNP map for signatures of natural selection. Genome Res. 2002;12(12):1805–14. doi: 10.1101/gr.631202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew T, Aviv A, Falchi M, Surdulescu G, Gardner J, Lu X, Kimura M, Kato B, Valdes A, Spector T. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am J Hum Genet. 2006;78:480–6. doi: 10.1086/500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet. 2009;10:45–61. doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M, Chen J, Cogan J, Alder J, Ingersoll R, Markin C, Lawson W, Xie M, Vulto I, Phillips JI. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. others. [DOI] [PubMed] [Google Scholar]
- Aubert G, Lansdorp P. Telomeres and aging. Physiol Rev. 2008;88(2):557–79. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- Aviv A, Chen W, Gardner J, Kimura M, Brimacombe M, Cao X, Srinivasan S, Berenson G. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169(3):323–9. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Fry B, Maller J, Daly M. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann. Statist. 2001;29:1165–1188. [Google Scholar]
- Blasco MALH, Hande MP, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- Bohr V. Rising from the RecQ-age: the role of human RecQ helicases in genome maintenance. Trends Biochem Sci. 2008;33:609–620. doi: 10.1016/j.tibs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado R, Regal J, Hills M, Yewdell W, Dalmazzo L, Zago M, Lansdorp P, Hogge D, Chanock S, Estey E. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc. Natl. Acad. Sci U.S.A. 2009;106:1187–1192. doi: 10.1073/pnas.0807057106. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon R. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd V, Mangino M, Harst P, Braund P, Kaiser M, Beveridge A, Rafert S, et al. Common variants near TERC are associated with mean telomere length. 2010;42:197–199. doi: 10.1038/ng.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins K, Mitchell J. Telomerase in the human organism. Oncogene. 2002;21:564–579. doi: 10.1038/sj.onc.1205083. [DOI] [PubMed] [Google Scholar]
- Cook BD, Dynek JN, Chang W, Shostak G, Smith S. Role for the related poly(ADP-Ribose) polymerases tankyrase 1 and 2 at human telomeres. Mol. Cell. Biol. 2002;22:332–342. doi: 10.1128/MCB.22.1.332-342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T. T-loops and the origin of telomeres. Nat Rev Mol Cell Biol. 2004;5:323–329. doi: 10.1038/nrm1359. [DOI] [PubMed] [Google Scholar]
- De Vivo I, Prescott J, Wong J, Kraft P, Hankinson S, Hunter D. A prospective study of relative telomere length and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1152–1156. doi: 10.1158/1055-9965.EPI-08-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C, Wright W, Shay J. Human diseases of telomerase dysfunction: insights into tissue aging. Nucleic Acids Res. 2007;35:7406–7416. doi: 10.1093/nar/gkm644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohagan J, Prorok P, Hayes R, Kramer B. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials. 2000;21:251–72S. doi: 10.1016/s0197-2456(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Griffith J, Comeau L, Rosenfield S, Stansel R, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- Hackett J, Greider C. Balancing instability: dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene. 2002;21(4):619–626. doi: 10.1038/sj.onc.1205061. [DOI] [PubMed] [Google Scholar]
- Hsiao SJ, Smith S. Tankyrase function at telomeres, spindle poles, and beyond. Biochimie. 2008;90:83–92. doi: 10.1016/j.biochi.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Hu Y, Raynard S, Sehorn M, Lu X, Bussen W, Zheng L, Stark J, Barnes E, Chi P, Janscak P. RECQL5/Recql5 helicase regulates homologous recombination and suppresses tumor formation via disruption of Rad51 presynaptic filaments. Genes Dev. 2007;21:3073–3084. doi: 10.1101/gad.1609107. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D, Kraft P, Jacobs K, Cox D, Yeager M, Hankinson S, Wacholder S, Wang Z, Welch R, Hutchinson A. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007;39:870–874. doi: 10.1038/ng2075. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanclos E, Schork N, Kyvik K, Kimura M, Skurnick J, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36:195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- Jin G, Xu L, Shu Y, Tian T, Liang J, Xu Y, Wang F, Chen J, Dai J, Hu Z. Common genetic variants on 5p15.33 contribute to risk of lung adenocarcinoma in a Chinese population. Carcinogenesis. 2009;30(6):987–90. doi: 10.1093/carcin/bgp090. others. [DOI] [PubMed] [Google Scholar]
- Kanoh J, Ishikawa F. Composition and conservation of the telomeric complex. Cell Mol Life Sci. 2003;60:2295–2302. doi: 10.1007/s00018-003-3245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi M, Chatterjee N, Yu K, Goldin L, Goldstein A, Rotunno M, Mirabello L, et al. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. American Journal of Human Genetics. 2009;85(5):679–691. doi: 10.1016/j.ajhg.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Neuhausen S, Hunt S, Kimura M, Hwang S, Chen W, Bis J, Fitzpatrick A, Smith E, Johnson A. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci U S A. 2010;107(20):9293–8. doi: 10.1073/pnas.0911494107. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Oestreich S, de Lange T. Identification of human Rap1: implications for telomere evolution. Cell. 2000;101:471–483. doi: 10.1016/s0092-8674(00)80858-2. [DOI] [PubMed] [Google Scholar]
- Lin S, Elledge S. Multiple tumor suppressor pathways negatively regulate telomerase. Cell. 2003;113:881–889. doi: 10.1016/s0092-8674(03)00430-6. [DOI] [PubMed] [Google Scholar]
- Mangino M, Brouilette S, Braund P, Tirmizi N, Vasa-Nicotera M, Thompson J, Samani N. A regulatory SNP of the BICD1 gene contributes to telomere length variation in humans. Hum Mol Genet. 2008;17:2518–2523. doi: 10.1093/hmg/ddn152. [DOI] [PubMed] [Google Scholar]
- Mangino M, Richards J, Soranzo N, Zhai G, Aviv A, Valdes A, Samani N, Deloukas P, Spector T. A genome-wide association study identifies a novel locus on chromosome 18q12.2 influencing white cell telomere length. J Med Genet. 2009;46:451–454. doi: 10.1136/jmg.2008.064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JD, Hung RJ, Gaborieau V, Boffetta P, Chabrier A, Byrnes G, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J. Lung cancer susceptibility locus at 5p15.33. Nat Genet. 2008;40(12):1404–6. doi: 10.1038/ng.254. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabello L, Huang W, Wong J, Chatterjee N, Reding D, Crawford E, De Vivo I, Hayes R, Savage S. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell. 2009;8(4):405–413. doi: 10.1111/j.1474-9726.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabello L, Xiao N, Deng X, Qi L, Wang Z, Savage SA. Worldwide genetic structure in 37 genes important for telomere maintenance using HGDP and HapMap data. The American Society of Human Genetics; Philadelphia, Pennsylvania: 2008. Abstract 2565. [Google Scholar]
- Moon I, Jarstfer M. The human telomere and its relationship to human disease, therapy, and tissue engineering. Frontiers in Bioscience. 2007;12:4595–4620. doi: 10.2741/2412. [DOI] [PubMed] [Google Scholar]
- Morlá M, Busquets X, Pons J, Sauleda J, MacNee W, Agustí A. Telomere shortening in smokers with and without COPD. Eur Respir J. 2006;27(3):525–8. doi: 10.1183/09031936.06.00087005. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Cech T. Reversing time: Origin of telomerase. Cell. 1998;92:587–590. doi: 10.1016/s0092-8674(00)81123-x. [DOI] [PubMed] [Google Scholar]
- Njajou O, Cawthon R, Damcott C, Wu S, Ott S, Garant M, Blackburn E, Mitchell B, Shuldiner A, Hsueh W. Telomere length is paternally inherited and is associated with parental lifespan. Proc Natl Acad Sci U S A. 2007;104:12135–9. doi: 10.1073/pnas.0702703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordfjáll K, Svenson U, Norrback K, Adolfsson R, Lenner P, Roos G. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. 2009;5(2):e1000375. doi: 10.1371/journal.pgen.1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen G, Amundadottir L, Fuchs C, Kraft P, Stolzenberg-Solomon R, Jacobs K, Arslan A, Bueno-de-Mesquita H, Gallinger S, Gross M. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010 doi: 10.1038/ng.522. others. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prorok P, Andriole G, Bresalier R, Buys S, Chia D, Crawford E, Fogel R, Gelmann E, Gilbert F, Hasson M. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21:273S–309S. doi: 10.1016/s0197-2456(00)00098-2. others. [DOI] [PubMed] [Google Scholar]
- Rafnar T, Sulem P, Stacey SN, Geller F, Gudmundsson J, Sigurdsson A, Jakobsdottir M, Helgadottir H, Thorlacius S, Aben KK. Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet. 2009;41(2):221–7. doi: 10.1038/ng.296. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rog O, Smolikov S, Krauskopf A, Kupiec M. The yeast VPS genes affect telomere length regulation. Curr Genet. 2005;47:18–28. doi: 10.1007/s00294-004-0548-y. [DOI] [PubMed] [Google Scholar]
- Rufer N, Migliaccio M, Antonchuk J, Humphries R, Roosnek E, Lansdorp P. Transfer of the human telomerase reverse transcriptase (TERT) gene into T lymphocytes results in extension of replicative potential. Blood. 2001;98:597–603. doi: 10.1182/blood.v98.3.597. [DOI] [PubMed] [Google Scholar]
- Savage S, Alter B. Dyskeratosis congenita. Hematol Oncol Clin North Am. 2009;23(2):215–31. doi: 10.1016/j.hoc.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage S, Stewart B, Eckert A, Kiley M, Liao J, Chanock S. Genetic variation, nucleotide diversity, and linkage disequilibrium in seven telomere stability genes suggest that these genes may be under constraint. Hum Mutat. 2005;26(4):343–50. doi: 10.1002/humu.20226. [DOI] [PubMed] [Google Scholar]
- Shete S, Hosking FJ, Robertson LB, Dobbins SE, Sanson M, Malmer B, Simon M, Marie Y, Boisselier B, Delattre JY. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009;41(8):899–904. doi: 10.1038/ng.407. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriver M, Kennedy G, Parra E, Lawson H, Sonpar V, Huang J, Akey J, Jones K. The genomic distribution of population substructure in four populations using 8,525 autosomal SNPs. Hum Genomics. 2004;1(4):274–86. doi: 10.1186/1479-7364-1-4-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriver M, Mei R, Parra E, Sonpar V, Halder I, Tishkoff S, Schurr T, Zhadanov S, Osipova L, Brutsaert T. Large-scale SNP analysis reveals clustered and continuous patterns of human genetic variation. Hum Genomics. 2005;2(2):81–89. doi: 10.1186/1479-7364-2-2-81. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagboom P, Droog S, Boomsma D. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- Smith S, de Lange T. Tankyrase promotes telomere elongation in human cells. Curr. Biol. 2000;10:1299–1302. doi: 10.1016/s0960-9822(00)00752-1. [DOI] [PubMed] [Google Scholar]
- The International HapMap Consortium The International HapMap Project. Nature. 2003;426(6968):789–96. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- The International HapMap Consortium A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–862. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi V, Brendle A, Brandt A, Johansson R, Enquist K, Henriksson R, Svenson U, Tavelin B, Roos G, Hemminki K. Polymorphisms in telomere-associated genes, breast cancer susceptibility and prognosis. Eur J Cancer Prev. 2009;45:3008–3016. doi: 10.1016/j.ejca.2009.08.012. others. [DOI] [PubMed] [Google Scholar]
- Vasa-Nicotera M, Brouilette S, Mangino M, Thompson J, Braund P, Clemitson J, Mason A, Bodycote C, Raleigh S, Louis E. Mapping of a major locus that determines telomere length in humans. Am J Hum Genet. 2004;76:147–151. doi: 10.1086/426734. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–44. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Weir B, Cardon L, Anderson A, Nielsen D, Hill W. Measures of human population structure show heterogeneity among genomic regions. Genome Res. 2005;15(11):1468–76. doi: 10.1101/gr.4398405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J, Crout R, McNeil D, Weyant R, Marazita M, Wenger S. Human telomere length correlates to the size of the associated chromosome arm. 2009;4:e6013. doi: 10.1371/journal.pone.0006013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrensch M, Jenkins R, Chang J, Yeh R, Xiao Y, Decker P, Ballman K, Berger M, Buckner J, Chang S. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. 2009;41:905–908. doi: 10.1038/ng.408. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Xiao S, Zhu X-D. MRE11-RAD50-NBS1 and ATM function as co-mediators of TRF1 in telomere length control. Nat Struct Mol Biol. 2007;14:832–840. doi: 10.1038/nsmb1286. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Calado R, Ly H, Kajigaya S, Baerlocher G, Chanock S, Lansdorp P, Young N. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N. Engl. J. Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- Yeager M, Orr N, Hayes R, Jacobs K, Kraft P, Wacholder S, Minichiello M, Fearnhead P, Yu K, Chatterjee N. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet. 2007;39:645–649. doi: 10.1038/ng2022. others. [DOI] [PubMed] [Google Scholar]
- Yu K, Li Q, Bergen A, Pfeiffer R, Rosenberg P, Caporaso N, Kraft P, Chatterjee N. Pathway analysis by adaptive combination of P-values. Genet Epidemiol. 2009;33:700–709. doi: 10.1002/gepi.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Kuster B, Mann M, Petrini J, de Lange T. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat. Genet. 2000;25:347–352. doi: 10.1038/77139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.