Abstract
Objective: The purpose of this study was to determine the unique and universal features of microsatellite instability-high (MSI-H) colorectal cancer (CRC) and MSI-H gastric cancer (GC) in the Chinese population. Methods: A new panel of mononucleotide MSI markers, BAT25, BAT26, NR21, NR24, and MONO-27, was used to define MSI status in 303 CRC and 288 GC subjects. Clinicopathological features of both types of MSI-H tumors were analyzed. Methylation analysis in the hMLH1 promoter region by methylation specific polymerase chain reaction (PCR) and mutation detection of hMSH2/hMLH1 genes by denaturing high-performance liquid chromatography (DHPLC) were carried out simultaneously. Results: MSI-H CRCs and MSI-H GCs account for 11.9% and 8.0% of unselected sporadic CRCs and GCs, respectively. MSI-H CRCs are strongly characterized by early onset, right-side location, low differentiation, mucinous tumor, less infiltration, less lymphatic metastasis, and more often familial tumor. MSI-H GCs only showed site preference for the antrum and less lymphatic metastasis. Genetic and epigenetic analyses were positive in 6/36 MSI-H CRCs and 0/23 MSI-H GCs with pathological mutation in major mismatch repair genes, and in 7/36 MSI-H CRCs and 18/23 MSI-H GCs with methylated hMLH1 promoter (P<0.01), respectively. Conclusions: Although there are many differences in the genetic basis and clinicopathological features between MSI-H CRC and MSI-H GC, when compared with their microsatellite stable (MSS) counterparts, site preference and lymphatic metastasis are features common to both types of MSI-H tumors.
Keywords: Microsatellite instability, Colorectal cancer, Gastric cancer, Mutation, DNA methylation
1. Introduction
Microsatellite instability (MSI) is the term given to deletions or insertions of short repeat nucleotides sequence in the genome (Oda et al., 2005). MSI can be divided into microsatellite instability-high (MSI-H) and microsatellite instability-low (MSI-L) according to the appearance of a number of unstable MSI markers (Pawlik et al., 2004). The causes of MSI-H tumors have been linked to several genetic and epigenetic changes in mismatch repair (MMR) genes, suggesting that an MSI-H tumor is caused by an impotent MMR system (Grady, 2004; Li and Lai, 2009). MSI-H tumors are often seen in gastrointestinal cancer. In fact, there are a number of studies of MSI-H colorectal cancer (CRC) and MSI-H gastric cancer (GC) in recent decades (Lubbe et al., 2009; Seo et al., 2009). However, to date, there have been no comparisons made of these two types of MSI-H cancers. Presumably, since the MSI-H tumor is caused by MMR gene deficiency, MSI-H tumors may share similar cancer genesis pathways, which may lead to similar clinical outcomes. If there exist common features in MSI-H tumors, this will help to understand better the relationship between MSI genotype and clinicopathological phenotypes. Moreover, it will help to distinguish MSI-H tumors from microsatellite stable (MSS) tumors more easily. In an attempt to discover similarities and differences between MSI-H CRC and MSI-H GC, we studied clinicopathological features as well as conducted genetic analyses of both MSI-H CRC and MSI-H GC in a Chinese population.
2. Materials and methods
2.1. Subjects and specimens
This study was approved by the ethics committee of our institution, following the ethical guidelines of the 1975 Declaration of Helsinki (Forster et al., 2001). In total, 303 patients diagnosed with CRC and 288 patients diagnosed with GC underwent curative surgical resection between 2000 and 2004 in the Second Affiliated Hospital, School of Medicine, Zhejiang University, China. These patients served as the study population. During surgical resection, about 1 cm3 of paired tumor and normal tissue were resected from the removed gastrointestinal tract and immediately preserved in −20 °C for future use. Each resection and preservation took place with the informed consent of the patients. Each tumor sample was pathologically verified by two experienced pathologists. Genomic DNA of each tissue was extracted by a standard phenol-chloroform method and was preserved in −20 °C.
2.2. MSI analysis by denaturing high-performance liquid chromatography (DHPLC)
The DHPLC instrument used was the WAVE system (Transgenomic Inc., Omaha, NE, USA). Five MSI markers, BAT25, BAT26, NR21, NR24, and MONO-27, were used to determine microsatellite status according to the methodology previously reported (Murphy et al., 2006; Berginc and Glavac, 2009).
Polymerase chain reaction (PCR) primers for these five MSI markers were designed. DNA was amplified in a 25-µl volume system containing 100 ng of sample DNA, 200 µmol/L deoxynucleotide triphosphates (dNTPs), 2 mmol/L MgCl2, 15 pmol of each primer, and 0.25 U Taq DNA polymerase in a transgenomic recommended buffer. The reaction was incubated at 95 °C for 5 min, followed by 35 cycles of 95 °C (primer specific annealing temperature) and 72 °C for 50 s each, and then 8 min of final extension at 72 °C. PCR products were sent to DHPLC analysis without any purification.
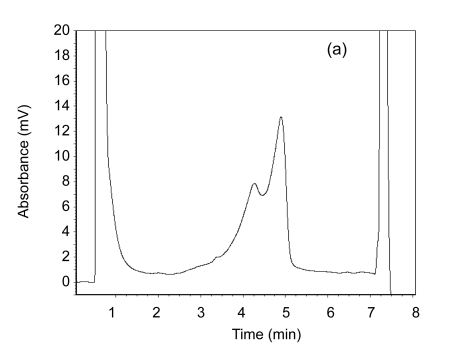
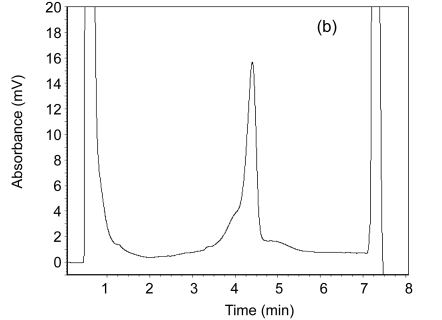
MSI analysis was carried out on an automated HPLC device equipped with a DNA separation column. In total, 6–10 µl of each PCR product was separated at a flow rate 0.9 ml/min by means of a linear acetonitrile gradient. The column temperature was 50 °C. The detection range was set as ±100 bp of specific DNA fragment. In the DHPLC, MSI-H sample shows a double peak and MSS one shows a single one (Fig. 1). A tumor sample was considered to be MSI-H if two or more of the five markers demonstrated instability, and was considered to be MSI-L if only one marker demonstrated instability.
Fig. 1.
A double peak for the MSI-H sample (a) and a single peak for the MSS sample (b) on DHPLC
2.3. Methylation analysis of hMLH1 promoter region
After MSI-H CRC and MSI-H GC were determined, methylation specific PCR (MSP) was used to determine the methylation status of the hMLH1 promoter region in both MSI-H tumor types. Methylation specific primers were designed as previously reported (Deng et al., 1999). The PCR amplification procedure was similar with that in MSI detection. Each promoter region amplified by PCR has decisive roles in gene expression. MSP product was separated by DHPLC instead of agarose gel. If the DNA sequence was methylated, MSP would amplify methylated alleles, which would appear on DHPLC as a peak. If the DNA sequence was not methylated, there was no appearance of peaks on DHPLC.
2.4. Mutation analyses of coding sequences of hMSH2 and hMLH1
For those MSI-H tumors without methylation in the hMLH1 promoter region, further detection of somatic mutations in coding sequences of hMSH2 and hMLH1 was carried out by DHPLC. A total of 35 exons of both genes for every single methylation-negative MSI-H tumor were examined according to the methodology previously reported (Yuan et al., 2004). When a mutation was found by DHPLC and was verified by DNA sequencing, it was soon sent to a verification procedure to determine if it was a pathological mutation. Verification was settled in two steps. The first step was to align the mutant sequence with published dbSNP database in order to exclude the possibility of being known single nucleotide polymorphisms (SNPs). If the mutation was unpublished, the second step was carried out, in which DNA from 100 healthy individuals was examined for the same mutation. If the positive rate of a specific mutation in healthy individuals was less than 1%, it was considered to be a pathological mutation; otherwise it was regarded as a single nucleotide polymorphism with no significant pathological meaning.
2.5. Data analysis
Medical data for every subject were collected. The database was designed to preserve clinical and pathological statistics, MSI status, and genetic and epigenetic information. χ 2 statistic analysis was conducted by SPSS software.
3. Results
3.1. Frequency of MSI tumors
MSI analyses of 303 CRCs resulted in 36 (11.9%) MSI-H and 262 (86.5%) MSS tumors. Three DNA fragments from BAT26 PCR products were sent to DNA sequencing, and all the repeated mononucleotides showed shortened (2–6 bp) alleles. Of the 288 GCs in which MSI analyses were completed, 23 (8.0%) were MSI-H and 253 (87.8%) were MSS.
The DHPLC evaluation of mononucleotide instability is convenient, effective, and timesaving. It took less than 8 min per sample, allowing rapid throughput of a large numbers of samples in minimal time. However, it is difficult to evaluate dinucleotide markers D5S346, D2S123, and D17S250 by DHPLC. Despite the comparison of DNA from both paired cancer and normal tissue to define MSI status in dinucleotide evaluation, it was difficult to distinguish instability from stability by DHPLC for these dinucleotide markers.
3.2. Clinicopathological features of MSI-H CRC and MSI-H GC
Statistical analysis showed (Table 1) that there were significant differences between MSI-H CRC and MSS CRC in several clinicopathological characteristics. However, differences between MSI-H GC and MSS GC were not apparent. MSI-H CRCs were more likely from younger subjects, were located in the right colon, and have a mucinous phenotype, less local aggressiveness, and less lymphatic metastasis. However, MSI-H GC tended only to locate in distal parts of the stomach.
Table 1.
Clinicopathological features of MSI-H CRC and MSI-H GC in a Chinese population
| Parameter | CRC |
GC |
||||
| MSI-H (36) | MSS (262) | P | MSI-H (23) | MSS (253) | P | |
| Sex | 0.517 | 0.695 | ||||
| Male | 24 | 160 | 15 | 175 | ||
| Female | 12 | 102 | 8 | 78 | ||
| Average age (year) | 54.6 | 60.9 | 0.035 | 62.3 | 59.3 | 0.415 |
| Site | ||||||
| Right side1 | 20 | 63 | 0.000 | − | − | − |
| Rectum | 5 | 126 | 0.031 | − | − | − |
| Antrum2 | − | − | − | 19 | 142 | 0.014 |
| Differentiation | 0.007 | 0.869 | ||||
| High | 21 | 147 | 3 | 38 | ||
| Middle | 10 | 107 | 10 | 96 | ||
| Low | 5 | 8 | 10 | 119 | ||
| Mucinous tumor3 | 0.002 | 0.645 | ||||
| + | 10 | 24 | 3 | 43 | ||
| − | 24 | 204 | 16 | 17 | ||
| Infiltration | 0.002 | 0.422 | ||||
| Muscle | 6 | 47 | 6 | 51 | ||
| Serosa | 28 | 131 | 11 | 101 | ||
| Invade through serosa | 2 | 84 | 6 | 101 | ||
| Lymphatic metastasis4 | 0.011 | 0.089 | ||||
| N0 | 29 | 144 | 3 | 56 | ||
| N1 | 6 | 79 | 10 | 81 | ||
| N2 | 1 | 39 | 9 | 73 | ||
| N3 | − | − | − | 1 | 43 | |
| TNM stage | 0.024 | 0.468 | ||||
| I | 6 | 37 | 3 | 48 | ||
| II | 23 | 105 | 7 | 53 | ||
| III | 6 | 102 | 11 | 106 | ||
| IV | 1 | 18 | 2 | 46 | ||
| Family history5 | 0.001 | 0.100 | ||||
| Yes | 8 | 11 | 3 | 11 | ||
| No | 28 | 251 | 20 | 242 | ||
Right side means that colorectal cancers (CRCs) were located in the cecum, ascending colon, and right half of transversum colon
19 microsatellite instability-high (MSI-H) gastric cancers (GCs) were located at antrum while four were located in another part of the stomach
Partial mucinous differentiation was excluded from mucinous tumor category
Number of metastatic lymph nodes (N0: no, N1: 1–6, N2: 7–15, N3: >15)
Family history includes hereditary nonpolyposis colorectal cancer related tumor in any of subject’s immediate relatives
3.3. Genetic and epigenetic alterations of MMR genes in MSI-H gastrointestinal tumors
hMLH1 promoter methylation was present in 18 out of 23 MSI-H GCs and 5 of 30 MSS GCs, while only 7 out of 36 MSI-H CRCs and 1 of 30 MSS CRCs showed methylated alleles in the hMLH1 promoter region, significantly lower (P<0.01) than those in GCs. After methylation analysis, 5 MSI-H GCs and 29 MSI-H CRCs without methylation were sent for further mutation detection in hMSH2/hMLH1 genes.
Mutation detection of hMSH2 and hMLH1 indicates 37 single nucleotide changes in 34 MSI-H tumors. However, only six mutations proved pathological in MSI-H CRC (Table 2). No pathological mutations were found in MSI-H GC.
Table 2.
Six pathological somatic mutations in MSI-H CRC
| Sample No. | Gene | HGVS description | Amino acid change | Population frequency |
| CRC03 | hMLH1 | GI:13905125 c.8+5 T>G | Splice site | <1% |
| CRC07 | hMLH1 | GI:13905125 c.244 G>A | Ala>Thr | <1% |
| CRC14 | hMLH1 | GI:13905125 c.1477 C>G | Pro>Ala | <1% |
| CRC15 | hMLH1 | GI:13905125 c.1449 delA | Frame shift | <1% |
| CRC20 | hMLH1 | GI:13905125 c.2250 G>C | Termination | <1% |
| CRC31 | hMSH2 | GI:18204305 c.2516 G>A | Asp>His | <1% |
MSI-H: microsatellite instability-high; CRC: colorectal cancer; HGVS: Human Genome Variation Society
4. Discussion
4.1. MSI frequencies of CRC and GC in Chinese patients
In 1997, a National Cancer Institute (NCI) workshop recommended the Bethesda panel markers for defining MSI tumor, these markers including two mononucleotide markers (BAT25 and BAT26) and three dinucleotide markers (D5S346, D2S123, and D17S250). If two of the five markers are unstable in a tumor, it is MSI-H. If only one marker is positive, it is MSI-L (Boland et al., 1998). In 2004, a follow-up NCI workshop further discussed MSI testing (Umar et al., 2004), and recognized the limitations of the original Bethesda panel due to the inclusion of dinucleotide repeats, which are less sensitive and specific than mononucleotide repeats for the identification of cancers with MMR deficiencies. Subsequent studies have shown that mononucleotide markers are sufficient to define MSI-H tumors (de la Chapelle, 1999; Suraweera et al., 2002; Buhard et al., 2004). Bacher et al. (2004) evaluated a set of 266 mono-, di-, tetra-, and penta-nucleotide repeats loci for potential use in MSI screening. They determined that mononucleotide loci are more sensitive and specific than dinucleotide loci for defining MSI tumors. Therefore, a new panel of markers for MSI testing was developed. This MSI analysis system includes five mononucleotide markers: BAT25, BAT26, NR21, NR24, and MONO-27. The system is now commercially available from Promega Corp. (Murphy et al., 2006).
In our study, a total of 303 CRCs and 288 GCs were screened by five mononucleotide markers. We confirmed 36 MSI-H CRCs and 23 MSI-H GCs. The observed MSI-H tumor frequency in CRC was 11.9%, which was similar with that reported in Korean (9%) and Australian (11%) populations (Lim et al., 2004). The frequency of MSI-H GC in our study was 8.0%, which was quite lower than that observed either in Japanese population (20%) or in European American (39%) (Theuer et al., 2002). Another study reported a frequency of 19% in GCs in Caucasians (An et al., 2005). However, a study in a Chinese population with a small sample (n=68) reported an 11% MSI-H frequency in GCs (Fang et al., 2003). A very recent study in a Korean population reported a 9.6% MSI-H GC frequency in sporadic GCs (Gu et al., 2009). We conclude that the frequency of MSI-H GC in Chinese populations is relatively low when compared with those in Japan and Western countries.
4.2. Universal and unique features of MSI-H gastrointestinal cancer
Many reports have provided information about clinicopathological features of MSI-H CRC. MSI-H CRC is strongly characterized by early onset, mucinous type tumors, right-side location of the colon, and better survival rates. In this study, we were interested in comparative features between MSI-H CRC and MSI-H GC. Hypothesizing that since all MSI tumors are caused by impotent DNA mismatch repair system, one may postulate that MSI tumors share similar oncogenesis pathways which in turn may lead to similar clinicopathological outcomes. However, the results turned out to be different. There were only two universal features between MSI-H CRC and MSI-H GC found in this study. First, both MSI-H CRC and MSI-H GC tend to be located at a particular site of the respective organs, though it is difficult to explain the relationships between these particular sites. Another universal feature which may exist between these two types of MSI-H tumors is local lymphatic metastasis. In an attempt to find out the relationship between lymphatic metastasis and MSI status, we first separated tumors into two groups in both the MSI-H tumor and MSS tumor groups. One was lymphatic metastasis positive and the other was free from lymphatic metastasis. As a result, MSI-H CRC showed statistically significantly less lymphatic metastasis than MSS CRC. Overall, 80% of MSI-H CRCs had no lymphatic involvement. Yet, the same feature failed to reach statistical significance for MSI-H GC, though there was a tendency that MSI-H GC had less lymphatic metastasis. Re-examining the data, we found that there was a significant difference of involved lymph node numbers between MSI-H GC and MSS GC. The average number of inspected lymph nodes per case in MSI-H GC was 23.4, almost the same as that for MSS GC (23.7). However, the average number of positive lymph nodes in MSI-H GC (5.8) was significantly (P=0.005) lower than that of MSS GC (8.3). The data were less persuasive, suggesting that both MSI-H CRC and MSI-H GC tend to have less lymphatic involvement. In a very recent study, Ma et al. (2009) observed a lower frequency of MSI-H in GC without lymph node metastasis when compared with that in lymph node metastasis positive GC. Xiao et al. (2006)’s study in a Chinese population also found that the frequency of MSI in GC without lymph node metastasis was significantly higher than that in GC with lymph node metastasis (66.7% vs. 34.3%, P<0.05). We conclude that there may exist a weak relationship between MSI-H and lymph node metastasis in gastrointestinal cancer.
Apart from site preference and local lymphatic metastasis, MSI-H GC failed to show any special features when compared with its MSS counterpart. However, unique features of MSI-H CRC are apparent and well-grounded.
4.3. Clinical and biological significance
In the newest version of practice guidelines for colon cancer from the National Comprehensive Cancer Network, MSI testing is recommended for all colon cancers because stage II colon cancer will not benefit from 5-FU (fluorouracil) adjuvant chemotherapy (Sargent et al., 2010). Selective adjuvant chemotherapeutic treatment of stage II CRC has long been a problem for oncologists. This problem also existed in GC (Vita et al., 2007), and induction adjuvant chemotherapy for stage II GC will possibly depend on MSI status of the cancer. From the results of our findings, it is unreasonable to make such a hypothesis, because MSI-H CRC and MSI-H GC are so often different in both clinicopathological and genetic features. From DNA mismatch repair deficiency to MSI and tumor formation, it is difficult to conclude that there exists a definite, common oncogenesis pathway in gastrointestinal tumors. In conclusion, MSI is a phenotypic phenomenon rather than a cause of these diseases.
Footnotes
Project (No. R2090353) supported by the Zhejiang Provincial Natural Science Foundation of China
References
- 1.An C, Choi IS, Yao JC, Worah S, Xie K, Mansfield PF, Ajani JA, Rashid A, Hamilton SR, Wu TT. Prognostic significance of CpG island methylator phenotype and microsatellite instability in gastric carcinoma. Clin Cancer Res. 2005;11(2):656–663. [PubMed] [Google Scholar]
- 2.Bacher JW, Flanagan LA, Smalley RL, Nassif NA, Burgart LJ, Halberg RB, Megid WM, Thibodeau SN. Development of a fluorescent multiplex assay for detection of MSI-high tumors. Dis Markers. 2004;20(4-5):237–250. doi: 10.1155/2004/136734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berginc G, Glavac D. Rapid and accurate approach for screening of microsatellite unstable tumours using quasimonomorphic mononucleotide repeats and denaturating high performance liquid chromatography (DHPLC) Dis Markers. 2009;26(1):19–26. doi: 10.3233/DMA-2009-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, et al. A national cancer institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58(22):5248–5257. [PubMed] [Google Scholar]
- 5.Buhard O, Suraweera N, Lectard A, Duval A, Hamelin R. Quasimonomorphic mononucleotide repeats for high-level microsatellite instability analysis. Dis Markers. 2004;20(4-5):251–257. doi: 10.1155/2004/159347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Chapelle A. Testing tumors for microsatellite instability. Eur J Hum Genet. 1999;7(4):407–408. doi: 10.1038/sj.ejhg.5200335. [DOI] [PubMed] [Google Scholar]
- 7.Deng G, Chen A, Hong J, Chae HS, Kim YS. Methylation of CpG in a small region of the hMLH1 promoter invariably correlates with the absence of gene expression. Cancer Res. 1999;59(9):2029–2033. [PubMed] [Google Scholar]
- 8.Fang DC, Wang RQ, Yang SM, Yang JM, Liu HF, Peng GY, Xiao TL, Luo YH. Mutation and methylation of hMLH1 in gastric carcinomas with microsatellite instability. World J Gastroenterol. 2003;9(4):655–659. doi: 10.3748/wjg.v9.i4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forster HP, Emanuel E, Grady C. The 2000 revision of the Declaration of Helsinki: a step forward or more confusion? Lancet. 2001;358(9291):1449–1453. doi: 10.1016/S0140-6736(01)06534-5. [DOI] [PubMed] [Google Scholar]
- 10.Grady WM. Genomic instability and colon cancer. Cancer Metastasis Rev. 2004;23(1-2):11–27. doi: 10.1023/A:1025861527711. [DOI] [PubMed] [Google Scholar]
- 11.Gu M, Kim D, Bae Y, Choi J, Kim S, Song S. Analysis of microsatellite instability, protein expression and methylation status of hMLH1 and hMSH2 genes in gastric carcinomas. Hepatogastroenterology. 2009;56(91-92):899–904. [PubMed] [Google Scholar]
- 12.Li FY, Lai MD. Colorectal cancer, one entity or three. J Zhejiang Univ-Sci B. 2009;10(3):219–229. doi: 10.1631/jzus.B0820273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim SB, Jeong SY, Lee MR, Ku JL, Shin YK, Kim WH, Park JG. Prognostic significance of microsatellite instability in sporadic colorectal cancer. Int J Colorectal Dis. 2004;19(6):533–537. doi: 10.1007/s00384-004-0596-2. [DOI] [PubMed] [Google Scholar]
- 14.Lubbe SJ, Webb EL, Chandler IP, Houlston RS. Implications of familial colorectal cancer risk profiles and microsatellite instability status. J Clin Oncol. 2009;27(13):2238–2244. doi: 10.1200/JCO.2008.20.3364. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, Wu L, Liu C, Xu L, Li D, Li JC. The correlation of genetic instability of PINX1 gene to clinicopathological features of gastric cancer in the Chinese population. J Cancer Res Clin Oncol. 2009;135(3):431–437. doi: 10.1007/s00432-008-0471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy KM, Zhang S, Geiger T, Hafez MJ, Bacher J, Berg KD, Eshleman JR. Comparison of the microsatellite instability analysis system and the Bethesda panel for the determination of microsatellite instability in colorectal cancers. J Mol Diagn. 2006;8(3):305–311. doi: 10.2353/jmoldx.2006.050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oda S, Zhao Y, Maehara Y. Microsatellite instability in gastrointestinal tract cancers: a brief update. Surg Today. 2005;35(12):1005–1015. doi: 10.1007/s00595-005-3125-1. [DOI] [PubMed] [Google Scholar]
- 18.Pawlik TM, Raut CP, Rodriguez-Bigas MA. Colorectal carcinogenesis: MSI-H versus MSI-L. Dis Markers. 2004;20(4-5):199–206. doi: 10.1155/2004/368680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, French AJ, Kabat B, Foster NR, Torri V, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28(20):3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo HM, Chang YS, Joo SH, Kim YW, Park YK, Hong SW, Lee SH. Clinicopathologic characteristics and outcomes of gastric cancers with the MSI-H phenotype. J Surg Oncol. 2009;99(3):143–147. doi: 10.1002/jso.21220. [DOI] [PubMed] [Google Scholar]
- 21.Suraweera N, Duval A, Reperant M, Vaury C, Furlan D, Leroy K, Seruca R, Iacopetta B, Hamelin R. Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology. 2002;123(6):1804–1811. doi: 10.1053/gast.2002.37070. [DOI] [PubMed] [Google Scholar]
- 22.Theuer CP, Campbell BS, Peel DJ, Lin F, Carpenter P, Ziogas A, Butler JA. Microsatellite instability in Japanese vs. European American patients with gastric cancer. Arch Surg. 2002;137(8):960–965. doi: 10.1001/archsurg.137.8.960. [DOI] [PubMed] [Google Scholar]
- 23.Umar A, Boland CR, Terdiman JP, Syngal S, Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (lynch syndrome) and microsatellite instabilit. J Natl Cancer Inst. 2004;96(4):261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vita FD, Giuliani F, Galizia G, Belli C, Aurilio G, Santabarbara G, Ciardiello F, Catalano G, Orditura M. Neo-adjuvant and adjuvant chemotherapy of gastric cancer. Ann Onc. 2007;18(Suppl. 6):120–123. doi: 10.1093/annonc/mdm239. [DOI] [PubMed] [Google Scholar]
- 25.Xiao YP, Wu DY, Xu L, Xin Y. Loss of heterozygosity and microsatellite instabilities of fragile histidine triad gene in gastric carcinoma. World J Gastroenterol. 2006;12(23):3766–3769. doi: 10.3748/wjg.v12.i23.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan Y, Huang YQ, Cai SR, Song YM, Zheng S, Zhang SZ. Genetic characterization of Chinese hereditary non-polyposis colorectal cancer by DHPLC and multiplex PCR. Jpn J Clin Oncol. 2004;34(11):660–666. doi: 10.1093/jjco/hyh121. [DOI] [PubMed] [Google Scholar]