Abstract
Sevoflurane postconditioning reduces myocardial infarct size. The objective of this study was to examine the role of the phosphatidylinositol-3-kinase (PI3K)/Akt pathway in anesthetic postconditioning and to determine whether PI3K/Akt signaling modulates the expression of pro- and antiapoptotic proteins in sevoflurane postconditioning. Isolated and perfused rat hearts were prepared first, and then randomly assigned to the following groups: Sham-operation (Sham), ischemia/reperfusion (Con), sevoflurane postconditioning (SPC), Sham plus 100 nmol/L wortmannin (Sham+Wort), Con+Wort, SPC+Wort, and Con+dimethylsulphoxide (DMSO). Sevoflurane postconditioning was induced by administration of sevoflurane (2.5%, v/v) for 10 min from the onset of reperfusion. Left ventricular developed pressure (LVDP), left ventricular end-diastolic pressure (LVEDP), maximum increase in rate of LVDP (+dP/dt), maximum decrease in rate of LVDP (−dP/dt), heart rate (HR), and coronary flow (CF) were measured at baseline, R30 min (30 min of reperfusion), R60 min, R90 min, and R120 min. Creatine kinase (CK) and lactate dehydrogenase (LDH) were measured after 5 min and 10 min reperfusion. Infarct size was determined by triphenyltetrazolium chloride staining at the end of reperfusion. Total Akt and phosphorylated Akt (phospho-Akt), Bax, Bcl-2, Bad, and phospho-Bad were determined by Western blot analysis. Analysis of variance (ANOVA) and Student-Newman-Keuls’ test were used to investigate the significance of differences between groups. The LVDP, ±dP/dt, and CF were higher and LVEDP was lower in the SPC group than in the Con group at all points of reperfusion (P<0.05). The SPC group had significantly reduced CK and LDH release and decreased infarct size compared with the Con group [(22.9±8)% vs. (42.4±9.4)%, respectively; P<0.05]. The SPC group also had increased the expression of phosphor-Akt, Bcl-2, and phospho-Bad, and decreased the expression of Bax. Wortmannin abolished the cardioprotection of sevoflurane postconditioning. Sevoflurane postconditioning may protect the isolated rat heart. Activation of PI3K and modulation of the expression of pro- and antiapoptotic proteins may play an important role in sevoflurane-induced myocardial protection.
Keywords: Sevoflurane, Postconditioning, Cardioprotection, Akt, Bcl-2, Bad
1. Introduction
Using volatile anesthetics in early reperfusion to protect against myocardial reperfusion injuries was first proposed in the 1990s (Schlack et al., 1998) and in vitro (Preckel et al., 1998; 1999). In recent years the method has been called “anesthetic postconditioning (APO)” (Chiari et al., 2005). Postconditioning is an effective therapeutic strategy for attaining myocardial protection against ischemia/reperfusion injuries, and from a clinical point of view, postconditioning is particularly promising to provide effective protection, as no previous information of the ischemic event is required.
Research has been focused on the cardio-protective mechanisms of pharmacological-induced postconditioning. However, the mechanisms underlying anesthetic postconditioning are complex and are not yet well understood. Previous research has mainly dealt with the following aspects: release of reactive oxygen species (ROS) (Tsutsumi et al., 2007), reduction of Ca2+ overload (Obal et al., 2005), attenuation of myocardial apoptosis (Venkatapuram et al., 2006), modulation of the phosphatidylinositol-3-kinase (PI3K)/Akt (Chiari et al., 2005) and its downstream component glycogen synthase kinase 3β (GSK3β) (Pagel et al., 2006), activation of extracellular signal-regulated kinase (ERK1/2) pathway (Krolikowski et al., 2006), opening of mitochondrial adenosine triphosphate (ATP)-sensitive potassium channels (mitoKATP), and inhibition of mitochondrial permeability transition pore (mPTP) opening (Krolikowski et al., 2005).
Due to its low tissue and blood/gas partition coefficient, sevoflurane, a new type of volatile anaesthetic, provides more controllable depth of anaesthesia and more rapid recovery than other volatile anaesthetics. Research has demonstrated that anesthetic postconditioning reduces ischemia/reperfusion myocardial infarction and attenuates cardiocyte apoptosis (Obal et al., 2001; 2005; Krolikowski et al., 2005; Weihrauch et al., 2005; Wang et al., 2006). Many genes have been reported to be linked to the regulation of apoptosis under physiological and pathological conditions. Among such genes, the Bcl-2 family has been suggested as a major one controlling the pathway to apoptotic cell death, especially the roles of antiapoptotic protein Bcl-2 and pro-apoptotic protein Bax. The antiapoptotic prosurvival kinase signaling cascade PI3K/Akt (protein kinase B) pathway has been demonstrated to provide protection against ischemia/reperfusion injuries (Fujio et al., 2000), and has also been proposed to play a central role in reducing apoptosis by modulating the Bcl-2 family (Takatani et al., 2004). Activation of the PI3K/Akt pathway has been shown to be essential for the antiapoptotic effects of hypoxic preconditioning (Uchiyama et al., 2004; Hausenloy et al., 2005) and isoflurane preconditioning (Raphael et al., 2006) in the protection of cardiomyocytes. Previous studies have indicated that adoption of sevoflurane at the onset of reperfusion may protect against myocardial reperfusion injuries by activating the PI3K/Akt pathway (Li et al., 2008). However, it is not clear whether PI3K/Akt signaling is also essential for sevoflurane postconditioning-induced reduction of myocardial apoptosis. In this study, we have investigated the hypothesis that sevoflurane-induced postconditioning attenuates myocardial apoptosis in isolated rat hearts via the PI3K/Akt signaling and the modulation of Bcl-2 family proteins.
2. Materials and methods
All experimental procedures and protocols used in this investigation were reviewed and approved by the Animal Care and Use Committee at the School of Medicine of Zhejiang University, China, and they also conformed to the Guiding Principles in the Care and Use of Laboratory Animals published by the National Institutes of Health of the USA (NIH publication 85-23, revised in 1996).
2.1. Langendorff heart preparation
Male Sprague-Dawley rats (230–250 g) were heparinized (500 U i.p.) and anesthetized (pentobarbital sodium, 60 mg/kg i.p.). The hearts were immediately excised and perfused in a non-recirculation Langendorff apparatus with Krebs-Henseleit buffer (155 mmol/L Na+, 5.6 mmol/L K+, 138 mmol/L Cl−, 2.1 mmol/L Ca2+, 1.2 mmol/L PO4 3−, 25 mmol/L HCO3 −, 0.56 mmol/L Mg2+, and 11 mmol/L glucose). The buffer (pH 7.4±0.5) was oxygenated with a mixture of 95% (v/v) O2 and 5% (v/v) CO2 [partial pressure of O2 (PO2): (381±8) mmHg; partial pressure of CO2 (PCO2): (19±2) mmHg], the temperature was maintained at (37±0.1) °C, and the perfusion pressure was kept at 80 mmHg throughout the experimental procedures. A small latex balloon connected to a pressure transducer (RM6240, Chengdu, China) was inserted into the left ventricle through the left atrium and the mitral valve. The balloon was filled with saline to achieve a left ventricular end-diastolic pressure (LVEDP) of 4–6 mmHg and the balloon volume remained unchanged (Li et al., 2008). Heart temperature was maintained at 37 °C.
2.2. Experimental protocol
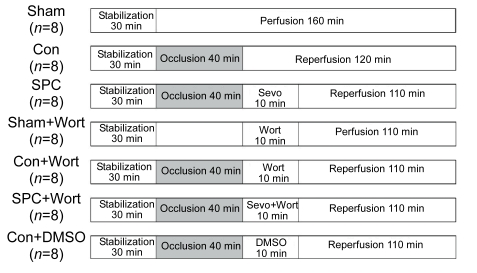
Each experiment lasted 190 min in total (Fig. 1), with 30 min equilibration, 40 min global ischemia, and then 120 min reperfusion in general. The prepared hearts were randomly assigned into the following seven groups (n=8 in each group): (1) Sham-operated group (Sham): 30 min equilibration and then direct perfusion with Krebs-Henseleit buffer for 160 min; (2) Control group (Con): global ischemia for 40 min followed by 120 min reperfusion; (3) Sevoflurane postconditioning group (SPC): administration of 2.5% (v/v) sevoflurane [1.0 minimum alveolar concentration (MAC) in rats at 37 °C] for 10 min right at the onset of reperfusion. The buffer solution was equilibrated with sevoflurane using a Sevotec 3 vaporizer (Datex-Ohmeda, Tewksbury, MA) with an air bubbler. Sevoflurane concentrations were measured in the buffer solution right before entering the aorta using a gas chromatograph [Perkin-Elmer, Norwalk, CT; (0.50±0.04) mmol/L]. All reservoirs were filled with buffer saturated with 1.0 MAC sevoflurane by adding sevoflurane to the perfusate 10 min before opening the three-way stopcock for reperfusion; (4) Sham+wortmannin group (Sham+Wort): administration of 100 nmol/L wortmannin, a selective PI3K inhibitor dissolved in dimethylsulphoxide (DMSO), for 10 min prior to 110 min of perfusion; (5) Control+wortmannin group (Con+Wort): administration of 100 nmol/L wortmannin (Tsang et al., 2004) for 10 min at the onset of reperfusion; (6) Sevoflurane+wortmannin group (Sevo+Wort): administration of both 100 nmol/L wortmannin in DMSO and 2.5% (v/v) sevoflurane for 10 min at the onset of reperfusion; and (7) Control+DMSO group (Con+DMSO): administration 0.02% (v/v) DMSO for 10 min at the onset of reperfusion. The sevoflurane was from Maruishi Pharmaceutical Co., Ltd., Osaka, Japan, and the wortmannin and DMSO were from Sigma-Aldrich, CA, USA.
Fig. 1.
Experimental protocol
Except the Sham and Sham+Wort groups, which were perfused constantly for 190 min, the remaining hearts were subjected to 40 min of global ischemia followed by 120 min of reperfusion. 1.0 MAC of sevoflurane was administered for 10 min at the onset of reperfusion. Wortmannin (100 nmol/L) was also administered for 10 min at the onset of reperfusion with sevoflurane absence or presence. Sevo: sevoflurane; Wort: wortmannin; DMSO: dimethylsulphoxide (vehicle of wortmannin)
2.3. Measurements of hemodynamics
The measured hemodynamic parameters were left ventricular developed pressure (LVDP), left ventricular end-diastolic pressure (LVEDP), maximum increase in the rate of LVDP (+dP/dt), maximum decrease in the rate of LVDP (−dP/dt), and heart rate (HR). Coronary flow (CF) was measured by timed collection of coronary effluent. These parameters were continuously measured throughout the experimental procedure.
2.4. Measurements of creatine kinase (CK) and lactate dehydrogenase (LDH) release
The severity of myocardial injuries can be indicated by concentrations of CK and LDH in the perfused buffer (He et al., 2008). To evaluate the myocardial injuries, 1 ml of effluent was first collected after 5 and 10 min reperfusion. The samples were analyzed by spectrophotometry to determine the CK and LDH (Cao et al., 2004). LDH activity was expressed as U/L (Li et al., 1999).
2.5. Determination of myocardial infarct size
After 120 min reperfusion, myocardial infarct size was determined by 2,3,5-triphenyltetrazolium chloride staining, as described by Feng et al. (2005). Hearts were frozen at −20 °C for 2 h and subsequently sliced as six 2-mm cross-sections across the long axis. The sections were incubated at 37 °C for 5 min with 1% (w/v) of 2,3,5-triphenyltetrazolium chloride in 0.1 mol/L phosphate buffer (pH 7.4), then fixed for 24 h in 10% (v/v) formaldehyde, and scanned. Tissues that were stained brick red were taken as viable; tissues that appeared pale or white were deemed to be necrotic. Planimetric analysis was performed using the ImageJ 1.38 analysis software. The size of the infarcted myocardium was calculated as an area ratio (necrotic area/total area of the myocardium×100%).
2.6. Western blot analysis
Seven similar experimental groups of rats (n=10 in each group) were subjected to the same experimental protocol. Left ventricular tissues were obtained and immediately frozen in liquid nitrogen (−70 °C) after 10 min (for total Akt and phosphorylated Akt (phospho-Akt) proteins, n=5) and 120 min (for Bcl-2 family proteins, n=5) reperfusion. Subsequently, the tissue samples were powdered by liquid nitrogen in mortars and homogenized in ice-cold lysis buffer (Beyotime Institute of Biotechnology, China) for 30 min. The lysis buffer contained 20 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1% (v/v) Triton X-100, 2.5 mmol/L sodium pyrophosphate, β-glycerophosphate, 1 mmol/L Na3VO4, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), leupeptin, 1 mmol/L phenylmethanesulfonyl fluoride (PMSF; Beyotime Institute of Biotechnology, China), and 1 mmol/L complete proteinase inhibitor cocktail (Sigma, St. Louis, MO, USA). The powdered samples were centrifuged at 13 000×g for 15 min at 4 °C to remove cellular debris and to isolate total proteins. The BCA protein assay kit (Beyotime Institute of Biotechnology, China) was used to determine protein concentrations in the supernatants. The protein samples were mixed with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample-loading buffer and denatured at 100 °C for 5 min. Equivalent amounts (80 μg) of protein samples were loaded and separated on 12% (v/v) SDS-PAGE and then electrophoretically transferred to a polyvinylidene difluoride membrane (Millipore Co., Billerica, MA, USA). In addition, gels were stained with Coomassie blue to confirm that equal amounts of proteins had been loaded onto each lane. The membranes were blocked with 5% (w/v) nonfat dry milk in phosphate-buffered saline containing 0.1% (v/v) Tween 20 (PBST) for 1 h at room temperature, and subsequently incubated overnight at 4 °C with the following primary antibodies: rabbit monoclonal anti-Akt, phospho-Akt (at Ser473), Bcl-2; mouse monoclonal anti-Bax, Bad, phospho-Bad (Ser136) (Cell Signaling Technology, Beverly, MA), 1:1000 dilution with 5% (w/v) bovine serum albumin in Tris-HCl buffered saline containing 0.1% (v/v) Tween 20 (TBST). The β-actin (1:2000 dilution; Protein Tech Group, Inc., Chicago, USA) was also detected as a loading control for protein quantity. After washing three times with PBST for 15 min, the membranes were subsequently incubated for 2 h in 5% (w/v) bovine serum albumin in TBST containing the appropriate secondary antibody of either a goat anti-rabbit or goat anti-mouse immunoglobulin G (IgG) conjugated to horseradish peroxidase (Beyotime Institute of Biotechnology, China) at 1:1000 dilution. Immune complexes were detected using the infrared imaging system (Odyssey, USA). Scanned images were imported into quantity one analyzer (Bio-Rad Laboratories, CA). Scanning densitometry was used for semi-quantitative analysis.
2.7. Statistical analysis
All data are presented as mean±standard error of the mean (SEM). The statistical analysis was performed using SPSS 16.0 (Chicago, USA). The analysis of variance (ANOVA) was used and followed by post-hoc Tukey test analysis for multiple comparisons to investigate the significance of differences between groups. The differences were considered significant when P<0.05.
3. Results
3.1. Hemodynamic parameters
The measured hemodynamic data are summarized in Table 1. Baseline hemodynamics among all experimental groups were similar (P>0.05), and there were no differences during the experiment in the Sham and Sham+Wort groups. After 40 min global ischemia, the LVDP, ±dP/dt, and CF were higher and the LVEDP was lower in the SPC group than in the Con group throughout reperfusion (P<0.05), and though the HR of the SPC group was higher than that of the Con group, this did not reach statistical significance (P>0.05). The recovery of myocardial function due to sevoflurane postconditioning was completely abolished by wortmannin, which did not influence postischemic recovery of myocardial function when given alone (P>0.05). DMSO had no effect on cardiac variables (P>0.05).
Table 1.
Hemodynamic parameters of all experimental groups
| Group | LVEDP (mmHg) | LVDP (mmHg) | +dP/dt (mmHg/s) | −dP/dt (mmHg/s) | HR (beat/min) | CF (ml/min) |
| Baseline | ||||||
| Sham | 5±1 | 97±4 | 3374±137 | 2077±85 | 320±18 | 8.3±0.3 |
| Con | 5±1 | 98±6 | 3164±163 | 1964±108 | 292±16 | 7.9±0.4 |
| SPC | 5±1 | 91±5 | 3196±188 | 2010±137 | 305±16 | 8.1±0.5 |
| Sham+Wort | 5±1 | 98±3 | 3414±139 | 2185±116 | 309±19 | 8.1±0.3 |
| Con+Wort | 5±1 | 89±6 | 2931±247 | 1916±74 | 319±21 | 8.4±0.3 |
| SPC+Wort | 5±1 | 87±6 | 2930±290 | 1985±140 | 299±22 | 7.8±0.3 |
| Con+DMSO | 5±1 | 98±4 | 2929±181 | 1902±94 | 291±18 | 8.2±0.5 |
| R30 min | ||||||
| Sham | 9±1 | 87±7 | 2677±156 | 1428±92 | 292±21 | 7.8±0.2 |
| Con | 50±3^ | 38±4^ | 1034±170^ | 673±92^ | 251±14 | 5.2±0.4^ |
| SPC | 31±1^* | 62±4^* | 2242±182* | 1082±50* | 273±13 | 7.4±0.4* |
| Sham+Wort | 15±2*# | 70±4* | 2558±127* | 1327±76* | 289±14 | 7.6±0.2* |
| Con+Wort | 52±3^#∆ | 32±6^#∆ | 982±187^#∆ | 660±108^#∆ | 253±15 | 5.2±0.3^#∆ |
| SPC+Wort | 50±3^#∆ | 34±5^#∆ | 1067±202^#∆ | 672±50^#∆ | 251±14 | 5.0±0.2^#∆ |
| Con+DMSO | 49±3^#∆ | 32±6^#∆ | 890±182^#∆ | 593±97^#∆ | 251±18 | 5.1±0.3^#∆ |
| R60 min | ||||||
| Sham | 11±2 | 84±7 | 2365±162 | 1367±139 | 280±21 | 6.8±0.3 |
| Con | 46±2^ | 37±4^ | 1248±137^ | 691±57^ | 242±13 | 4.6±0.4^ |
| SPC | 26±1^* | 63±5* | 2064±127* | 1095±43* | 268±16 | 6.7±0.4* |
| Sham+Wort | 15±2*# | 65±4* | 2309±116* | 1152±68* | 276±13 | 6.8±0.2* |
| Con+Wort | 44±3^#∆ | 38±5^#∆ | 1214±158^#∆ | 676±73^#∆ | 244±13 | 4.5±0.3^#∆ |
| SPC+Wort | 43±4^#∆ | 40±5^#∆ | 1243±156^#∆ | 765±47^#∆ | 243±15 | 4.6±0.2^#∆ |
| Con+DMSO | 42±2^#∆ | 38±4^#∆ | 1248±193^#∆ | 706±57^#∆ | 242±12 | 4.5±0.3^#∆ |
| R90 min | ||||||
| Sham | 13±2 | 78±6 | 2158±150 | 1191±103 | 278±21 | 6.2±0.2 |
| Con | 42±3^ | 36±2^ | 1177±112^ | 655±49^ | 238±17 | 4.0±0.4^ |
| SPC | 25±1^* | 59±5* | 1949±112* | 1009±45* | 264±16 | 6.0±0.3* |
| Sham+Wort | 15±2*# | 61±3* | 2052±120* | 1054±57* | 269±11 | 6.1±0.2* |
| Con+Wort | 41±3^#∆ | 33±5^#∆ | 1142±146^#∆ | 631±66^#∆ | 240±7 | 4.0±0.2^#∆ |
| SPC+Wort | 40±3^#∆ | 36±5^#∆ | 1145±155^#∆ | 685±47^#∆ | 239±13 | 4.1±0.2^#∆ |
| Con+DMSO | 39±1^#∆ | 39±5^#∆ | 1170±164^#∆ | 674±57^#∆ | 239±10 | 4.1±0.3^#∆ |
| R120 min | ||||||
| Sham | 14±2 | 72±5 | 1948±142 | 1109±117 | 269±19 | 5.5±0.2 |
| Con | 41±2^ | 33±3^ | 1094±118^ | 565±52^ | 240±17 | 3.4±0.4^ |
| SPC | 25±1^* | 56±5* | 1839±115* | 958±49* | 265±16 | 5.1±0.2* |
| Sham+Wort | 14±1*# | 59±3* | 1868±119* | 943±53* | 266±9 | 5.5±0.3* |
| Con+Wort | 39±3^#∆ | 30±5^#∆ | 1039±141^#∆ | 586±75^#∆ | 235±5 | 3.5±0.2^#∆ |
| SPC+Wort | 38±2^#∆ | 33±5^#∆ | 1198±167^#∆ | 602±37^#∆ | 232±14 | 3.4±0.3^#∆ |
| Con+DMSO | 36±1^#∆ | 36±4^#∆ | 1153±196^#∆ | 628±57^#∆ | 233±9 | 3.4±0.3^#∆ |
Data are presented as mean±SEM, n=8 in each group.
P<0.05 compared with the Sham group
P<0.05 compared with the Con group
P<0.05 compared with the SPC group
P<0.05 compared with the Sham+Wort group
Sham: Sham-operation group; Con: ischemia/reperfusion group; SPC: sevoflurane postconditioning group; Sham+Wort: Sham+wortmannin group; Con+Wort: Con+wortmannin group; SPC+Wort: SPC+wortmannin group; Con+DMSO: Con+dimethylsulphoxide group. LVDP: left ventricular developed pressure; LVEDP: left ventricular end-diastolic pressure; +dP/dt: maximum increase in rate of LVDP; −dP/dt: maximum decrease in rate of LVDP; HR: heart rate; CF: coronary flow
3.2. Effects on CK and LDH release and myocardial infarct size
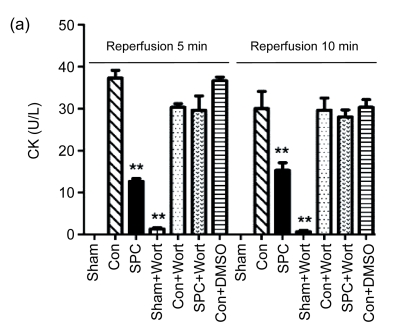
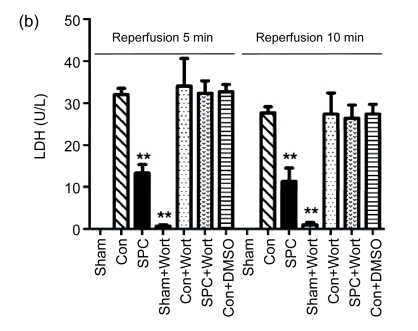
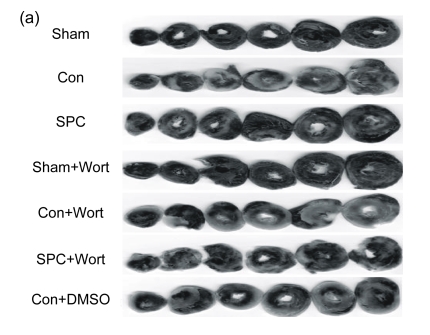
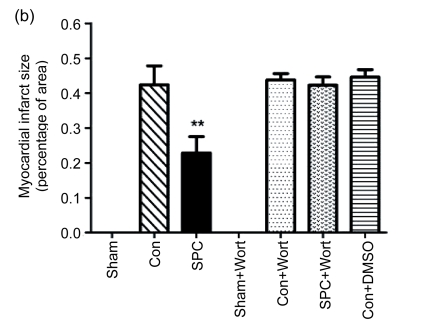
The measurements of CK and LDH release and the myocardial infarct size were shown in Figs. 2 and 3, respectively. After 120 min of reperfusion, the SPC group significantly reduced the infarct size compared with the Con group [(22.9±8)% vs. (42.4±9.4)%, P<0.05]. The PI3K inhibitor alone did not influence infarct size [(43.8±3.1)% in the Con+Wort group), but eliminated the cardioprotection produced by sevoflurane [(42.3±4)%, P<0.05 compared with the SPC group). No myocardial infarctions were measured in the Sham and Sham+Wort groups. The reduction of CK and LDH release was consistent with the decrease of the myocardial infarct size.
Fig. 2.
Measurements of CK (a) and LDH (b) release during reperfusion 5 and 10 min in each experimental group
Data are presented as mean±SEM. ** P<0.01 compared with the Con group
Fig. 3.
Myocardial infarct size in each experimental group
(a) Representative photographs of heart slices with 1% (w/v) triphenyltetrazolium chloride staining in each group; (b) Average infarct size in six hearts for each group. The size of the infarcted myocardium was calculated as an area ratio (necrotic area/total area of the myocardium×100%). ** P<0.01 compared with the Con group
3.3. Effects on expression of total Akt and phospho-Akt
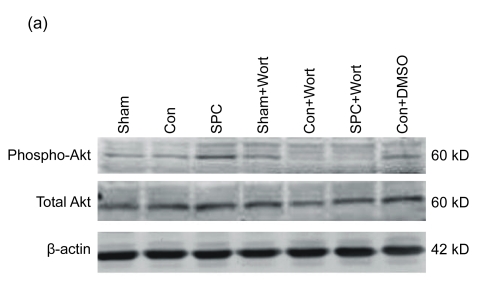
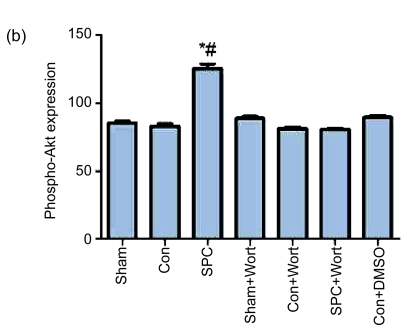
As shown in Fig. 4, the expression of total Akt and its activated, phosphorylated form (phospho-Akt) at Ser473 was measured by Western blot after 10 min reperfusion. There were no differences in total Akt expression observed among groups, whereas the phospho-Akt expression was significantly increased in the SPC group compared with the Con group. Wortmannin inhibited the increased expression of phospho-Akt in the SPC group.
Fig. 4.
Western blot analysis of total Akt and phospho-Akt at Ser473
(a) Western blotting analysis of phospho-Akt at Ser473 and total Akt. β-actin was used to demonstrate equal protein loading; (b) Graphic presentation of phospho-Akt normalized against total Akt expression. Values in the graphs are presented as mean±SEM. * P<0.05 compared with the Con group; # P<0.05 compared with the SPC+Wort group
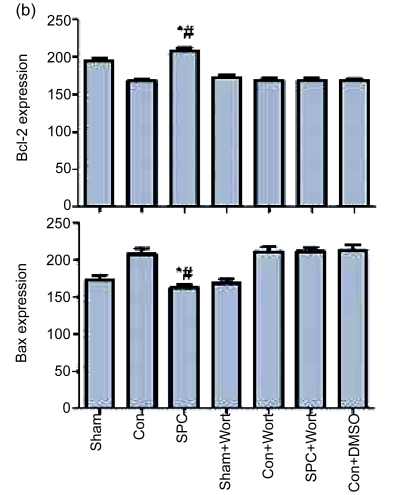
3.4. Effects on expression of Bcl-2 and Bax proteins
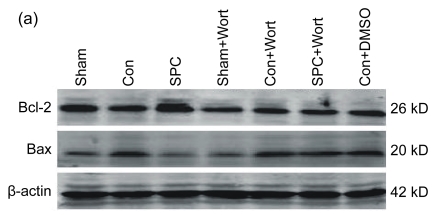
The expression of Bcl-2 and Bax proteins extracted by Western blot analysis from left ventricular necrotic myocardia is shown in Fig. 5. The expression of the antiapoptotic protein Bcl-2 was significantly decreased and that of the pro-apoptotic protein Bax was significantly increased in the Con group compared with the Sham group. The SPC group significantly increased the expression of Bcl-2 and decreased the expression of Bax (P<0.05 compared with the Con group) to a level similar to that of the Sham group. Wortmannin counteracted the effects of up-regulation of the Bcl-2 and the down-regulation of the Bax due to the sevoflurane postconditioning. It also decreased the Bcl-2 expression and increased the Bax expression to a degree similar to that of the Con group.
Fig. 5.
Western blot analysis of Bcl-2 and Bax
(a) Western blotting analysis of Bcl-2 and Bax. β-actin was used to demonstrate equal protein loading; (b) Graphic presentations of Bcl-2 and Bax expression. Values are expressed as mean±SEM. * P<0.05 compared with the Con group; # P<0.05 compared with the SPC+Wort group
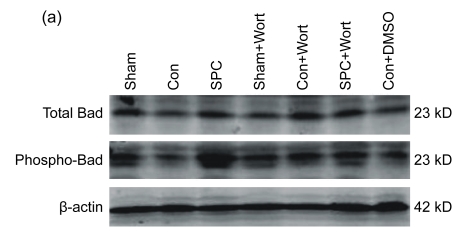
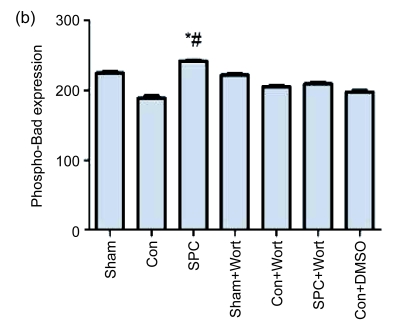
3.5. Effects on expression of total Bad and phospho-Bad
No significant difference among all the groups was found in the expression of total Bad. However, the phospho-Bad expression at Ser136 was significantly decreased in the Con group compared with the SPC group (P<0.05). Wortmannin abolished the effect of sevoflurane postconditioning on the phospho-Bad and caused the phospho-Bad expression to decrease to a level similar to that of the Con group (Fig. 6).
Fig. 6.
Western blot analysis of total Bad and phospho-Bad
(a) Western blotting analysis of total Bad and phospho-Bad at Ser136. β-actin was used to demonstrate equal protein loading; (b) Graphic presentation of phospho-Bad normalized against total Bad expression. Values are expressed as mean±SEM. * P<0.05 compared with the Con group; # P<0.05 compared with the SPC+Wort group
4. Discussion
The current study has shown several salient findings in isolated rat hearts. Firstly, sevoflurane postconditioning improves the recovery of myocardial function, attenuates myocardial apoptosis, and reduces infarct size after ischemia/reperfusion injuries. Secondly, the activation of the PI3K/Akt prosurvival pathway plays an important role on cardioprotection contributed by the sevoflurane postconditioning. Thirdly, the cardiocyte apoptosis inhibited by sevoflurane postconditioning may influence the modulation of the expression of pro- and antiapoptotic proteins, specifically Bcl-2, Bax, and phosphor-Bad. The PI3K/Akt signaling pathway may participate in the modulation of the expression of pro- and antiapoptotic proteins in sevoflurane postconditioning.
The purpose of using the isolated rat heart model in the current study is to exclude the effects of neuroendocrine system on the hearts. Studies have reported that infarct size did not decrease when administrating 0.5 MAC volatile anaesthetic at the onset of reperfusion and the cardioprotective effects were greater when administrating 1.0 MAC volatile anaesthetic after ischemia (Obal et al., 2001; Krolikowski et al., 2005; Weihrauch et al., 2005; Wang et al., 2006; Deyhimy et al., 2007).
The pathology associated with myocardial ischemia and reperfusion is cardiocyte necrosis and apoptosis. Apoptosis is also called programmed cell death. Its chief morphologic features include cell shrinkage, nuclear chromatin condensation, DNA fragmentation, formation of cytoplasmic blebs, and apoptotic bodies, no loss of membrane integrity and no inflammatory response (Bartling et al., 1998; Freude et al., 1998; Haunstetter and Izumo, 1998; van Heerde et al., 2000). Apoptosis mainly appears in myocardia subjected to a brief period of ischemia followed by reperfusion, and it does not appear in ischemic tissues without reperfusion (Freude et al., 2000; Zhao et al., 2000). Research has demonstrated that anesthetic postconditioning reduces ischemia/reperfusion myocardial infarction and attenuates cardiocyte apoptosis (Obal et al., 2001; 2005; Krolikowski et al., 2005; Weihrauch et al., 2005; Wang et al., 2006).
Mechanisms that the Bcl-2 may adopt to regulate the antiapoptotic pathway have been proposed as: direct antioxidant effect, inhibition of release of pro-apoptotic proteins, and attenuation of cytotoxic effect of pro-apoptotic regulators such as Bax (Freude et al., 1998; Haunstetter and Izumo, 1998). Misao et al. (1996) showed that increased Bcl-2 expression occurs in salvaged myocytes in acute infarction, while Bax was expressed only in the infarcted area in matured myocardial infarction, which suggests that Bcl-2 may play an important role in salvage of ischemic tissue. Kirshenbaum and de Moissac (1997) demonstrated that the Bcl-2 was expressed in isolated rat myocytes to prevent apoptosis. Wang et al. (2006) reported that inhibition of the Bcl-2 activity decreased the protective effects against myocardial infarction produced by isoflurane postconditioning that occurred as a result of indirect modulation of mitochondrial permeability transition pore (mPTP) by Bcl-2 in vivo, which suggests that the Bcl-2 family proteins regulate apoptosis by modulating mitochondrial permeability.
Ischemia/reperfusion decreases the expression of Bcl-2 and increases the expression of Bax in the ischemic/reperfused myocardium (Nakamura et al., 2000; Zhao et al., 2000). The findings of our study are consistent with the conclusions that the expression of Bcl-2 and Bax regulates the apoptotic cell death, and that the ischemic/reperfusion significantly decreases the expression of Bcl-2 and increases the expression of Bax in the left ventricular tissues of the isolated rat hearts. Sevoflurane postconditioning prevented these changes, and inhibited the decreased expression of Bcl-2 and the increased expression of Bax to a level similar to that of the Sham group. The protection accordingly led to an increased Bcl-2/Bax ratio, which has been suggested to determine the survival or death after an apoptotic stimulus. PI3K inhibitor wortmannin counteracted the effect of sevoflurane on the expression of Bcl-2 and Bax, suggesting that the reduction of myocardial apoptosis by sevoflurane postconditioning was attenuated, at least partially, by activation of PI3K/Akt signaling pathway.
Bad, another pro-apoptotic protein, is also a cell survival target of Akt. This protein can be sequestered in the cytosol if being maintained in a phosphorylated state on either of the two serine residues (Ser112 and Ser136) embedded in the 14-3-3 consensus binding sites (Zha et al., 1996). Activation of Bad by dephosphorylation results in translocation of Bad to the mitochondria with subsequent heterodimerization with Bcl-xl or Bcl-2 to promote cell death (Zha et al., 1996; 1997; Adams and Cory, 1998). The translocation is inhibited by phosphorylation of Bad, leading to its cytosolic sequestration. Thus, phosphorylation of Bad may promote cell survival (Steenbergen et al., 2003). Previous data have demonstrated that Akt is capable of phosphorylating Ser136 and thereby inactivating Bad (Datta et al., 1997). Our study showed that the expression of total Bad among the various experimental groups was similar. However, the ischemia/reperfusion reduced the expression of phospho-Bad. The administration of the sevoflurane at reperfusion did maintain Bad in its nonactivated form (phospho-Bad), but prevented the decrease in Bad phosphorylation. Wortmannin abolished the effect of sevoflurane, and decreased the expression of the phospho-Bad.
Akt activation at the time of reperfusion confers cardioprotection against reperfusion-induced injuries by reducing apoptosis (Fujio et al., 2000; Matsui et al., 2001). The PI3K/Akt pathway participates in numerous cellular processes by phosphorylating a diverse array of substrates: PI3K converts phosphatidylinositol-4,5-bisphosphate to phosphatidylinositol-3,4,5-trisphosphate (Hausenloy and Yellon, 2004), and then phosphorylation of the serine/threonine kinase Akt by phosphoinositide-dependent kinase 1 inhibits the formation of the pro-apoptotic proteins Bad and Bax (Cantley, 2002), and, in the meantime, maintains the level of the antiapoptotic protein Bcl-2 (Uchiyama et al., 2004). The results of the current study are consistent with that of Li et al. (2008). Further, this study has demonstrated that sevoflurane postconditioning may inhibit myocardial apoptosis, at least partially, through activating the PI3K/Akt pathway and modulating the expression of the pro- and antiapoptotic proteins.
The process of ischemia/reperfusion has been shown to activate the antiapoptotic pro-survival kinase signaling cascades: PI3K/Akt and ERK1/2, both of which are related to cellular survival and play a pivotal role in reperfusion injury salvage kinase cascade (RISK) pathway. Activating the RISK pathway at the time of reperfusion has been demonstrated to provide protection against reperfusion-induced injuries. It has been reported that ERK1/2 is involved in targeting the apoptotic components of reperfusion-induced cell death through modulation of pro- and antiapoptotic protein levels (Hausenloy and Yellon, 2004). Inamura et al. (2010) reported that sevoflurane postconditioning prevents the activation of caspase-3 and -9 via phosphorylation of Akt and ERK, mediators of apoptosis in ischemia/reperfusion injury. The mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase 1/2 (ERK1/2)/p70S6K signaling pathway participates in the cardioprotection of anesthetic postconditioning on myocardial infarction during early reperfusion (Chen et al., 2008; Yao et al., 2010). However, whether ERK1/2 participates in modulating the expression of Bcl-2 family proteins is not clear. Therefore, further studies are necessary to unveil the relationship between ERK1/2 and anesthetic postconditioning-induced inhibition of cardiocyte apoptosis, and to identify other signaling elements involved in anesthetic postconditioning and the relationships among them.
The limitations of this study are as follows: Firstly, we used an isolated heart model. Secondly, we did not determine the area at risk. We determined the infarct size by the radio of necrotic area to total area of the myocardium as described previously (Feng et al., 2005). Determining the necrotic area by the area at risk can be more accurate. Thirdly, we did not confirm direct morphological features of apoptotic cells.
In summary, this investigation has shown that sevoflurane postconditioning attenuates myocardial apoptosis and reduces infarct size, and the effects of sevoflurane postconditioning may be modulated by activation of the PI3K/Akt signaling pathway and modulation of the expression of pro- and antiapoptotic proteins.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30772090), the Natural Science Foundation of Zhejiang Province (Nos. Y204141 and R2090259), the Foundation from Science and Technology Department of Zhejiang Province (No. 2007R10034), and the Foundation from the Health Bureau of Zhejiang Province (No. 2007QN007), China
References
- 1.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281(5381):1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Bartling B, Holtz J, Darmer D. Contribution of myocyte apoptosis to myocardial infarction? Basic Res Cardiol. 1998;93(2):71–84. doi: 10.1007/s003950050065. [DOI] [PubMed] [Google Scholar]
- 3.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 4.Cao CM, Xia Q, Tu J, Chen M, Wu S, Wong TM. Cardioprotection of interleukin-2 is mediated via kappa-opioid receptors. J Pharmacol Exp Ther. 2004;309(2):560–567. doi: 10.1124/jpet.103.061135. [DOI] [PubMed] [Google Scholar]
- 5.Chen HT, Yang CX, Li H, Zhang CJ, Wen XJ, Zhou J, Fan YL, Huang T, Zeng YM. Cardioprotection of sevoflurane postconditioning by activating extracellular signal-regulated kinase 1/2 in isolated rat hearts. Acta Pharmacol Sin. 2008;29(8):931–941. doi: 10.1111/j.1745-7254.2008.00824.x. [DOI] [PubMed] [Google Scholar]
- 6.Chiari PC, Bienengraeber MW, Pagel PS, Krolikowski JG, Kersten JR, Warltier DC. Isoflurane protects against myocardial infarction during early reperfusion by activation of phosphatidylinositol-3-kinase signal transduction: evidence for anesthetic-induced postconditioning in rabbits. Anesthesiology. 2005;102(1):102–109. doi: 10.1097/00000542-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of Bad couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–241. doi: 10.1016/S0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 8.Deyhimy DI, Fleming NW, Brodkin IG, Liu H. Anesthetic preconditioning combined with postconditioning offers no additional benefit over preconditioning or postconditioning alone. Anesth Analg. 2007;105(2):316–324. doi: 10.1213/01.ane.0000267524.71445.e7. [DOI] [PubMed] [Google Scholar]
- 9.Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3beta. Anesthesiology. 2005;103(5):987–995. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Freude B, Masters TN, Kostin S, Robicsek F, Schaper J. Cardiomyocyte apoptosis in acute and chronic conditions. Basic Res Cardiol. 1998;93(2):85–89. doi: 10.1007/s003950050066. [DOI] [PubMed] [Google Scholar]
- 11.Freude B, Masters TN, Robicsek F, Fokin A, Kostin S, Zimmermann R, Ullmann C, Lorenz-Meyer S, Schaper J. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J Mol Cell Cardiol. 2000;32(2):197–208. doi: 10.1006/jmcc.1999.1066. [DOI] [PubMed] [Google Scholar]
- 12.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101(6):660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998;82(11):1111–1129. doi: 10.1161/01.res.82.11.1111. [DOI] [PubMed] [Google Scholar]
- 14.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res. 2004;61(3):448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288(2):H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 16.He W, Zhang FJ, Wang SP, Chen G, Chen CC, Yan M. Postconditioning of sevoflurane and propofol is associated with mitochondrial permeability transition pore. J Zhejiang Univ Sci B. 2008;9(2):100–108. doi: 10.1631/jzus.B0710586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inamura Y, Miyamae M, Sugioka S, Domae N, Kotani J. Sevoflurane postconditioning prevents activation of caspase 3 and 9 through antiapoptotic signaling after myocardial ischemia-reperfusion. J Anesth. 2010;24(2):215–224. doi: 10.1007/s00540-010-0877-6. [DOI] [PubMed] [Google Scholar]
- 18.Kirshenbaum LA, de Moissac D. The Bcl-2 gene product prevents programmed cell death of ventricular myocytes. Circulation. 1997;96(5):1580–1585. doi: 10.1161/01.cir.96.5.1580. [DOI] [PubMed] [Google Scholar]
- 19.Krolikowski JG, Bienengraeber M, Weihrauch D, Warltier DC, Kersten JR, Pagel PS. Inhibition of mitochondrial permeability transition enhances isoflurane-induced cardioprotection during early reperfusion: the role of mitochondrial KATP channels. Anesth Analg. 2005;101(6):1590–1596. doi: 10.1213/01.ANE.0000181288.13549.28. [DOI] [PubMed] [Google Scholar]
- 20.Krolikowski JG, Weihrauch D, Bienengraeber M, Kersten JR, Warltier DC, Pagel PS. Role of Erk1/2, p70s6K, and eNOS in isoflurane-induced cardioprotection during early reperfusion in vivo. Can J Anaesth. 2006;53(2):174–182. doi: 10.1007/BF03021824. [DOI] [PubMed] [Google Scholar]
- 21.Li DY, Zhang YC, Philips MI, Sawamura T, Mehta JL. Upregulation of endothelial receptor for oxidized low-density lipoprotein (LOX-1) in cultured human coronary artery endothelial cells by angiotensin II type 1 receptor activation. Circ Res. 1999;84(9):1043–1049. doi: 10.1111/j.1440-1681.2008.04952.x. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Wang JK, Zeng YM, Yang CX, Chen HT, Wen XJ, Shui CL, Liang H. Sevoflurane post-conditioning protects against myocardial reperfusion injury by activation of phosphatidylinositol-3-kinase signal transduction. Clin Exp Pharmacol Physiol. 2008;35(9):1043–1051. doi: 10.1111/j.1440-1681.2008.04952.x. [DOI] [PubMed] [Google Scholar]
- 23.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104(3):330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 24.Misao J, Hayakawa Y, Ohno M, Kato S, Fujiwara T, Fujiwara H. Expression of Bcl-2 protein, an inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in ventricular myocytes of human hearts with myocardial infarction. Circulation. 1996;94(7):1506–1512. doi: 10.1161/01.cir.94.7.1506. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura M, Wang NP, Zhao ZQ, Wilcox JN, Thourani V, Guyton RA, Vinten-Johansen J. Preconditioning decreases Bax expression, PMN accumulation and apoptosis in reperfused rat heart. Cardiovasc Res. 2000;45(3):661–670. doi: 10.1016/S0008-6363(99)00393-4. [DOI] [PubMed] [Google Scholar]
- 26.Obal D, Preckel B, Scharbatke H, Müllenheim J, Höterkes F, Thämer V, Schlack W. One MAC of sevoflurane provides protection against reperfusion injury in the rat heart in vivo. Br J Anaesth. 2001;87(6):905–911. doi: 10.1093/bja/87.6.905. [DOI] [PubMed] [Google Scholar]
- 27.Obal D, Dettwiler S, Favoccia C, Scharbatke H, Precke B, Schlack W. The influence of mitochondrial KATP-channels in the cardioprotection of preconditioning and postconditioning by sevoflurane in the rat in vivo. Anesth Analg. 2005;101(5):1252–1260. doi: 10.1213/01.ANE.0000181336.96511.32. [DOI] [PubMed] [Google Scholar]
- 28.Pagel PS, Krolikowski JG, Neff DA, Weihrauch D, Bienengraeber M, Kersten JR, Warltier DC. Inhibition of glycogen synthase kinase enhances isoflurane-induced protection against myocardial infarction during early reperfusion in vivo. Anesth Analg. 2006;102(5):1348–1354. doi: 10.1213/01.ane.0000202379.61338.37. [DOI] [PubMed] [Google Scholar]
- 29.Preckel B, Schlack W, Comfère T, Obal D, Barthel H, Thämer V. Effects of enflurane, isoflurane, sevoflurane and desflurane on reperfusion injury after regional myocardial ischaemia in the rabbit heart in vivo. Br J Anaesth. 1998;81(6):905–912. doi: 10.1093/bja/81.6.905. [DOI] [PubMed] [Google Scholar]
- 30.Preckel B, Thamer V, Schlack W. Beneficial effects of sevoflurane and desflurane against myocardial reperfusion injury after cardioplegic arrest. Can J Anaesth. 1999;46(11):1076–1081. doi: 10.1007/BF03013206. [DOI] [PubMed] [Google Scholar]
- 31.Raphael J, Abedat S, Rivo J, Meir K, Beeri R, Pugatsch T, Zuo Z, Gozal Y. Volatile anesthetic preconditioning attenuates myocardial apoptosis in rabbits after regional ischemia and reperfusion via Akt signaling and modulation of Bcl-2 family proteins. J Pharmacol Exp Ther. 2006;318(1):186–194. doi: 10.1124/jpet.105.100537. [DOI] [PubMed] [Google Scholar]
- 32.Schlack W, Preckel B, Stunneck D, Thämer V. Effects of halothane, enflurane, isoflurane, sevoflurane and desflurane on myocardial reperfusion injury in the isolated rat heart. Br J Anaesth. 1998;81(6):913–919. doi: 10.1093/bja/81.6.913. [DOI] [PubMed] [Google Scholar]
- 33.Steenbergen C, Afshari CA, Petranka JG, Collins J, Martin K, Bennett L, Haugen A, Bushel P, Murphy E. Alterations in apoptotic signaling in human idiopathic cardiomyopathic hearts in failure. Am J Physiol Heart Circ Physiol. 2003;284(1):H268–H276. doi: 10.1152/ajpheart.00707.2002. [DOI] [PubMed] [Google Scholar]
- 34.Takatani T, Takahashi K, Uozumi Y, Matsuda T, Ito T, Schaffer SW, Fujio Y, Azuma J. Taurine prevents the ischemia-induced apoptosis in cultured neonatal rat cardiomyocytes through Akt/caspase-9 pathway. Biochem Biophys Res Commun. 2004;316(2):484–489. doi: 10.1016/j.bbrc.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 35.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95(3):230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 36.Tsutsumi YM, Yokoyama T, Horikawa Y, Roth DM, Patel HH. Reactive oxygen species trigger ischemic and pharmacological postconditioning: in vivo and in vitro characterization. Life Sci. 2007;81(15):1223–1227. doi: 10.1016/j.lfs.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uchiyama T, Engelman RM, Maulik N, Das DK. Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation. 2004;109(24):3042–3049. doi: 10.1161/01.CIR.0000130647.29030.90. [DOI] [PubMed] [Google Scholar]
- 38.van Heerde WL, Robert-Offerman S, Dumont E, Hofstra L, Doevendans PA, Smits JF, Daemen MJ, Reutelingsperger CP. Markers of apoptosis in cardiovascular tissues: focus on annexin V. Cardiovasc Res. 2000;45(3):549–959. doi: 10.1016/S0008-6363(99)00396-X. [DOI] [PubMed] [Google Scholar]
- 39.Venkatapuram S, Wang C, Krolikowski JG, Weihrauch D, Kersten JR, Warltier DC, Pratt PFJr, Pagel PS. Inhibition of apoptotic protein p53 lowers the threshold of isoflurane-induced cardioprotection during early reperfusion in rabbits. Anesth Analg. 2006;103(6):1400–1405. doi: 10.1213/01.ane.0000240903.63832.d8e. [DOI] [PubMed] [Google Scholar]
- 40.Wang C, Neff DA, Krolikowski JG, Weihrauch D, Bienengraeber M, Warltier DC, Kersten JR, Pagel PS. The influence of B-cell lymphoma 2 protein, an antiapoptotic regulator of mitochondrial permeability transition, on isoflurane-induced and ischemic postconditioning in rabbits. Anesth Analg. 2006;102(5):1355–1360. doi: 10.1213/01.ane.0000202463.28618.64. [DOI] [PubMed] [Google Scholar]
- 41.Weihrauch D, Krolikowski JG, Bienengraeber M, Kersten JR, Warltier DC, Pagel PS. Morphine enhances isoflurane-induced postconditioning against myocardial infarction: the role of phosphatidylinositol-3-kinase and opioid receptors in rabbits. Anesth Analg. 2005;101(4):942–949. doi: 10.1213/01.ane.0000171931.08371.a2. [DOI] [PubMed] [Google Scholar]
- 42.Yao YT, Li LH, Chen L, Wang WP, Li LB, Gao CQ. Sevoflurane postconditioning protects isolated rat hearts against ischemia-reperfusion injury: the role of radical oxygen species, extracellular signal-related kinases 1/2 and mitochondrial permeability transition pore. Mol Biol Rep. 2010;37(5):2439–2446. doi: 10.1007/s11033-009-9755-4. [DOI] [PubMed] [Google Scholar]
- 43.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87(4):619–628. doi: 10.1016/S0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 44.Zha J, Harada H, Osipov K, Jockel J, Waksman G, Korsmeyer SJ. BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity. J Biol Chem. 1997;272(39):24101–24104. doi: 10.1074/jbc.272.39.24101. [DOI] [PubMed] [Google Scholar]
- 45.Zhao ZQ, Nakamura M, Wang NP, Wilcox JN, Shearer S, Ronson RS, Guyton RA, Vinten-Johansen J. Reperfusion induces myocardial apoptotic cell death. Cardiovasc Res. 2000;45(3):651–660. doi: 10.1016/S0008-6363(99)00354-5. [DOI] [PubMed] [Google Scholar]