Abstract
Objective: To explore the molecular mechanism by which cordycepin inhibits cell proliferation and induces apoptosis of human colorectal cancer cells. Methods: Cell counting and MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfopheny)-2H-tetrazolium, inner salt) method were used to monitor the effects of cordycepin on cell proliferation. Flow cytometry (FCM) was used to analyze the effects of cordycepin on the cell cycle progress. Annexin V-fluorescein isothiocyanate (FITC) analysis was used to detect apoptosis at a very early stage. Caspase-Glo was used to determine caspase activity and Western blot was used to measure protein expression levels of c-Jun N-terminal kinase (JNK), p38, and Bcl-2 pro-apoptosis family. Results: The numbers of viable SW480 and SW620 cells and the proliferation of these cells were significantly reduced with increases in cordycepin concentration (P<0.01). The cell cycle progression of SW480 and SW620 was arrested at the G0/G1 phase by the addition of cordycepin, and apoptosis rates of cordycepin treatments were increased compared with the control group. Cordycepin-treated cells showed phosphatidylserine valgus, suggesting the existence of early apoptosis. Caspase-3/7 and -9 activity significantly increased and the protein expression levels of JNK, p38, and Bax, Bid, Bim, and Puma from Bcl-2 pro-apoptosis molecules also increased after the treatment with cordycepin. Conclusions: Cordycepin can inhibit SW480 and SW620 cell proliferation and induce apoptosis. Apoptosis might be induced by enhancing JNK and p38 kinase activity and increasing the protein expression of Bcl-2 pro-apoptotic molecules.
Keywords: Colorectal cancer, Cordycepin, Proliferation, Apoptosis, Bcl-2 molecules
1. Introduction
Cordyceps sinensis is a rare herb traditionally used in Chinese medicine. It has been widely used in China for anti-aging and nourishing, and has been used for the treatment of various diseases such as cancer, hypertension, hepatitis, hyperglycemia, and hypercholesterolemia. It has also been reported to possess anti-tumor properties as well as the ability to scavenge free radicals and stimulate immunity (Lo et al., 2004; Wang et al., 2005; Wu et al., 2007; Paterson, 2008). Cordycepin (3-deoxyadenosine, 3′-dA) is the main active ingredient. Early studies have shown that cordycepin has various biological activities, which include inhibiting inflammation (Kim et al., 2006), inhibiting platelet aggregation (Cho et al., 2007), strengthening the immune system (Zhou et al., 2008), inhibiting the polyadenylation of mRNA (Lallas et al., 2004), and inhibiting human immunodeficiency virus infection (Müller et al., 1991). Studies also have shown that cordycepin has significant anti-tumor effects such as inhibiting cell proliferation and metastasis (Nakamura et al., 2005; Chang et al., 2008; Shi et al., 2008), inducing apoptosis (Wehbe-Janek et al., 2007; Wu et al., 2007; Chen et al., 2008; Thomadaki et al., 2008), and interfering with cell signaling pathways (Lee et al., 2009; Wong et al., 2010). Cordycepin is one of the 18 new anti-cancer drugs currently being investigated and developed by the National Cancer Institute in the USA (Kodama et al., 2000). However, the molecular mechanism by which cordycepin inhibits tumor cell proliferation and induces apoptosis remains unclear.
Proteins in the Bcl-2 family are key regulators involved in cell apoptosis. The Bcl-2 family includes anti-apoptotic and pro-apoptotic members which regulate the stabilities of mitochondrial structure and function. In other words, they act as “on/off switches” of the apoptosis pathway at the mitochondrial level (Cory et al., 2003; Willis and Adams, 2005). The growth factor receptor-mediated mitogen-activated protein kinase (MAPK) signal transduction pathway has been found to be activated at the occurrence of colorectal cancer, and it is also closely related to cell proliferation and differentiation (Schwartsmann et al., 2005; Roberts and Der, 2007). Three MAPK pathways have been found so far: the extracellular signal-regulated protein kinase (ERK) pathway, c-Jun N-terminal kinase (JNK) pathway, and p38 MAPK pathway. However, since it has not been proven that cordycepin affected Bcl-2 family expression and activation of MAPK pathways, further study is needed.
This study analyzed the effects of cordycepin on colorectal cancer cell proliferation and apoptosis, and also studied the relationship between caspase and the Bcl-2 family.
2. Materials and methods
2.1. Main reagents
Leibovitz L-15 culture medium and trypsin were purchased from Gibco Inc., USA. Phosphate buffered saline (PBS), dimethylsulfoxide (DMSO), trypan blue dye, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfopheny)-2H-tetrazolium inner salt (MTS), and fetal bovine serum (FBS) were purchased from Hangzhou Sijiqing Biological Engineering Materials Co., Ltd., Hangzhou, Zhejiang, China. Propidium iodide (PI) was purchased from Sigma Chemical Co., USA. Annexin V apoptosis detection kit and monoclonal antibodies for Bcl-2 pro-apoptotic family and the MAPK family were purchased from the Cell Signaling Inc., USA. Caspase-Glo ratio colorimetric analysis kit was purchased from Promega Co., USA. Cordycepin (purity ≥98%) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (NICPBP) of China, dissolved in DMSO at 0.5 mol/L as stock solution, and stored at −20 °C. During the experiment, stock cordycepin was diluted with culture medium based on the required final concentration. The final concentration of DMSO from the treatment group was less than 0.2% (v/v) versus 0.2% (v/v) from the control group.
2.2. Cell culture
Human colorectal cancer cells SW480 and SW620 from the Cancer Institute of Zhejiang University were cultured in Leibovitz L-15 with 10% (v/v) FBS (37 °C, saturated humidity, 5% (v/v) CO2). Cells were collected at the logarithmic growth phase at a density of (0.5–1.0)×108 cells/L. Before use in each experiment, cell growth was synchronized by overnight serum starvation.
2.3. Cell viability determination
For the determination of cell proliferation, 8×103 cells per well were seeded in 96-well plates (100 μl/well). Cordycepin was added after 24 h at final concentrations of 1000, 100, 10, 1, and 0.1 μmol/L versus the control group with 0.2% (v/v) DMSO medium. After 72-h incubation, 20 µl MTS (5 g/L) was added and mixed well, and then the plates were read by a microplate reader (Bio-Rad 450, Hercules, CA, USA) at 0 h (background value) and 3 h at 490 nm. Cell growth inhibition rate was calculated by the following equation:
| (1−ODexp/ODcon)×100% |
, where ODexp is the experimental group optical density (OD) value and ODcon is the control group OD value. For cell counts, 2×105 cells per well were seeded in six-well plates (2 ml/well). Cordycepin was added after 24 h at final concentrations of 1000, 100, and 10 μmol/L versus the control group with 0.2% (v/v) DMSO medium. After 24-, 48-, and 72-h treatments, cells were counted after applied by Trypan blue.
2.4. Cell cycle analysis and apoptosis detection
A total of 1×106 cells were treated for 48 h with 0.25 and 0.50 mmol/L in SW620 cells and 0.65 and 1.30 mmol/L in SW480 cells (1/3 and 2/3 of the half maximal inhibitory concentration (IC50) of appropriate cells). Then cells were washed twice with 0.01 mol/L pH 7.4 PBS, and mixed well with 10 ml 70% (v/v) cold ethanol. After being fixed at 4 °C overnight, cells were centrifuged and the pellets were washed with PBS. Ribonuclease (RNase; final concentration 25 mg/L) was added to the pellets, which were then incubated for 30 min at 37 °C. PI (final concentration at 50 mg/L) was added to the cells, which were incubated at 4 °C in dark for 30 min. Flow cytometry (FCM; BD Co., USA) was used to determine the proportion of dead cells by measuring cell numbers and DNA content at different cell cycle phases.
2.5. Annexin V-fluorescein isothiocyanate (FITC) assay
Around 1×106 cells were treated with cordycepin, then processed with ethylenediamine tetraacetic acid (EDTA)-free trypsin, and washed twice with PBS. After centrifugation, cells were double-stained with Annexin V-FITC and PI, and then detected at excitation wavelength of 488 nm and emission wavelength of 530 nm. All the analyses were done by FCM (BD Co., USA).
2.6. Caspase enzyme activity
A total of 1×104 cells were seeded per well on a 96-well plate. After 24-h incubation, different concentrations (1/3 and 2/3 of IC50 of appropriate cells) of cordycepin were added as treatment group concentrations versus 0.2% (v/v) DMSO medium as the control group. Following the caspase-Glo kit instruction, caspase-3/7, -8, and -9 enzyme activity was measured after another 24-h incubation.
2.7. Western blot
Cells were collected, washed with PBS, and lysed at 100 °C for 10 min in buffer. Protein was extracted from SW480 cells after the treatment with cordycepin for 48 h. Western blot was performed according to the Cell Signaling Technology Company’s instruction with slight adjustments. Protein expression levels were measured for Bax, Bid, Bim and Puma (Bcl-2 pro-apoptotic members), and also for ERK1/2, p38, and JNK. Blots were reacted at 4 °C overnight with appropriate primary and secondary antibodies (Zhu et al., 2005).
2.8. Statistical analysis
Comparison among groups was performed by using analysis of variance (ANOVA) at a level of 0.05 probabilities with SPSS 16.0. Results are presented as mean±standard deviation (SD).
3. Results
3.1. Cordycepin inhibits the proliferation of colorectal cancer cells
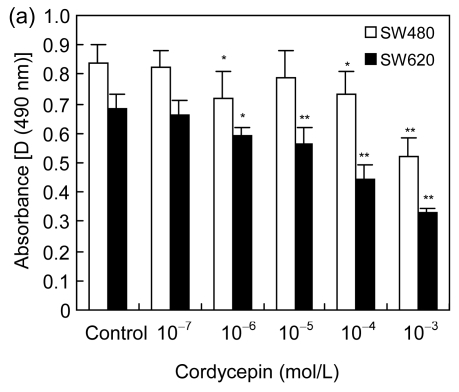
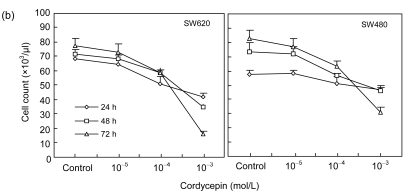
To study the effects of cordycepin on cell proliferation, we used MTS dye assay to analyze the proliferation of SW480 and SW620 cells. The results showed that the proliferation of SW480 (IC50 is 2 mmol/L) and SW620 (IC50 is 0.72 mmol/L) cells was significantly inhibited with increasing concentration of cordycepin (Fig. 1a) (P<0.05 or P<0.01). Additionally, we counted the numbers of SW480 and SW620 cells after the treatment of cordycepin. The results showed that the cell numbers were significantly reduced with cordycepin in dose- and time-dependent manners (Fig. 1b) (P<0.01). Our combined results imply that cordycepin inhibited the proliferation of colorectal cancer cells.
Fig. 1.
Effects of cordycepin on the proliferation of colorectal cancer cells in dose- and time-dependent manners in vitro
(a) Cordycepin inhibits the proliferation of SW480 and SW620 cells measured by MTS method. Cordycepin was added after 24 h at final concentrations of 1000, 100, 10, 1, and 0.1 μmol/L verses the control group with 0.2% (v/v) DMSO medium. MTS method was performed after 72-h treatment. * P<0.05 and ** P<0.01, compared with the control group; (b) Cordycepin inhibits the proliferation of SW620 and SW480 cells measured by cell counting. Cordycepin was added after 24 h at final concentrations of 1000, 100, and 10 μmol/L versus the control group with 0.2% (v/v) DMSO medium. After 24-, 48-, and 72-h treatments, Trypan blue was applied and cells were counted
3.2. Cordycepin arrests cell cycle progression and promotes apoptosis
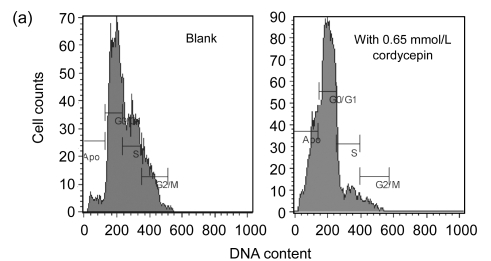
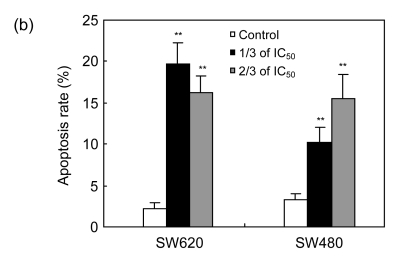
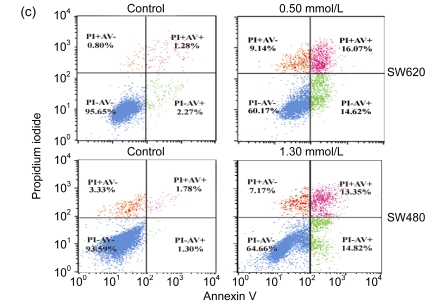
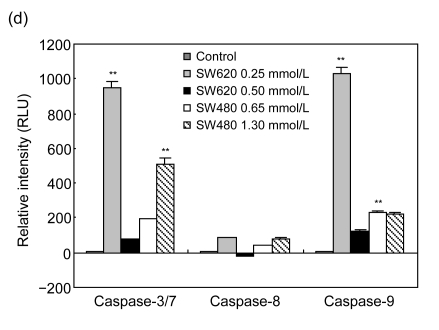
To study the molecular mechanism by which cordycepin inhibits the proliferation of colorectal cancer cells, we used FCM to analyze the effects of cordycepin on cell cycle progression and apoptosis, and we also measured the caspase enzyme activity of the cells treated with cordycepin. The results showed that the cell cycle progression was significantly inhibited in the G0/G1 phase (Fig. 2a) and apoptosis rates increased significantly after the treatment with cordycepin (Fig. 2b). Annexin V assay showed early apoptosis induced by cordycepin in both SW480 and SW620 cells (Fig. 2c). Caspase enzyme activity assay showed that the increase in caspase-3/7 and -9 activity was significant (Fig. 2d), which agreed with other report (Jen et al., 2009). All of these data suggest that cordycepin arrests cell cycle progression and promotes apoptosis. Compared with the control group, the difference was statistically significant (P<0.01).
Fig. 2.
Effects of cordycepin on cell cycle progression, apoptosis and caspase-3/7, -8, and -9 activity in colorectal cancer cells
(a) The cell cycle progression of SW480 was arrested at G0/G1 phase after 48-h treatment with cordycepin. The same effect was also observed in SW620 (data not shown); (b) Cordycepin caused apoptotic death in SW620 and SW480 cells after 48-h treatment. Flow cytometry was used to determine the proportion of dead cells by measuring cell numbers and DNA contents at different cell cycle phases; (c) Cordycepin induced apoptosis in SW620 and SW480 cells. Cells were collected after 24-h treatment with cordycepin, stained with annexin V/propidium iodide (PI), and evaluated by fluorescence activated cell sorter (FACS) analysis; (d) Cordycepin caused a significant increase in caspase-3/7 and -9 activity. Different concentrations of cordycepin were added as treatment group concentrations versus 0.2% (v/v) DMSO medium as a control group. Following the caspase-Glo kit instruction, caspase-3/7, -8, and -9 enzyme activity was measured after 24-h incubation. RLU: relative light unit. ** P<0.01, compared with the control group
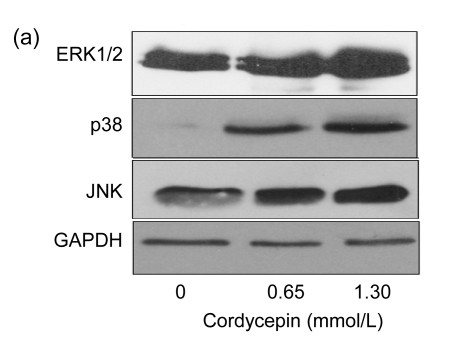
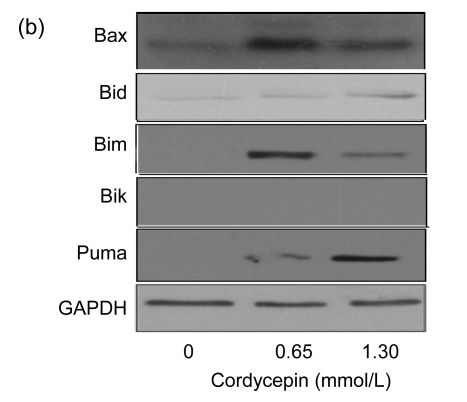
3.3. Cordycepin enhances the protein expression levels of JNK, p38 kinase, and Bcl-2 pro-apoptotic molecules
To study the mechanism by which cordycepin inhibits cell cycle progression and induces cell apoptosis, SW480 cells were used for protein expression analysis. MAPK and Bcl-2 pro-apoptotic members were analyzed as target proteins by Western blot. The results showed that the protein expression levels of JNK and p38 kinase increased significantly after the treatment with cordycepin, while the expression level of ERK1/2 (Fig. 3a) was not affected. The protein expression levels of Bcl-2 pro-apoptotic molecules (Bax, Bid, Bim, and Puma) also changed. Bid and Puma protein expression levels were positively correlated with cordycepin concentration, while the increases of Bid and Puma protein levels were more obvious in lower-dose cordycepin-treated cells (Fig. 3b). Together, these results imply that cordycepin induced cell apoptosis through activation of JNK and p38 kinase pathways and increases of the expression levels of Bcl-2 pro-apoptotic members such as Bim, Bid, Bax, and Puma.
Fig. 3.
Effects of cordycepin on the protein expression levels of JNK, p38 kinase, and Bcl-2 pro-apoptotic molecules
Protein was extracted from SW480 cells after the treatment with cordycepin 0.65 and 1.30 mmol/L for 48 h. The results show that cordycepin enhanced the protein expression levels of JNK, p38 kinase, and Bcl-2 pro-apoptotic molecules. (a) Blots were developed as indicated with antibodies specific for ERK1/2, p38, and JNK; (b) Blots were developed as indicated with antibodies specific for Bax, Bid, Bim, Bik, and Puma
4. Discussion
Recent studies have indicated that many of the biological effects of cordycepin are correlated with mammalian target of rapamycin (mTOR) and adenosine monophosphate-activated protein kinase (AMPK) signaling pathways. Cordycepin inhibits mTOR signal transduction and protein synthesis through activation of AMPK (Wong et al., 2010). It also can induce apoptosis through activation of caspase-9, -3, and -7 (Jen et al., 2009), change the expression of metabolism-related proteins (Shi et al., 2008), activate adenosine A3 receptors, reduce the expression of cell-cycle regulating protein cyclin D1 (Yoshikawa et al., 2008), increase p21WAF1 expression, and activate JNK (Lee et al., 2009). However, the molecular mechanism underlying cordycepin-induced apoptosis needs to be further understood.
There are three known ways to induce apoptosis: the death receptor pathway, the mitochondrial pathway, and endoplasmic reticulum stress-induced apoptosis. The mitochondrial pathway is the one which has been relatively well studied and understood. Bcl-2 family is the key regulator of the mitochondrial pathway, which includes anti-apoptotic members and pro-apoptotic members (Cory et al., 2003; Niquet and Wasterlain, 2004). When cells get stimulated by external signals, a protease causes the conformational changes of pro-apoptotic proteins (Bax, Bid, Bim, etc.) which then cause the functionality loss of organelles and the loss of the apoptosis inhibitory activities of anti-apoptotic proteins, leading to the release of various pro-apoptotic factors such as cytochrome c, apoptosis-inducing factors, and Smac. Afterwards, caspase activation breaks important intracellular structural proteins and functional molecules, and ultimately leads to cell apoptosis (Cory et al., 2003; Niquet and Wasterlain, 2004). Our research shows that, after cordycepin treatment, the protein expression levels of Bax, Bid, Bim, and Puma (Bcl-2 pro-apoptotic members) changed, which suggests that cordycepin might induce apoptosis by increasing the expression levels of Bcl-2 pro-apoptotic members such as Bax, Bid, Bim, and Puma.
The MAPK cell signaling pathway is important for gene regulation since it brings extracellular signals to the nucleus extracellular stimuli (Xia et al., 1995). The growth factor receptor-mediated MAPK signal transduction pathway was found to be activated at the occurrence of colorectal cancer, and was also found to be closely related to cell proliferation and differentiation; therefore, it could be a potential drug target to prevent and treat cancers (Schwartsmann et al., 2005; Roberts and Der, 2007). From our study, since cordycepin increased JNK and p38 kinase activity, it indicated that activating JNK and p38 kinase pathways could possibly be a mechanism for cordycepin to induce apoptosis. It has been reported that the activation of MAPK involved a signal transduction pathway that is potentially correlated with Bcl-2 family proteins involved in apoptosis, and JNK and p38 kinase can induce Bax translocation (van Laethem et al., 2004; Gao et al., 2005); however, it needs to be further studied whether cordycepin affects the Bc1-2 family to induce apoptosis through MAPK-involved signaling pathway.
Our research has shown the following conclusions: (1) Cordycepin inhibits the proliferation of colorectal cancer cells SW480 and SW620; (2) Cordycepin inhibits cell cycle progression; thus, the number of G0/G1 phase cells increased; (3) Cordycepin activates JNK and p38 kinase and increases protein expression levels of Bcl-2 pro-apoptotic molecules such as Bax, Bid, Bim, and Puma. This suggests that cordycepin can directly inhibit cell proliferation of SW480 and SW620 cells and induce cell apoptosis. It may activate JNK and p38 kinase MAPK pathways and increase the Bcl-2 family protein expression, then eventually increase the activity of caspase.
Acknowledgments
We thank Dr. Estrellita BERMUDEZ and Zheng-yu ZHANG of the Pennington Biomedical Research Center, Baton Rouge, LA, USA for proofreading this paper.
Footnotes
Project supported by the Department of Education of Zhejiang Province (No. Y200804636), the Department of Science and Technology of Zhejiang Province (Nos. 2008C23049, 2007C23027, and 2009C33081), and the Natural Science Foundation of Zhejiang Province (No. Y206174), China
References
- 1.Chang W, Lim S, Song H, Song BW, Kim HJ, Cha MJ, Sung JM, Kim TW, Hwang KC. Cordycepin inhibits vascular smooth muscle cell proliferation. Eur J Pharmacol. 2008;597(1-3):64–69. doi: 10.1016/j.ejphar.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 2.Chen LS, Stellrecht CM, Gandhi V. RNA-directed agent, cordycepin, induces cell death in multiple myeloma cells. Br J Haematol. 2008;140(6):682–691. doi: 10.1111/j.1365-2141.2007.06955.x. [DOI] [PubMed] [Google Scholar]
- 3.Cho HJ, Cho JY, Rhee MH, Kim HS, Lee HS, Park HJ. Inhibitory effects of cordycepin (3'-deoxy-adenosine), a component of Cordyceps militaris, on human platelet aggregation induced by thapsigargin. J Microbiol Biotechnol. 2007;17(7):1134–1138. [PubMed] [Google Scholar]
- 4.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22(53):8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y, Signore AP, Yin W, Cao G, Yin XM, Sun F, Luo Y, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J Cereb Blood Flow Metab. 2005;25(6):694–712. doi: 10.1038/sj.jcbfm.9600062. [DOI] [PubMed] [Google Scholar]
- 6.Jen CY, Lin CY, Leu SF, Huang BM. Cordycepin induced MA-10 mouse Leydig tumor cell apoptosis through caspase-9 pathway. Evid Based Complement Alternat Med. 2009 doi: 10.1093/ecam/nen084. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HG, Shrestha B, Lim SY, Yoon DH, Chang WC, Shin DJ, Han SK, Park SM, Park JH, Park HI, et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol. 2006;545(2-3):192–199. doi: 10.1016/j.ejphar.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 8.Kodama EN, McCaffrey RP, Yusa K, Mitsuya H. Antileukemic activity and mechanism of action of cordycepin against terminal deoxynucleotidyl transferase-positive (TdT+) leukemic cells. Biochem Pharmacol. 2000;59(3):273–281. doi: 10.1016/S0006-2952(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 9.Lallas GC, Courtis N, Havredaki M. K562 cell sensitization to 5-fluorouracil- or interferon-alpha-induced apoptosis via cordycepin (3′-deoxyadenosine): fine control of cell apoptosis via poly(A) polymerase upregulation. Int J Biol Markers. 2004;19(1):58–66. doi: 10.1177/172460080401900108. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, Kim SK, Choi WS, Kim WJ, Moon SK. Cordycepin causes p21WAF1-mediated G2/M cell-cycle arrest by regulating c-Jun N-terminal kinase activation in human bladder cancer cells. Arch Biochem Biophys. 2009;490(2):103–109. doi: 10.1016/j.abb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Lo HC, Tu ST, Lin KC, Lin SC. The anti-hyperglycemic activity of the fruiting body of cordyceps in diabetic rats induced by nicotinamide and streptozotocin. Life Sci. 2004;74(23):2897–2908. doi: 10.1016/j.lfs.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Müller WEG, Weiler BE, Charubala R, Pfleiderer W, Leserman L, Sobol RW, Suhadolnik RJ, Schröder HC. Cordycepin analogues of 2′,5′-oligoadenylate inhibit human immunodeficiency virus infection via inhibition of reverse transcriptase. Biochemistry. 1991;30(8):2027–2033. doi: 10.1021/bi00222a004. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura K, Konoha K, Yoshikawa N, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Effect of cordycepin (3′-deoxyadenosine) on hematogenic lung metastatic model mice. In Vivo. 2005;19(1):137–141. [PubMed] [Google Scholar]
- 14.Niquet J, Wasterlain CG. Bim, Bad, and Bax: a deadly combination in epileptic seizures. J Clin Invest. 2004;113(7):960–962. doi: 10.1172/JCI21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paterson RR. Cordyceps: a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry. 2008;69(7):1469–1495. doi: 10.1016/j.phytochem.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 17.Schwartsmann G, Di Leone LP, Dal Pizzol F, Roesler R. MAPK pathway activation in colorectal cancer: a therapeutic opportunity for GRP receptor antagonists. Lancet Oncol. 2005;6(7):444–445. doi: 10.1016/S1470-2045(05)70226-6. [DOI] [PubMed] [Google Scholar]
- 18.Shi P, Huang Z, Tan X, Chen G. Proteomic detection of changes in protein expression induced by cordycepin in human hepatocellular carcinoma BEL-7402 cells. Methods Find Exp Clin Pharmacol. 2008;30(5):347–353. doi: 10.1358/mf.2008.30.5.1186085. [DOI] [PubMed] [Google Scholar]
- 19.Thomadaki H, Scorilas A, Tsiapalis CM, Havredaki M. The role of cordycepin in cancer treatment via induction or inhibition of apoptosis: implication of polyadenylation in a cell type specific manner. Cancer Chemother Pharmacol. 2008;61(2):251–265. doi: 10.1007/s00280-007-0467-y. [DOI] [PubMed] [Google Scholar]
- 20.van Laethem A, van Kelst S, Lippens S, Declercq W, Vandenabeele P, Janssens S, Vandenheede JR, Garmyn M, Agostinis P. Activation of p38 MAPK is required for Bax translocation to mitochondria, cytochrome c release and apoptosis induced by UVB irradiation in human keratinocytes. Faseb J. 2004;18(15):1946–1948. doi: 10.1096/fj.04-2285fje. [DOI] [PubMed] [Google Scholar]
- 21.Wang BJ, Won SJ, Yu ZR, Su CL. Free radical scavenging and apoptotic effects of Cordyceps sinensis fractionated by supercritical carbon dioxide. Food Chem Toxicol. 2005;43(4):543–552. doi: 10.1016/j.fct.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Wehbe-Janek H, Shi Q, Kearney CM. Cordycepin/hydroxyurea synergy allows low dosage efficacy of cordycepin in MOLT-4 leukemia cells. Anticancer Res. 2007;27(5A):3143–3146. [PubMed] [Google Scholar]
- 23.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17(6):617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong YY, Moon A, Duffin R, Barthet-Barateig A, Meijer HA, Clemens MJ, de Moor CH. Cordycepin inhibits protein synthesis and cell adhesion through effects on signal transduction. J Biol Chem. 2010;285(4):2610–2621. doi: 10.1074/jbc.M109.071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu JY, Zhang QX, Leung PH. Inhibitory effects of ethyl acetate extract of Cordyceps sinensis mycelium on various cancer cells in culture and B16 melanoma in C57BL/6 mice. Phytomedicine. 2007;14(1):43–49. doi: 10.1016/j.phymed.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270(5240):1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 27.Yoshikawa N, Yamada S, Takeuchi C, Kagota S, Shinozuka K, Kunitomo M, Nakamura K. Cordycepin (3′-deoxyadenosine) inhibits the growth of B16-BL6 mouse melanoma cells through the stimulation of adenosine A3 receptor followed by glycogen synthase kinase-3beta activation and cyclin D1 suppression. Naunyn-Schmiedeberg’s Arch Pharmacol. 2008;377(4-6):591–595. doi: 10.1007/s00210-007-0218-y. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Luo L, Dressel W, Shadier G, Krumbiegel D, Schmidtke P, Zepp F, Meyer CU. Cordycepin is an immunoregulatory active ingredient of Cordyceps sinensis . Am J Chin Med. 2008;36(5):967–980. doi: 10.1142/S0192415X08006387. [DOI] [PubMed] [Google Scholar]
- 29.Zhu YL, Zhong X, Zheng S, Du Q, Xu WZ. Transformed immortalized gastric epithelial cells by virulence factor CagA of Helicobacter pylori through Erk mitogen-activated protein kinase pathway. Oncogene. 2005;24(24):3886–3895. doi: 10.1038/sj.onc.1208551. [DOI] [PubMed] [Google Scholar]