Abstract
The fungal pathogen Histoplasma capsulatum evades the innate and adaptive immune responses and thrives within resting macrophages (Mφ). Cytokines that induce antimicrobial activity such as granulocyte macrophage-colony stimulating factor (GM-CSF) inhibit H. capsulatum growth in Mφ. Conversely, interleukin 4 (IL-4) inhibits the killing of intracellular pathogens. Using inductively coupled plasma mass spectrometry, we examined alterations in metal homeostasis of murine H. capsulatum-infected Mφ infected that were exposed to activating cytokines. Restriction of iron (Fe2+/3+) and zinc (Zn2+) was observed in infected, GM-CSF-treated Mφ compared to infected controls. IL-4 reversed the anti-fungal activity of GM-CSF-activated Mφ and was associated with increased intracellular Zn2+. Chelation of Zn2+ inhibited yeast replication both in the absence and presence of Mφ. Treatment of cells with GM-CSF altered the host Zn2+ binding species profile. These results establish that Zn2+ deprivation may be a host defense mechanism utilized by Mφ.
Keywords: Histoplasma capsulatum, macrophages, mass spectrometry, zinc, cytokines
Introduction
H. capsulatum infection is initiated by inhalation of fungal spores followed by conversion to the pathogenic yeast phase. Infection is controlled in most immunocompetent individuals but establishes a persistent state. Histoplasmosis can be life-threatening for individuals suffering from immune defects arising from HIV infection or receiving drugs for malignancy, graft rejection, or au-toimmune diseases. More recently, tumor necrosis factor (TNF)-α antagonists have been linked to higher incidence of histoplasmosis [1].
Alveolar macrophages (Mφ) are the presumed first line of cellular defense H. capsulatum encounters in the host. Mφ are the only professional phagocytic cell population in which H. capsula-tum replicates freely [2], although growth is inhibited following cytokine activation of Mφ. Interfe-ron gamma (IFN)-γ and granulocyte macrophage-colony stimulating factor (GM-CSF) restrict growth in mouse and human Mφ respectively [3, 4]. One mechanism by which IFN-γ inhibits H. capsulatum intracellular growth is by restricting iron (Fe2+/3+ ) [3]. Much of this data has been accrued by adding exogenous Fe to cells or restricting access to Fe2+/3+. A missing analysis has been a direct measurement of Fe or any metal present within phagocytes or pathogens harbored by these cells.
Since metals are critical to the functional integrity of cells, we sought to determine if there was a correlation between metal uptake of H. capsulatum infected Mφ activated by cytokines. We observed that Zn2+ and Fe2+/3+ levels were restricted inside GM-CSF-treated Mφ infected with H. capsulatum compared to resting Mφ. Zn2+ binding species were differentially regulated within resting Mφ versus GM-CSF-treated cells. Chelating Zn2+ reduced H. capsulatum growth in medium and within resting Mφ. Moreover, pretreating GM-CSF-activated Mφ with IL-4 reversed growth inhibition, and partially replenished Zn2+ levels. These data support an important role for Zn2+ restriction as a host defense strategy to H. capsulatum infection.
Methods
Mice
Six to eight week old C57BL/6 mice were purchased from Jackson Laboratories, Bar Harbor, Maine. Animals were housed in isolator cages and maintained by the Department of Laboratory Animal Medicine, University of Cincinnati, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All animal experiments were performed in accordance with the Animal Welfare Act guidelines of the National Institutes of Health. All protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Reagents
Sodium dodecyl sulfate (SDS), high performance liquid chromatography (HPLC)-grade water, methanol, diethylenetriaminepenta-acetic acid (DTPA), ethylene glycol tetraacetic acid (EGTA), N,N,N′,N′-Tetrakis-(2-pyridylmethyl) ethylenediamine (TPEN), ZnSO4, FeSO4, and F-12 Ham’s were purchased from Sigma Aldrich (St. Louis, MO). Recombinant mouse GM-CSF, IFN-γ, and IL-4 were acquired from PeproTech (Rocky Hill, NJ). Arginase, YM1, and FIZZ1 primers for RT-PCR analysis were purchased from Applied Biosystems (Foster City, CA) and TRIzol was purchased from Invitrogen (Carlsbad, CA). Diff-Quik stain kit was obtained from IMEB Inc. (San Marcos, CA).
All solutions for ICPMS analysis were prepared in 18 MΩ cm−1 double deionized water (Sy-bron Barnstead, Boston, MA), in which no metal was detected. The mobile phase for size-exclusion chromatography (SEC) was made by dissolving tris(hydroxymethyl)aminomethane (Tris) in double deionized water and adjusting the pH with hydrochloric acid. The SEC standard (Bio-Rad Laboratories, Inc., Hercules, CA) is a lyophilized mixture of molecular weight markers ranging from 1,300 to 670,000 Da.
H. capsulatum intracellular growth assays
Murine alveolar (A) Mφ, bone marrow derived (BM) Mφ, and peritoneal (P) Mφ were used. AMφ were prepared by consecutive lung lavages, rinsing 5x with 1 ml of phosphate buffered saline (PBS). PMφ were acquired by lavaging peritoneal cavities with 10 ml of PBS. BMMφ were generated by extracting bone marrow cells from the femurs of mice, and cells were incubated in RPMI-1640 growth media at 37°C in the presence of GM-CSF (10 ng/ml) and 5% CO2 for 5–6 days. In some experiments, cells were incubated with 10 ng/ml of IL-4 for another 24 hr following 5 day incubation with GM-CSF. PMφ were incubated with 10 ng/ml of either GM-CSF or IFN-γ for 24 hr. All Mφ were plated at 1×105 cells/well in a 96-well plate.
H. capsulatum strain G217B yeasts were grown as previously described [5]. Mφ were infected with H. capsulatum at a multiplicity of infection of 5X yeasts/Mφ; growth inside Mφ was quantified by plating. Infected Mφ were lysed in sterile water and lysates plated onto Mycosel agar 5 (Becton Dickinson) plates containing 5% sheep blood and 5% glucose. Plates were incubated at 30°C for 1 wk.
The leucine assay was used for metal chelation and supplementation experiments [4]. For metal supplementation experiments ZnSO4 or FeSO4 were added (100 μM) to media (RPMI-1640), and for metal chelation experiments TPEN or DTPA was added prior to infection. Toxicity of TPEN, DTPA, and ZnSO4 was measured using trypan blue stain.
Sample preparation for ICPMS analysis of infected Mφ and intracellular yeasts
Mφ were plated at 1×106 per well in a 12-well plate and infected with H. capsulatum at a multiplicity of infection of 5X. After 24 hr, cells were washed with HBSS. Infected Mφ were treated (~1 min) with 250 μl of 0.1% SDS in water per well. Metal concentrations in 0.1% SDS and wash buffer (HBSS) were less than 10 ppb (μg L−1) for all metals except sodium,.
For metal analysis of intracellular H. capsulatum, yeasts were isolated from infected Mφ following 0.1% SDS and centrifugation. Each group of intracellular yeasts isolated were set at 1×108/10 μl using a hemacytometer.
Yeasts and infected Mφ were further diluted 1:1.25 to set at a concentration of 20% HNO3. Before ICPMS analysis of infected Mφ and isolated organisms, each sample was subjected to microwave digestion using a closed-vessel CEM Discover-Explorer microwave digestion system (CEM Corporation, Matthews, NC, USA). The digestion process was performed at 150 °C for 2 min by maintaining a pressure below 250 psi.
For SEC and ICPMS analysis samples were not subjected to microwave digestion. Infected Mφ were immediately centrifuged following 0.1% SDS treatment, and H. capsulatum removed. The supernatant was stored at −70 °C before ICPMS analysis.
ICPMS analysis
ICPMS-based quantification has been commonly used to determine accurate concentration of multiple metals simultaneously in biological samples [6, 7]. ICPMS metal analysis was performed on an Agilent 7500ce ICPMS (Agilent Technologies, Santa Clara, CA,). A conventional Meinhard nebulizer, a Peltier-cooled spray chamber (2°C) and a shield torch constitute the sample introduction system under standard plasma conditions.
HPLC was performed using flow injection, and SEC were carried out with an Agilent 1100 liquid chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a binary HPLC pump, an autosampler, a vacuum degasser system, a temperature column compartment and a diode array detector. The outlet of the HPLC/UV detector was connected to a sample inlet of the ICPMS nebulizer using 0.25 mm i.d. polyether ether ketone tubing of 30 cm in length. Both UV (wavelength = 280 nm). ICPMS signals were collected online.
A Superdex 200 10/300 GL column (10 mm × 300 mm, 13 μm) (Amersham Pharmacia Bio-tech, Uppsala, Sweden) was used for SEC analysis. The column was calibrated with a UV detector (wavelength = 280 nm) using a gel filtration standard mixture (thyroglobulin MW=670 kDa, γglobulin MW=158 kDa, ovalbumin MW=44 kDa, myoglobin MW=17 kDa, vitamin B12 MW=1.3kDa), purchased from Bio-Rad Laboratories, Inc. Lysed Mφ (0.1% SDS treated) described above were injected onto the column. ICPMS flow injection analysis and SEC conditions are shown in Table 1.
Table 1.
Operating conditions for HPLC-ICPMS
| ICPMS parameters | |
| Forward power (W) | 1500 |
| Plasma gas flow rate (L min−1) | 15.0 |
| Carrier gas flow rate (L min−1) | 1.01 |
| Isotopes monitored for SEC | 66Zn |
| Isotopes monitored for flow injection | 23Na, 24Mg, 39K, 44Ca, 55Mn, 57Fe, 60Ni, 63Cu, 66Zn |
| Collision gas (mL H2 min−1) | 3.2 |
| Quadrupole bias (V) | −16 |
| Octopole bias (V) | −18 |
| SEC chromatographic parameters | |
| Column | Superdex 200 10/300 GL |
| Mobile phase | 30 mM Tris-HCl buffer, pH 7.5 |
| Flow rate (mL min−1) | 0.7 |
| Injection volume (μL) | 100 |
| Flow injection parameters | |
| Column | N/A |
| Mobile phase | 0.1 % nitric acid |
| Flow rate (mL min−1) | 0.8 |
| Injection volume (μL) | 50 |
Intracellular H. capsulatum counting
The number of organisms within each cytokine treated Mφ population following 5X yeasts/Mφ ratio was counted by staining cells with Diff-Quik and counting at least 100 infected Mφ.
Flow cytometry
Two hundred thousand Mφ were treated with IL-4 for 24hr and then exposed to H. capsulatum. Cells were then stained with Peridinin Chlorophyll Protein Complex (PerCP) - conjugated CD11b and allophycocyanin (APC)-CD71 (BD Biosciences, San Jose, CA). Cells were then stained with APC-conjugated CD71 antibodies. Flow cytometry analysis was performed on Mφ infected with H. capsulatum (5X yeasts/Mφ ratio) expressing green fluorescent protein [8] and PerCP-CD11b to determine the percentage of infected cells. Staining for both groups was done at 4°C for 15 min in HBSS containing 1% BSA and 0.01% sodium azide. Cells were washed and resuspended in 1% paraformaldehyde to fix. Isotype controls were performed in parallel. Fluorescence intensity was assessed using a FACS Caliber (BD Biosciences) and analyzed using FCS Express Software.
Real-time reverse transcriptase polymerase chain reaction (qRT-PCR)
A total of 5 × 105 Mφ were infected with 5X yeasts/Mφ ratio. Following 24hr total RNA from Mφ was isolated using TRIzol. Oligo(dT)-primed cDNA was prepared using the reverse transcriptase system (Pro-mega, Madison, WI). qRT-PCR analysis for was performed using Taq-Man Master Mix and primers; HPRT, Arginase, FIZZ1 (Retna), YM1 (Chi3-L3), and calprotectin (S100). Samples were analyzed on an ABI Prism 7500 (Applied Biosystems). In each experiment, HPRT was used as an internal control. The conditions for amplification were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min.
Nitric oxide detection
Nitric oxide levels in infected Mφ (5X yeasts/Mφ ratio) were measured using the Cayman (Ann Arbor, MI) Nitrate/Nitrite colorimetric assay kit. Experiments were performed in triplicate using 5×105 BMMφ per well in a 96-well plate.
Statistical analysis
T-test was used to compare two groups while ANOVA analysis was used to compare multiple groups. Differences with P<.05 were considered significant.
Results
Intracellular yeast growth in resting vs. GM-CSF-activated Mφ
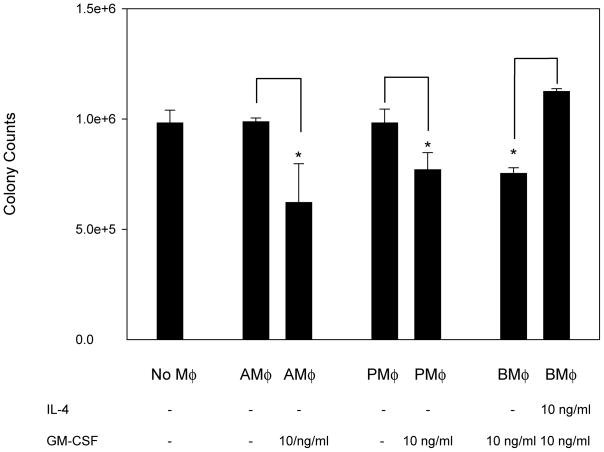
We previously reported that GM-CSF enhanced host defenses to H. capsulatum [9]. Hence, we sought to determine if it acted upon Mφ. Exposure of several populations of Mφ to GM-CSF inhibited the intracellular growth of H. capsulatum (Fig. 1). IL-4 blunts immunity to H. capsulatum and is associated with the emergence of alternatively activated Mφ [10]. We asked if pre-treatment of Mφ with IL-4 alters intra-cellular growth in activated Mφ [11]. Exposure of Mφ for 24 hr to IL-4 enhanced yeast growth in BMMφ (Fig. 1).
Figure 1.
GM-CSF activates Mφ to exert antifungal activity and IL-4 inhibits activation. Colony counts from H. capsulatum in culture alone, resting AMφ, GM-CSF treated AMφ, resting PMφ, GM-CSF treated PMφ, and GM-CSF BMMφ pretreated with IL-4 for 24hr. Data represent mean ± SEM from 3 experiments. * P< .05 (student’s t test) compared to resting PMφ.
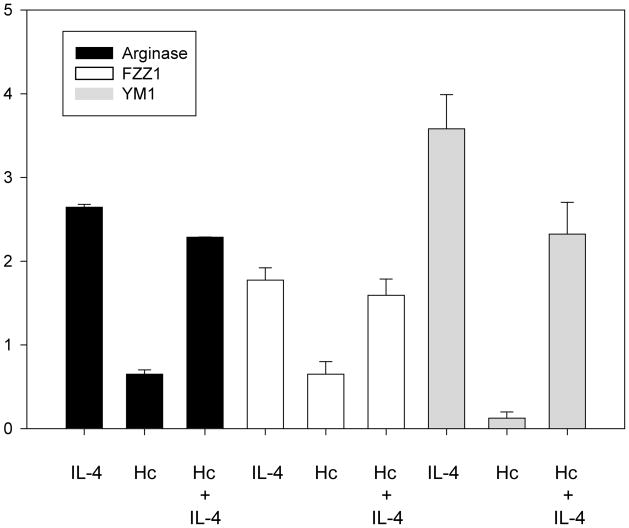
We examined if IL-4 alternatively activated infected Mφ. We assessed the expression of argi-nase-1, FIZZ1, and YM1 [12]. Each gene manifested enhanced expression following exposure of BMMφ cells to IL-4 and infection with H. capsulatum (Fig. 2). The abundance of another marker of IL-4 activation, transferrin receptor (TfR), was analyzed by flow cytometry. IL-4 treatment of Mφ 24 hr before H. capsulatum infection did not increase total TfR expression as determined by the mean fluorescent intensity (MFI). The MFI of Mφ exposed to medium (926.6) did not differ from that of cells exposed to 10ng/ml of IL-4 (952.3). Moreover, no differences in the percentage of cells expressing TfR were observed between the two groups (data not shown). IL-4 did enhance TfR expression on uninfected cells in a dose dependent manner. The MFI of cells exposed to medium only (2055.2) was less than that of cells exposed to 0.1 ng/ml of IL-4 (3763.4), 1 ng/ml (4378.7) or 10 ng/ml (4333.9). Likewise there was an increase in the percentage of cells expressing TfR; 79.5% of cells incubated in medium only were TfR+ whereas those values were 81.5%, 86.1%, 88.1% for 0.1 ng/ml, 1 ng/ml, and 10 ng/ml of IL-4 respectively.
Figure 2.
Expression of IL-4-regulated genes in BMMφ. qRT-PCR of arginase, FIZZ1, and YM1 expression in BMMφ pretreated with 10 ng of IL-4 for 24hr, infected with H. capsulatum (without IL-4), or IL-4 treated and infected. Expression levels were normalized to uninfected BMMφ without IL-4. Data represent mean ± SEM from 3 experiments.
We did not detect a significant difference in nitric oxide (NO) production between IL-4 treated (5.3 ± .2 μM nitrite) versus untreated Mφ (5.7 ± .4 μM nitrite).
GM-CSF regulates Mφ metal levels
We queried if GM-CSF altered metal levels in cells. We undertook an unbiased approach using ICPMS to assess levels of Mg2+, Ca2+, Fe2+/3+, Zn2+, Cu2+, and Mn2+ during infection of Mφ.
We determined metal levels in uninfected and infected PMφ and in those pre-treated with [4]. Total metal concentrations were highest in resting uninfected PMφ compared to all other populations (Table 2). We observed decreases in the overall concentrations of metals upon infection, but this restriction was enhanced for Fe2+/3+ and Zn2+ by GM-CSF activation (Table 2). GM-CSF did induce a decrease in Zn2+ in uninfected PMφ, but this was not statistically significant (Table 2).
Table 2.
Metal Levels
| Metal levels from 1×105 uninfected and H. capsulatum-infected Mφ cells | ||||||||
|---|---|---|---|---|---|---|---|---|
| Resting PMφ | GM-CSF PMφ | GM-CSF BMMφ | GM-CSF BMMφ + IL-4 | |||||
| AVG | RSD | AVG | RSD | AVG | RSD | AVG | RSD | |
| Mg | 2240 | 2% | 790 | 2% | 1210 | 3% | 1270 | 2% |
| Ca | 3700 | 3% | 1750 | 5% | 2220 | 5% | 2044 | 2% |
| Fe | 1360 | 2% | 664a | 5% | 932 | 3% | 1002 | 5% |
| Zn | 190 | 6% | 89 | 6% | 179 | 5% | 153 | 2% |
| Cu | 57 | 5% | 23 | 6% | 22 | 3% | 20 | 1% |
| Mn | 8 | 9% | <1 | 3% | <1 | 4% | <1 | 2% |
| Resting PMφ + Hc | GM-CSF PMφ + Hc | GM-CSF BMMφ +Hc | GM-CSF BMMφ + IL-4 + Hc | |||||
|---|---|---|---|---|---|---|---|---|
| AVG | RSD | AVG | RSD | AVG | RSD | AVG | RSD | |
| Mg | 778 | 2% | 954 | 2% | 1300 | 1% | 1310 | 3% |
| Ca | 1780 | 8% | 1350 | 4% | 2110 | 1% | 2032 | 2% |
| Fe | 680 | 6% | 275a | 7% | 566 | 3% | 597 | 7% |
| Zn | 131 | 8% | 28a | 6% | 63a | 2% | 162b | 10% |
| Cu | 24 | 11% | 22 | 2% | 26 | 3% | 24 | 8% |
| Mn | <1 | 9% | <1 | 12% | <1 | 2% | <1 | 1% |
| H. capsulatum metal levels from 1×107 cells following isolation from infected Mφ and culture alone. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Resting PMφ | GM-CSF PMφ | GM-CSF BMMφ | GM-CSF BMMφ + IL-4 | Culture Alone | ||||||
| AVG | RSD | AVG | RSD | AVG | RSD | AVG | RSD | AVG | RSD | |
| K | 25500 | 1% | 14000 | 1% | 13400 | 1% | 28200 | 2% | 13400 | 1% |
| Mg | 17000 | 1% | 3400 | 9% | 2400 | 8% | 4100 | 12% | 1800 | 9% |
| Ca | 38300 | 1% | 11800a | 7% | 5600a | 12% | 12500b | 10% | 4400 | 15% |
| Fe | 6400 | 4% | 4300 | 3% | 1700a | 5% | 2160 | 7% | 4650 | 2% |
| Zn | 1900 | 1% | 1200a | 2% | 500a | 1% | 1100b | 1% | 600 | 1% |
| Cu | 406 | 2% | 219 | 2% | 73 | 6% | 50 | 25% | 46 | 3% |
| Mn | 42 | 16% | 53 | 15% | 21 | 26% | 55 | 2% | 36 | 15% |
| Ni | 175 | 9% | 147 | 15% | 59 | 23% | 102 | 7% | 56 | 19% |
NOTE: Data are a mean of 3 experimental replicates represented in parts per billion (ppb) (1 ppb=1 μg L−1). RSD, Relative standard deviation, Hc, Histoplasma capsulatum, PMφ, peritoneal macrophages, BMMφ, bone marrow derived macrophages.
P<.05 (ANOVA analysis) compared to resting infected (Hc) PMφ, and
compared to infected (Hc) GM-CSF BMMφ
Mφ from various organs may not regulate metals similarly; hence, Zn2+ concentrations in BMMφ and AMφ were measured. Similar to infected PMφ exposed to GM-CSF, infected BMMφ grown in the presence of GM-CSF yielded lower Zn2+ and Fe2+/3+ levels compared to that of infected resting PMφ (Table 2). A Zn2+ concentration of 246 ppb (RSD = 3%, n = 3) was measured in resting AMφ while in GM-CSF treated AMφ was124 ppb (RSD = 6%, n = 3). Fe2+/3+ levels were 1200 ppb (RSD = 1.9%, n = 3) in resting AMφ while in GM-CSF treated AMφ levels were 540 ppb (RSD = 4%, n = 3).
To ensure that altered metal levels observed in this study were not a result of different numbers of yeast initially ingested by the Mφ, we counted the number of yeasts within each cytokine treated Mφ population. We observed that following infection of Mφ for 1 hr, each Mφ population contained a mean (± SEM) of 12 ± 3 yeasts. Moreover, utilizing H. capsulatum expressing green fluorescent protein, we found that that ~90% of Mφ were infected after 24 hr.
We questioned whether our overall for cellular metal concentrations were acceptable. The cy-tosolic pool of free Zn in a cell is estimated to range from 10−5-10−12 M; our measurements were in the 10−7 M range [13, 14].
Metal levels within intracellular yeast cells
Total Mφ metal analysis may not dictate the amount within ingested yeasts. Thus, we analyzed metal concentrations of K+, Mg2+, Ca2+, Fe2+/3+, Zn2+, Cu2+, Mn2+ and Ni2+ within H. capsulatum isolated from Mφ. Lower amounts of Zn2+ and Ca2+ (P < .05) were detected in yeasts isolated from BMMφ and GM-CSF-exposed PMφ compared to resting PMφ or H. capsulatum grown in culture alone (Table 2).
To validate our methodology, we assessed if IFN-γ pretreatment of PMφ decreased Fe2+/3+ levels. A Fe2+/3+ concentration with a mean of 2440 ppb (RSD = 7%, n = 3) was detected for H. capsulatum isolated from IFN-γ pretreated PMφ compared to resting PMφ mean of 6240 ppb (RSD = 4%, n = 3). Thus, these data support the utility of this method for assaying intracellular metals. IFN-γ also decreased Zn2+ levels. Cytokine treated cells contained 600 ppb (RSD = 6%, n = 3) and resting cells had 1900 ppb, (RSD = 6%, n = 3).
Increases in Zn levels in IL-4 treated Mφ and intracellular yeast cells
We asked if IL-4 enhancement of fungal growth was associated with a change in intracellular metal abundance. Zn2+ levels increased >2X inside infected BMMφ treated with IL-4 while Fe2+/3+ concentrations did not (Table 2). In addition, higher Zn2+ and Ca2+ levels were detected in H. capsulatum isolated from IL-4 pretreated BMMφ compared to that of untreated cells (Table 2).
Zn influences yeast growth
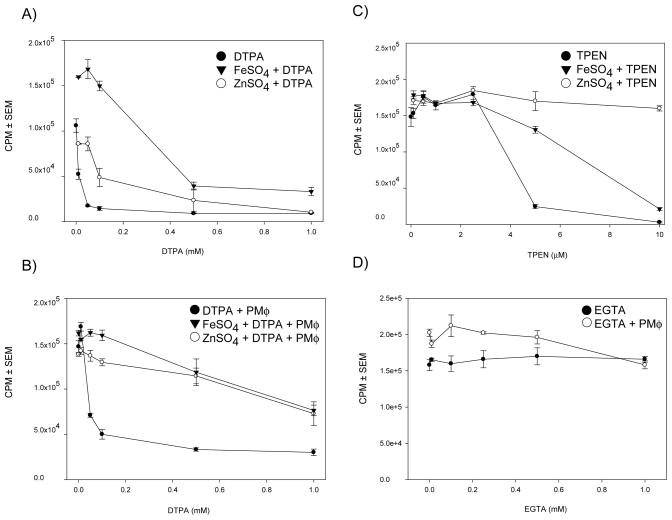
A decrease of Zn2+ levels in activated Mφ coupled with an increase following IL-4 treatment led us to hypothesize that restriction of Zn2+ is a host defense mechanism against H. capsulatum. We sought to determine the effects of the Zn2+ chelators DTPA and TPEN on H. capsulatum growth.
DTPA inhibited H. capsulatum growth in a dose dependent manner in the presence and absence of PMφ (Fig. 3A and 3B). DTPA was not toxic at the concentrations used in this study. TPEN inhibited H. capsulatum growth in a dose dependent manner in the presence and absence of PMφ (Fig. 3C). TPEN is toxic to Mφ at low concentrations therefore we could not adequately interpret TPEN’s influence on intracellular yeast growth.
Figure 3.
Zn chelation inhibits H. capsulatum growth. CPM from tritiated leucine assays of H. capsulatum in A) media containing DTPA (filled circle), in the presence of DTPA + FeSO4 (filled triangle), or TPEN + ZnSO4 (unfilled circle) B.) PMφ. C.) media containing TPEN (filled circle), in the presence of TPEN + FeSO4 (filled triangle), or TPEN + ZnSO4 (unfilled circle) D) media containing EGTA (filled circle) and media containing EGTA + PMφ (unfilled circle). Data represent the mean ± SEM of triplicates from 1 representative experiment of 3.
Because TPEN and DTPA bind Fe2+ [15] we determined if Fe2+ chelation by DTPA and TPEN influenced our results. In the presence of DTPA, FeSO4 and ZnSO4 partially rescue H. capsulatum growth in culture alone or inside resting peritoneal Mφ. In the presence of TPEN, the addition of FeSO4 partially enhanced H. capsulatum growth whereas ZnSO4 completely restored it.
We examined whether Ca2+ had an effect on yeast growth inside Mφ because Ca2+ levels were diminished in H. capsulatum isolated from GM-CSF treated Mφ and increased following IL-4 treatment. Chelation of Ca2+ with EGTA did not significantly influence yeast growth in medium or inside PMφ (Fig. 3D).
GM-CSF regulation of Mφ Zn species
Zn2+ is a cofactor and required for numerous host processes [16]. We hypothesized that the changes in total Zn2+would need to be tightly regulated. Thus, we should detect a change in the total Zn2+ binding species of GM-CSF PMφ and BMMφ compared to resting PMφ. Figure 4 shows SEC chromatograms followed by ICPMS analysis representing the total Zn2+ binding species of infected and uninfected resting PMφ versus GM-CSF treated PMφ. A higher amount of Zn2+ binding species were consistently detected in GM-CSF PMφ compared to that of resting PMφ especially Zn2+ species eluting at 27 min..
Figure 4.
Mφ activation and H. capsulatum infection regulate host Zn binding species. Size exclusion chromatography followed by ICPMS analysis of Zn species in A.) resting PMφ B.) resting PMφ infected with H. capsulatum C.) GM-CSF treated PMφ D) infected GM-CSF treated PMφ. Yeasts were removed before SEC and ICPMS analysis. Molecular weight standards are listed above A and B.
Effect of GM-CSF is independent of modulation of calprotectin
Calprotectin has been shown to bind Zn2+ and possess anti-fungal activity [17]. We asked if GM-CSF altered expression of it. Exposure of cells to GM-CSF did not enhance significantly (p > 0.05, t-test) calprotectin expression (Fig. 5).
Figure 5.
GM-CSF does not increase calprotectin expression in PMφ. qRT-PCR of calprotectin expression of H. capsulatum infected resting PMφ, uninfected PMφ pretreated with 10 ng of GM-CSF for 24hr, no infection or GM-CSF infected PMφ. Expression levels were normalized to un-infected PMφ without GM-CSF treatment. Data represent mean ± SEM from 3 experiments. Hc = H. capsulatum.
Discussion
In this study, GM-CSF-activated Mφ restricted intracellular growth of H. capsulatum as well as the concentrations of Zn2+ and Fe2+/3+. Moreover, IL-4 reversed the growth inhibitory properties of activated Mφand increased [Zn2+] in Mφ and yeast cells. Among the metals analyzed, Zn2+ was the most heavily influenced by GM-CSF and IL-4 treatment. These findings prompted us to examine the role of Zn2+ in the growth of H. capsulatum. Zn2+ chelation restricted growth both in medium alone and within Mφ. Furthermore GM-CSF activation was accompanied by changes in the abundance and number of Zn2+ binding species. The data provide a link between Zn2+ abundance and H. capsulatum intracellular survival.
Zn2+ is a cofactor for over 300 proteins and required for the survival of most microorganisms [16]. Salmonella enterica and Aspergillus fumigatus utilize a Zn2+ uptake system that is required for Zn2+ homeostasis and full virulence [18, 19]. Several pathogenic fungi including Candida al-bicans and A. fumigatus display growth impairment to Zn2+ deprivation [20]. Using Zn2+ chelators DTPA and TPEN our studies reveal that Zn2+ is required for the growth of H. capsulatum in medium and can influence intracellular growth. Although our data suggests this chelator also binds Fe2+, a clear advantage exists for the host to restrict both Fe2+ and Zn2+ during infection with H. capsulatum.
Activation of murine Mφ with IFN-γ stimulates the production of NO which is believed to promote degradation of several intracellular pathogens including H. capsulatum [21–23]. Indirect and direct evidence also exist for IFN-γ-mediated host restriction of Fe2+/3+ availability [3, 24–26]. We detected lower levels of Fe2+/3+ and Zn2+ in IFN-γ and GM-CSF-treated Mφ. GM-CSF is a pleiotropic cytokine that induces myelopoiesis, acts as pro-inflammatory agent, and arms phagocytes to express anti microbial activity [27]. Much of the literature indicates that GM-CSF enhances reactive oxygen intermediates as one mechanism of host defenses. Our data reveal that another antimicrobial defense mechanism is limitation of metals [28, 29]. We discovered that Zn2+ levels were less in GM-CSF treated Mφ infected with H. capsulatum compared to resting infected Mφ.
Host intracellular Zn2+ concentrations are controlled by Zn2+ importers, exporters, and metal binding proteins such as metallothionens [30]. Zn2+ is an essential element required for mammalian cell function, but high concentrations of Zn2+ can be toxic therefore regulation of this metal must be tightly controlled [31]. Thus fluctuations in intracellular Zn2+ levels must be initiated and/or responded to by the host. Accordingly we detected changes in Zn2+ species abundance to accompany GM-CSF induced Zn2+ restriction and IL-4 induced Zn2+ replenishment. Thus the changes in the number of Zn2+ species add further support to the Zn2+ level alterations detected. These data also suggest the participation of a host Zn regulatory mechanism influenced by different cytokines during H. capsulatum infection.
IL-4 alternatively activates Mφ and thereby inhibits the killing of intracellular and some extra-cellular organisms in vitro [11, 32, 33]. Overproduction of IL-4 under conditions of altered immunity accelerates H. capsulatum infection, but the mechanism remains unclear [10, 34, 35]. One mechanism which IL-4 may contribute to organism survival during infection is by reducing NO production [36], but in this study IL-4 reversal of GM-CSF activity was independent of NO production. Another potential mechanism of organism growth enhancement by IL-4 is the alteration of metal homeostasis. IL-4 increases Ca2+ movement and accumulation in Mφ [37, 38]. Likewise we detected increases in Ca2+ levels in H. capsulatum isolated from IL-4 exposed Mφ. However, Ca2+ chelation had only a minimal effect on intracellular yeast growth, thus suggesting that this metal is not important in the effect of IL-4. Treatment of Mφ with IL-4 has been suggested to make Fe more available to Mycobacterium tuberculosis in Mφ [39]. In this study increases in Mφ Fe2+/3+ levels were not detected. In contrast, IL-4 treatment partially restored intracellular yeast growth in associating with increasing intracellular Zn2+ levels. Thus, Zn2+ rather than Fe replenishment following IL-4 treatment of Mφ may contribute to a more permissive environment.
In summary we have shown that cytokine activated Mφ manifest a modulation of metal levels. The data support a crucial role for Zn2+ limitation as a key element of host defenses.
Acknowledgments
This work was supported by a Merit Review from the Veterans Affairs and grants AI-73337, and AI-83313 from the National Institute of Allergy and Infectious Diseases
Footnotes
The authors report no conflicts.
This manuscript was not presented at a meeting.
References
- 1.Wadman M. Poor trial design leaves gene therapy death a mystery. Nat Med. 2007;13:1124. doi: 10.1038/nm1007-1124a. [DOI] [PubMed] [Google Scholar]
- 2.Howard DH. Intracellular Behavior Of Histoplasma capsulatum. J Bacteriol. 1964;87:33–8. doi: 10.1128/jb.87.1.33-38.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane TE, Wu-Hsieh BA, Howard DH. Iron limitation and the gamma interferon-mediated antihistoplasma state of murine macrophages. Infect Immun. 1991;59:2274–8. doi: 10.1128/iai.59.7.2274-2278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman SL, Gootee L. Colony-stimulating factors activate human macrophages to inhibit intracellular growth of Histoplasma capsulatum yeasts. Infect Immun. 1992;60:4593–7. doi: 10.1128/iai.60.11.4593-4597.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allendoerfer R, Deepe GS., Jr Intrapulmonary response to Histoplasma capsulatum in gamma interferon knockout mice. Infect Immun. 1997;65:2564–9. doi: 10.1128/iai.65.7.2564-2569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang CC, Yang MH, Shih TS. Automated on-line sample pretreatment system for the determination of trace metals in biological samples by inductively coupled plasma mass spectrometry. Anal Chem. 1997;69:3930–9. doi: 10.1021/ac970284e. [DOI] [PubMed] [Google Scholar]
- 7.Sarmiento-Gonzalez A, Marchante-Gayon JM, Tejerina-Lobo JM, Paz-Jimenez J, Sanz-Medel A. High-resolution ICP-MS determination of Ti, V, Cr, Co, Ni, and Mo in human blood and urine of patients implanted with a hip or knee prosthesis. Anal Bioanal Chem. 2008;391:2583–9. doi: 10.1007/s00216-008-2188-4. [DOI] [PubMed] [Google Scholar]
- 8.Deepe GS, Jr, Gibbons RS, Smulian AG. Histoplasma capsulatum manifests preferential invasion of phagocytic subpopulations in murine lungs. J Leukoc Biol. 2008;84:669–78. doi: 10.1189/jlb.0308154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deepe GS, Jr, Gibbons R. Recombinant murine granulocyte-macrophage colony-stimulating factor modulates the course of pulmonary histoplasmosis in immunocompetent and immunodeficient mice. Antimicrob Agents Chemother. 2000;44:3328–36. doi: 10.1128/aac.44.12.3328-3336.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szymczak WA, Deepe GS., Jr The CCL7-CCL2-CCR2 axis regulates IL-4 production in lungs and fungal immunity. J Immunol. 2009;183:1964–74. doi: 10.4049/jimmunol.0901316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vouldoukis I, Becherel PA, Riveros-Moreno V, et al. Interleukin-10 and interleukin-4 inhibit intracellular killing of Leishmania infantum and Leishmania major by human macrophages by decreasing nitric oxide generation. Eur J Immunol. 1997;27:860–5. doi: 10.1002/eji.1830270409. [DOI] [PubMed] [Google Scholar]
- 12.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canzoniero LM, Sensi SL, Choi DW. Measurement of intracellular free zinc in living neurons. Neurobiol Dis. 1997;4:275–9. doi: 10.1006/nbdi.1997.0160. [DOI] [PubMed] [Google Scholar]
- 14.Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–92. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 15.O’Sullivan WJ, Smithers GW. Stability constants for biologically important metal-ligand complexes. Methods Enzymol. 1979;63:294–336. doi: 10.1016/0076-6879(79)63014-8. [DOI] [PubMed] [Google Scholar]
- 16.Rink L, Haase H. Zinc homeostasis and immunity. Trends Immunol. 2007;28:1–4. doi: 10.1016/j.it.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Urban CF, Ermert D, Schmid M, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ammendola S, Pasquali P, Pistoia C, et al. High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect Immun. 2007;75:5867–76. doi: 10.1128/IAI.00559-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno MA, Ibrahim-Granet O, Vicentefranqueira R, et al. The regulation of zinc homeostasis by the ZafA transcriptional activator is essential for Aspergillus fumigatus virulence. Mol Microbiol. 2007;64:1182–97. doi: 10.1111/j.1365-2958.2007.05726.x. [DOI] [PubMed] [Google Scholar]
- 20.Lulloff SJ, Hahn BL, Sohnle PG. Fungal susceptibility to zinc deprivation. J Lab Clin Med. 2004;144:208–14. doi: 10.1016/j.lab.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Lane TE, Otero GC, Wu-Hsieh BA, Howard DH. Expression of inducible nitric oxide synthase by stimulated macrophages correlates with their antihistoplasma activity. Infect Immun. 1994;62:1478–9. doi: 10.1128/iai.62.4.1478-1479.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura LT, Wu-Hsieh BA, Howard DH. Recombinant murine gamma interferon stimulates macrophages of the RAW cell line to inhibit intracellular growth of Histoplasma capsulatum. Infect Immun. 1994;62:680–4. doi: 10.1128/iai.62.2.680-684.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kagaya K, Watanabe K, Fukazawa Y. Capacity of recombinant gamma interferon to activate macrophages for Salmonella-killing activity. Infect Immun. 1989;57:609–15. doi: 10.1128/iai.57.2.609-615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrd TF, Horwitz MA. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Invest. 1989;83:1457–65. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nairz M, Fritsche G, Brunner P, Talasz H, Hantke K, Weiss G. Interferon-gamma limits the availability of iron for intramacrophage Salmonella typhimurium. Eur J Immunol. 2008;38:1923–36. doi: 10.1002/eji.200738056. [DOI] [PubMed] [Google Scholar]
- 26.Wagner D, Maser J, Moric I, et al. Changes of the phagosomal elemental concentrations by Mycobacterium tuberculosis Mramp. Microbiology. 2005;151:323–32. doi: 10.1099/mic.0.27213-0. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–44. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 28.Denis M, Ghadirian E. Granulocyte-macrophage colony-stimulating factor restricts growth of tubercle bacilli in human macrophages. Immunol Lett. 1990;24:203–6. doi: 10.1016/0165-2478(90)90049-v. [DOI] [PubMed] [Google Scholar]
- 29.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–12. [PubMed] [Google Scholar]
- 30.Murakami M, Hirano T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008;99:1515–22. doi: 10.1111/j.1349-7006.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugarman B. Zinc and infection. Rev Infect Dis. 1983;5:137–47. doi: 10.1093/clinids/5.1.137. [DOI] [PubMed] [Google Scholar]
- 32.Cenci E, Romani L, Mencacci A, et al. Interleukin-4 and interleukin-10 inhibit nitric oxide-dependent macrophage killing of Candida albicans. Eur J Immunol. 1993;23:1034–8. doi: 10.1002/eji.1830230508. [DOI] [PubMed] [Google Scholar]
- 33.Denis M, Gregg EO, Ghandirian E. Cytokine modulation of Mycobacterium tuberculosis growth in human macrophages. Int J Immunopharmacol. 1990;12:721–7. doi: 10.1016/0192-0561(90)90034-k. [DOI] [PubMed] [Google Scholar]
- 34.Allen HL, Deepe GS., Jr Apoptosis modulates protective immunity to the pathogenic fungus Histoplasma capsulatum. J Clin Invest. 2005;115:2875–85. doi: 10.1172/JCI25365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gildea LA, Gibbons R, Finkelman FD, Deepe GS., Jr Overexpression of interleukin-4 in lungs of mice impairs elimination of Histoplasma capsulatum. Infect Immun. 2003;71:3787–93. doi: 10.1128/IAI.71.7.3787-3793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutschman R, Lang R, Hesse M, Ihle JN, Wynn TA, Murray PJ. Cutting edge: Stat6-dependent substrate depletion regulates nitric oxide production. J Immunol. 2001;166:2173–7. doi: 10.4049/jimmunol.166.4.2173. [DOI] [PubMed] [Google Scholar]
- 37.Sharma P, Chakraborty R, Wang L, et al. Redox regulation of interleukin-4 signaling. Immunity. 2008;29:551–64. doi: 10.1016/j.immuni.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albuquerque PC, Nakayasu ES, Rodrigues ML, et al. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol. 2008;10:1695–710. doi: 10.1111/j.1462-5822.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahnert A, Seiler P, Stein M, et al. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol. 2006;36:631–47. doi: 10.1002/eji.200535496. [DOI] [PubMed] [Google Scholar]