Abstract
Malignant gliomas are treated with a combination of surgery, radiation and temozolomide (TMZ), but these therapies ultimately fail due to tumor recurrence. In glioma cultures, TMZ treatment significantly decreases neurosphere formation; however, a small percentage of cells survive and repopulate the culture. A promising target for glioma therapy is the Notch signaling pathway. Notch activity is upregulated in many gliomas and can be suppressed using gamma-secretase inhibitors (GSIs). Using a neurosphere recovery assay and xenograft experiments, we analyzed if the addition of GSIs with TMZ treatment could inhibit repopulation and tumor recurrence. We demonstrate that TMZ+GSI treatment decreased neurosphere formation and inhibited neurosphere recovery. This enhancement of TMZ treatment occurred through inhibition of the Notch pathway and depended on the sequence of drug administration. In addition, ex vivo TMZ+GSI treatment of glioma xenografts in immunocompromised mice extended tumor latency and survival, and in vivo TMZ+GSI treatment blocked tumor progression in 50% of mice with pre-existing tumors. These data demonstrate the importance of the Notch pathway in chemoprotection and repopulation of TMZ-treated gliomas. The addition of GSIs to current treatments is a promising approach to decrease brain tumor recurrence.
Keywords: glioma, neurosphere, temozolomide, Notch, gamma-secretase inhibitor
Introduction
Glioblastoma multiforme (GBM) is the most frequent and malignant form of brain tumor, making up 17% of all primary brain tumors in the United States, with an incidence of 3.17 cases per 100,000 persons/year (1). The current five- and ten-year survival rates for GBM patients are 4.5% and 2.7%, respectively (1). GBM clinical treatment consists of a combination of surgical resection, radiotherapy and chemotherapy. The chemotherapy drug, temozolomide (TMZ), is an alkylating agent that readily penetrates the blood-brain barrier (2). TMZ is administered as both concomitant and adjuvant treatments to radiotherapy. This aggressive treatment increases the two-year survival rate for GBM patients from 10.4% with radiotherapy alone, to 26.5% (3). Cells that escape radiotherapy- and chemotherapy-induced cell death eventually re-enter the cell cycle and contribute to local tumor recurrence. Despite advances in chemotherapy regimens, the median progression-free survival, which measures the time until tumor recurrence, is 6.9 months, and the median overall survival is 14.6 months with temozolomide and radiotherapy (3). Hence, there is a dire need to target the cells that evade current treatments.
Neurosphere cultures were originally developed for propagation of normal neural stem cells (4), and these methods are now applied to tumors (5–7). Neurosphere cultures maintain genetic profiles similar to the patients’ tumors and form invasive intracranial xenografts in immunocompromised mice (8–10). Our lab developed a neurosphere recovery assay that measures neurosphere formation at three time points to assess the capacity of the culture to repopulate after chemotherapy (11). First, we assess the ability of the cells to form neurospheres shortly after treatment. Second, we count the number of neurospheres that form during a one-week recovery period to determine if the surviving cells resume neurosphere formation. Third, we dissociate the neurospheres and count the number of secondary neurospheres that form to measure self-renewal (12). This neurosphere recovery assay provides a quantitative assay for culture repopulation following drug treatment. We previously demonstrated that TMZ drastically diminished initial neurosphere formation in many glioma cultures; however, these cultures eventually recovered and formed a robust number of secondary neurospheres (11). The ability of TMZ-treated neurospheres to recover and repopulate the culture suggests that some cells undergo a transient cell cycle arrest, allowing them to evade cell death and eventually resume proliferation.
Notch signaling is a promising pathway to target glioma cells. The Notch receptors, their ligands, and downstream targets, include members of the Hairy enhancer of split (Hes) and Hes-related protein (Hey) families, are commonly over-expressed in glioma tissue and cell lines (13–15). Gamma-secretase inhibitors (GSIs) are used to inhibit the Notch pathway in basic research and clinical trials (16). In glioma cultures, GSI treatment suppressed cell growth and decreased neurosphere formation and tumor growth of CD133+ cells (17). Correspondingly, increased Notch signaling enhanced glioma cell survival (18). GSIs were also shown to sensitize glioma neurosphere cultures to radiation, thereby, increasing the efficacy of radiotherapy (19).
In this study, we analyze if the combination of TMZ and GSIs enhances glioma therapy by inhibiting tumor repopulation and recurrence. In contrast to TMZ-only treatment, the TMZ+GSI treatment strongly inhibited neurosphere recovery. This was confirmed by the loss of secondary neurosphere formation in cultures treated with both TMZ and GSIs. In subcutaneous xenografts, ex vivo and in vivo TMZ+GSI treatment decreased tumor progression and increased survival. These data demonstrate the importance of the Notch pathway for chemoprotection in malignant gliomas. The addition of GSIs to the current care regimens for GBM patients is a promising new approach to decrease brain tumor recurrence.
Materials and Methods
Cell Culture
Glioma cell lines converted to neurosphere cultures, U87NS and U373NS, and primary GBM lines, GS7-2 and GS8-26, were grown in serum-free defined medium consisting of DMEM/F12 1:1 (GIBCO, Carlsbad, CA), B27 (GIBCO, Carlsbad, CA), 15 mM HEPES (GIBCO, Carlsbad, CA), 20 ng/ml EGF (Invitrogen, Carlsbad, CA), and 20 ng/ml bFGF (Invitrogen, Carlsbad, CA) and 1% penicillin-streptomycin (GIBCO, Carlsbad, CA). Cultures were passaged using a pH dissociation method (20). Details of the converted and primary lines are described in Supplementary Materials and Methods.
Drug Treatment
TMZ and N-[N-(3,5-difluorophenacetyl)-L-alanyl]-5-phenylglycine t-butyl ester (DAPT) were purchased from Sigma-Aldrich (St. Louis, MO). N-2((2S)-2-(3,5-difluorophenyl)-2-hydroxyethanoyl)-N1-((7S)-5-methyl-6-oxo-6,7-dihydro-5H-dibenzo[b,d]azepin-7-yl)-L-alaninamide (LY411,575) (21) was a gift from Lisa Minter and Barbara Osborne (UMass, Amherst). Details of drug dosage are in Supplementary Materials and Methods.
Neurosphere Recovery and Secondary Neurosphere Assays
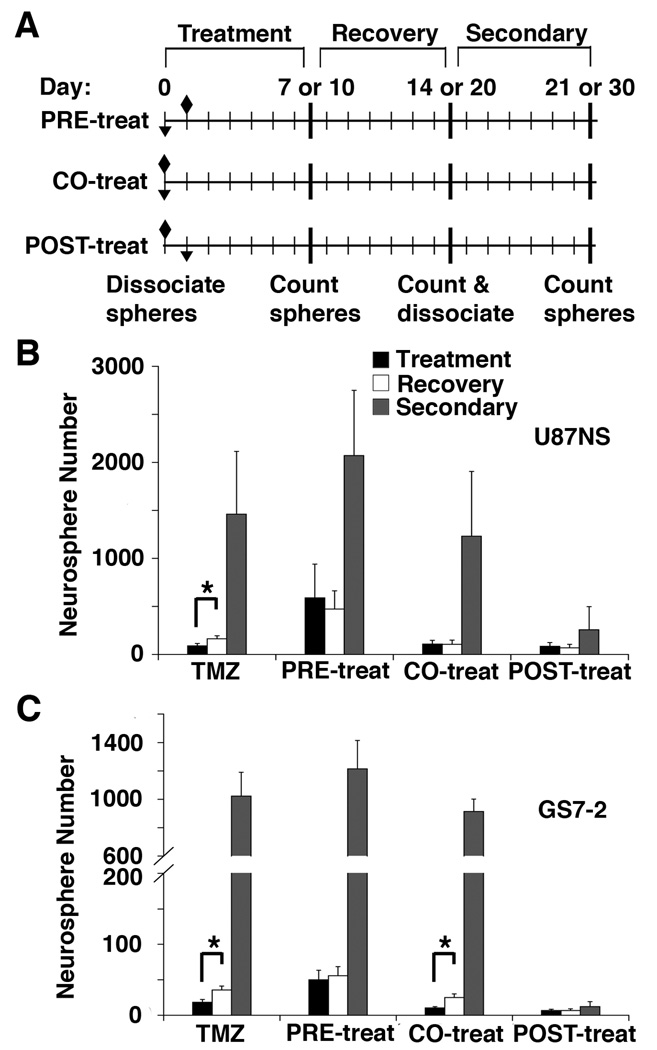
For the neurosphere assay, cells were plated as previously described (11). Immediately after plating, cells were treated with DMSO, DAPT-only, LY411,575 (LY)-only TMZ-only, TMZ+DAPT or TMZ+LY. The initial neurospheres were counted on day 7 for the converted cell lines and on day 10 for the slower growing primary lines. Neurosphere recovery was measured on day 14 or 20. The neurospheres were dissociated, re-plated and secondary neurosphere formation was measured on day 21 or 30. Details are described in Supplementary Materials and Methods.
For the samples labeled ‘PRE-treat’, a single dose of DAPT was administered when the cells were plated, and then TMZ was added to the medium 24 hours later. For the ‘CO-treat’ samples, single doses of TMZ and DAPT were added simultaneously when the cells were plated. Finally, samples labeled ‘POST-treat’ were treated with TMZ, and then DAPT was added 24 hours later.
Virus Infections
NICD-pMIG (22) or pMIG vectors were co-transfected with retrovirus envelope and gag-pol vectors into HEK293T cells, with FuGENE 6 (Roche Applied Science, Indianapolis, IN). Retrovirus was collected after 48 hours. Neurosphere cultures were infected in non-coated bacterial dishes to avoid the cells becoming adherent in the presence of serum. Cells were incubated with virus and 8 µg/ml polybrene (Sigma-Aldrich, St. Louis, MO) at 37°C for 6 hours. GFP-positive cells were sorted on a FACS Aria (BD Biosciences, Franklin Lakes, NJ).
Subcutaneous Xenografts: ex vivo Drug Treatment
U87NS and U373NS neurospheres were dissociated and 2.5×104 cells/ml were plated in defined media and treated with DMSO, TMZ-only (200 µM), DAPT-only (1 µM or 5 µM), or TMZ+DAPT as described for recovery assays. After 7 days, 2.5×105 or 3×106 live cells were counted using trypan blue and re-suspended in 100 µl PBS. Cells were subcutaneously injected into the flanks of nude mice. Mice were monitored for tumor formation for up to 120 days post-injection and euthanized when tumors reached volumes of 1.5 to 2 cm3.
Subcutaneous Xenografts: in vivo Drug Treatment
For the in vivo experiments, we used LY411,575 incorporated into 7012 Teklad LM-485 rodent chow (LY chow) at a concentration of 0.0275 g/kg (Harlan Laboratories Inc, Madison, WI)(23). 106 U87NS cells re-suspended in 100 µl PBS were subcutaneously injected into the flanks of male nude mice. When the tumor reached approximately 150 mm3 (volume=(¾)(π)(length/2)(width/2)2), we began the following treatments: 1) DMSO control: two days of 100 µl DMSO/PBS (1:1) intraperitoneal (i.p.) injections; 2) TMZ-only: i.p. injections of TMZ (20 mg/kg) in 100 µl DMSO/PBS on days one and two; 3) LY chow-only: two days of 100 µl DMSO/PBS i.p. injections. The mice were fed LY chow from day 3 to 12; 4) TMZ+LY chow: i.p. injections of TMZ (20 mg/kg) in 100 µl DMSO/PBS on days one and two. The mice were fed LY chow from day 3 to 12. Mice were observed for up to 150 days and euthanized when the tumor reached 1.5 to 2 cm3.
Results
Glioma Neurosphere Cell Lines Express Notch Receptors and Downstream Targets
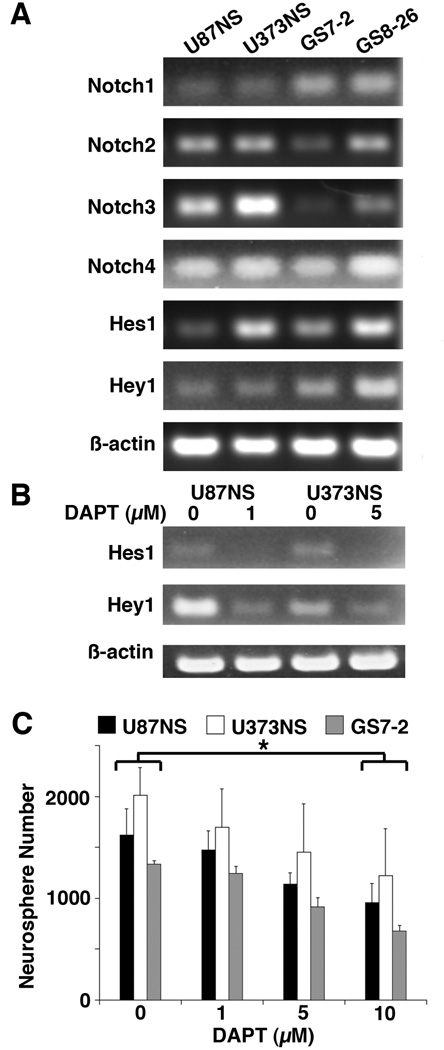
Converted cell lines (U87NS, U373NS) and primary neurosphere cultures established from patients’ GBMs (GS7-2, GS8-26) express the mRNAs for Notch1-4 and the downstream targets, Hes1 and Hey1 (Fig. 1A). Treatment with DAPT downregulated the mRNA levels of Hes1 and Hey1 (Fig. 1B). The DAPT concentration used was determined based on a 50% or greater knockdown of Notch targets. For subsequent experiments, U87NS and GS7-2 cultures were treated with 1 µM DAPT, while U373NS and GS8-26 cultures were treated with 5 µM DAPT.
Figure 1.
The Notch pathway is active in neurosphere cultures and is blocked with DAPT treatment. A, mRNA levels of the Notch receptors and downstream targets were measured by RT-PCR. Notch1-4, Hes1, and Hey1 were detected in each neurosphere culture. β-Actin was used for the loading control. B, Hes1 and Hey1 expression were analyzed by RT-PCR 48 hours after DAPT treatment. DAPT treatment decreased Hes1 and Hey1 mRNA levels by 72% and 76% in U87NS (1 µM), and by 76% and 51% in U373NS (5 µM). C, The DAPT titration curve demonstrated that low concentrations (1–5 µM) of DAPT-only treatment had minimal inhibition of neurosphere formation (mean ± SD) in U87NS, U373NS, or GS7-2 cultures. DAPT administered at higher concentrations (10 µM) significantly decreased neurosphere formation. Neurospheres were counted on day 7 for U87NS and U373NS and on day 10 for GS7-2. The t-test was used to calculate statistical significance. *=P<0.001.
TMZ+DAPT Treatment Inhibits Neurosphere Recovery and Secondary Neurosphere Formation
When administered alone, low concentrations of DAPT (1–5 µM) decreased Notch pathway signaling (Fig. 1B), but had little to no affect on the number of neurospheres (Fig. 1C). In addition, low concentrations of DAPT did not affect the size of the neurospheres (Fig. 2B). In U87NS, U373NS, and GS7-2 cultures, treatment with 10 µM DAPT decreased neurosphere formation by 41%, 39%, and 49%, respectively, compared to DMSO controls (Fig. 1C); however, the DAPT treated cells resumed proliferation and formed secondary neurospheres (Supplementary Fig. S1).
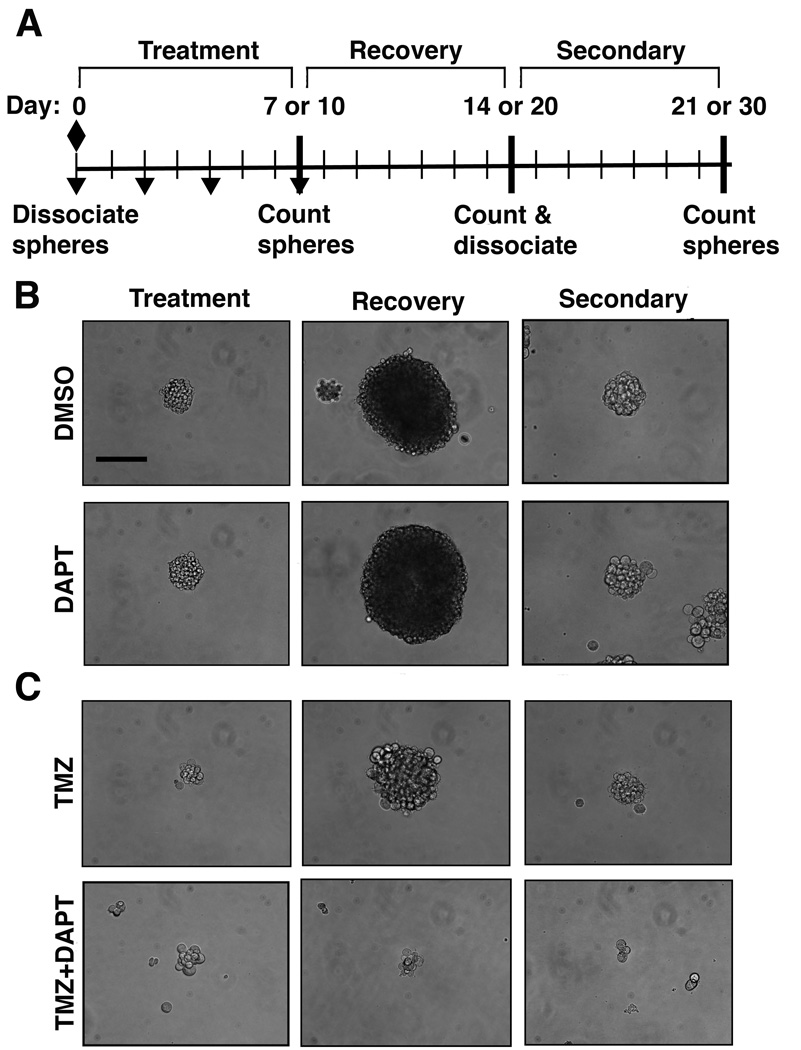
Figure 2.
TMZ+DAPT treatment inhibits neurospheres from increasing in size. A, Schematic for the neurosphere recovery assay. TMZ (diamond) is administered on day 0 after cells are dissociated and plated, DAPT (inverted triangle) is administered on days 0, 2, 4, and 7. B, Representative micrographs of U87NS neurospheres with control DMSO and DAPT (1 µM) treatments. Neurospheres treated with DAPT display a similar size compared to DMSO control cultures at treatment (day 7), recovery (day 14) and secondary (day 21) time points. C, Representative micrographs of U87NS neurospheres with TMZ (200 µM) treatment and TMZ (200 µM) combined with DAPT (1 µM) treatment. After the initial treatment (day 7), TMZ-only and TMZ+DAPT treated neurospheres are smaller than the control cultures. TMZ-only treated neurospheres increased in size during the recovery period (day 14) and formed secondary neurospheres (day 21), while TMZ+DAPT treated neurospheres did not increase in size or form secondary neurospheres. Bar=50 µm.
To determine if DAPT enhances TMZ therapy, we examined the effect of combined treatment on neurosphere recovery (Fig. 2A). After treatment with TMZ-only and TMZ+DAPT, cultures had similar decreases in the number of initial neurospheres formed (Fig. 3A–D). TMZ-only and TMZ+DAPT treatments decreased initial neurosphere formation by 80–98% and 83–99%, respectively. Cultures were given an additional 7 or 10 days to recover in the absence of drugs. During this recovery period, the neurospheres that formed after TMZ-only treatment increased in size; however, the TMZ+DAPT treated neurospheres remained the same size (Fig. 2C). The number of neurospheres also increased after recovery in the TMZ-only treated cultures, but this recovery was not observed in the TMZ+DAPT treated cultures. After recovery from the TMZ-only treatment, U87NS showed a 2-fold increase and U373NS showed a 1.5-fold increase in the number of neurospheres (Fig. 3A and B). The primary neurosphere cultures also showed a recovery from the TMZ-only treatment: the number of GS7-2 neurospheres increased by 1.8-fold, and GS8-26, by1.6-fold (Fig. 3C and D). In contrast, TMZ+DAPT effectively inhibited recovery for U87NS, U373NS, GS7-2 and GS8-26 (Fig. 3A–D). The number of neurospheres in these cultures was essentially the same after recovery on day 14 or 20 relative to the number of initial neurospheres counted on day 7 or 10.
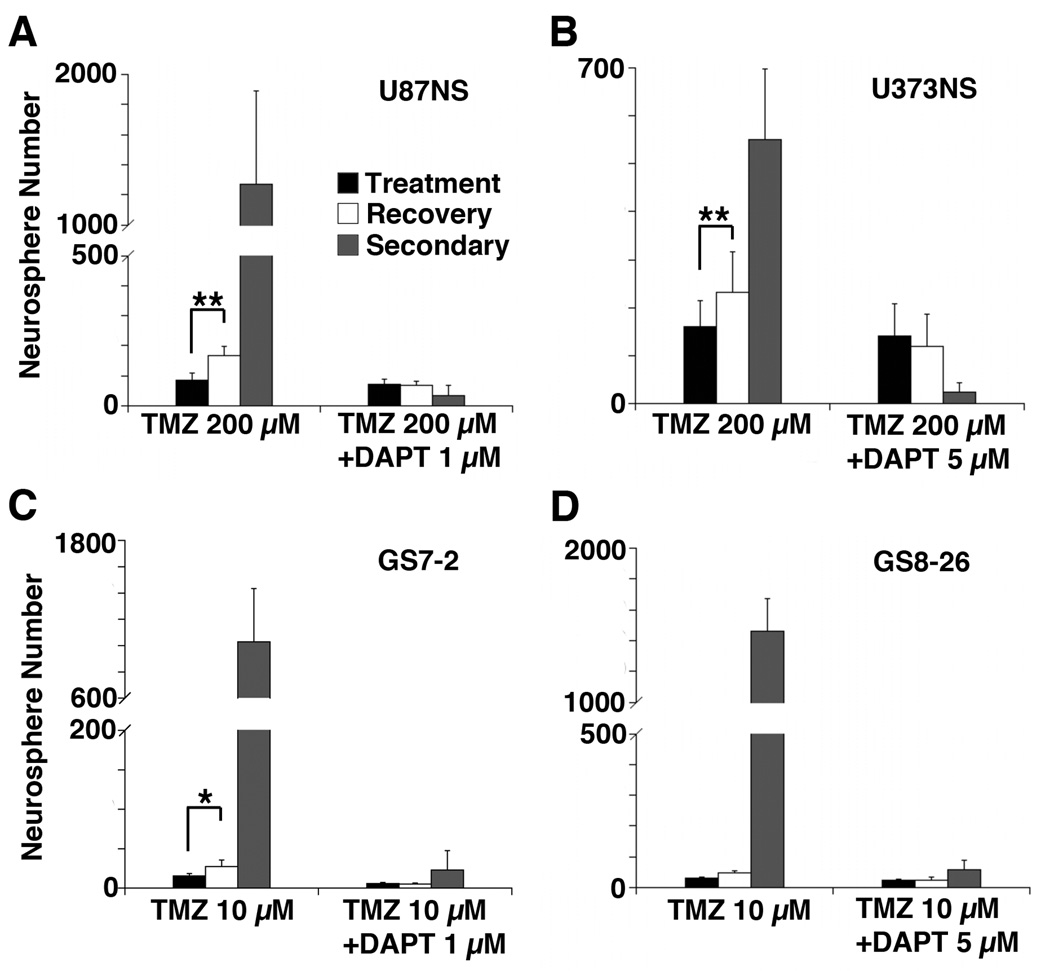
Figure 3.
The neurosphere recovery assay demonstrates that TMZ+DAPT treatment inhibits recovery and secondary neurosphere formation. Initial neurospheres (mean ± SD) were counted on day 7 for A, U87NS; and B, U373NS cultures; or on day 10 for C, GS7-2, and; D, GS8-26 cultures. Recovery neurospheres were counted on day 14 or 20, and secondary neurospheres were counted on day 21 or 30. *=P<0.001. **=P<0.0001.
To assess if the cultures retained cells capable of self-renewal, the initial neurospheres were dissociated to single cells and re-plated to measure secondary neurosphere formation. TMZ-only treated cultures readily formed secondary neurospheres, but secondary neurosphere formation for TMZ+DAPT treated cultures was significantly diminished. U87NS secondary neurosphere formation in the TMZ-only treated culture was 36-fold greater (P<0.0001) than secondary neurosphere formation in the TMZ+DAPT treated culture (Fig. 3A), and U373NS secondary neurosphere formation in the TMZ-only treated culture was 23-fold greater (P<0.001) than in the TMZ+DAPT treated culture (Fig. 3B). The primary cultures also had profuse secondary neurosphere formation after TMZ-only treatments, but minimal secondary neurosphere formation after TMZ+DAPT treatments. Secondary neurosphere formation was 45-fold greater (P<0.001) in the GS7-2 TMZ-only treated culture (Fig. 3C) and 25-fold greater (P<0.001) in the GS8-26 TMZ-only treated culture (Fig. 3D).
The number of cells in each neurosphere capable of self-renewal can be calculated by dividing the number of secondary neurospheres by the number of neurospheres formed during the recovery period. After recovery from TMZ-only treatment, there were an average of 8 and 3 cells per neurosphere that maintained self-renewal properties in the U87NS and U373NS cultures, respectively; however, in the TMZ+DAPT treated cultures there were only approximately 0.5 cells per neurosphere that were capable of self-renewal after the recovery period. In the primary lines treated with TMZ-only, each neurosphere from the GS7-2 and GS8-26 cultures contained a large number of cells capable of self-renewal, an average of 38 and 31 cells, respectively. In contrast, the average number of cells capable of self-renewal after TMZ+DAPT treatment decreased to only 2 cells per neurosphere in the GS7-2 and GS8-26 cultures.
To demonstrate that the lack of recovery and secondary neurosphere formation after TMZ+DAPT treatment was a specific response to the inhibition of gamma-secretase activity, we repeated the neurosphere recovery assay with LY411,575 (LY) (21). When LY was administered to U87NS and U373NS cultures at various concentrations, there was a dose-dependent decrease in neurosphere formation (Supplementary Fig. S2A); however, the LY-only treated cultures retained the ability to form secondary neurospheres (Supplementary Fig. S2B). In contrast, the combination of TMZ+LY significantly repressed recovery and secondary neurosphere formation (Supplementary Fig. S2C and D).
Constitutive Expression of NICD Protects Neurosphere Cultures from TMZ+DAPT Treatment
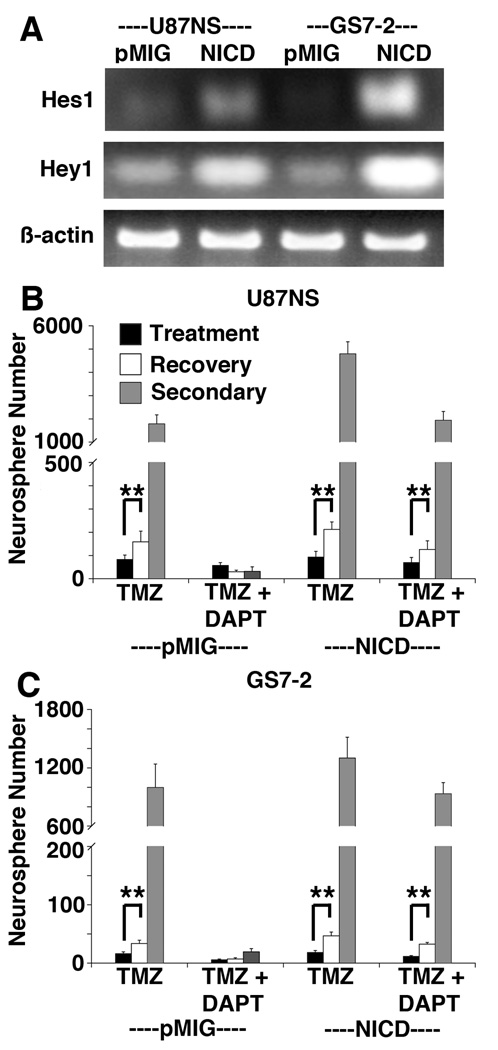
Gamma-secretase cleaves other substrates, in addition to the Notch receptors (24). To establish that DAPT enhances TMZ treatment by targeting the Notch pathway, we infected U87NS and GS7-2 cells with a retrovirus to express the constitutively active Notch1 Intracellular Domain (NICD) (22). Expression of functional NICD was confirmed by measuring increased mRNA levels of the downstream targets, Hes1 and Hey1 (Fig. 4A). When NICD is constitutively expressed, the Notch pathway is not inhibited by GSI treatment (Supplementary Fig. S3). NICD-expressing U87NS and GS7-2 cells treated with TMZ-only were capable of recovery and robust secondary neurosphere formation, similar to the control cells expressing the empty vector (pMIG) (Fig. 4B and C). Importantly, NICD expression attenuated the effects of TMZ+DAPT treatment, and the culture demonstrated neurosphere recovery and robust secondary neurosphere formation. The control U87NS-pMIG TMZ-only treated cells had a 1.9-fold recovery, but no increase was seen in the TMZ+DAPT treated culture (Fig. 4B). U87NS-NICD cells showed a 2.3–fold recovery in TMZ-only treated cultures and a 1.8-fold recovery in TMZ+DAPT treated cultures. GS7-2-pMIG TMZ-only treated cells showed a 2.1-fold increase in neurospheres during recovery, while TMZ+DAPT treated cells showed no recovery (Fig. 4C). GS7-2-NICD cells showed a 2.6–fold recovery after TMZ-only a 2.8–fold recovery after TMZ+DAPT treatment.
Figure 4.
NICD-expressing cultures resume neurosphere formation following TMZ+DAPT treatment. A, RT-PCR analysis of Hes1 and Hey1 expression was used to confirm NICD activity. Hes1 and Hey1 expression increased in U87NS-NICD and GS7-2-NICD cultures compared to cultures expressing the empty vector (pMIG). B, Treating U87NS-pMIG with TMZ+DAPT inhibited recovery, but U87NS-NICD cultures recovered after both TMZ-only and TMZ+DAPT treatment. C, In GS7-2-pMIG and GS7-2-NICD cultures treated with TMZ-only or TMZ+DAPT, cultures constitutively expressing NICD, but not the empty vector, were able to recover after TMZ+DAPT treatment. **=P<0.0001.
Similar to the parental lines (Fig. 3A, C), U87NS-pMIG and GS7-2-pMIG cultures treated with TMZ-only had robust secondary neurosphere formation, but cultures treated with TMZ+DAPT had minimal secondary neurosphere formation (Fig. 4B, C). In contrast, U87NS-NICD and GS7-2-NICD cultures had robust secondary neurosphere formation for both TMZ-only and TMZ+DAPT treatments. When treated with TMZ+DAPT, U87NS-NICD secondary neurosphere formation was 61.2-fold greater (P<0.0001) than U87NS-pMIG secondary neurosphere formation (Fig. 4B). In the GS7-2-NICD TMZ+DAPT treated cultures, secondary neurosphere formation was 47.8-fold greater (P<0.0001) than secondary neurosphere formation in the GS7-2-pMIG TMZ+DAPT treated cultures (Fig. 4C). Hence, constitutive NICD expression eliminates GSI enhancement of TMZ therapy, identifying the Notch pathway as the relevant GSI target.
Treatment Schedules for Single Doses of TMZ and DAPT Affect Neurosphere Recovery
We tested if single DAPT doses administered before, during, or after TMZ treatment would have distinct effects. TMZ and DAPT were administered to U87NS and GS7-2 neurosphere cultures with three treatment schedules (Fig. 5A). Interestingly, PRE-treatment with DAPT decreased the efficacy of TMZ. Initial neurosphere formation was 7.2-fold and 2.7-fold greater than neurosphere formation in TMZ-only treated U87NS and GS7-2 cultures, respectively (Fig. 5B and C). When dissociated, the PRE-treated and CO-treated samples formed a large number of secondary neurospheres; however, POST-treated samples had minimal secondary neurosphere formation (Fig. 5B and C). Secondary neurosphere formation was significantly greater in TMZ-only, PRE-treated and CO-treated cultures compared to POST-treated cultures. Secondary neurosphere formation in U87NS cultures was 5.7-fold greater (P<0.001) with TMZ-only treatment, 8.1-fold greater (P<0.001) with DAPT PRE-treatment, and 4.8-fold greater (P<0.01) with CO-treatment, relative to secondary neurosphere formation after DAPT POST-treatment (Fig. 5B). The inhibition of GS7-2 secondary neurosphere formation was also greatest with POST-treatment. Secondary neurosphere formation in the GS7-2 cultures was 85.7-fold greater (P<0.0001) with TMZ-only treatment, 98.5-fold greater (P<0.0001) with DAPT PRE-treatment, and 72.8-fold greater (P<0.0001) with CO-treatment, when compared to the DAPT POST-treatment (Fig. 5C). These results led to two observations. First, TMZ+DAPT treatment acts through a specific, sequence-dependent mechanism. Second, these results provide insight for in vivo treatment schedule.
Figure 5.
The treatment schedule of DAPT and TMZ affects neurosphere recovery. A, Schematic of the recovery assay treatment schedules. TMZ (diamond) and DAPT (inverted triangle) treatments were either administered with DAPT 24 hours prior to TMZ (PRE-treat), simultaneously with TMZ (CO-treat), or 24 hours after TMZ (POST-treat). B, U87NS cultures and C, GS7-2 cultures were treated with different DAPT treatment schedules and recovery was analyzed. Initial neurospheres were counted on day 7 or 10 (mean ± SD), recovery neurospheres were counted on day 14 or 20, and secondary neurospheres were counted on day 21 or 30. Secondary neurosphere formation was inhibited only in cultures treated with the POST-treat schedule. The t-test was used to calculate statistical significance. *=P<0.001.
TMZ+DAPT Ex vivo Treatment Greatly Reduces Tumor Initiation
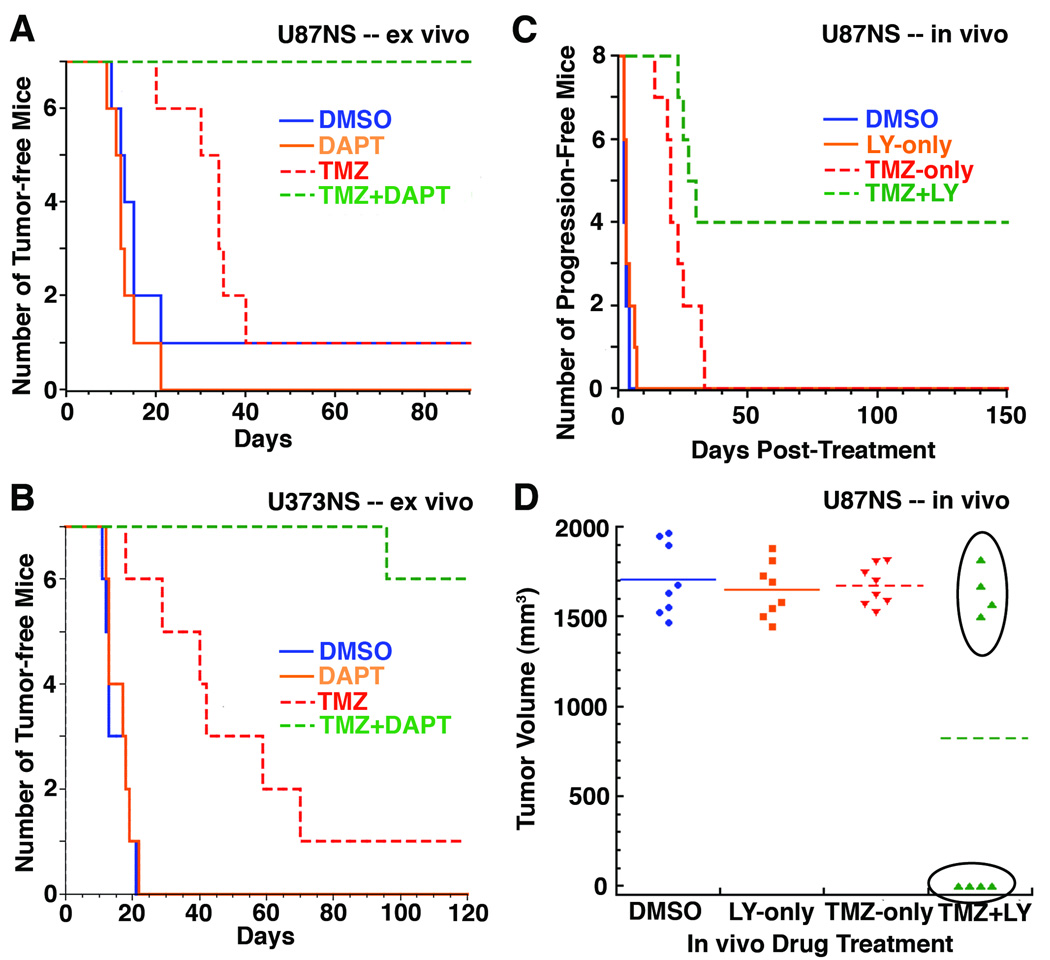
We tested if neurosphere recovery correlated with the ability of cells to initiate tumors in a subcutaneous xenograft model. U87NS cells were treated in vitro with DMSO, DAPT-only, TMZ-only or TMZ+DAPT (Fig. 6A and Supplementary Table S1). 2.5×105 live cells were subcutaneously injected into nude mice, and tumor initiation was observed when a palpable tumor formed. DMSO and DAPT-only ex vivo treated cells showed similar tumor incidence (6/7 and 7/7, respectively) and average latencies of 15 and 14 days, respectively. TMZ-only treated cells had an increased tumor latency of 32 days, but the tumor incidence (6/7 mice) was similar to control xenografts. Impressively, none of the mice injected with TMZ+DAPT treated cells (0/7 mice) formed tumors, even after 90 days. When a higher number of live U87NS cells (3×106) were injected, we saw a similar trend (Supplementary Table S1). Mice with 3×106 cells for U87NS DMSO (2/2 mice) and DAPT-only (2/2 mice) xenografts developed palpable tumors at 3 and 4 days, respectively, and 3/4 mice formed tumors in TMZ-only treated cells with an average latency of 25 days. With this higher number of cells injected, U87NS TMZ+DAPT xenografts formed tumors in only 1/4 mice with a longer latency of 43 days.
Figure 6.
TMZ+DAPT treatment decreases tumorigenicity. A, U87NS cells were treated ex vivo with DMSO, DAPT 1 µM, TMZ 200 µM or TMZ 200 µM + DAPT 1 µM, and 7 days post-treatment, mice were subcutaneously injected with 2.5×105 live cells. TMZ treatment increased latency, but xenografts still formed. TMZ+DAPT inhibited xenograft formation. B, U373NS cells were treated ex vivo, and mice were subcutaneously injected with 3×106 live cells. TMZ 200 µM treatment increased latency, but tumors still formed. TMZ 200 µM + DAPT 5 µM treatment decreased xenograft formation. C, In vivo TMZ treatment increased the tumor latency in U87NS xenografts, but 100% of the mice succumbed to progression. TMZ+LY chow treatment completely blocked progression in 50% of the mice. D, Tumor volumes at the time sacrifice. The U87NS TMZ+LY chow treated xenografts that lacked progression had no palpable tumor at 150 days post-treatment.
U373NS cultures were treated with DMSO, DAPT-only, TMZ-only or TMZ+DAPT, and 3×106 live cells were injected subcutaneously into nude mice (Fig. 6B and Supplementary Table S1). The control DMSO cells formed palpable tumors in an average of 15 days for 7/7 xenografts, and DAPT-only treated cells formed tumors in an average of 16 days for 7/7 xenografts. Ex vivo treatment with TMZ-only increased the latency of tumor formation; however, the tumor incidence was similar to the DMSO control xenografts. Palpable tumors formed for 6/7 TMZ-treated U373NS xenografts in an average of 43 days. Ex vivo treatment with TMZ+DAPT greatly reduced tumor formation in mice. Only 1/7 mice formed a tumor in the TMZ+DAPT U373NS xenografts with an extended latency of 96 days. The tumor-free mice were observed for up to 120 days before sacrifice. These ex vivo experiments demonstrate the potency of TMZ+DAPT combined treatment in reducing tumor formation.
TMZ+LY In Vivo Treatment Inhibits Tumor Regrowth
We tested the effect of in vivo TMZ+GSI treatments on pre-existing subcutaneous glioma xenografts using LY chow (23). A ten-day diet of LY chow significantly decreased the mRNA levels of the Notch targets Hes1 and Hey1 (Supplementary Fig. S4). Mice were subcutaneously injected with 106 U87NS cells and treated when the tumors reached a volume of approximately 150 mm3. When the tumor volume was double the original volume from the start of the drug treatments, we judged the xenograft as progressing. The DMSO control and LY chow-only cohorts did not have any delay in tumor progression (Fig. 6C). TMZ treatment initially had decreased tumor volumes (Supplementary Fig. S5A). However, the TMZ-only treated tumors progressed in 8/8 xenografts, and tumor volume doubled in an average of 23±7 days after treatment (Fig. 6C). These tumors had a normal growth rate and were sacrificed between 23 to 39 days post-treatment. Impressively, 4/8 the mice treated with TMZ+LY chow displayed no tumor progression (Fig. 6C). In the other 4/8 mice treated with TMZ+LY chow, tumor progression occurred in an average of 26±3 days (Supplementary Fig. S5B), and mice were euthanized between 24 to 33 days post-treatment. The TMZ+LY chow mice that did not have tumor progression displayed a complete loss of a palpable tumor and remained tumor-free until euthanized at 150 days (Fig. 6D). In these mice, no tumor masses were evident by gross dissection and examination of H&E stained sections (Supplementary Fig. S6A). Hence, the TMZ+LY chow treatment had a dramatic effect on pre-existing tumors by curing 50% of the mice. During drug administration, toxicity was determined by weight loss. TMZ-only and TMZ+LY chow cohorts initially showed a slight weight loss after TMZ injections (Supplementary Fig. S6B). However, the TMZ-only and TMZ+LY chow mice returned to their starting body weight, and no significant weight difference was observed throughout the remainder of the treatment. This demonstrates that the mice tolerated the LY chow alone and the combination of the TMZ +LY chow. The lack of overall weight loss also suggests that the mice on LY chow diets did not significantly reduce their food consumption compared to control mice and received the estimated average dose of 5 mg/kg/day of LY411,575 (23).
Discussion
With current GBM treatment, tumor recurrence is highly probably. Our laboratory’s neurosphere recovery assay demonstrates that the glioma cells that survive chemotherapy can repopulate neurosphere cultures and form tumors (11). Neurosphere cultures are useful in vitro to study glioma response to drug treatments, since the neurospheres resemble the phenotypes and genotypes of the patients’ tumors (8, 10). In addition, we found that the adherent glioma cell lines grown as serum cultures are more sensitive to TMZ than the neurosphere cultures and do not recover (data not shown). In contrast, when neurospheres are treated with clinically relevant concentrations of TMZ, a small number of cells survive, recover from the chemotherapy and repopulate the cultures.
The Notch pathway is active in gliomas, and is inhibited with GSI treatment. Low concentrations of GSIs alone (1 µM and 5 µM) did not have a significant effect on neurosphere formation (Fig. 1C). These results are consistent with Wang et al. (19), which demonstrated that low concentrations of DAPT or L685,458 only moderately reduced cell growth. However, a recent publication demonstrated that the potent GSI-18 inhibited neurosphere formation and xenograft growth (25). In the presence of higher concentrations of DAPT and LY411,575, a dose-dependent response was observed. At 10 µM, the GSIs had a moderate effect on initial neurosphere formation, but these cells retained their ability to form secondary neurospheres. It appears that GSI-only treatment initially impedes the proliferation of neurosphere cells, but these cells are capable of recovery. On the other hand, we demonstrated that low concentrations of two GSIs, DAPT and LY411,575, enhanced TMZ treatment (Fig. 2, 3 and Supplementary Fig. S2). Neurosphere recovery was inhibited, and tumor formation was greatly reduced with TMZ+GSI treatment. Additionally, when the remaining neurospheres were dissociated and re-plated, we found that the cells from TMZ+GSI treated cultures were no longer capable of self-renewal, based on their inability to form secondary neurospheres. The mechanism for the permanent suppression of neurosphere formation with TMZ+GSI treatment is under study in our laboratory. The specific population of cells that are targeted by TMZ+GSI treatment is unknown. Research in the growing cancer stem cell field demonstrates that GBM stem cells exhibit chemo- and radio-resistance (26–28). Since Notch activity is associated with GBM stem cell function and survival (13, 29), and the cells that survive TMZ-only treatment are capable of self-renewal (Fig. 3) and tumor initiation (Fig. 6), it is probable that the cells targeted by TMZ+GSI treatment possess a cancer stem cell phenotype. There is controversy about the current GBM stem cell markers, such as CD133 (30), and further progress in this field will be necessary to determine if the TMZ+GSI responsive cells and the cancer stem cells are the same population.
Inhibiting the repopulation of the neurosphere cultures is dependent on the sequence of TMZ and GSI treatments (Fig. 5). With single doses of DAPT, secondary neurosphere formation was inhibited only when DAPT was administered after TMZ (POST-treated). Simultaneous treatment of TMZ and DAPT did not significantly inhibit self-renewal. When DAPT was administered before TMZ (PRE-treated), secondary neurosphere formation was similar to the TMZ-only treated cells. PRE-treatment also resulted in a higher number of initial neurospheres forming. These results were important in determining in vivo treatment schedules for mice, and will be valuable to translate this research into the clinic. Since current treatments also include radiotherapy, we ultimately need to add radiation to our TMZ+GSI treatment schedule. Recently, it was found that TMZ and radiation are additive when TMZ is administered prior to radiation (31). GSIs can also enhance radiation-induced cell death when administered within 24 hours before or after radiotherapy (19). However, our results demonstrate the GSI treatment before TMZ can diminish the efficacy of the chemotherapy, and to inhibit neurosphere and tumor formation TMZ should be administered prior to GSIs. Further experiments are necessary to determine how irradiation will contribute to combination therapy with TMZ and GSIs and the sequence of treatments that will provide the most efficient therapy.
Our mouse experiments demonstrate that the addition of GSIs to TMZ treatment can significantly enhance the survival of mice with glioma xenografts. Ex vivo treatment of U87NS and U373NS cultures with TMZ+DAPT greatly decreased tumorigenicity (Fig. 6A and B). The in vivo treatments demonstrated that TMZ-only treatment of pre-existing tumors was not a sufficient therapy, because it only temporarily blocked tumor progression. In 50% of the treated mice, TMZ+LY chow treatment completely halted tumor progression and culminated with the loss of a palpable tumor (Fig. 6C and D). In the other 50% of treated mice, there was substantial tumor volume at the time of sacrifice. This variability may result from several sources. In the mice that have a shorter latency the TMZ concentration may not be high enough to induce a cell cycle arrest in all of the cells capable of recovery, which could hinder GSI enhancement (Supplementary Fig. S5A and B). Also, a slight variability in the food consumption between mice in the TMZ+LY chow cohort could explain the heterogeneous response. These observations emphasize the need for personalized treatment in regards to drug dosing. The response to the in vivo treatment schedule was analogous to the DAPT POST-treatment schedule in the neurosphere recovery assay, which demonstrated that TMZ+GSI treatments permanently blocked culture repopulation and tumor regrowth. These studies suggest a role for TMZ+GSI therapy to reduce recurrences in patients with low tumor burden after surgical resection of the bulk tumor.
We believe that these studies have great potential for clinical translation because most or all GBMs have active Notch signaling (13, 14, 18) (Fig. 1A), and all the lines in this study responded to the TMZ+GSI treatment (Fig. 3, 6, and Supplementary Fig. S2). In addition, TMZ is already the chemotherapy drug of choice for GBMs (3), and GSIs are in clinical trials (16, 32–34). An additional benefit of the combined treatment with TMZ+GSI is that lower concentrations of the GSI can be used, and in culture, a single dose of GSI is sufficient to enhance TMZ therapy. These may be important clinical factors, because GSIs can cause cytotoxicity in the gastrointestinal tract (35); however, low GSI doses and intermittent treatment schedules diminish these side effects (16). It is also possible that more specific inhibitors, such as anti-Notch receptor antibodies (36), could be utilized in conjunction with TMZ. In contrast to the reversible effects of GSI-only or TMZ-only treatments, TMZ+GSI has an apparently permanent effect on neurosphere and tumor formation. This response has the potential to enhance clinical TMZ therapy by inhibiting glioma recurrence.
Supplementary Material
Acknowledgements
Financial support: National Institutes of Health (NS021716 and HD04147) and the CVIP Technology Development Fund
We thank Alicia Mihaliak for comments on this manuscript. Brent Cochran (Tufts Medical School) provided the GS7-2 culture. We thank Lisa Minter and Barbara Osborne (UMass Amherst) for the LY411,575 and LY411,575 chow. We thank Steve Lyle (UMass Medical School) and Tom Smith (UMass Neuropathology) for their help with pathology and Ted Giehl (UMass Flow Cytometry Core) for the FACS. The NICD vector was provided by Michelle Kelliher’s lab.
Footnotes
Conflict of Interest: None of the authors have a conflict of interest.
References
- 1.CBTRUS. Hinsdale, IL: Central Brain Tumor Registry of the United States; CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2006. 2010
- 2.Ostermann S, Csajka C, Buclin T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10:3728–3736. doi: 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 5.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 6.Ponti D, Costa A, Zaffaroni N, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 7.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 10.Ernst A, Hofmann S, Ahmadi R, et al. Genomic and expression profiling of glioblastoma stem cell-like spheroid cultures identifies novel tumor-relevant genes associated with survival. Clin Cancer Res. 2009;15:6541–6550. doi: 10.1158/1078-0432.CCR-09-0695. [DOI] [PubMed] [Google Scholar]
- 11.Mihaliak AM, Gilbert CA, Li L, et al. Clinically relevant doses of chemotherapy agents reversibly block formation of glioblastoma neurospheres. Cancer Lett. 2010 doi: 10.1016/j.canlet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 13.Shih AH, Holland EC. Notch signaling enhances nestin expression in gliomas. Neoplasia. 2006;8:1072–1082. doi: 10.1593/neo.06526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanamori M, Kawaguchi T, Nigro JM, et al. Contribution of Notch signaling activation to human glioblastoma multiforme. J Neurosurg. 2007;106:417–427. doi: 10.3171/jns.2007.106.3.417. [DOI] [PubMed] [Google Scholar]
- 15.Fischer A, Gessler M. Delta-Notch--and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res. 2007;35:4583–4596. doi: 10.1093/nar/gkm477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27:5124–5131. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 17.Fan X, Khaki L, Zhu TS, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purow BW, Haque RM, Noel MW, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Wakeman TP, Lathia JD, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells. 28:17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sen A, Kallos MS, Behie LA. New tissue dissociation protocol for scaled-up production of neural stem cells in suspension bioreactors. Tissue Eng. 2004;10:904–913. doi: 10.1089/1076327041348554. [DOI] [PubMed] [Google Scholar]
- 21.Fauq AH, Simpson K, Maharvi GM, Golde T, Das P. A multigram chemical synthesis of the gamma-secretase inhibitor LY411575 and its diastereoisomers. Bioorg Med Chem Lett. 2007;17:6392–6395. doi: 10.1016/j.bmcl.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pui JC, Allman D, Xu L, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 23.Samon JB, Champhekar A, Minter LM, et al. Notch1 and TGFbeta1 cooperatively regulate Foxp3 expression and the maintenance of peripheral regulatory T cells. Blood. 2008;112:1813–1821. doi: 10.1182/blood-2008-03-144980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beel AJ, Sanders CR. Substrate specificity of gamma-secretase and other intramembrane proteases. Cell Mol Life Sci. 2008;65:1311–1334. doi: 10.1007/s00018-008-7462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan X, Khaki L, Zhu TS, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang MK, Kang SK. Tumorigenesis of chemotherapeutic drug-resistant cancer stem-like cells in brain glioma. Stem Cells Dev. 2007;16:837–847. doi: 10.1089/scd.2007.0006. [DOI] [PubMed] [Google Scholar]
- 28.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 29.Jeon HM, Jin X, Lee JS, et al. Inhibitor of differentiation 4 drives brain tumor-initiating cell genesis through cyclin E and notch signaling. Genes Dev. 2008;22:2028–2033. doi: 10.1101/gad.1668708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert CA, Ross AH. Cancer stem cells: cell culture, markers, and targets for new therapies. J Cell Biochem. 2009;108:1031–1038. doi: 10.1002/jcb.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chalmers AJ, Ruff EM, Martindale C, Lovegrove N, Short SC. Cytotoxic effects of temozolomide and radiation are additive- and schedule-dependent. Int J Radiat Oncol Biol Phys. 2009;75:1511–1519. doi: 10.1016/j.ijrobp.2009.07.1703. [DOI] [PubMed] [Google Scholar]
- 32.Fleisher AS, Raman R, Siemers ER, et al. Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch Neurol. 2008;65:1031–1038. doi: 10.1001/archneur.65.8.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson RE, Albright CF. Recent progress in the medicinal chemistry of gamma-secretase inhibitors. Curr Top Med Chem. 2008;8:17–33. doi: 10.2174/156802608783334088. [DOI] [PubMed] [Google Scholar]
- 34.Kreft AF, Martone R, Porte A. Recent advances in the identification of gamma-secretase inhibitors to clinically test the Abeta oligomer hypothesis of Alzheimer's disease. J Med Chem. 2009;52:6169–6188. doi: 10.1021/jm900188z. [DOI] [PubMed] [Google Scholar]
- 35.Barten DM, Meredith JE, Jr, Zaczek R, Houston JG, Albright CF. Gamma-secretase inhibitors for Alzheimer's disease: balancing efficacy and toxicity. Drugs R D. 2006;7:87–97. doi: 10.2165/00126839-200607020-00003. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Cain-Hom C, Choy L, et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.