Abstract
Background
Type 2 diabetes mellitus (T2DM) has a strong genetic component, and its prevalence is notably increased in the family members of T2DM patients. However, there are few studies about the family history of T2DM. We carried out this study to assess the influences of family history on clinical characteristics in T2DM patients.
Methods
This is a cross-sectional study involving 651 T2DM patients. Patient history and physical examination were performed and fasting blood was taken. If any first degree relative was diabetic, a family history of diabetes was considered to exist.
Results
Among the total 621 patients, 38.4% had a family history of diabetes. Patients with a family history had a younger age, higher weight, younger age at diagnosis and higher triglyceride level than did those without a family history. Dyslipidemia medication and metabolic syndrome were more prevalent in familial diabetes. Sex, blood pressure, previous treatment for diabetes, HbA1c, C-peptide, total cholesterol, high density lipoprotein cholesterol, and low density lipoprotein cholesterol were not different between familial and non-familial diabetes. Upon multiple linear regression analysis, the family history of diabetes remained significantly associated with serum triglyceride level.
Conclusion
In T2DM patients with a family history of diabetes, the disease tended to develop earlier. Metabolic syndrome and cardiovascular risk factors are more prevalent in familial T2DM than they were in non-familial T2DM. These results support the necessity of earlier screening for diabetes in family members of T2DM patients and more active prevention against cardiovascular disease in T2DM patients with a family history.
Keywords: Diabetes mellitus, type 2; Family history; Metabolic syndrome; Triglyceride
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a disease characterized by increased insulin resistance, decreased beta cell function [1], and is known to develop due to various factors. Its prevalence has grown continuously around the world, and its complications have provoked much social and economic loss. Around 7% of the total world population has T2DM, and the number of patients is expected to increase up to 300 million persons by 2025 [2].
Although prevalence of T2DM is generally high in older adults, young T2DM patients are increasingly being reported [3]. Many studies on the etiologic factors of T2DM have been conducted, and, out of many factors, genetic predispositions are found to be important [4,5]. For family history of T2DM, insulin resistance and deficiency in insulin secretion tend to be inherited by descendants of T2DM patients [6,7], and the incidence rate of T2DM becomes higher in said descendants [8,9]. In addition, metabolic syndrome, an important risk factor of diabetes and a predictor of cardiovascular disease [10], was observed to be inherited in some studies [11-13]; however, there are few studies on its association with a family history of diabetes.
Although many diabetologists have supposed that a family history of diabetes has influence on the metabolic aspects of patients, only a few studies have been performed on its effects on metabolic and clinical factors, except for studies for the influence of family history of diabetes on T2DM development. Therefore, this study aims to determine whether a family history of diabetes is related to age at diagnosis, cardiovascular, factors, and metabolic syndrome in T2DM patients.
METHODS
Participants
This study included 651 T2DM patients (341 males and 309 females) aged 20 to 70 years hospitalized in Jeju National University Hospital and Konyang University Hospital between June 2004 and January 2010.
Type 1 diabetes presented by ketoacidosis at diagnosis, serum C-peptide level of < 0.6 ng/mL, or higher anti-GAD antibody titer and secondary diabetes caused by medication, pancreatic diseases, or endocrine diseases were excluded. Patients with a serum creatinine level greater than 2 mg/dL, a leukocyte count higher than 10,000/µL, aspartate aminotransferase and alanine aminotransferase levels more than three times the upper normal limit, and those diagnosed as having malignant tumor were also excluded.
Methods
Detailed medical histories including smoking history, family history of diabetes, duration of diabetes, and other associated diseases were investigated through patient interviews. Physical measurements, as well as and blood and urine tests, were performed using the same standards and methods in each university hospital, and their data were prospectively collected. Height and body weight were measured with an electronic scale. Waist circumference was measured midway between the costal margin and the iliac crest during mid-respiration with a loosely applied tapeline by an investigator sitting next to the standing participant with legs spread 25 cm to 30 cm and evenly distributed body weight. Hip circumference was measured at its widest part (greater trochanter). Family history of diabetes was considered to be present if any first degree relative (parents, brother, sister, and child) had diabetes. Fasting blood glucose and other blood tests were performed after a 12-hour fast. Total cholesterol, triglyceride, high density lipoprotein cholesterol (HDL-C), apolipoprotein B (apoB), apolipoprotein AI (apoAI), creatinine, aspartate aminotransferase, and alanine aminotransferase were measured with a TBA 200FR (Toshiba, Tokyo, Japan) in Jeju National University Hospital and with an AU 5400 (Olympus, Shizuoka-ken, Japan) and a BN ProSpec (Dade Behring, Marburg, Germany) in Konyang University Hospital. Low density lipoprotein cholesterol (LDL-C) was also measured directly using auto-analyzers. HbA1c was examined with a VARIANT II™ (Bio-Rad, Hercules, CA, USA) and a Tosoh G7 (Tosoh Co., Tokyo, Japan) in Jeju National University Hospital and in Konyang University Hospital, respectively. C-peptide was measured with an Elecsys 2010™ (Roche, Mannheim, Germany).
Metabolic syndrome was diagnosed based on the criteria of the International Diabetes Federation (IDF). Abdominal obesity was defined by the criteria of the Korean Society for the Study of Obesity (90 cm for males and 85 cm for females). As all participants of this study were diabetic patients, they were considered to meet the blood glucose criteria (≥ 100 mg/dL). Therefore, metabolic syndrome was diagnosed when a participant had two or more of the following four factors:
Abdominal obesity: waist circumference; male ≥ 90 cm, female ≥ 85 cm
Triglyceride ≥ 150 mg/dL
Blood pressure ≥ 130/85 mm Hg
HDL-C: male < 40 mg/dL, female < 50 mg/dL
Statistical analysis
Statistical analysis was performed with SPSS version 15.0 (SPSS Inc., Chicago, IL, USA), and data were presented as mean ± standard deviation. Means and frequencies of groups were compared using Student's t-test and χ2 test, respectively. Variables not showing a normal distribution, including total cholesterol, triglyceride, HDL-C, LDL-C, apoB, and apoAI were analyzed through log conversion, and multivariate analysis was conducted with linear and logistic regression analysis. All P values < 0.05 were considered to be statistically significant.
RESULTS
Clinical characteristics
The participants consisted of 341 males and 309 females, and their age was 53.8 ± 11.7 years. In total, 38.4% of the study members had a family history of diabetes. Out of the participants, 39.1% had hypertension (familial T2DM group, 38.4%; non-familial T2DM group, 39.7%), and the systolic and diastolic blood pressures were 127 ± 16 mm Hg and 78 ± 10 mm Hg, respectively.
The body weight and body mass index (BMI) were 65.3 ± 12.6 kg and 24.7 ± 4.2 kg/m2, respectively, indicating that these patients tended to be overweight. The age at diagnosis was 45.3 ± 11.6 years, and the duration of diabetes was relatively long at 8.4 ± 7.9 years. Their glycemic control was poor, with a HbA1c value of 9.6 ± 3.8%.
Serum creatinine, total cholesterol, triglyceride, and LDL-C were 0.9 ± 0.2 mg/dL, 183.6 ± 52.2 mg/dL, 177.4 ± 149.8 mg/dL, and 114.3 ± 40.8 mg/dL, respectively, which tended to be higher than the recommended NCEP-ATP III guideline. Out of the total participants, 33.5% were found to have metabolic syndrome (Table 1).
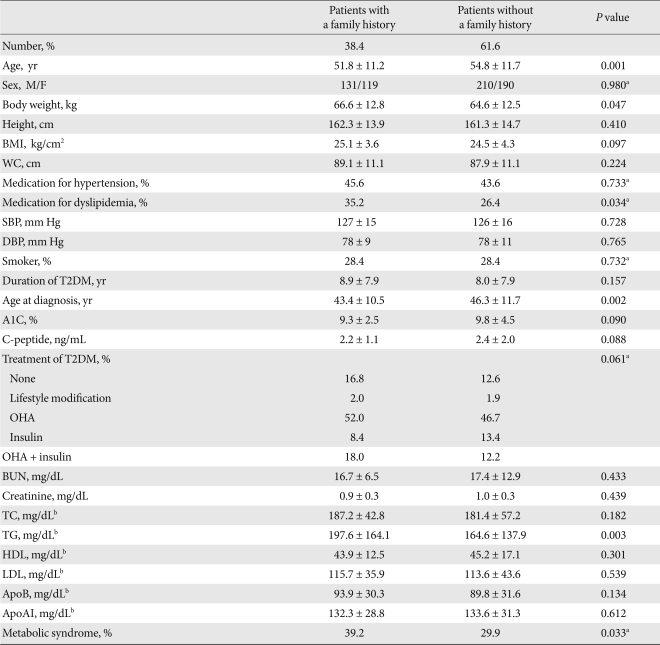
Table 1.
Baseline characteristics of the study population
Values are presented as mean ± standard deviation.
BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; T2DM, type 2 diabetes mellitus; A1C, hemoglobin A1C; OHA, oral hypoglycemic agent; BUN, blood urea nitrogen; TC, total cholesterol; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein; apoAI, apolipoprotein AI; apoB, apolipoprotein B.
aSex, medication for hypertension, medication for dyslipidemia, smoking and treatment for T2DM were analyzed using the χ2 test, bP value was expressed after each variable was logarithmically transformed.
Differences in clinical characteristics by family history
The familial T2DM group had a younger overall age, higher body weight, younger age at diagnosis, higher blood triglyceride level, and more often took medication for dyslipidemia compared to the non-familial T2DM group. Moreover, the familial group recorded higher prevalence of metabolic syndrome. However, systolic blood pressure, diastolic blood pressure, smoking history, HbA1c, C-peptide, total cholesterol, HDL-C, LDL-C, apoB, apoAI, and creatinine levels were not significantly different between the two groups. In addition, therapeutic modalities for diabetes before participation in this study were not different between the groups (Table 1).
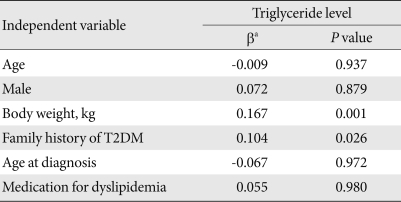
To investigate whether family history had significant associations with various metabolic factors even after adjusting for other variables, multiple regression analysis was conducted by including age, gender, and other variables showing a significant difference in univariate analysis such as body weight, age at diagnosis, treatment for dyslipidemia, and family history of diabestes. According to multiple linear regression analysis with triglyceride level, one of the components of metabolic syndrome, as a dependant variable, age, gender, treatment for dyslipidemia, and age at diagnosis were not statistically associated, and only family history and body weight had a significant influence (Table 2). However, regression analysis with body weight and metabolic syndrome as dependant variables revealed that there was no statistical association with family history of T2DM for either variable.
Table 2.
Multiple linear regression analysis for the associations between triglyceride level and other factors
Triglyceride level was the dependent variable.
T2DM, type 2 diabetes mellitus.
aRegression coefficient.
DISCUSSION
A genetic predisposition is known to be a critical factor related to the development and clinical characteristics of T2DM. In particular, the genetic factor is considered to be important in relation to insulin secretion defect [14], insulin resistance [15,16], or both, which are related to early onset of the disease [17,18]. Therefore, studies on family history of diabetes are necessary to determine the genetic factors of T2DM and to establish effective preventive measures for high risk groups [19].
T2DM develops at various ages, but its incidence rate, prevalence rate, and mortality tend to increase sharply with age [19-22]. Therefore, age at diagnosis and duration of the disease are considered to be very important indexes for determining treatment and prognosis. In this regard, family history of T2DM is thought to have a profound impact on age of onset, duration of the disease, and other factors. However, until now, there have been very few reports about its influence, except for the finding that family history increases the incidence of T2DM. In this study, we found that patients with family history were diagnosed at younger ages, so the younger age of onset was expected to lead to longer duration and more complications compared to those in patients without family history. From a metabolic aspect, the familial T2DM group showed a heavier body weight and a higher blood triglyceride level. However, systolic and diastolic blood pressures, smoking history, HbA1c, C-peptide, total cholesterol, HDL-C, LDL-C, and creatinine were not different between the two groups. Although the treatment rate of hypertension was not different between the groups, the familial T2DM group received more treatment for dyslipidemia than did the other group.
Metabolic syndrome is a condition that includes several risk factors of cardiovascular disease that occur together in an individual [23], and in patients with metabolic syndrome, incidence rate and mortality of cardiovascular disease are increased greatly [24]. Although there are several criteria to diagnose the syndrome, the IDF criteria used in this study emphasize the importance of abdominal obesity over the other criteria [25] since the correlation between obesity and sensitivity to cardiovascular disease is known to be high [26]. Actually, patients diagnosed with the criteria showed a markedly higher incidence rate of cardiovascular disease than did normal persons [26]. Some studies have reported no correlation between obesity-related factors and family history of T2DM [27,28], but others [12,29,30] have shown a close relation between obesity and family history. This current study also found that body weight and blood triglyceride level, which are known to be related to obesity and metabolic syndrome, were significantly higher in the familial T2DM group. This finding, along with those of other studies, suggests that more complications related with metabolic syndrome were expected in T2DM patients with a family history of diabetes. In addition, analysis on the metabolic profile related with metabolic syndrome revealed that, although blood pressure and HDL-C were not significantly associated with family history of T2DM, the prevalence of metabolic syndrome was significantly higher in the familial T2DM group. Consequently, the higher prevalence rate of metabolic syndrome in patients with family history would provoke a higher risk of cardiovascular disease.
To determine whether family history had a significant association with metabolic factors even after adjustment for variables like age and gender, multiple regression analysis was conducted using variables found to be significant in univariate analysis as dependant variables. Age at diagnosis, body weight, and treatment rate of dyslipidemia, which were significant in univariate analysis, were included as independent variables in addition to age, gender and family history. According to the results, a family history of T2DM was significantly associated with blood triglyceride level, a variable related with diabetes and metabolic syndrome. Metabolic syndrome also did not show any statistical significance after the adjustment for other factors, probably because the diagnosis of metabolic syndrome depended upon various factors, including gender, abdominal obesity, and triglyceride, used for the adjustment. The findings that metabolic factors such as waist circumference, BMI, and duration of diabetes were not significantly associated in univariate analysis, and that body weight was not statistically significant in multiple regression analysis, may be due to the participant selection. Patients with diabetes generally have more complications and worse health conditions for a longer duration. However, this study limited the age of the patients to the range of 20 to 70 in order to investigate an accurate association of family history with waist circumference, abdominal obesity, and metabolic syndrome and excluded all patients with a creatinine level greater than 2 mg/dL to avoid confounding factors such as edema caused by diabetic nephropathy. Because patients who were expected to have poor health conditions with longer durations of diabetes or complications such as diabetic nephropathy were excluded in this study, waist circumference, BMI, and duration may not be significantly different.
In this study, family history of diabetes was defined as having a diabetic patient among one's first-degree relatives (parents, brother or sister, and child), which was thought to be a limitation in accurately quantifying the influence of a genetic predisposition. However, previous studies on family history of diabetes also defined the family history only with first degree relatives [31], and inclusion of relatives more distant than first degree could provoke confounding effects, as the disease is very common. Therefore, the definition of family history used this study could be considered appropriate for the investigation of its influence.
Because this study was cross-sectional and the duration of diabetes was long, the results of this study could have limitations with regard to the determination of a causal relationship. However, therapeutic modalities for diabetes were not different between the two groups, so the difference between the groups was considered to be caused by differences in family history itself rather than by differences in treatment process or long duration of the disease. Moreover, to investigate a direct relationship between family history and metabolic syndrome, analysis including the T2DM patients and non-diabetic participants was necessary, but was not conducted in this study. This was believed to be a limitation of this study. However, the various differences according to family history that were shown in this study may be clinically meaningful for the management of T2DM patients. In addition, the finding that body weight and triglyceride level among the IDF criteria of metabolic syndrome were related to family history could be applied usefully in management of T2DM patients with family history.
Most previous studies on the family history of diabetes were conducted with healthy family members of diabetes patients, and there had been no report on family history conducted with T2DM patients in Korea. In particular, this study was different from previous ones as it investigated differences according to family history only with T2DM patients.
In conclusion, patients with a family history of diabetes were diagnosed at younger ages and they showed a heavier body weight, a higher blood triglyceride level, and a higher prevalence of metabolic syndrome compared to those without a family history. These results suggested that family history could affect the development of cardiovascular disease due to a deteriorating metabolic profile and early onset of diabetes. Therefore, different guidelines of treatment and follow-up should be applied according to existence of a family history. Patients with a family history of diabetes in particular should undergo early screening for metabolic syndrome and should take more active measures to prevent cardiovascular disease. For example, T2DM patients with a family history require lower levels of thresholds for cardiovascular risk factor managements, and through more in-depth analysis on each criterion, the use of anti-platelet agent, anti-hypertensive agent, and treatment for dyslipidemia can be started at a relatively young age. In addition, a large-scaled clinical study is necessary to determine a specific screening time for diagnosing diabetes and to make management guidelines for the prevention of cardiovascular disease for family members of diabetic patients in the future.
References
- 1.Li H, Isomaa B, Taskinen MR, Groop L, Tuomi T. Consequences of a family history of type 1 and type 2 diabetes on the phenotype of patients with type 2 diabetes. Diabetes Care. 2000;23:589–594. doi: 10.2337/diacare.23.5.589. [DOI] [PubMed] [Google Scholar]
- 2.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–354. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 4.Simmons D, Gatland BA, Leakehe L, Fleming C. Frequency of diabetes in family members of probands with non-insulin-dependent diabetes mellitus. J Intern Med. 1995;237:315–321. doi: 10.1111/j.1365-2796.1995.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 5.Klein BE, Klein R, Moss SE, Cruickshanks KJ. Parental history of diabetes in a population-based study. Diabetes Care. 1996;19:827–830. doi: 10.2337/diacare.19.8.827. [DOI] [PubMed] [Google Scholar]
- 6.Groop L, Forsblom C, Lehtovirta M, Tuomi T, Karanko S, Nissen M, Ehrnstrom BO, Forsen B, Isomaa B, Snickars B, Taskinen MR. Metabolic consequences of a family history of NIDDM (the Botnia study): evidence for sex-specific parental effects. Diabetes. 1996;45:1585–1593. doi: 10.2337/diab.45.11.1585. [DOI] [PubMed] [Google Scholar]
- 7.Vauhkonen I, Niskanen L, Vanninen E, Kainulainen S, Uusitupa M, Laakso M. Defects in insulin secretion and insulin action in non-insulin-dependent diabetes mellitus are inherited: metabolic studies on offspring of diabetic probands. J Clin Invest. 1998;101:86–96. doi: 10.1172/JCI716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SC, Ko GT, Li JK, Chow CC, Yeung VT, Critchley JA, Cockram CS, Chan JC. Factors predicting the age when type 2 diabetes is diagnosed in Hong Kong Chinese subjects. Diabetes Care. 2001;24:646–649. doi: 10.2337/diacare.24.4.646. [DOI] [PubMed] [Google Scholar]
- 9.Molyneaux L, Constantino M, Yue D. Strong family history predicts a younger age of onset for subjects diagnosed with type 2 diabetes. Diabetes Obes Metab. 2004;6:187–194. doi: 10.1111/j.1462-8902.2004.00330.x. [DOI] [PubMed] [Google Scholar]
- 10.Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002;156:1070–1077. doi: 10.1093/aje/kwf145. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Moran M, Guerrero-Romero F. The parental phenotype of diabetes, but not of essential hypertension, is linked to the development of metabolic syndrome in Mexican individuals. Acta Diabetol. 2001;38:87–91. doi: 10.1007/s005920170019. [DOI] [PubMed] [Google Scholar]
- 12.Sargeant LA, Wareham NJ, Khaw KT The European Prospective Investigation into Cancer. Family history of diabetes identifies a group at increased risk for the metabolic consequences of obesity and physical inactivity in EPIC-Norfolk: a population-based study. Int J Obes Relat Metab Disord. 2000;24:1333–1339. doi: 10.1038/sj.ijo.0801383. [DOI] [PubMed] [Google Scholar]
- 13.Van Dam RM, Boer JM, Feskens EJ, Seidell JC. Parental history of diabetes modifies the association between abdominal adiposity and hyperglycemia. Diabetes Care. 2001;24:1454–1459. doi: 10.2337/diacare.24.8.1454. [DOI] [PubMed] [Google Scholar]
- 14.O'Rahilly SP, Nugent Z, Rudenski AS, Hosker JP, Burnett MA, Darling P, Turner RC. Beta-cell dysfunction, rather than insulin insensitivity, is the primary defect in familial type 2 diabetes. Lancet. 1986;2:360–364. doi: 10.1016/s0140-6736(86)90052-8. [DOI] [PubMed] [Google Scholar]
- 15.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Increased insulin concentrations in nondiabetic offspring of diabetic parents. N Engl J Med. 1988;319:1297–1301. doi: 10.1056/NEJM198811173192001. [DOI] [PubMed] [Google Scholar]
- 16.Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340:925–929. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson J, Franssila-Kallunki A, Ekstrand A, Saloranta C, Widen E, Schalin C, Groop L. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Engl J Med. 1989;321:337–343. doi: 10.1056/NEJM198908103210601. [DOI] [PubMed] [Google Scholar]
- 18.Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 19.WHO Study Group. Diabetes mellitus: report of a WHO Study Group. World Health Organ Tech Rep Ser. 1985;727:1–113. [PubMed] [Google Scholar]
- 20.King H, Rewers M WHO Ad Hoc Diabetes Reporting Group. Global estimates for prevalence of diabetes mellitus and impaired glucose tolerance in adults. Diabetes Care. 1993;16:157–177. doi: 10.2337/diacare.16.1.157. [DOI] [PubMed] [Google Scholar]
- 21.Nagai M, Sakata K, Yanagawa H, Sueta H, Tanaka T, Shirahama S. Prevalence estimates for non-insulin dependent diabetes mellitus (NIDDM) in Japan from National Survey of Circulatory Disorders 1990 data. Nippon Koshu Eisei Zasshi. 1994;41:720–723. [PubMed] [Google Scholar]
- 22.Ohmura T, Ueda K, Kiyohara Y, Kato I, Iwamoto H, Nakayama K, Nomiyama K, Ohmori S, Yoshitake T, Shinkawu A, Hasuo Y, Fujishima M. Prevalence of type 2 (non-insulin-dependent) diabetes mellitus and impaired glucose tolerance in the Japanese general population: the Hisayama Study. Diabetologia. 1993;36:1198–1203. doi: 10.1007/BF00401066. [DOI] [PubMed] [Google Scholar]
- 23.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 24.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 25.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 26.Lorenzo C, Williams K, Hunt KJ, Haffner SM. The National Cholesterol Education Program - Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30:8–13. doi: 10.2337/dc06-1414. [DOI] [PubMed] [Google Scholar]
- 27.Boer JM, Feskens EJ, Kromhout D. Characteristics of non-insulin-dependent diabetes mellitus in elderly men: effect modification by family history. Int J Epidemiol. 1996;25:394–402. doi: 10.1093/ije/25.2.394. [DOI] [PubMed] [Google Scholar]
- 28.Osei K, Cottrell DA, Bossetti B. Relationships of obesity indices to serum insulin and lipoproteins in relatives of black patients with noninsulin-dependent diabetes mellitus (NIDDM) Int J Obes. 1991;15:441–451. [PubMed] [Google Scholar]
- 29.Mykkanen L, Laakso M, Uusitupa M, Pyorala K. Prevalence of diabetes and impaired glucose tolerance in elderly subjects and their association with obesity and family history of diabetes. Diabetes Care. 1990;13:1099–1105. doi: 10.2337/diacare.13.11.1099. [DOI] [PubMed] [Google Scholar]
- 30.Mohan V, Shanthirani CS, Deepa R. Glucose intolerance (diabetes and IGT) in a selected South Indian population with special reference to family history, obesity and lifestyle factors: the Chennai Urban Population Study (CUPS 14) J Assoc Physicians India. 2003;51:771–777. [PubMed] [Google Scholar]
- 31.The Diabetes Control and Complications Trial Research Group. Clustering of long-term complications in families with diabetes in the diabetes control and complications trial. Diabetes. 1997;46:1829–1839. [PubMed] [Google Scholar]