Abstract
The dopamine system is under multiple forms of regulation, and in turn provides effective modulation of system responses. Dopamine neurons are known to exist in several states of activity. The population activity, or the proportion of dopamine neurons firing spontaneously, is controlled by the ventral subiculum of the hippocampus. In contrast, burst firing, which is proposed to be the behaviorally salient output of the dopamine system, is driven by the brainstem pedunculopontine tegmentum. When an animal is exposed to a behaviorally salient stimulus, the pedunculopontine tegmentum elicits a burst of action potentials in the dopamine neurons. However, this bursting only occurs in the portion of the dopamine neuron population that is firing spontaneously. This proportion is regulated by the ventral subiculum. Therefore, the ventral subiculum provides the gain, or the amplification factor, for the behaviorally salient stimulus. The ventral subiculum itself is proposed to carry information related to the environmental context. Thus, the ventral subiculum will adjust the responsivity of the dopamine system based on the needs of the organism and the characteristics of the environment. However, this finely tuned system can be disrupted in disease states. In schizophrenia, a disruption of interneuronal regulation of the ventral subiculum is proposed to lead to an overdrive of the dopamine system, rendering the system in a constant hypervigilant state. Moreover, amphetamine sensitization and stressors also appear to cause an abnormal dopaminergic drive. Such an interaction could underlie the risk factors of drug abuse and stress in the precipitation of a psychotic event. On the other hand, this could point to the ventral subiculum as an effective site of therapeutic intervention in the treatment or even the prevention of schizophrenia.
Schizophrenia is a devastating disorder that arises during late adolescence/early adulthood. This disorder is characterized by a spectrum of disruptions, including disruption of perceptions and hallucinations, thought disorder, and cognitive disturbances. Schizophrenia has been shown to be genetically linked, in that the propensity for an individual to develop schizophrenia is related to the proportion of shared genetic material (Gottesman and Shields, 1971, 1976). Nonetheless, it is clear that this disorder is not completely genetically determined. Thus, investigators have found a number of risk factors that predispose an individual to schizophrenia, or alternately can lead to exacerbation of psychosis or relapse in those individuals in which the schizophrenia is in remission, including stress, perinatal factors, environmental factors, and drug abuse (Jones et al., 1994; Hultman et al., 1999; McDonald and Murray, 2000). However, how such risk factors can interact with a genetic predisposition to facilitate transition to psychosis, or what common neurobiological substrates underlie this comorbidity, is unknown. Using studies in a rat model of schizophrenia, we found that risk factors such as stress and psychostimulant abuse affect the mesolimbic dopamine (DA) system in a similar fashion. Moreover, preliminary data suggest that interruption of the cycle of system disruption set in motion as a consequence of uncontrolled stress early in life may serve to circumvent the transition to psychosis (Fox and Grace, 2009).
The dopamine system and schizophrenia
Substantial evidence exists implicating the dopamine system in schizophrenia. Thus, drugs that increase DA release will exacerbate psychosis (Angrist et al., 1975) and may cause a schizophrenia-like psychosis in normal individuals (Angrist et al., 1974; Friedman and Sienkiewicz, 1991; Giladi et al., 2000). Moreover, all antipsychotic drugs in current use attenuate DA transmission (Carlsson and Lindqvist, 1963; Creese et al., 1976; Seeman et al., 1976; Kapur and Remington, 2001). Finally, studies using in vivo imaging in humans demonstrate that amphetamine-induced raclopride displacement (which is an index of phasic DA release;(Grace, 1991; Laruelle, 1998; Wong et al., 2006) is significantly higher in the brains of schizophrenia patients, and moreover the magnitude of the increase above control levels is proportional to the ability of the amphetamine to exacerbate the psychosis of the individual tested (Laruelle and Abi-Dargham, 1999; Laruelle et al., 1999). Thus, there is substantial correlative evidence that the DA system is involved at least in the psychotic features of schizophrenia. Nonetheless, there is little direct evidence that it is the DA system that is abnormal in schizophrenia (Post et al., 1975; van Kammen et al., 1986; Beuger et al., 1996). Instead, evidence has pointed to a dysfunction in the regulation of the DA system – i.e., that the DA system is in a hyper-responsive state due to alterations in its afferent regulation.
Regulation of dopamine system activity
We have performed extensive studies into the mechanisms that control DA neuron activity states. First, it is known that the mesolimbic DA neurons can exist in several activity states. First, the neurons can either be spontaneously firing or in a quiescent, non-firing “in reserve” state (Bunney and Grace, 1978; Grace and Bunney, 1984). The proportion of neurons firing spontaneously has been termed the “population activity” of the DA neurons (West and Grace, 2000; Floresco et al., 2001b; Moore et al., 2001a). Once the DA neurons are firing spontaneously, they can demonstrate different patterns of activity. In the baseline, unstimulated state, DA neurons fire in a slow, irregular patter, of about 3–5 Hz. However, if the DA system is activated, either by antagonist administration (Bunney and Grace, 1978; Grace et al., 1997), glutamate activation (Grace and Bunney, 1984; Lodge and Grace, 2006b), or afferent stimulation (Smith and Grace, 1992; Floresco et al., 2003b; Lodge and Grace, 2006a, b), the DA neurons will begin to fire in bursts of action potentials. These bursts are also emitted by DA neurons in awake behaving animals in response to a behaviorally activating stimulus, such as reward (Schultz, 1998; Anstrom and Woodward, 2005; Zweifel et al., 2009). We have found that a brainstem nucleus known as the pedunculopontine tegmentum (PPTg) is the most potent activator of mesolimbic DA neuron burst firing in the rodent brain (Floresco et al., 2003a; Lodge and Grace, 2006b), with an associated region, the laterodorsal tegmentum, gating the ability of the PPTg to drive bursting (Lodge and Grace, 2006a) (see Fig. 1). Burst firing in DA neurons occurs via stimulation of N-methyl-D-aspartate (NMDA) receptors on the DA neuron somatodendritic tree (Chergui et al., 1993; Zweifel et al., 2009). It is known that a neuron must be in a depolarized state for the NMDA receptor to be capable of activation; otherwise, there is a magnesium blockade of the NMDA channel (Mayer et al., 1984). As a result, only DA neurons that are spontaneously firing can be driven to burst fire (Lodge and Grace, 2006c).
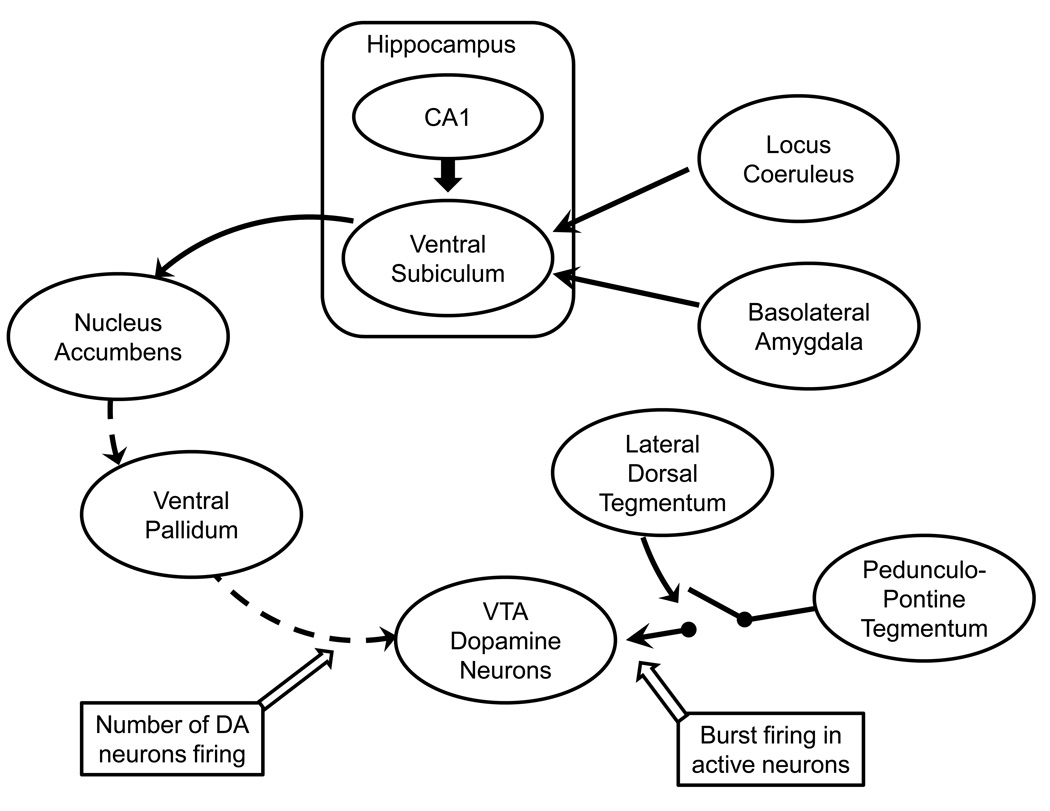
Figure 1.
Diagram illustrating the interconnections of the brain regions regulating dopamine neuron firing. Dopamine neurons in the ventral tegmental area (VTA) are differentially regulated by two projection systems. Spatial location is integrated with limbic and stress-related information from the basolateral amygdala (emotion and stress) and noradrenergic locus coeruleus (attention and stress) within the ventral subiculum, providing a contextual representation of the behavioral contingencies. The ventral subiculum then drives nucleus accumbens neuron activity which, in turn, inhibits the ventral pallidum. The ventral pallidum provides a powerful inhibition to the VTA dopamine neurons, causing a portion to be inhibited and non-firing. By controlling the ventral pallidum, this system determines the number of VTA dopamine neurons that are firing spontaneously. In contrast, the pedunculopontine tegmentum provides a glutamatergic drive over the dopamine neurons that causes only the spontaneously active neurons (determined by the ventral pallidum) to burst fire in response to a phasic stimulus. The ability of the pedunculopontine tegmentum to drive burst firing is gated by the lateral dorsal tegmentum; inhibition of the lateral dorsal tegmentum prevents burst activity altogether. These systems interact to provide phasic behaviorally salient dopamine release (via the pedunculopontine tegmentum) in response to a stimulus, with the amplitude of the phasic release dependent on the number of dopamine neurons firing (via the ventral subiculum). Solid lines = excitatory/facilitatory projections; dashed lines = inhibitory projections
Our studies show that these two activity patterns; i.e., population activity and burst firing, are driven by distinct processes. With respect to population activity, in vivo intracellular recording studies have revealed that DA neurons are constantly bombarded with high-amplitude GABAergic inhibitory postsynaptic potentials (IPSPs) arriving at a rapid pace (Grace and Bunney, 1985). These IPSPs presumably arise from GABAergic afferents derived from the ventral pallidum, a region containing neurons that are GABAergic, fire at rapid rates (Tsai et al., 1989), and project to the midbrain DA neuron population. We have posited that the ventral pallidum, via high discharge rates and direct GABAergic projections to midbrain DA neurons, holds subsets of these neurons in a hyperpolarized, non-firing state (Floresco et al., 2001a). The primary source of input to the ventral pallidum arises from the nucleus accumbens which, in turn, is potently driven by glutamatergic afferents from the ventral subiculum of the hippocampus. We have found that the most potent control of DA neuron population activity arises from this ventral subicular input (Floresco et al., 2001a; Floresco et al., 2003a). Thus, when the ventral subiculum is activated, it provides a powerful glutamatergic drive over the nucleus accumbens, which in turn inhibits the ventral pallidum and releases the DA neuron population activity from inhibitory control (Fig. 1).
What is the purpose of modulating DA neuron population activity? We have shown that the population activity of the DA system will have a minor influence on DA transmission in the striatum; i.e., increases in the number of neurons firing appears to cause a linear increase in the low level, tonic DA levels present in the striatum (Floresco et al., 2003a). However, controlling the population activity is likely to have a much more important consequence when it comes to regulating the responsivity of the DA system. Thus, as outlined above, burst firing is believed to be the behaviorally salient output of the DA system. Furthermore, burst firing is driven directly by glutamatergic afferents arising from the PPTg; a region that is known to respond to conditioned responses and behaviorally salient cues (Pan and Hyland, 2005).
Thus, a salient stimulus will activate burst firing in spontaneously firing DA neurons via activation of the PPTg. However, the number of DA neurons that are driven to burst fire will depend on the population activity. Therefore, the more DA neurons firing, the larger the amplitude of the phasic response (Lodge and Grace, 2006b). As a result, the ventral subiculum, by controlling the number of DA neurons firing, will set the amplification factor, or the gain, of the phasic burst firing response driven by the PPTg.
What is the role of the ventral subiculum, beyond its influence over DA neuron activity? Studies have shown that the ventral subiculum (which is the rodent homolog of the anterior hippocampus in humans) receives afferent input from a number of structures, including the dorsal hippocampus CA1 region (Amaral et al., 1991) as well as areas related to limbic system information processing (French et al., 2003; Herman and Mueller, 2006). It is well-known that dorsal regions of the hippocampus are involved in place learning, and contain place cells believed to code one’s location in space (O'Keefe, 1976). However, as one moves ventrally through the rodent hippocampus, the place information appears to be more integrated with limbic input. I propose that this represents a layering of affective input on top of the location, which would transform information from “where am I in space” to “what is the emotional significance of my location?” The emotional salience of a location would, by definition, be its behaviorally salient context. This is highly consistent with studies showing that the ventral subiculum is involved in context-dependent fear conditioning (Fanselow, 2000) as well as other context-related processes (Jarrard, 1995; Maren, 1999; Sharp, 1999). This would also be consistent with its role in determining DA neuron population activity; i.e., the amplitude of the stimulus-driven phasic response of the DA system should be a function of the behavioral context of the event. Thus, in conditions in which the context is safe and innocuous, stimuli should cause little activation of the DA system and promote minor behavioral modifications. However, in conditions in which the organism is in an environment in which rapid and massive responses are required to obtain reward (e.g., identifying prey) or reacting to threat, the context should maintain the DA system at a high state of responsivity (Fig 2).
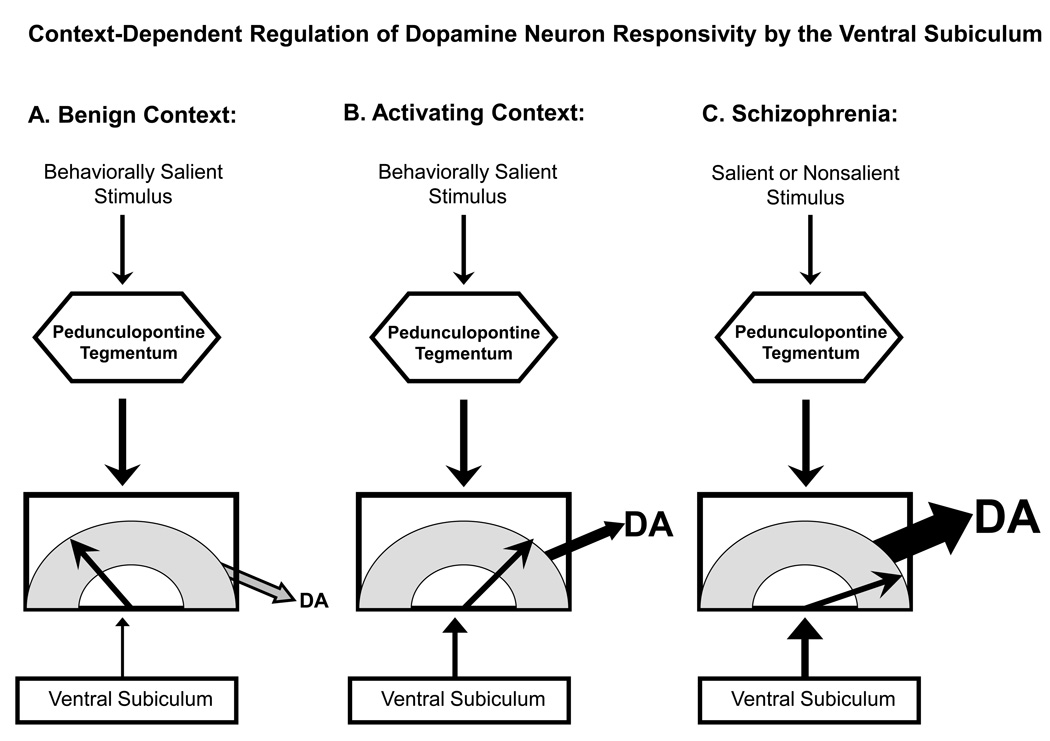
Figure 2.
Dopamine neuron activity states are regulated by the ventral subiculum of the hippocampus and the pedunculopontine tegmentum. Stimuli that are behaviorally salient activate the pedunculopontine tegmentum, causing glutamate release onto mesolimbic dopamine neurons and leading them to burst fire. The amplitude of this phasic dopamine signal is dependent on the number of dopamine neurons that the pedunculopontine can activate, since it can only cause burst firing in dopamine neurons that are already spontaneously firing. By controlling the population activity (i.e., proportion of dopamine neurons firing spontaneously), the ventral subiculum regulates the gain of the phasic signal. A) In a safe, benign context, novel salient stimuli activate the pedunculopontine tegmentum. However, because of the benign environmental context, the ventral subiculum only allows a small proportion of dopamine neurons to be firing spontaneously. As a result, the dopamine signal is small, garnering little behavioral activation of the subject. B) In an activating context, stimuli would likely have a strong motivating or threatening association, such as when searching for prey (rewarding) or in dangerous situations (threatening). In this case, the ventral subiculum causes a large proportion of dopamine neurons to be spontaneously firing. Now when a salient stimulus activates the pedunculopontine tegmentum, the dopamine signal is large in amplitude. C) In schizophrenia, a dysfunctional and overactive ventral subiculum overdrives the dopamine system, causing essentially all of the dopamine neurons to fire spontaneously. In this condition, even minor activation of the pedunculopontine tegmentum by salient or even nonsalient stimuli still results in massive activation of the dopamine signal. As a result, the subject is forced to attend to every signal it receives as if it were a life-threatening situation, clamoring for attention.
Abnormal drive of the dopamine system in schizophrenia
As outlined above, the gain of the DA system is regulated by drive from the ventral subiculum in a context-dependent manner. In other words, the system is modulated to be highly vigilant and reactive to stimuli under conditions of high threat/high reward, but to return to a baseline state when in a more safe context. Such modulation would be necessary in order to maintain proper perspective over situations, and to modulate responses appropriately. It would not be behaviorally advantageous to be in a constant low-active state, particularly when a rapid reaction to a threat is required. However, on the other hand, being in a maintained high-vigilance state at all times would be disadvantageous for other reasons – one would be constantly over-reacting to non-threatening or benign/non-salient stimuli, with no ability to screen out events. In other words, the normal filter that tells the brain what is important and screens out everything else is turned off, as has been described by schizophrenia patients (Saks, 2007). Such a condition appears to be present in the brain of schizophrenia patients, as well as in animal models of schizophrenia. Thus, as reviewed above, in the schizophrenia patient the DA system is in a hyper-responsive state, with abnormal levels of DA release occurring to stimuli when contrasted with control conditions. Moreover, there is increasing evidence that the anterior hippocampus (the human homolog of the ventral subiculum) is overactive in schizophrenia patients (Malaspina et al., 1999; Kegeles et al., 2000; Heckers, 2001; Medoff et al., 2001; Meyer-Lindenberg et al., 2005).
A similar condition appears to exist in animal models of schizophrenia. One model which was developed in my laboratory (Moore and Grace, 1997; Grace and Moore, 1998) and has been extensively characterized (Moore et al., 2001b; Flagstad et al., 2004; Gourevitch et al., 2004; Le Pen et al., 2006; Moore et al., 2006) is based on administration of the mitotoxin methylazoxymethanol acetate (MAM). We have found that administration of MAM to pregnant rats during gestational day 17 creates in the adult offspring a set of characteristics consistent with what one would predict for an animal model of schizophrenia, including limbic cortical thinning with increased cell packing density, disruption of prepulse inhibition of startle and latent inhibition, deficits in social interactions, reversal learning and hyper-responsivity to PCP and amphetamine, all characteristics shared with the MAM rat model and human schizophrenia patients (Talamini et al., 1999; Flagstad et al., 2004; Gourevitch et al., 2004; Le Pen et al., 2006; Moore et al., 2006; Lodge et al., 2009). In these animals, we found that there is a substantial increase in the population activity of the DA neurons, being nearly 2.5× higher than that observed in controls. Moreover, this occurs in concert with hyperactivity of ventral subicular neurons (Lodge and Grace, 2007). In these animals, both the increased DA neuron population activity and the hyper-responsivity to amphetamine can be restored to baseline upon inactivation of the ventral subiculum (Lodge and Grace, 2007). The source of this hyper-activity in the ventral subiculum appears to correlate with a loss of parvalbumin GABAergic interneuron staining observed in the ventral subiculum and in the prefrontal cortex of MAM rats (Lodge et al., 2009) as well as in schizophrenia patients (Lewis et al., 2001; Zhang and Reynolds, 2002). Furthermore, the diminished parvalbumin staining corresponds to a disruption of gamma band rhythmic activity in these regions in the MAM-treated rat (Lodge et al., 2009); a characteristic also found in schizophrenia patients (Gonzalez-Hernandez et al., 2003; Gallinat et al., 2004; Cho et al., 2005; Basar-Eroglu et al., 2007). Thus, these studies suggest that, in both animal models of schizophrenia and in the human schizophrenia patient, a loss of parvalbumin-GABAergic regulation of hippocampal activity leads to hyper-responsivity of the DA system and loss of control over appropriate responses to stimuli.
Risk factors in the development of schizophrenia: stress and drug abuse
Schizophrenia is a genetically linked disorder. However, there are a number of environmental factors that can increase the probability that a particular person will convert to psychosis. Studies have shown that substance abuse can play a significant role in susceptibility to schizophrenia. Thus, studies by Murray and collaborators (Arseneault et al., 2002) have shown that abuse of cannabis, particularly during adolescence, can render an individual more susceptible to schizophrenia, particularly when this is combined with other risk factors such as a polymorphism at the site for catechol-O-methyl-transferase enzyme responsible for metabolism of DA in the frontal cortex (Caspi et al., 2005). Cannabinoids themselves are known to be endogenous transmitters that modulate transmission, particularly when it involves GABA and glutamate synapses (Schlicker and Kathmann, 2001). GABA interneurons receive potent afferent drive from long-loop projections, such as those arising from the amygdala. Studies have shown that cannabinoids will potently modulate emotional learning mediated by amygdala afferents to the prefrontal cortex (Laviolette and Grace, 2006a, b). Thus, cannabinoid agonists will potentiate emotional learning to the point where it will enable learning and conditioning to stimuli that otherwise would be below threshold for mediating a learned response (Laviolette and Grace, 2006b). Interestingly, although GABAergic neurons that contain parvalbumin do not express cannabinoid receptors (Katona et al., 1999; Marsicano and Lutz, 1999; Hajos et al., 2000), these neurons are potently modulated by cannabinoid-receptor containing cholecystokinin (CCK)-expressing (Marsicano and Lutz, 1999; Tsou et al., 1999) GABAergic interneurons (Karson et al., 2009). Indeed, studies show that cannabinoid agonists can disrupt the ability of parvalbumin interneuron-driven gamma rhythmicity (Hajos et al., 2000; Robbe et al., 2006). Therefore, cannabinoids are positioned to interfere with the same neuronal subtype that was shown above to be disrupted in schizophrenia frontal cortical regions.
In addition to cannabinoids, other factors are known to play a prominent role in susceptibility to schizophrenia. Thus, other drugs of abuse, such as psychostimulants, are known to exacerbate the symptoms of schizophrenia (Angrist et al., 1975), and abuse of these drugs can render an individual more susceptible to transition to psychosis (Chen et al., 2003). Another factor that has been associated with schizophrenia susceptibility is stress. Stress is known to lead to relapse in schizophrenia patients that are in remission (Benes, 1997), and early adolescent stress is known to be a risk factor in the development of schizophrenia (Benes, 1997; Tsuang, 2000). Indeed, studies by Johnstone and colleagues have shown that in children at genetic risk for developing schizophrenia, those that show abnormally high responses to stressors tend to be those that convert to psychosis (Johnstone et al., 2002). Thus, there is an interesting relationship between stress and schizophrenia susceptibility.
Stress is a complex variable that is mediated via a number of brain circuits. Interestingly, the ventral subiculum is posited as a primary central integrator of the stress response (O'Mara, 2005; Herman and Mueller, 2006). Moreover, the prefrontal cortex has been shown to be a potent regulator of the stress response, in part via attenuation of responses in the amygdala (Rosenkranz and Grace, 2002a, b; Rosenkranz et al., 2003), which is the brain region in which fear and anxiety are expressed (LeDoux, 2000). We have posited that a potential etiological factor in schizophrenia may relate to an ineffective prefrontal cortical suppression of the stress response (Grace, 2004; Thompson et al., 2004). Thus, an initial deficit in prefrontal cortical function could interfere with the normal suppression of stress responses, leading to the heightened activation seen in those that transition to schizophrenia. A brain region that has been shown to be particularly susceptible to the deleterious effects of maintained stress is the hippocampus (Sapolsky et al., 1990; Sapolsky, 2000; Lee et al., 2002). Thus, it may be that deficits in prefrontal cortical modulation of the stress response could lead to a condition of overdrive of stress circuits, resulting in hippocampal interneuron damage and hyperactivity leading to psychosis (Grace, 2004; Thompson et al., 2004). If such a condition were indeed present, one would posit that limiting the stress response during the periadolescent period, when the at-risk individuals are showing heightened stress responses, may indeed circumvent this transition and prevent the onset of schizophrenia. In preliminary experiments, we have found that if the anti-anxiety drug diazepam is administered in the peri-pubertal period of MAM-treated rats, we prevent the increase in DA neuron population activity observed in the adult that we posit is related to psychosis (Fox and Grace, 2009). It may be that such an intervention in humans, in which the abnormal stress response in children at risk is treated, may actually prevent schizophrenia from emerging later in life.
Stress and drug abuse have been shown to be risk factors in schizophrenia; moreover, studies show that stress is a risk factor in the onset or relapse to drug-taking behavior (O'Doherty, 1991; Koob and Le Moal, 2001). This can occur via long-term stress-induced hippocampal damage, as mentioned above. However, there is also evidence that both stress and psychostimulant abuse exert common actions on the DA system.
Psychostimulants, stress and dopamine system dysregulation
In the animal model of schizophrenia, we found that the increased behavioral response to amphetamine was correlated with an increase in the DA neuron population activity, or the number of DA neurons firing spontaneously (Lodge and Grace, 2008). There is another condition in which animals exhibit abnormally heightened responses to psychostimulants, and this is behavioral sensitization. Thus, when a rat is administered amphetamine or cocaine for a period of 5 days and then is withdrawn from the amphetamine for an equivalent period, the rat will show an abnormally large, or sensitized, response when tested with a single dose of amphetamine (Post, 1980; Robinson and Berridge, 2000; Lodge and Grace, 2008). We found that animals treated in this manner exhibited a substantial increase in DA neuron population activity (Lodge and Grace, 2008), in a similar manner (but less magnitude) than was observed in the schizophrenia model (Lodge and Grace, 2007; Fig. 3).
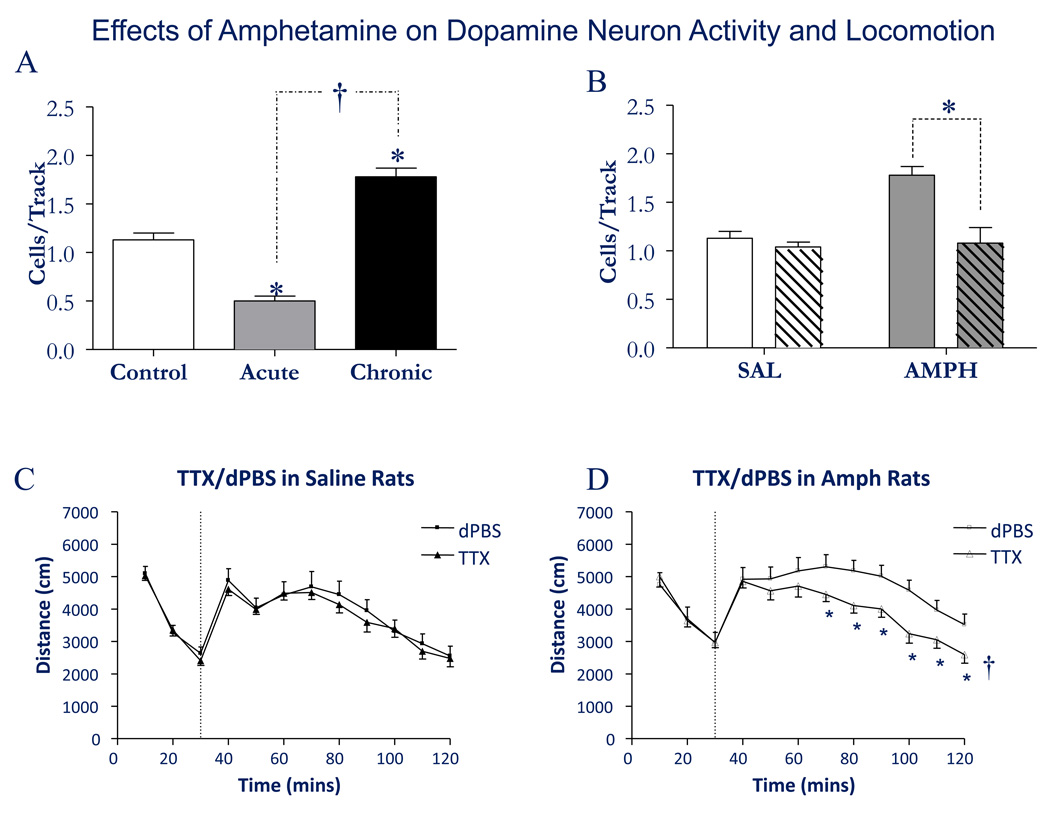
Figure 3.
Amphetamine sensitization increases dopamine neuron population activity via activation of the ventral subiculum. A) Acute administration of amphetamine (1.5 mg/kg i.p.) causes a decrease in the population activity of dopamine neurons in the midbrain ventral tegmental area. In contrast, after 5 days repeated amphetamine and 5 days withdrawal, there is a substantial increase in the number of dopamine neurons firing spontaneously. B) Inactivation of the ventral subiculum (cross-hatched) does not produce a strong effect on the population activity in control animals, likely due to its low level of activity in anesthetized rats. In contrast, inactivation of the ventral subiculum in amphetamine sensitized rats restores the increased population activity back to control levels. C) Inactivation of the ventral subiculum with TTX injection does not affect the locomotor response of control rats to amphetamine injection (1.5 mg/kg i.v., vertical dashed line). D) In contrast, in amphetamine-sensitized rats inactivation of the ventral subiculum restores the locomotor response to amphetamine back to control levels. Adapted from (Lodge and Grace, 2008). dPBS = distilled phosphate buffered saline; †Significant group effect of treatment
What is the source of the increased DA neuron population activity that is present following sensitization? An observation that may yield a clue to this is the finding that the sensitization is strongest when the behavior is tested in the same cage in which the sensitization occurred (Vezina et al., 1989; Badiani et al., 1995; Crombag et al., 2000). Thus, sensitization is a context-dependent phenomenon, and context is mediated via the ventral subiculum. Inactivation of the ventral subiculum in the amphetamine-sensitized rats was found to reverse the increase in DA neuron population activity, bringing the number of DA neurons firing spontaneously back to control levels. Moreover, following ventral subicular inactivation, the sensitized behavioral response to amphetamine was reversed (Lodge and Grace, 2008). Note that the rats still responded with behavioral activation to amphetamine administration, but the magnitude of the response was now identical to that observed in controls (Fig. 3).
Stress is also a phenomenon that demonstrates a number of characteristics that are similar in nature to what is observed in these other models. Thus, stress has been shown to cross-sensitize with amphetamine, in that an animal exposed to a stressor will show a heightened behavioral response to subsequent administration of amphetamine (Antelman et al., 1980; Pacchioni et al., 2002). Moreover, stress is a context-dependent phenomenon, in that a rat will exhibit anxiety-like behaviors when returned to an environmental context in which it had been previously stressed (Fanselow, 2000). The ventral subiculum, as mentioned above, is a region that is proposed to mediate central effects of stress (O'Mara, 2005; Herman and Mueller, 2006), and has afferent input from numerous stress-related structures, such as the amygdala and the noradrenergic locus coeruleus (Oleskevich et al., 1989; Schroeter et al., 2000; French et al., 2003), each of which we found will activate ventral subicular firing (Lipski and Grace, 2008). Stress itself has been shown to increase levels of dopamine in postsynaptic targets (Abercrombie et al., 1989; Finlay et al., 1995; Castro and Zigmond, 2001). What is the state of the DA system following an acute stressor? To examine this, we employed a 2-hour restraint protocol, which has been reported to lead to an increased behavioral response to amphetamine (Pacchioni et al., 2002). We found that, following 2 hours of restraint, there was a significant increase in DA neuron population activity. Moreover, as shown in the other paradigms, inactivation of the ventral subiculum reversed the increase in DA neuron population activity and normalized the behavioral response to amphetamine (Valenti and Grace, 2008).
Conclusions
The DA system plays a critical role in regulating responses to environmental stimuli. In particular, the DA system adjusts depending on the needs of the organism and the demands of the environment to modulate how an organism responds to stimuli. Thus, the responsivity of the DA system itself must be adjusted in order to enable a contextually-appropriate response to occur. Behaviorally salient stimuli can evoke different responses in different environmental contexts. Provided that the system is functioning in a normal fashion, the organism can efficiently interact with the environment and other organisms, yet be ready to alter their response pattern as the demands change. Being in a low responsive state when confronted with threat, or being in a highly vigilant state even in a very benign setting will lead to inappropriate and perhaps deleterious responses. For this reason, a complex system is in place to adjust the amplitude of the DA response based on a number of mood- and location-related stimuli. However, if this system is disrupted, such as is proposed to occur with conditions of ventral subiculum hyperactivity, then the responses will not match the environmental contingencies, leading the organism to over-attribute salience to benign stimuli, to be overwhelmed with attention-demanding stimuli without the ability to prioritize their responses, or to behave in a manner not consistent with effectively achieving their goals. The ability to pharmacologically restore normal activity levels within a damaged hippocampal circuit could provide an effective means of alleviating this deficit state at the source of disruption.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Dolorfo C, Alvarez-Royo P. Organization of CA1 projections to the subiculum: a PHA-L analysis in the rat. Hippocampus. 1991;1:415–435. doi: 10.1002/hipo.450010410. [DOI] [PubMed] [Google Scholar]
- Angrist B, Sathananthan G, Wilk S, Gershon S. Amphetamine psychosis: behavioral and biochemical aspects. Journal of Psychiatric Research. 1974;11:13–23. doi: 10.1016/0022-3956(74)90064-8. [DOI] [PubMed] [Google Scholar]
- Angrist B, Thompson H, Shopsin B, Gershon S. Clinical studies with dopamine-receptor stimulants. Psychopharmacologia. 1975;44:273–280. doi: 10.1007/BF00428906. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology. 2005;30:1832–1840. doi: 10.1038/sj.npp.1300730. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: Longitudinal prospective study. British Medical Journal. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Browman KE, Robinson TE. Influence of novel versus home environments on sensitization to the psychomotor stimulant effects of cocaine and amphetamine. Brain Res. 1995;674:291–298. doi: 10.1016/0006-8993(95)00028-o. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Brand A, Hildebrandt H, Karolina Kedzior K, Mathes B, Schmiedt C. Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol. 2007;64:39–45. doi: 10.1016/j.ijpsycho.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Benes FM. The role of stress and dopamine-GABA interactions in the vulnerability for schizophrenia. J Psychiatr Res. 1997;31:257–275. doi: 10.1016/s0022-3956(96)00044-1. [DOI] [PubMed] [Google Scholar]
- Beuger M, van Kammen DP, Kelley ME, Yao J. Dopamine turnover in schizophrenia before and after haloperidol withdrawal. CSF, plasma, and urine studies. Neuropsychopharmacology. 1996;15:75–86. doi: 10.1016/0893-133X(95)00158-A. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Grace AA. Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sciences. 1978;23:1715–1727. doi: 10.1016/0024-3205(78)90471-x. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M. Effect of chlorpromazine or haloperidol on formation of 3-methoxytyramine and normetanephrine in mouse brain. Acta Pharmacologica et Toxicologica. 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, Poulton R, Craig IW. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Castro SL, Zigmond MJ. Stress-induced increase in extracellular dopamine in striatum: Role of glutamatergic action via N-methyl-D-aspartate receptors in substantia nigra. Brain Res. 2001;901:47–54. doi: 10.1016/s0006-8993(01)02229-6. [DOI] [PubMed] [Google Scholar]
- Chen CK, Lin SK, Sham PC, Ball D, Loh EW, Hsiao CC, Chiang YL, Ree SC, Lee CH, Murray RM. Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. Psychol Med. 2003;33:1407–1414. doi: 10.1017/s0033291703008353. [DOI] [PubMed] [Google Scholar]
- Chergui K, Charlety PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G. Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo. European Journal of Neuroscience. 1993;5:137–144. doi: 10.1111/j.1460-9568.1993.tb00479.x. [DOI] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in gamma band syncrhonization and context processing in schizophrenia. Schizophrenia Bulletin. 2005;31:450–451. [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res. 2000;116:1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- Flagstad P, Mork A, Glenthoj BY, van Beek J, Michael-Titus AT, Didriksen M. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology. 2004;29:2052–2064. doi: 10.1038/sj.npp.1300516. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001a;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001b;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nature Neuroscience. 2003a;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003b;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Fox KM, Grace AA. 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience; 2009. Peripubertal administration of diazepam prevents dopaminergic pathophysiology in the MAM developmental model of schizophrenia. 2009 Online Program 341.6. [Google Scholar]
- French SJ, Hailstone JC, Totterdell S. Basolateral amygdala efferents to the ventral subiculum preferentially innervate pyramidal cell dendritic spines. Brain Res. 2003;981:160–167. doi: 10.1016/s0006-8993(03)03017-8. [DOI] [PubMed] [Google Scholar]
- Friedman A, Sienkiewicz J. Psychotic complications of long-term levodopa treatment of Parkinson's disease. Acta Neurol Scand. 1991;84:111–113. doi: 10.1111/j.1600-0404.1991.tb04918.x. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Giladi N, Treves TA, Paleacu D, Shabtai H, Orlov Y, Kandinov B, Simon ES, Korczyn AD. Risk factors for dementia, depression and psychosis in long-standing Parkinson's disease. J Neural Transm. 2000;107:59–71. doi: 10.1007/s007020050005. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez JA, Cedeno I, Pita-Alcorta C, Galan L, Aubert E, Figueredo-Rodriguez P. Induced oscillations and the distributed cortical sources during the Wisconsin card sorting test performance in schizophrenic patients: new clues to neural connectivity. Int J Psychophysiol. 2003;48:11–24. doi: 10.1016/s0167-8760(03)00019-9. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Schizophrenia: geneticism and environmentalism. Hum Hered. 1971;21:517–522. doi: 10.1159/000152447. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. A critical review of recent adoption, twin, and family studies of schizophrenia: behavioral genetics perspectives. Schizophr Bull. 1976;2:360–401. doi: 10.1093/schbul/2.3.360. [DOI] [PubMed] [Google Scholar]
- Gourevitch R, Rocher C, Le Pen G, Krebs MO, Jay TM. Working memory deficits in adult rats after prenatal disruption of neurogenesis. Behavioral Pharmacology. 2004;15:287–292. doi: 10.1097/01.fbp.0000135703.48799.71. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA. Developmental dysregulation of the dopamine system and the pathophysiology of schizophrenia. In: Keshavan MS, Kennedy JL, Murray RM, editors. Neurodevelopment and Schizophrenia. Cambridge, UK: Cambridge University Press; 2004. pp. 273–294. [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Opposing effects of striatonigral feedback pathways on midbrain dopamine cell activity. Brain Res. 1985;333:271–284. doi: 10.1016/0006-8993(85)91581-1. [DOI] [PubMed] [Google Scholar]
- Grace AA, Moore H. Regulation of information flow in the nucleus accumbens: A model for the pathophysiology of schizophrenia. In: Lenzenweger MF, Dworkin RH, editors. Origins and development of schizophrenia: Advances in experimental psychopathology. Washington D.C: American Psychological Association Press; 1998. pp. 123–157. [Google Scholar]
- Grace AA, Bunney BS, Moore H, Todd CL. Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. Trends in Neurosciences. 1997;20:31–37. doi: 10.1016/S0166-2236(96)10064-3. [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Hultman CM, Sparén P, Takei N, Murray RM, Cnattingus S. Prenatal and perinatal risk factors for schizophrenia, affective psychosis, and reactive psychosis of early onset: Case-control study. British Medical Journal. 1999;318:421–426. doi: 10.1136/bmj.318.7181.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE. What does the hippocampus really do? Behavioural Brain Research. 1995;71:1–10. doi: 10.1016/0166-4328(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Lawrie SM, Cosway R. What does the Edinburgh High-Risk Study tell us about schizophrenia? American Journal of Medical Genetics (Neuropsychiatric Genetics) 2002;114:906–912. doi: 10.1002/ajmg.b.10304. [DOI] [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M. Child developmental risk factors for adult schizophrenia in the British 1946 birth cohort study. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry. 2001;50:873–883. doi: 10.1016/s0006-3223(01)01251-3. [DOI] [PubMed] [Google Scholar]
- Karson MA, Tang AH, Milner TA, Alger BE. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci. 2009;29:4140–4154. doi: 10.1523/JNEUROSCI.5264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Shungu DC, Anjilvel S, Chan S, Ellis SP, Xanthopoulos E, Malaspina D, Gorman JM, Mann JJ, Laruelle M, Kaufmann CA. Hippocampal pathology in schizophrenia: magnetic resonance imaging and spectroscopy studies. Psychiatry Res. 2000;98:163–175. doi: 10.1016/s0925-4927(00)00044-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Quarterly Journal of Nuclear Medicine. 1998;42:211–221. [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of psychotic fire: new evidence from brain imaging studies. Journal of Psychopharmacology. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell Mol Life Sci. 2006a;63:1597–1613. doi: 10.1007/s00018-006-6027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala Inputs. J Neurosci. 2006b;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pen G, Gourevitch R, Hazane F, Hoareau C, Jay TM, Krebs MO. Peri-pubertal maturation after developmental disturbance: a model for psychosis onset in the rat. Neuroscience. 2006;143:395–405. doi: 10.1016/j.neuroscience.2006.08.004. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee AL, Ogle WO, Sapolsky RM. Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disord. 2002;4:117–128. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbuminimmunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Lipski WJ, Grace AA. Neurons in the ventral subiculum are activated by noxious stimuli and are modulated by noradrenergic afferents; Program No 1951, 2008 Neuroscience Meeting Planner; Washington, DC: Society for Neuroscience; 2008. 2008 Online. [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proceedings of the National Academy of Sciences of the United States of America. 2006a;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006b;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuopsychopharmacology. 2006c;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci. 2008;28:7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Storer S, Furman V, Esser P, Printz D, Berman A, Lignelli A, Gorman J, Van Heertum R. SPECT study of visual fixation in schizophrenia and comparison subjects. Biol Psychiatry. 1999;46:89–93. doi: 10.1016/s0006-3223(98)00306-0. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci. 1999;113:283–290. doi: 10.1037//0735-7044.113.2.283. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McDonald C, Murray RM. Early and late environmental risk factors for schizophrenia. Brain Res Brain Res Rev. 2000;31:130–137. doi: 10.1016/s0165-0173(99)00030-2. [DOI] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Moore H, Grace AA. Anatomical changes in limbic structures produced by methylazoxymethanol acetate (MAM) during brain development are associated with changes in physiological interactions among afferents to the nucleus accumbens. Society for Neuroscience Abstracts. 1997;23:2378. [Google Scholar]
- Moore H, Rose HJ, Grace AA. Chronic cold stress reduces the spontaneous activity of ventral tegmental dopamine neurons. Neuropsychopharmacology. 2001a;24:410–419. doi: 10.1016/S0893-133X(00)00188-3. [DOI] [PubMed] [Google Scholar]
- Moore H, Ghajarnia M, Geyer M, Jentsch JD, Grace AA. Selective disruption of prefrontal and limbic corticostriatal circuits by prenatal exposure to the DNA methylation agent methylazoxymethanol acetate (MAM): Anatomical, neurophysiological and behavioral studies. Schizophrenia Research. 2001b;49:48. [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty F. Is drug use a response to stress? Drug Alcohol Depend. 1991;29:97–106. doi: 10.1016/0376-8716(91)90026-u. [DOI] [PubMed] [Google Scholar]
- O'Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- O'Mara S. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. Journal of anatomy. 2005;207:271–282. doi: 10.1111/j.1469-7580.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleskevich S, Descarries L, Lacaille JC. Quantified distribution of the noradrenaline innervation in the hippocampus of adult rat. J Neurosci. 1989;9:3803–3815. doi: 10.1523/JNEUROSCI.09-11-03803.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacchioni AM, Gioino G, Assis A, Cancela LM. A single exposure to restraint stress induces behavioral and neurochemical sensitization to stimulating effects of amphetamine: involvement of NMDA receptors. Ann N Y Acad Sci. 2002;965:233–246. doi: 10.1111/j.1749-6632.2002.tb04165.x. [DOI] [PubMed] [Google Scholar]
- Pan WX, Hyland B. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. Journal of Neuroscience (Online) 2005;25:4725–4732. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM. Intermittent versus continuous stimulation: effect of time interval on the development of sensitization or tolerance. Life Sciences. 1980;26:1275–1282. doi: 10.1016/0024-3205(80)90085-5. [DOI] [PubMed] [Google Scholar]
- Post RM, Fink E, Carpenter WT, Jr, Goodwin FK. Cerebrospinal fluid amine metabolites in acute schizophrenia. Arch Gen Psychiatry. 1975;32:1063–1069. doi: 10.1001/archpsyc.1975.01760260127011. [DOI] [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95 Suppl 2:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002a;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002b;22:324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saks ER. The Center Cannot Hold: My Journey Through Madness. New York, NY: Hyperion; 2007. [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD. Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. J Comp Neurol. 2000;420:211–232. [PubMed] [Google Scholar]
- Schultz W. The phasic reward signal of primate dopamine neurons. Advances in Pharmacology. 1998;42:686–690. doi: 10.1016/s1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- Sharp PE. Complimentary roles for hippocampal versus subicular/entorhinal place cells in coding place, context, and events. Hippocampus. 1999;9:432–443. doi: 10.1002/(SICI)1098-1063(1999)9:4<432::AID-HIPO9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Smith ID, Grace AA. Role of the subthalamic nucleus in the regulation of nigral dopamine neuron activity. Synapse. 1992;12:287–303. doi: 10.1002/syn.890120406. [DOI] [PubMed] [Google Scholar]
- Talamini LM, Koch T, Luiten PG, Koolhaas JM, Korf J. Interruptions of early cortical development affect limbic association areas and social behaviour in rats; possible relevance for neurodevelopmental disorders. Brain Res. 1999;847:105–120. doi: 10.1016/s0006-8993(99)02067-3. [DOI] [PubMed] [Google Scholar]
- Thompson JL, Pogue-Geile MF, Grace AA. The interactions among developmental pathology, dopamine, and stress as a model for the age of onset of schizophrenia symptomatology. Schizophr Bull. 2004;30:875–900. doi: 10.1093/oxfordjournals.schbul.a007139. [DOI] [PubMed] [Google Scholar]
- Tsai CT, Mogenson GJ, Wu M, Yang CR. A comparison of the effects of electrical stimulation of the amygdala and hippocampus on subpallidal output neurons to the pedunculopontine nucleus. Brain Res. 1989;494:22–29. doi: 10.1016/0006-8993(89)90139-x. [DOI] [PubMed] [Google Scholar]
- Tsou K, Mackie K, Sanudo-Pena MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- Tsuang M. Schizophrenia: genes and environment. Biol Psychiatry. 2000;47:210–220. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- Valenti O, Grace AA. Acute and Repeated Stress Induce a Pronounced and Sustained Activation of VTA DA Neuron Population Activity; program No 47911, 2008 Neuroscience Meeting Planner; Washington, DC: Society for Neuroscience; 2008. 2008 in press. [Google Scholar]
- van Kammen DP, van Kammen WB, Mann LS, Seppala T, Linnoila M. Dopamine metabolism in the cerebrospinal fluid of drug-free schizophrenic patients with and without cortical atrophy. Arch Gen Psychiatry. 1986;43:978–983. doi: 10.1001/archpsyc.1986.01800100072010. [DOI] [PubMed] [Google Scholar]
- Vezina P, Giovino AA, Wise RA, Stewart J. Environment-specific cross-sensitization between the locomotor activating effects of morphine and amphetamine. Pharmacol Biochem Behav. 1989;32:581–584. doi: 10.1016/0091-3057(89)90201-3. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. Striatal nitric oxide signaling regulates the neuronal activity of midbrain dopamine neurons in vivo. J Neurophysiol. 2000;83:1796–1808. doi: 10.1152/jn.2000.83.4.1796. [DOI] [PubMed] [Google Scholar]
- Wong DF, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbuminimmunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, Phillips PE, Palmiter RD. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]