Abstract
Transfection of mammalian cell lines is a widely used technique that requires significant optimization, including transfection method or product used, DNA vector, cell density, media composition and incubation time. Generation and isolation of stable transfectants from the large pool of untransfected or only transiently transfected cells can be laborious and time-consuming. Transfection of DNA is usually performed with a non-linearized plasmid, since it is assumed that cutting the plasmid beforehand leads to a lower efficiency of transfection or the degradation of linearized DNA by cytosolic nucleases. However, the transfected circular plasmid will be linearized by a random cut within the cell and it might be possible that sensitive parts of the plasmid such as the resistance gene or the gene of interest are destroyed upon linearization. On the other hand, linearizing a plasmid before transfection by a single, defined cut with a selected restriction enzyme in a non-coding area of the gene has the advantage of ensuring the integrity of all necessary gene elements of the plasmid. In this study, we have compared these different methods in order to increase both transient and stable transfection efficiency in mammalian cells. We report that linearization of plasmid DNA prior to transfection can increase both the efficiency of stable clone generation and target gene expression, but is dependant on the site of linearization within the vector.
Keywords: Transfection, Enzymatic restriction, Linearization, Stable clones, Neuron, EGFP
Introduction
Transfection, the introduction of foreign DNA into mammalian cells, is a widely used technique in molecular and cellular biology. Several methods, including calcium phosphate precipitation/transfection, electroporation and liposome-mediated transfection, allow foreign DNA to pass through the lipid bilayer membrane of mammalian cells. Any cell that harbors foreign DNA not incorporated into the chromosomes is transiently transfected, whereby the DNA is able to be transcribed, but cannot be copied and therefore will be degraded over time and diluted during mitosis. Transient transfection is a useful tool, primarily used in short-term reporter assays. It is often a necessity however, to obtain a cell line that continually expresses the foreign gene of interest, known as stable transfection, which requires the integration of the foreign DNA into the chromosomal DNA of the host cell. It is well documented that the efficiency of transient transfection, particularly for liposome-mediated transfection, is cell-specific and affected by several factors. Transfection efficiencies of approximately 20–30% are generally observed, but can be optimized to achieve efficiency rates of 70–98% (Kovala et al. 2000; Nikcevic et al. 2003; Ohmiya et al. 2002; Kaiser and Toborek 2001; Goomer et al. 2001). Stable transfection however, relies on insertion of the foreign DNA into the genome, a process which occurs infrequently, thus resulting in low numbers of stable clones.
The topology of DNA is known to affect transfection efficiency, as supercoiled or open-circular DNA provides greater efficiency than linear DNA (Cherng et al.1999). Greater transfection efficiency of circular DNA potentially increases the chance of stable integration, but through a random cut in the vector stable clones that do not express the gene of interest could be generated. Green fluorescent protein (GFP) has previously been shown to be an effective method for quantifying transient efficiency (Dandekar et al. 2005). Thus, in this study, we used the enhanced-GFP-expressing vector pEGFP-N1 to measure both transient and stable transfection efficiency of Neuro2a and HT22 cells with circular/supercoiled and linearized vector by microscopic analysis. We demonstrate that although linearized DNA may result in similar transient transfection efficiency, it gives rise to a greater number of stable transfected cells.
Methods
Cell culture
Murine Neuro2a and HT22 cells were maintained in Dulbecco’s modified eagle’s medium (DMEM) containing 5% foetal calf serum (FCS), supplemented with penicillin (200 U/mL), streptomycin (200 μg/mL) and fungizone (2.6 μg/mL; all GIBCO). Cells were maintained at 37 °C, containing 5% CO2, in a humid environment. Cells were removed from flasks using rubber scrapers (Sarstedt) and counted in a counting chamber (Neubauer), then dispensed at a density of 1.2 × 106cells/well in 6-well plates (Sarstedt). The cells were then grown for 24 h in antibiotic-free DMEM containing 5% FCS prior to transfection.
Vector preparation
The 4.7 kb vector, pEGFP-N1 (Clontech) was purified using the Pureyield Plasmid Midiprep System (Promega). DNA purity was >1.6, as determined by the spectrophotometric 260/280 nm ratio. In duplicate, 2.5 μg of pEGFP-N1 was cut with BsaI or SspI (New England Biolabs) and the appropriate buffer for 2 h at 37 °C. A pseudo-digestion, containing buffer only, was also performed as uncut control. Restriction enzymes were then inactivated at 65 °C for 15 min and a sample run on a 1% agarose gel with untreated DNA as control, to confirm vector digestion. Three independent experiments in duplicate were performed (n = 6).
Transfection
Transfections were performed using Lipofectamine-LTX (Invitrogen). Each 2.5 μg of linearized and enzyme inactivated DNA was diluted to a volume of 500 μl with FCS and antibiotic-free Opti-MEM (Invitrogen), then combined with 2.5 μl of Plus Reagent at room temperature for 10 min. 6.25 μL of Lipofectamine-LTX was then added and allowed to complex at room temperature for 90 min. This extended incubation time was employed to ensure complete complexation of all fragments in the presence of added protein contamination post-digestion. Antibiotic-free DMEM containing 5% FCS was then added to a final volume of 2 mL, before addition to the cells in 6-well plates. Cells were transfected at 37 °C for 24 h before the Lipofectamine-containing medium was replaced with fresh DMEM containing 5% FCS.
Analysis of transient transfection
A 24 h after removal of the Lipofectamine-containing medium, cell number and eGFP expression were measured using an FL MZIII Stereomicroscope (Leica). Images of three randomly selected fields in each well were captured at 10× magnification, under both bright and UV light with an eGFP filter. Images were analyzed using Cell Profiler software and the number of eGFP-positive and negative cells were then counted. Fluorescent intensity of transfected cells was determined using Image J software.
Analysis of stable transfection
Following transient transfection analysis, cells were exposed to 600 μg/mL Geneticin (GIBCO) in the original six-well plate for 3 days. Cells of each well were then removed by scraping and transferred to a 10 cm cell culture dish containing 900 μg/mL of Geneticin for 10 days, with medium changed every 3 days. The number of eGFP-positive and negative colonies was then counted using the MZIII Stereomicroscope.
Statistical analysis
Results were graphed and analyzed using Graphpad Prism 4. A one-way ANOVA and Dunnett’s post test were performed using transfections with uncut vector as controls. Independent experiments were performed three times in duplicate (n = 6).
Results
Transient transfection efficiency
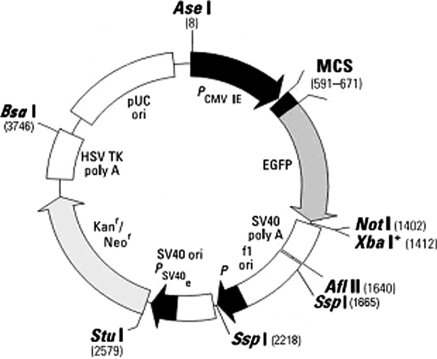
In this experiment, we wanted to determine whether transient transfection efficiency was affected by previous linearization of the pEGFP-N1 vector (Fig. 1). A 2.5 μg of vector was either left uncut or was linearized with BsaI or SspI. These two enzymes were selected because neither of them would cut essential components of the vector, necessary for transcription in mammalian cells and would also result in blunt ended fragments. BsaI cuts at position 3,746 bp and SspI at 1,665 bp and 2,218 bp. Linearization was confirmed by electrophoresis (data not shown), following heat inactivation of restriction enzymes. The plasmids were then transfected into 1.2 × 106 Neuro2a or HT22 cells for 24 h using Lipofectamine-LTX in six-well plates. After a further 24 h with medium change, three random fields of each well were imaged at 10× magnification using a fluorescent stereomicroscope and eGFP-expressing cells counted using Cell Profiler software.
Fig. 1.
Restriction enzyme digestion sites of pEGFP-N1. BsaI linearizes pEGFP-N1 by a single digestion at 3,746 bp at the terminal end of the poly A-tail of the Neomycin resistance gene. SspI linearizes by removal of a small fragment from 1,665 to 2,218 bp, in the bacterial promoter region of the Neomycin resistance gene
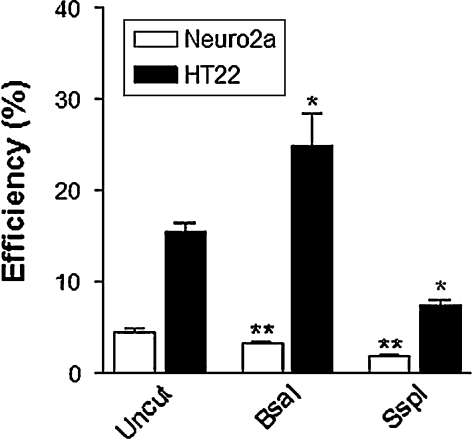
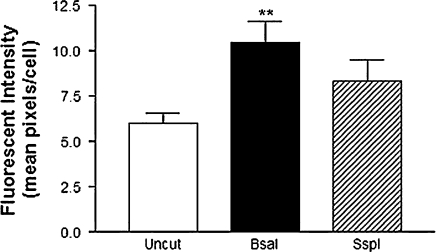
There was a marked difference between the two cell lines. Transfection efficiency was significantly higher in HT22 cells than Neuro2a cells, with 15.5 and 4.5% average efficiency for uncut vector (Fig. 2). There were also differences in efficiency between uncut and cut vectors. However, there was no clear transfection advantage for the circular plasmid compared with the linearized plasmid. Whereas the uncut plasmid provided the greatest efficiency for Neuro2a cells, digestion with BsaI resulted in the greatest number of transiently transfected HT22 cells. It remained consistent between the two cell lines however, that digestion with SspI decreased transfection efficiency (Fig. 2). Examination of the fluorescent images also demonstrates that cutting with BsaI, but not SspI, results in brighter fluorescence than undigested vector, as depicted in Fig. 3.
Fig. 2.
Transient transfection efficiency of Neuro2a and HT22 neuronal cells. A 24 h after transfection with pEGFP-N1, using Lipofectamine-LTX, cells were visualized and GFP-positive cells identified. (* p < 0.05, ** p < 0.01 compared to uncut controls)
Fig. 3.
Fluorescent intensity of transiently transfected HT22 neurons. pEGP-N1 vector was left untreated, or digested with BsaI or SspI prior to a 24 h transfection with Lipofectamine-LTX. A 24 h post-transfection, cells were imaged with a fluorescent microscope and analyzed with Image J software. Data are represented as mean pixel intensity per cell. (* p < 0.05, ** p < 0.01 compared to uncut controls)
Stable transfection efficiency
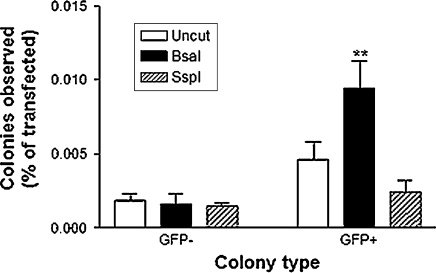
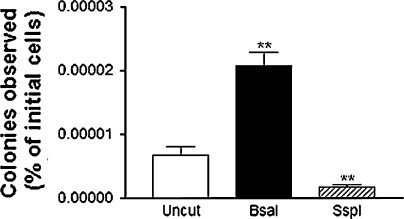
In this experiment, we aimed to determine whether the generation of stable clones was affected by previous linearization of the pEGFP-N1 vector. Following determination of transient transfection efficiency, Neuro2a and HT22 cells were treated with 600 μg/mL of Geneticin for 3 days, then 900 μg/mL for 10 day. eGFP-expressing colonies were then counted using a stereomicroscope with and without a GFP filter. No colonies were observed for any of the transfections performed using Neuro2a cells. The HT22 cells, however, yielded a significant number of stable colonies expressing eGFP. The highest number of eGFP-expressing HT22 colonies observed, from the initial 1.2 × 106 cells transfected, was 30, arising from cells transfected with BsaI-digested vector in a single well of a six-well plate. This was significantly higher than stable colonies resulting from either uncut vector or vector linearized with SspI, which obtained a maximum of 11 and 3 colonies per well respectively, in a single experiment. After transient transfection efficiency is taken into account, Fig. 4 demonstrates that stable integration of BsaI digested vector was significantly greater than and approximately twice as efficient as undigested vector and was nearly four times more efficient than SspI digested vector. Despite the differences in eGFP-expressing colonies between the three digestion treatments, the number of non-fluorescent stable colonies was similar. The overall efficiency of each transfection treatment is depicted in Fig. 5, where it is evident that given the same number of cells and transfection conditions, digestion of pEGFP-N1 with BsaI prior to transfection of HT22 cells, is three times more efficient than transfection with undigested vector and twelve times more efficient than prior digestion with SspI.
Fig. 4.
Stable integration efficiency of uncut and digested pEGFP-N1. 1.2 × 106 HT22 cells were transfected using Lipofectamine-LTX. Stable colonies were counted after 13 days of selection with G418 antibiotic. Values are represented in relation to positive transient transfectants. (* p < 0.05, ** p < 0.01 compared to uncut controls)
Fig. 5.
Stable transfection efficiency of pEGFP-N1 in HT22 neurons. 1.2 × 106 HT22 cells were transfected using Lipofectamine-LTX. Stable colonies were counted after 13 days of selection with G418 antibiotic (* p < 0.05, ** p < 0.01 compared to uncut controls)
Discussion
The generation of clonal cell lines is a vital technique that can often be laborious and time consuming. Several factors, including cell type, transfection technique, carrier vector and the desired sequence can all dramatically affect both transient and stable transfection efficiency. Previous studies have found that little or no transfection was obtained with linear DNA (von Groll et al. 2006). During the development of neuronal high throughput screening assays, we have found that efficient transient transfection of a neuronal cell line can be achieved with linear DNA and also increases the number of stable integrations. Both of these events however, are dependant on the site of vector digestion. BsaI digests pEGFP-N1 immediately prior to the mammalian eGFP promoter, in the pUC bacterial origin, while SspI linearizes the vector by cutting at two sites between eGFP and the antibiotic resistance gene, in the f1 bacterial origin, giving rise to a small 550 bp secondary fragment. This fragment will likely compete for liposomal complexation and transfection with the larger eGFP encoding fragment, which may have caused the observed decrease in transient transfection efficiency with SspI. Moreover, digestion immediately following the encoded eGFP protein may inhibit expression and therefore transfection detection. Such an effect may, in part, explain why von Groll et al. (2006) observed no efficiency with linearized vector, as their chosen enzyme (BamHI) also digested immediately after the encoded detection protein. Although comparative efficiencies between digestion sites in Neuro2a cells were similar to HT22, no stable Neuro2a colonies were obtained due to low transient efficiency, a feature of Neuro2a cells that has been previously reported (Koticha et al. 2002).
Incorporation of the foreign DNA into the chromosome of the host cell is required for the generation of a clonal cell line. This process is most likely to occur during nuclear replication, as fragments of linear DNA can be randomly copied into new chromosomes. It is therefore not surprising that restriction digestion of DNA prior to transfection greatly affects the integration into host chromosomes. By taking into account the number of cells initially transiently transfected, thereby removing the effect of variation in transfection efficiency, the integration efficiency of restriction enzyme treatment can be observed in Fig. 4. This difference in chromosomal integration and eGFP expression is not due to a difference in single stranded overhangs of the vector, as both restriction enzymes are blunt end cutters. The site of digestion however, may alter expression of the eGFP and antibiotic resistence genes, thereby effecting clonal selection. This is supported by the observation that transient transfections with BsaI resulted in greater eGFP expression, as displayed by measurably higher fluorescence intensity than other treatments, and also resulted in more numerous stable colonies (Fig. 3).
Interestingly, despite the variation in the number of eGFP-expressing colonies, all three transfection treatments resulted in a similar number of stable colonies that did not express eGFP (Fig. 4), indicating a consistent cellular digestion event and chromosomal insertion that conferred antibiotic resistance, but not eGFP expression.
Linearization of a vector is commonly used in transfections that have previously been difficult to isolate stable clones with uncut vectors. We have confirmed that linearization, via restriction enzyme digestion, of a vector prior to transfection affects the transfection and stable integration efficiency. In this report we have demonstrated that it is possible to increase the number of stable colonies by up to three-fold through vector linearization. It was also found however, that the effect of linearization is highly dependant on the restriction region chosen in the vector. To increase the likelihood of obtaining stably transfected cells, we suggest that the vector should be linearized with a restriction enzyme, which cuts in or after the polyA tail (at the end of the gene) and before the mammalian promotor (at the beginning of the gene).
Acknowledgements
We thank A. E. Maczurek for inspiring and stimulating discussions. This work was supported by grants from Furfural Espanol-Nutrafur, the JO & JR Wicking Trust (to GM), Alzheimer’s Australia (to GS) and by the ANZ trustees (Ph. D. fellowship to GS).
Conflicts of interest There are no actual or potential conflicts of interest.
References
- Cherng JY, Schuurmans-Nieuwenbroek NM, Jiskoot W, Talsma H, Zuidam NJ, Hennink WE, Crommelin DJ. Effect of DNA topology on the transfection efficiency of poly((2-dimethylamino)ethyl methacrylate)-plasmid complexes. J Control Release. 1999;60:343–353. doi: 10.1016/S0168-3659(99)00089-9. [DOI] [PubMed] [Google Scholar]
- Dandekar DH, Kumar M, Ladha JS, Ganesh KN, Mitra D. A quantitative method for normalization of transfection efficiency using enhanced green fluorescent protein. Anal Biochem. 2005;342:341–344. doi: 10.1016/j.ab.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Goomer RS, Deftos LJ, Terkeltaub R, Maris T, Lee MC, Harwood FL, Amiel D. High-efficiency non-viral transfection of primary chondrocytes and perichondrial cells for ex-vivo gene therapy to repair articular cartilage defects. Osteoarthritis Cartilage. 2001;9:248–256. doi: 10.1053/joca.2000.0382. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Toborek M. Liposome-mediated high-efficiency transfection of human endothelial cells. J Vasc Res. 2001;38:133–143. doi: 10.1159/000051040. [DOI] [PubMed] [Google Scholar]
- Koticha DK, McCarthy EE, Baldini G (2002) Plasma membrane targeting of SNAP–25 increases its local concentration and is necessary for SNARE complex formation and regulated exocytosis. J Cell Sci 115:3341–3351 [DOI] [PubMed]
- Kovala AT, Harvey KA, McGlynn P, Boguslawski G, Garcia JG, English D. High-efficiency transient transfection of endothelial cells for functional analysis. Faseb J. 2000;14:2486–2494. doi: 10.1096/fj.00-0147com. [DOI] [PubMed] [Google Scholar]
- Nikcevic G, Kovacevic-Grujicic N, Stevanovic M. Improved transfection efficiency of cultured human cells. Cell Biol Int. 2003;27:735–737. doi: 10.1016/S1065-6995(03)00143-4. [DOI] [PubMed] [Google Scholar]
- Ohmiya N, Emi N, Niwa Y, Goto H, Hayakawa T. Insulin-enhanced liposome-mediated gene transfer into a gastric carcinoma cell line. Clin Exp Pharmacol Physiol. 2002;29:544–548. doi: 10.1046/j.1440-1681.2002.03696.x. [DOI] [PubMed] [Google Scholar]
- Groll A, Levin Y, Barbosa MC, Ravazzolo AP. Linear DNA low efficiency transfection by liposome can be improved by the use of cationic lipid as charge neutralizer. Biotechnol Prog. 2006;22:1220–1224. doi: 10.1021/bp060029s. [DOI] [PubMed] [Google Scholar]