Thiazolyl peptides are ribosomally encoded, highly post-translationally modified peptide antibiotics with potent activity against gram-positive bacteria.1 Common structural motifs include several typically non-ribosomal moieties: dehydroalanines (Dhas), dehydrobutyrines (Dhbs), oxazolines/oxazoles, and thiazolines/thiazoles. However, the most broadly distinguishing attribute of thiazolyl peptides is the presence of a core nitrogenous heterocycle, which serves as the lynchpin of a highly constrained macrocycle. Three different macrocycle sizes have been discovered thus far: 26-atoms (thiocillins,2a–c nosiheptide, 2d–f and thiostrepton2g–i), 29-atoms (GE2270A2j,k), and 35-atoms (berninamycin2l–n). Amongst members of the 29-atom and 35-atom subclasses, only fully aromatized pyridines have been observed in the lynchpin position, while pyridines, dehydropiperidines, and piperidines have all been observed in members of the 26-atom subclass. Early feeding studies demonstrated that the constituents of these lynchpin heterocycles are derived from the amino acid serine in what has long been postulated as a tail-to-tail condensation proceeding via the corresponding dehydroalanine moities.3 The work presented herein demonstrates that, in case of the thiocillins, this biosynthetic hypothesis is operative and that a fully modified peptide bearing two Dhas at positions 1 and 10 is the acyclic precursor in thiocillin biosynthesis. Further, we identify TclM, a 39 kDa protein with no discernable homology to proteins of known function, as responsible for the putative cycloaddition reaction that combines the two residues.
To date, only two enzymes have been purified to homogeneity and demonstrated to catalyze reactions, wherein the substrates are consistent with a [4+2] cycloaddition: lovastatin nonaketide synthase (LovB) and macrophomate synthase.4 Such putative “Diels-Alderases” have been suggested in the biosyntheses of a number of other natural products, but the question remains as to whether or not these transformations require enzyme catalysis. Nevertheless, the “hetero-Diels-Alderase” that would be necessary for construction of the thiocillin pyridine moiety is unprecedented in the catalog of enzymology. Total syntheses of amythiamycin, thiostrepton, and GE2270A and T have made use of “biomimetic” heteroannulations, however, in all instances, the transformation has been used in early stage ring formations, free from constraint by an incipient macrocycle, and require alternative catalysis such as heat or metals. 5 Thus it is of interest to determine if that heterocyclization would be enzyme catalyzed.
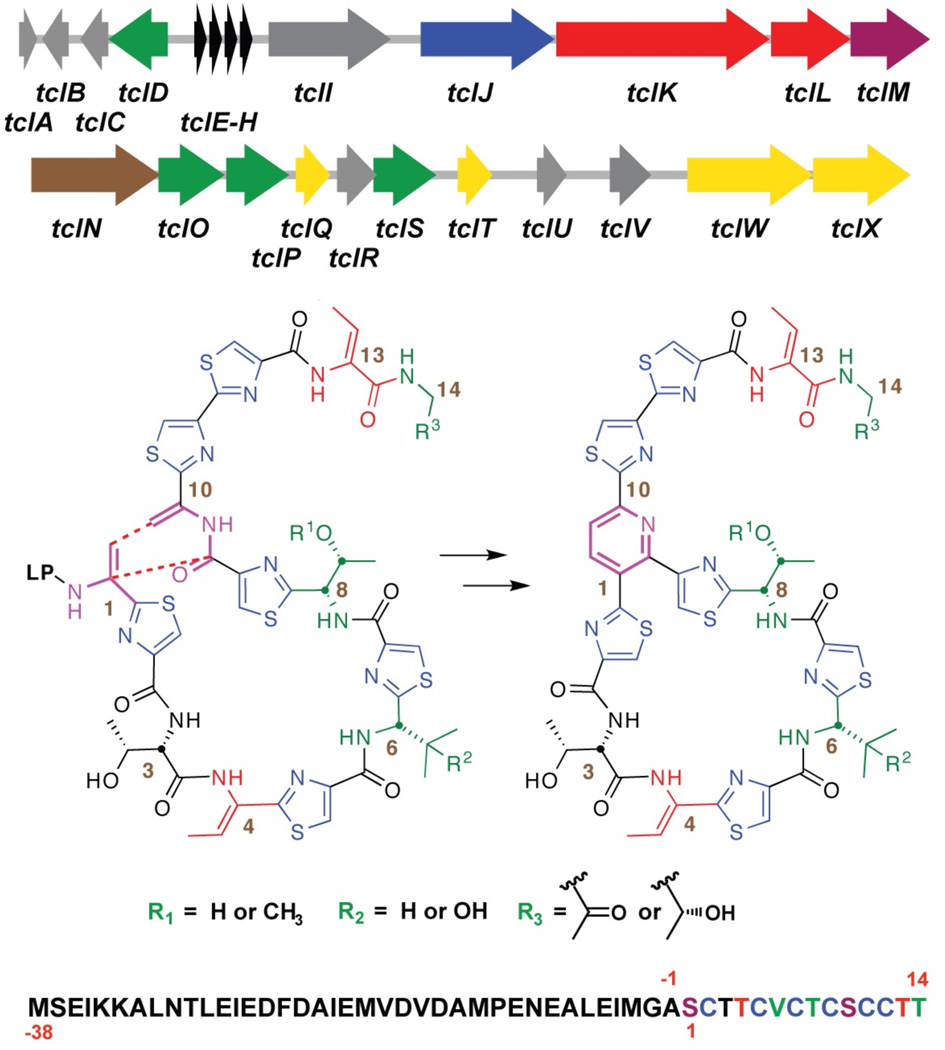
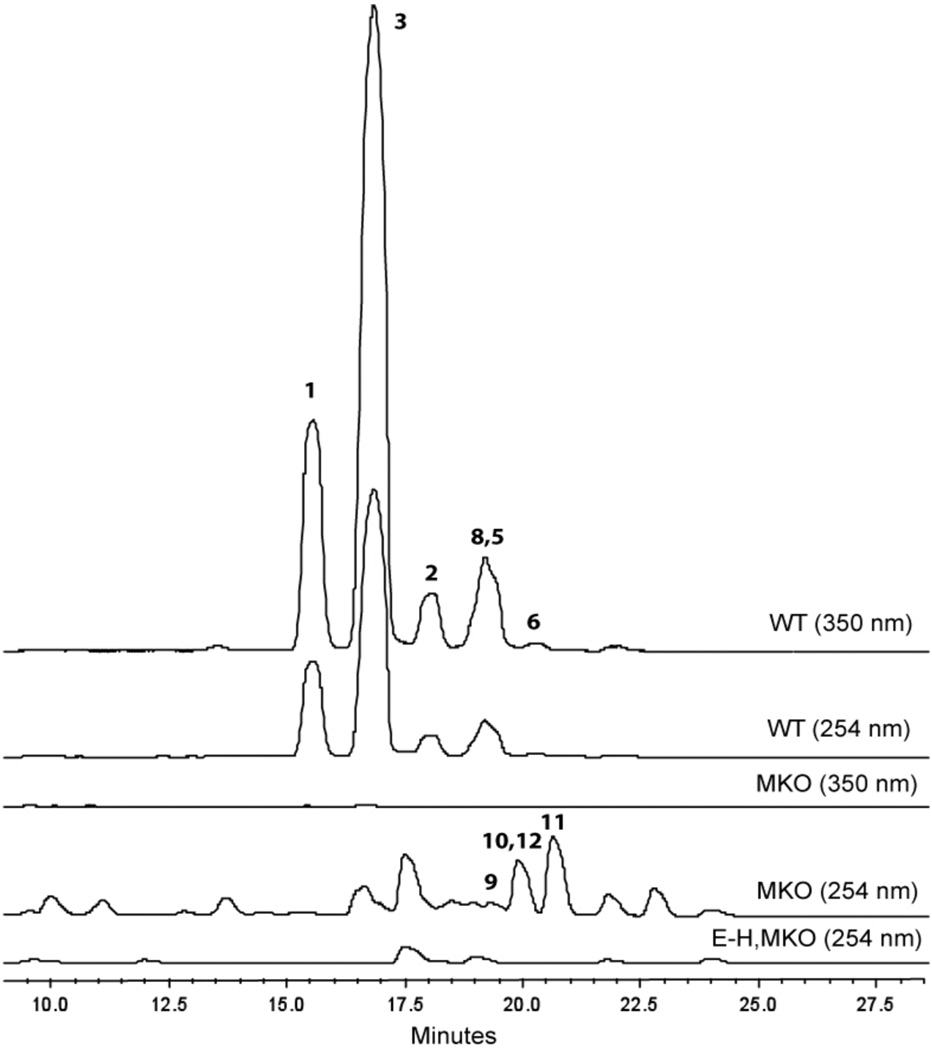
Thiocillin biosynthesis in B. cereus ATCC 14579 proceeds via ribosomal translation of a 52-amino acid prepeptide, which contains both a 38-residue leader sequence and the 14-amino acids that end up in the mature natural product after a cascade of posttranslational modifications (figure 1). 6 In addition to the four copies of the prepeptide encoding gene (tclE-H), the gene cluster contains two lantibiotic-type dehydratases (tclK and L),7a,b a McbBC-like cyclodehydratase and associated dehydrogenase (tclJ and N, respectively),7c,d several proposed resistance elements (tclQ, T, W, and X), and a number of tailoring enzymes that effect stochastic changes at residues 6, 8, and 14 (tclD, O, P, and S). Combinations of modifications effected by the latter set of enzymes give rise to eight thiocilin variants all of which can be isolated from this strain of B. cereus: thiocillin I, II, III, and IV Micrococcin P1 and P2, and YM-266183 and 266184 (compounds 1–8, respectively). Of the unassigned ORFs in the gene cluster, tclM first garnered our attention due to the presence of homologs in several other recently reported gene clusters: tsrL (thiostrepton),8a,b nosO (Nosiheptide),8c and tpdD (GE2270A)8d (see Supporting Information for sequences and alignment). Moreover, an M homolog was conspicuously absent from the genome of Streptomyces TP-A0584, which produces goadsporin, an acyclic thiazolyl variant.9 For these reasons, our initial efforts in characterization of a putative hetero-Diels-Alderase for B. cereus ATCC 14579 have focused on the 975 bp gene tclM. Due to the complexity of the substrates involved in thiocillin biosynthesis, we had previously made use of a double crossover knockout of the structural genes tclsE-H to probe the assembly line in vivo.10 For initial investigation of tclM, we again relied on this strategy. The tclM knockout (MKO) was prepared via homologous recombination with a plasmid bearing sequence homology to areas adjacent to, but not containing tclM. The same plasmid was employed in conjunction with our previously reported knockout of the structural genes to prepare a tandem EH, M knockout (E-H,MKO), into which was next introduced a single copy of tclE via Campbell integration (EinMKO). All three strains, MKO, E-H,MKO, and EinMKO were grown and extracted in the same manner as for wild-type. Analysis was performed by hi-res ESI-LC/MS, with UV monitoring at 254 and 350 nm (figure 2). Due to the conjugated aromatic system at core of the thiocillins, wild type compounds absorb across both wavelengths. In contrast, extracts from both the MKO and EinMKO strains exhibited peaks that absorbed at 254 nm, but not at 350 nm. Four significant masses corresponded to the new peaks in the isolates: 1421.3447 (C58H69N16O15S6+ 9), 1364.3232 (C56H66N15O14S6+ 10), 1194.2541 (C49H56N13O11S6+ 11), and 1196.1697 (C49H58N13O11S6+ 12). These peaks and associated masses were absent from traces and mass spectra of both the wild type producer and the tandem E-H,MKO.
Figure 1.
Annotated gene cluster responsible for production of thiocillins from a 52-residue prepeptide. Formation of the pyridine ring illustrated as the final step. (LP = leader peptide).
Figure 2.
HPLC traces at 254 and 350 nm of wild type thiocillins, MKO, and E-H,MKO isolates.
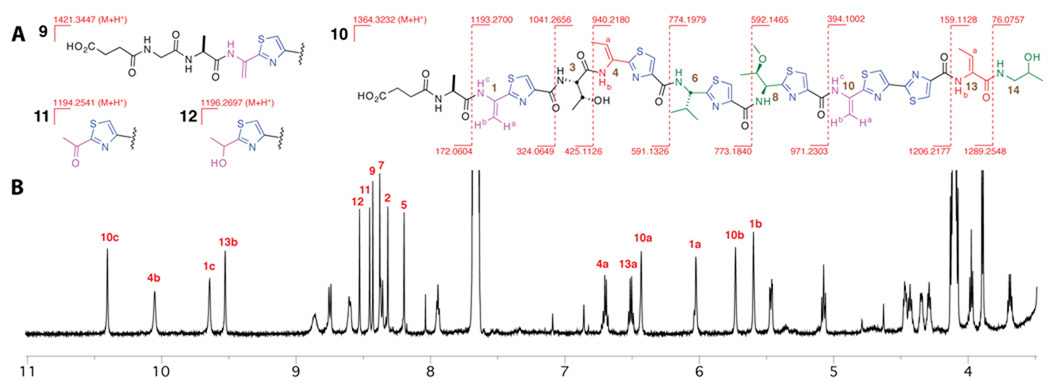
By combination of hi-res MS/MS and 1D and 2D 1H and 13C-NMR spectra (figure 3 and Supporting Information) we were able to assign the products from the tclM knockout cultures as the fully modified, linear, and successively proteolyzed 16–14 residues at the C-termini of the structural peptide; in instances where residues from the leader peptide remained, their N-termini had been succinylated. Thus, compound 9, with mass 1421.3447 represents the fully modified 14-mer with a glycine and alanine from the leader peptide remaining at the N-terminus and the glycine nitrogen N-succinylated. Similarly, compound 10, with mass 1364.3232 contains only the alanine residue of the leader peptide and is again N-succinylated, this time on alanine (Figure 2). We had previously observed N-succinylation in the E-HKO system, with prepeptide genes that encode lysines for incorporation into the macrocycle at positions 3, 4, and 8. 10a In those cultures, with a functional TclM, cyclization was effected satisfactorily, but the lysine distal nitrogen was succinylated, perhaps in self-protection, as may be the case here. Lastly, compounds 11 and 12, with masses 1194.2541 and 1196.2697 appear to have been degraded back to the first residue, serine 1, of the 14-mer product peptide. In this case, cleavage of the Ala−1-Ser1 amide bond would leave Dha1 as a primary enamine, which rather than be captured by a presumed succinyl transferase has tautomerized and hydrolyzed to the methyl ketone (11). This ketone is further reduced to the secondary alcohol (12) in fashion similar to the threonine at residue 14 of the natural product (see SI for structures and scheme).
Figure 3.
(A) Structure of linear thiocillin precursors isolated from fermentations of the tclM knock-out strain and primary mass spectral fragmentation pattern of 10. (B) 1H NMR of 10, characteristic peaks labeled in red (600 MHz, DMSO-d6; see SI for complete assignment).
To reconstitute thiocillin production, tclM was reintroduced via homologous recombination: peaks at 254 nm in the LC trace were again replaced by peaks at 350 nm, with masses corresponding to the fully cyclized and aromatized natural product. As an additional confirmation that the intermediate peaks were on track from the thiocillin gene cluster, a threonine-3 to alanine prepeptide mutant was introduced into the E-H,MKO and a corresponding −30 mass shift was observed in the MS-trace of the resultant compounds: 1391.3341 (C57H67N16O14S6+ 13), 1334.3126 (C55H64N15O13S6+ 14), 1164.2435 (C48H54N13O10S6+ 15), and 1166.1591 (C48H56N13O10S6+ 16) (see Supporting Information for structures).
Isolation of these derivatives from the tclM knockout strain demonstrates that key post-translational modifications in thiocillin biosynthesis, thiazole formation, serine and threonine dehydration, as well as C-terminal oxidation/decarboxylation can, and perhaps must, precede central pyridine ring formation. Moreover, it strongly suggests that the pyridine ring is formed through a late stage cycloaddition (either stepwise or concerted) between the two dehydroalanines formed at serines 1 and 10. Although it cannot be conclusively ruled out that these modifications regularly succeed ring formation and that disruption of cyclization has allowed persistence long enough to permit non-specific modification, this seems highly unlikely given the level of production and amount of peptidase-affected degradation. The conversion of ten residues, six cysteines to planar thiazoles, two threonines and two serines to sp2-containing Dhb and Dha residues, may impose conformational constraints that allow Dha1 and Dha10 to come within bonding distance in the active site of TclM. These results clearly implicate a specific class of enzyme, TclM and its homologs, in pyridine/piperidine formation.
Moreover, the N-terminal peptide degradative trimming to −2 and −1 tails, as well as their N-succinylation, raises questions regarding the mode of cleavage of the leader peptide in biosynthesis of the mature natural products. On the one hand, the presence of both glycine and alanine cleavage products suggests action of non-specific peptidases. However, the gly-ala motif itself recalls the specific peptidases involved in maturation of the lantibiotics11 and the cyanobactins.12 N-Succinylation may be an effort on part of the bacteria to detoxify uncyclized substrates, which could explain the presence of tclV, an as yet uncharacterized acetyltransferase homolog, in the gene cluster. Alternatively, a role for such succinylation in cyclization, perhaps to enhance a final elimination step of the upstream peptide cannot be ruled out.
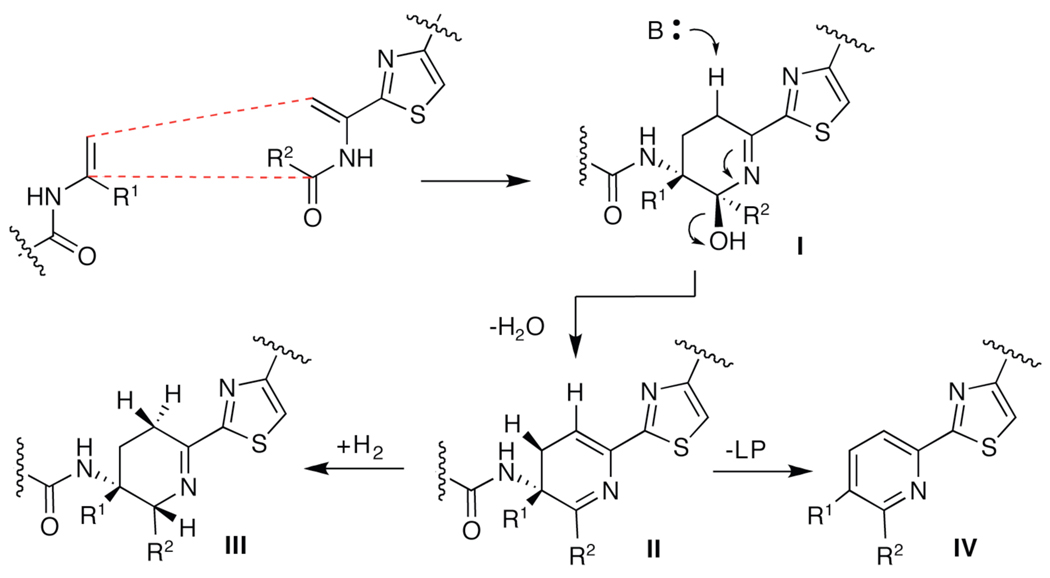
The overall transformation of two serine residues to either a trisubstituted pyridine or dehydropiperidine in the final maturation step of the thiazolyl peptide antibiotics is a remarkable chemical outcome. If the mechanism depicted in figure 4 is assumed, it seems clear that aromatization of intermediate II would be thermodynamically favored. It is unclear if, in case of the dehydropiperidine cores, where again there are TclM homologs, this intermediate is sustained and then reduced or if an alternate mechanism is in effect. Answers to these questions, as well as insight into the more precise timing and mechanism of cyclization to the core heterocycle, whether stepwise or concerted, awaits further work in vivo and in vitro.
Figure 4.
Possible routes for conversion of cycloadduct II to dehydropiperidine III or pyridine IV.
Supplementary Material
Acknowledgement
We thank Michael Fischbach for his tremendous help and insight throughout the project. We also thank Brian Ames for aid with bioinformatic analysis. This work was supported by NIH NIAID Grant nos. AI057159 and GM20011 (C.T.W.) and the New England Regional Center of Excellence (NIAID 057159). Support for instrumentation was provided by the Taplin Funds for Discovery Program (Suzanne Walker, PI). A.A.B. is supported by the NIH National Cancer Institute (CA136283). M.G.A. is a fellow of the Helen Hay Whitney Foundation.
Footnotes
Supporting Information Available: Supporting figures and tables, experimental procedures, and spectral data. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.(a) Bagley MC, Dale JW, Merritt EA, Xiong X. Cem. Rev. 2005;105:685–714. doi: 10.1021/cr0300441. [DOI] [PubMed] [Google Scholar]; (b) Arndt H-D, Schoof S, Lu J-Y. Angew. Chem. Intl. Ed. Engl. 2009;48:6770–6773. doi: 10.1002/anie.200901808. [DOI] [PubMed] [Google Scholar]
- 2.Thiocillin: Su TL. Br. J. Exp. Pathol. 1948;29:473–481. Bycroft BW, Gowland MS. J. Chem. Soc., Chem. Commun. 1978:256–258. Fenet B, Pierre F, Cundliffe E, Ciufolini MA. Tetrahedron Lett. 2002;43:2367–2370. Nosiheptide: Depaire H, Thomas JP, Brun A, Lukacs G. Tetrahedron Lett. 1977:1395–1396. Pascard C, Ducruix A, Lunel J, Prange T. J. Am. Chem. Soc. 1977;99:6418–6423. doi: 10.1021/ja00461a039. Prange T, Ducruix A, Pascard C, Lunel J. Nature. 1977;265:189–190. doi: 10.1038/265189a0. Thiostrepton: Anderson B, Hodgkin DC, Viswamitra MA. Nature. 1970;225:233–235. doi: 10.1038/225233a0. Hensens OD, Albers-Schonberg G. J. Antibiot. 1983;36:832–845. doi: 10.7164/antibiotics.36.832. Trejo WH, Dean LD, Pluscec J, Meyers E, Brown WE. J. Antibiot. 1977;30:639–643. doi: 10.7164/antibiotics.30.639. GE2270A: Kettenring J, Colombo L, Ferrari P, Tavecchia P, Nebuloni M, Vekey K, Gallo GG, Selva E. J. Antibiot. 1991;44:702–715. doi: 10.7164/antibiotics.44.702. Selva E, Beretta G, Montanini N, Saddler GS, Gastaldo L, Ferrari P, Lorenzetti R, Landini P, Ripamonti F, et al. J. Antibiot. 1991;44:693–701. doi: 10.7164/antibiotics.44.693. Berninamycin: Abe H, Kushida K, Shiobara Y, Kodama M. Tetrahedron Lett. 1988;29:1401–1404. Liesch JM, Millington DS, Pandey RC, Rinehart KL., Jr J. Am. Chem. Soc. 1976;98:8237–8249. doi: 10.1021/ja00441a058. Liesch JM, Rinehart KL., Jr. J. Am. Chem. Soc. 1977;99:1645–1646. doi: 10.1021/ja00447a061.
- 3.(a) Mocek U, Beale JM, Floss HG. J. Antibiotics. 1989;42:1649–1652. doi: 10.7164/antibiotics.42.1649. [DOI] [PubMed] [Google Scholar]; (b) Mocek U, Knaggs AR, Tsuchiya R, Nguyen T, Beale JM, Floss HG. J. Am. Chem. Soc. 1993;115:7557–7568. [Google Scholar]; (c) Mocek U, Zeng Z, O#x00027;Hagan D, Zhou P, Fan LDG, Beale JM, Floss HG. J. Am. Chem. Soc. 1993;115:7992–8001. [Google Scholar]
- 4.(a) Kelly WL. Org. Biomol. Chem. 2008;6:4483–4493. doi: 10.1039/b814552k. [DOI] [PubMed] [Google Scholar]; (b) Auclair K, Sutherland A, Kennedy J, Witter DJ, Van den Heever JP, Hutchinson CR, Vederas JC. J. Am. Chem. Soc. 2000;122:11519–11520. [Google Scholar]; (c) Watanabe K, Oikawa H, Yagi K, Ohashi S, Mie T, Ichihara A, Honma M. J. Biochem. 2000;127:467–473. doi: 10.1093/oxfordjournals.jbchem.a022629. [DOI] [PubMed] [Google Scholar]
- 5.(a) Nicolaou KC, Dethe DH, Leung GY, Zou B, Chen DY. Chem. Asian J. 2008;3:413–429. doi: 10.1002/asia.200700361. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Zak M, Rahimipour S, Estrada AA, Lee SH, O'Brate A, Giannakakou P, Ghadiri MR. J. Am. Chem. Soc. 2005;127:15042–15044. doi: 10.1021/ja0552803. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC, Zou B, Dethe DH, Li DB, Chen DY. Angew. Chem. Int. Ed. Engl. 2006;45:7786–7792. doi: 10.1002/anie.200602798. [DOI] [PubMed] [Google Scholar]; (d) Delgado O, Muller HM, Bach T. Chemistry. 2008;14:2322–2339. doi: 10.1002/chem.200701823. [DOI] [PubMed] [Google Scholar]; (e) Muller HM, Delgado O, Bach T. Angew. Chem. Int. Ed. Engl. 2007;46:4771–4774. doi: 10.1002/anie.200700684. [DOI] [PubMed] [Google Scholar]; (f) Lefranc D, Ciufolini MA. Angew. Chem. Int. Ed. Engl. 2009;48:4198–4201. doi: 10.1002/anie.200900621. [DOI] [PubMed] [Google Scholar]
- 6.Brown LC, Acker MG, Clardy J, Walsh CT, Fischbach MA. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2549–2553. doi: 10.1073/pnas.0900008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Xie L, Miller LM, Chatterjee C, Averin O, Kelleher NL, van der Donk WA. Science. 2004;303:679–681. doi: 10.1126/science.1092600. [DOI] [PubMed] [Google Scholar]; (b) Willey JM, van der Donk WA. Annu. Rev. Microbiol. 2007;61:477–501. doi: 10.1146/annurev.micro.61.080706.093501. [DOI] [PubMed] [Google Scholar]; (c) Hughes RA, Moody CJ. Angew. Chemie Int. Ed. Engl. 2007;46:7930–7954. doi: 10.1002/anie.200700728. [DOI] [PubMed] [Google Scholar]; (d) Li YM, Milne JC, Madison LL, Kolter R, Walsh CT. Science. 1996;274:1188–1193. doi: 10.1126/science.274.5290.1188. [DOI] [PubMed] [Google Scholar]
- 8.(a) Kelly WL, Pan L, Li C. J. Am. Chem. Soc. 2009;131:4327–4334. doi: 10.1021/ja807890a. [DOI] [PubMed] [Google Scholar]; (b) Liao R, Duan L, Lei C, Pan H, Ding Y, Zhang Q, Chen D, Shen B, Yu Y, Liu W. Chem. Biol. 2009;16:141–147. doi: 10.1016/j.chembiol.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yu Y, Duan L, Zhang Q, Liao R, Ding Y, Pan H, Wendt-Pienkowski E, Tang G, Shen B, Liu W. ACS Chem. Biol. 2009;4:855–864. doi: 10.1021/cb900133x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Onaka H, Tabata H, Igarashi Y, Sato Y, Furumai T. J. Antibiotics. 2001;54:1036–1044. doi: 10.7164/antibiotics.54.1036. [DOI] [PubMed] [Google Scholar]; (b) Igarashi Y, Kan Y, Fujii K, Fujita T, Harada K, Naoki H, Tabata H, Onaka H, Furumai T. J. Antibiotics. 2001;54:1045–1053. doi: 10.7164/antibiotics.54.1045. [DOI] [PubMed] [Google Scholar]; (c) Onaka H, Nakaho M, Hayashi K, Igarashi Y, Furumai T. Microbiology. 2005;151(Pt 12):3923–3933. doi: 10.1099/mic.0.28420-0. [DOI] [PubMed] [Google Scholar]; (d) Onaka H. Biosci Biotech Biochem. 2009;73:2149–2155. doi: 10.1271/bbb.90263. [DOI] [PubMed] [Google Scholar]
- 10.(a) Acker MG, Bowers AA, Walsh CT. J. Am. Chem. Soc. 2009;131:17563–17565. doi: 10.1021/ja908777t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bowers AA, Acker MG, Koglin A, Walsh CT. J. Am. Chem. Soc. 2010;132:7319–7327. doi: 10.1021/ja102339q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furgerson Ihnken LA, Chaterjee C, van der Donk W. Biochemistry. 2008;47:7352–7363. doi: 10.1021/bi800278n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Donia MS, Hathaway BJ, Sudek S, Haygood MG, Rosovitz MJ, Ravel J, Schmidt EW. Nat. Chem. Biol. 2006;2:729–735. doi: 10.1038/nchembio829. [DOI] [PubMed] [Google Scholar]; (b) Donia MS, Ravel J, Schmidt EW. Nat. Chem. Biol. 2008;4:341–343. doi: 10.1038/nchembio.84. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lee J, McIntosh J, Hathaway BJ, Schmidt EW. J. Am. Chem. Soc. 2009;131:2122–2124. doi: 10.1021/ja8092168. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) McIntosh JA, Donia MS, Schmidt EW. J. Am. Chem. Soc. 2010;132:4089–4091. doi: 10.1021/ja9107116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.