Abstract
Rats subcutaneously implanted with AH109A hepatoma cells show hyperlipidemia with high concentrations of serum triglyceride and nonesterified fatty acid, suppression of lipoprotein lipase (LPL), and elevation of hormone-sensitive lipase (HSL) activities during the growth of the hepatoma. Supplementation of the diet with sulfur amino acids such as l-methionine (Met) and l-cystine (Cys) improved hyperlipidemia by restoring LPL and HSL activities. In the present study, we have attempted to examine the effects of sulfur amino acids on the activity and mRNA level of LPL and the activity of HSL using 3T3-L1 cells, which are known to differentiate to adipocytes. The adipocytes were incubated with various concentrations of Met, Cys or l-cysteine (CysH) in the absence or presence of tumor necrosis factor-α (TNF-α). LPL activity was suppressed by TNF-α. In the absence of TNF-α, Met, Cys and CysH did not change the LPL activity. In the presence of TNF-α, Met and Cys significantly increased the LPL activity, and Met also enhanced the LPL mRNA level. HSL activity was also suppressed by TNF-α. In the absence of TNF-α, Met enhanced the HSL activity. In the presence of TNF-α, Met, Cys and CysH suppressed the HSL activity. Sulfur amino acids such as Met, Cys and CysH affected the LPL activity, mRNA level, and HSL activity in 3T3-L1 adipocytes. Some of these effects of sulfur amino acids were different between LPL and HSL, between the absence and the presence of TNF-α, and between 3T3-L1 adipocytes and the adipose tissue from rats.
Keywords: l-cysteine, l-cystine, Hormone-sensitive lipase, Lipoprotein lipase, l-methionine, Tumor necrosis factor-α
Introduction
Various types of cancers affect lipid metabolism (Barclay et al. 1970; Muntoni et al. 2009; Nydegger and Butler 1972; Qadir and Malik 2008). Previous studies have reported that Donryu rats subcutaneously implanted with AH109A hepatoma cells show hyperlipidemia (Irikura et al. 1985) with high concentrations of serum triglyceride and nonesterified fatty acid (NEFA) as well as suppression of lipoprotein lipase (LPL) and elevation of hormone-sensitive lipase (HSL) activities in the epididymal adipose tissue during the growth of the hepatoma (Kawasaki et al. 2004, 2010).
Sulfur amino acids are well known to affect lipid metabolism. l-methionine (Met) (Sugiyama et al. 1984; Yagasaki et al. 1984) and l-cystine (Cys) (Sugiyama et al. 1984) have changed the serum or plasma cholesterol concentration in rats in a normal state. We have also reported that in AH109A-implanted rats, the supplementation of a diet with Met and Cys improved the AH109A-induced hypertriglyceridemia (Yagasaki et al. 1986) by restoring epididymal adipose tissue LPL activity (Kawasaki et al. 2010). Dietary Met and Cys also suppressed the serum NEFA concentration via the suppression of the HSL activity (Kawasaki et al. 2010).
In the present study, to inspect the above-mentioned effects of sulfur amino acids such as Met and Cys on LPL and HSL in adipose tissue in AH109A-bearing rats, we have attempted to examine the effects of these sulfur amino acids on the activity and mRNA level of LPL and the activity of HSL, which are key enzymes in triglyceride hydrolysis in serum and adipose tissue, respectively, using 3T3-L1 cells, which are known to differentiate to adipocytes. In AH109A-bearing rats, the serum tumor necrosis factor-α (TNF-α) concentration was significantly increased as compared with that of the normal rats (Kawasaki et al. 2004). TNF-α is a cytokine and is well known to have various functions in the lipid metabolism. Thus, the differentiated adipocytes were incubated with various concentrations of Met, Cys or l-cysteine (CysH) in the absence or presence of TNF-α at its concentration in serum from AH109A-bearing rats (Kawasaki et al. 2004) or at its 50% inhibitory concentration (IC50) against LPL or HSL activity.
Materials and methods
Cell culture
3T3-L1 preadipocytes were cultured in a 12-well tissue culture plate (7.5 × 103 cells per well) for LPL and HSL activity measurements, or in a culture dish with a diameter of 60 mm (6.0 × 104 cells per dish) for LPL mRNA level measurement in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum (FCS) in a humid atmosphere of 5% CO2 at 37 °C. After the attainment of a confluent state, the cells were placed in DMEM supplemented with 10% FCS, 0.5 mM 3-isobutyl-1-methylxanthine, 0.25 μM dexamethasone and 10 μg insulin per mL of medium. After 48 h, the medium was removed and replaced with DMEM supplemented with FCS and insulin alone. The cells were cultured for a further 3 weeks and converted to adipocytes. The differentiated adipocytes were incubated for indicated times with various concentrations of Met (0–600 μM), Cys (0–400 μM) or CysH (0–1,200 μM) in the absence or presence of TNF-α at its concentration in serum from AH109A-bearing rats (=2.35 pM) (Kawasaki et al. 2004) or at its IC50 against LPL or HSL activity. LPL and HSL were then extracted, and their activities (LPL and HSL) and mRNA level (LPL) were measured.
Measurement of LPL activity
LPL was extracted from 3T3-L1 adipocytes as previously described (Kawakami et al. 1982). LPL activity was then estimated as described previously (Kawasaki et al. 1995; Nakai et al. 1979; Nilsson-Ehle and Schotz 1976). Briefly, 3T3-L1 adipocytes were cultured for indicated times, and then 5 U of heparin in a volume of 5 μL was added to each well and incubated for 1 h to release the intracellular LPL into the medium. The above-mentioned enzyme source (0.1 mL) was mixed with 0.1 mL of substrate solution (arabic gum emulsified 200 mM Tris–HCl buffer, pH = 8.0, containing rat serum to supply adequate apolipoprotein C-II as a LPL activator) containing 925 Bq (= 55,500 dpm)/μmol/assay of [carboxyl-14C]triolein (original specific radioactivity = 4.1 GBq/mmol, NEN Research Products, Boston, MA, USA). The mixture was incubated at 37 °C for 1 h, followed by the extraction and counting of hydrolyzed [14C]oleic acid. One unit of LPL activity was defined as 1 μmol of fatty acid released per min per mg cellular protein.
Measurement of LPL mRNA level
The LPL mRNA level of 3T3-L1 adipocytes was estimated using a slot blot assay (Sambrook et al. 1989). Total RNA from 3T3-L1 adipocytes was isolated by the acid phenol-guanidinium thiocyanate-chloroform extraction method (Chomczynski and Sacchi 1987). One sample was prepared from 3 dishes. After the total RNA had been purified, 10 μg of each RNA sample was dissolved in 10 μL of dietylpyrocarbonate-treated water and then denatured. Each denatured RNA sample was loaded into a slot, and gentle suction was then applied. After the samples had been passed through the membrane, each of the slots was rinsed, and the membrane was dried. RNA was fixed to the membrane by baking at 80 °C for 3 h. The immobilized RNA was pre-hybridized at 42 °C for 20 h, and then RNA hybridization with cDNA probes of LPL (Wion et al. 1987) and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) (Fort et al. 1985) was carried out at 42 °C for 20 h. The hybridized blots were washed and exposed to X-ray film. The developed blots were quantified by densitometry. The estimated of LPL mRNA level is expressed as the ratio of LPL mRNA to GAPDH mRNA (LPL/GAPDH).
Measurement of HSL activity
HSL from 3T3-L1 adipocytes was extracted as previously described (Kawamura et al. 1981). HSL activity was then measured as previously described (Kawasaki et al. 1995; Nakai et al. 1979; Nilsson-Ehle and Schotz 1976). After the 3T3-L1 adipocytes were incubated with the experimental medium for designated times, 5 U of heparin in a volume of 5 μL was added to each well and further incubated for 1 h to remove the intracellular LPL. After the medium was withdrawn, the cells were washed with Ca2+- and Mg2+-free phosphate buffered saline (PBS(−)), and then 0.5 mL of extraction buffer (10 mM Tris–HCl, pH = 7.4, containing 1 mM EDTA and 250 mM sucrose (final concentration)) was added. The cells were scraped, and HSL was extracted using an ultrasonic generator. After the suspension was centrifuged, the supernatant was used for the measurement of HSL activity. The enzyme solution (0.1 mL) previously incubated with 1 M NaCl (final concentration) for 10 min to inactivate the LPL activity was mixed with 0.1 mL of substrate solution (arabic gum-emulsified 200 mM phosphate buffer, pH = 7.0) containing 925 Bq/0.1 μmol/assay of [carboxyl-14C]triolein, and the mixture was incubated at 30 °C for 1 h. At the end of the incubation period, hydrolyzed [14C]oleic acid was extracted, and the radioactivity was measured after the addition of Insta-Gel (Packard Instrument Co. Inc., Meriden, CT, USA). One unit of HSL activity was defined as 1 μmol of fatty acid released per min per mg cellular protein.
Measurement of cellular protein content
Cellular protein content of 3T3-L1 adipocytes was measured by the Bradford method (Bio-Rad Protein Assay kit, Bio-Rad Laboratories, Richmond, CA, USA) (Bradford 1976).
Statistical analysis
Results were expressed as means ± standard errors. Differences between groups were compared by Student’s t test. Multiple comparisons were carried out by one-way analysis of variance followed by Dunnett’s pairwise multiple comparison t test. Differences were considered significant at p < 0.05.
Results
Effect of TNF-α on LPL activity
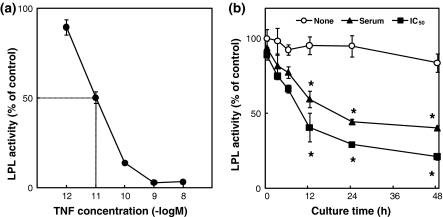
We first examined the effect on LPL activity. The effect of the addition of TNF-α to 3T3-L1 adipocytes and the effect of the culture time of 3T3-L1 adipocytes on LPL activity were tested. TNF-α suppressed the LPL activity in a dose-dependent manner at concentrations of 1 pM to 1 nM. The IC50 of TNF-α against LPL activity was 10.0 pM (Fig. 1a). In the absence of TNF-α, the LPL activity did not change over 48 h incubation, whereas in the presence of TNF-α, the LPL activity was suppressed by TNF-α at its concentration in serum from AH109A-bearing rats and at its IC50 against LPL activity as compared with the absence of the TNF-α group after more than 12 h incubation following the addition of TNF-α (Fig. 1b).
Fig. 1.
a Effect of the addition of tumor necrosis factor-α (TNF-α) on LPL activity in 3T3-L1 adipocytes. Each value represents % of control (=No addition of TNF-α group). Each point and vertical bar represents the means and SEM for 4 wells. A total of 50% inhibitory concentration (IC50) of TNF-α against LPL activity is 10.0 pM. b Effect of the culture time on LPL activity in 3T3-L1 adipocytes without (None) or with (concentration in serum from AH109A-bearing rats (Serum) or IC50 against LPL activity (IC50)) TNF-α. Each value represents % of control (=TNF-α-free and 0 h group). Each point and vertical bar represents the means and SEM for 4 wells. In a same culture time, * represents significant difference from the TNF-α-free group at p < 0.05 by Dunnett’s pairwise multiple comparison t test
Effect of sulfur amino acids on LPL activity
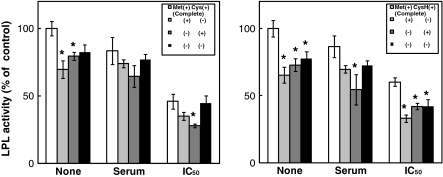
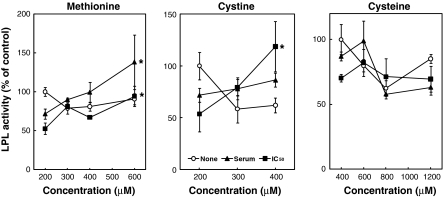
To investigate whether or not the sulfur amino acids affect the LPL activity in 3T3-L1 adipocytes, the effects of sulfur amino acids on LPL activity under the conditions of the deprivation of and the addition of sulfur amino acids were tested. The 3T3-L1 adipocytes were incubated for 24 h with various concentrations of Met, Cys or CysH in the absence or presence of TNF-α. Figure 2 shows the effect of the deprivation of sulfur amino acids on LPL activity. LPL activity in the absence of sulfur amino acids was lower than that in the presence of sulfur amino acids in both the absence and presence of TNF-α. Figure 3 represents the effect of the addition of sulfur amino acids on LPL activity. In the absence of TNF-α, Met, Cys and CysH did not change the LPL activity. However, Met and Cys significantly increased the LPL activity at the concentrations of 600 and 400 μM, respectively, with the addition of TNF-α at its IC50 against LPL activity. Met also significantly increased the LPL activity at the concentration of 600 μM with the addition of TNF-α at its concentration in serum from AH109A-bearing rats, whereas Cys did not change the LPL activity under this condition. Although CysH did not change the LPL activity, the LPL activity tended to increase due to CysH at the concentration of 600 μM with the addition of TNF-α both at its concentration in serum from AH109A-bearing rats and at its IC50 against LPL activity.
Fig. 2.
Effect of the deprivation of sulfur amino acids (l-methionine (Met) and l-cystine (Cys) or Met and l-cysteine (CysH)) on LPL activity in 3T3-L1 adipocytes without (None) or with (concentration in serum from AH109A-bearing rats (Serum) or IC50 against LPL activity (IC50)) TNF-α. Each value represents % of control (=TNF-α-free and complete group). Each value and vertical bar represents the means and SEM for 4 wells. * Represents significant difference from the complete group at p < 0.05 by Dunnett’s pairwise multiple comparison t test
Fig. 3.
Effect of the addition of sulfur amino acids (l-methionine (Met), l-cystine (Cys) or l-cysteine (CysH)) on LPL activity in 3T3-L1 adipocytes without (None) or with (concentration in serum from AH109A-bearing rats (Serum) or IC50 against LPL activity (IC50)) TNF-α. Each value represents % of control (=TNF-α-free and 200 μM (Met and Cys) or 400 μM (CysH) of sulfur amino acid-containing group). Each point and vertical bar represents the means and SEM for 4 wells. In a same line, * represents significant difference from the basal group (200 μM (Met and Cys) or 400 μM (CysH) of sulfur amino acid-containing group) at p < 0.05 by Dunnett’s pairwise multiple comparison t test
Effect of sulfur amino acids on LPL mRNA level
Sulfur amino acids affected the LPL activity in 3T3-L1 adipocytes, so we next examined the effect of sulfur amino acids on LPL mRNA level. Sulfur amino acids were added at the most effective concentration against LPL activity, and 3T3-L1 adipocytes were incubated for 24 h. Met enhanced the LPL mRNA level as well as the LPL activity at the concentration of 600 μM with the addition of TNF-α at its concentration in serum from AH109A-bearing rats and at its IC50 against LPL activity. In the TNF-α-free condition, the LPL mRNA level was not affected by Met supplementation, nor was the LPL activity. Cys and CysH did not change the LPL mRNA levels at the concentrations of 400 and 600 μM, respectively, in both the absence and presence of TNF-α (Fig. 4).
Fig. 4.
Effect of sulfur amino acids (l-methionine (Met), l-cystine (Cys) or l-cysteine (CysH)) on LPL mRNA level in 3T3-L1 adipocytes without (None) or with (concentration in serum from AH109A-bearing rats (Serum) or IC50 against LPL activity (IC50)) TNF-α. Each value represents % of control (=TNF-α-free and 200 μM (Met and Cys) or 400 μM (CysH) of sulfur amino acid-containing group). Each point and vertical bar represents the means and SEM for 4 samples. In a same line, * represents significant difference from the basal group (200 μM (Met and Cys) or 400 μM (CysH) of sulfur amino acid-containing group) at p < 0.05 by Student’s t test
Effect of TNF-α on HSL activity
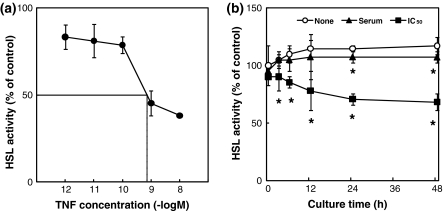
We second examined the effect on HSL activity. Figure 5a shows the effect of the addition of TNF-α to 3T3-L1 adipocytes on HSL activity. TNF-α suppressed the HSL activity as well as the LPL activity. The IC50 of TNF-α against HSL activity was 0.7 nM. Figure 5b shows the effect of the culture time of 3T3-L1 adipocytes on HSL activity. In the absence of TNF-α, there was no change in HSL activity over a 48-h incubation period. However, the HSL activity was suppressed by the addition of TNF-α at its concentration in serum from AH109A-bearing rats and at its IC50 against HSL activity as compared with the absence of the TNF-α group over incubation periods of more than 24 h and more than 3 h incubation, respectively, after the addition of TNF-α.
Fig. 5.
a Effect of the addition of tumor necrosis factor-α (TNF-α) on HSL activity in 3T3-L1 adipocytes. Each value represents % of control (=No addition of TNF-α group). Each point and vertical bar represents the means and SEM for 4 wells. A total of 50% inhibitory concentration (IC50) of TNF-α against HSL activity is 0.7 nM. b Effect of the culture time on HSL activity in 3T3-L1 adipocytes without (None) or with (concentration in serum from AH109A-bearing rats (Serum) or IC50 against HSL activity (IC50)) TNF-α. Each value represents % of control (=TNF-α-free and 0 h group). Each point and vertical bar represents the means and SEM for 4 wells. In a same culture time, * represents significant difference from the TNF-α-free group at p < 0.05 by Dunnett’s pairwise multiple comparison t test
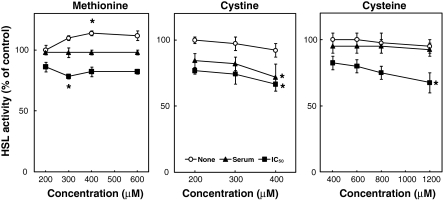
Effect of sulfur amino acids on HSL activity
The effects of sulfur amino acids on HSL activity in the conditions of the deprivation of and the addition of sulfur amino acids were examined. The 3T3-L1 adipocytes were incubated for 24 h with various concentrations of Met, Cys or CysH in the absence or presence of TNF-α. Figure 6 shows the effect of the deprivation of sulfur amino acids on HSL activity. HSL activity in the absence of sulfur amino acids was lower than that in the presence of sulfur amino acids in both the absence and presence of TNF-α. Figure 7 shows the effect of the addition of sulfur amino acids on HSL activity. In the absence of TNF-α, Met significantly increased the HSL activity at the concentration of 400 μM, whereas Cys and CysH exerted no influence on the activity. However, Met and CysH significantly suppressed the HSL activity at the concentrations of 300 and 1,200 μM, respectively, with the addition of TNF-α at its IC50 against HSL activity. Cys also significantly suppressed the HSL activity at the concentration of 400 μM with the addition of TNF-α at both its concentration in serum from AH109A-bearing rats and its IC50 against HSL activity.
Fig. 6.
Effect of the deprivation of sulfur amino acids (l-methionine (Met) and l-cystine (Cys) or Met and l-cysteine (CysH)) on HSL activity in 3T3-L1 adipocytes without (None) or with (concentration in serum from AH109A-bearing rats (Serum) or IC50 against HSL activity (IC50)) TNF-α. Each value represents % of control (=TNF-α-free and complete group). Each value and vertical bar represents the means and SEM for 4 wells. * Represents significant difference from the complete group at p < 0.05 by Dunnett’s pairwise multiple comparison t test
Fig. 7.
Effect of the addition of sulfur amino acids (l-methionine (Met), l-cystine (Cys) or l-cysteine (CysH)) on HSL activity in 3T3-L1 adipocytes without (None) or with (concentration in serum from AH109A-bearing rats (Serum) or IC50 against HSL activity (IC50)) TNF-α. Each value represents % of control (=TNF-α-free and 200 μM (Met and Cys) of or 400 μM (CysH) of sulfur amino acid-containing group). Each point and vertical bar represents the means and SEM for 4 wells. In a same line, * represents significant difference from the basal group (200 μM (Met and Cys) of or 400 μM (CysH) of sulfur amino acid-containing group) at p < 0.05 by Dunnett’s pairwise multiple comparison t test
Discussion
Our previous study reported that the LPL activity in the epididymal adipose tissue was suppressed in AH109A-bearing rats as well as in lipopolysaccharide-injected rats with an increase in the serum TNF-α concentration (Kawasaki et al. 2004). Another study has reported that the administration of TNF-α suppressed the LPL activity in adipose tissue in vivo (Grunfeld et al. 1989). In the present study, TNF-α suppressed the LPL activity in 3T3-L1 adipocytes. These results indicate that the AH109A-induced suppression of LPL activity may be caused by TNF-α, and that TNF-α may regulate the LPL activity in the adipose tissue directly in AH109A-bearing rats. It has been reported that TNF-α regulated the LPL mRNA level and its synthesis in addition to its activity (Price et al. 1986). The suppression of LPL activity by TNF-α might be regulated in the translational or posttranslational pathway of LPL protein.
Sulfur amino acids affected LPL activity in 3T3-L1 adipocytes. In the experiment involving sulfur amino acids deprivation, the LPL activity in the absence of both Met and Cys or both Met and CysH was lower than that in the presence of both Met and Cys or both Met and CysH in both the absence and presence of TNF-α. Furthermore, the LPL activity was suppressed even in the presence of either Met or Cys or in the presence of either Met or CysH in both the absence and presence of TNF-α. These results suggest that both Met and Cys or both Met and CysH are necessary to express the LPL activity.
In the experiment involving sulfur amino acids addition, the LPL activity was not changed by Met, Cys or CysH supplementation in the absence of TNF-α. On the other hand, Met and Cys supplementation significantly elevated the LPL activity in the presence of TNF-α. This finding was consistent with the result that diet supplementation with Met and Cys restored the LPL activity in AH109A-bearing rats (Kawasaki et al. 2010) in which the serum TNF-α concentration was significantly increased. Furthermore, Met enhanced the LPL mRNA level as well as its activity at the concentration of 600 μM in the presence of TNF-α. However, this finding was inconsistent with the result in AH109A-bearing rats that the LPL mRNA level in the adipose tissue was not changed by the Met-supplemented diet (Kawasaki et al. 2010). These results suggest that the elevation of LPL activity by Met may be accompanied by an increase in the expression of the LPL mRNA, and that Met may affect the expression of the LPL mRNA directly in 3T3-L1 adipocytes.
TNF-α suppressed the HSL activity in 3T3-L1 adipocytes. This finding was inconsistent with the result in LPS-injected and AH109A-bearing rats that the HSL activity in the adipose tissue was significantly elevated (Kawasaki et al. 2004). These two results suggest that the elevation of serum NEFA concentration accompanied by the significant enhancement of adipose tissue HSL activity in LPS-injected and AH109A-bearing rats may be little related or not related at all to TNF-α.
Sulfur amino acids also affected the HSL activity as well as the LPL activity in 3T3-L1 adipocytes. With the deprivation of sulfur amino acids, the HSL activity in the absence of both Met and Cys or both Met and CysH was lower than that in the presence of both Met and Cys or both Met and CysH in both the absence and presence of TNF-α. Furthermore, the HSL activity was suppressed even in the presence of either Met or Cys or in the presence of either Met or CysH in both the absence and presence of TNF-α. These are the same findings as for the LPL activity, and these results suggest that the expression of the HSL activity requires the presence of both Met and Cys or both Met and CysH.
There was no effect on the HSL activity with the addition of Cys and CysH and an elevation effect with the addition of Met in the absence of TNF-α. On the other hand, the HSL activity was suppressed by the addition of Met, Cys and CysH in the presence of TNF-α. This finding was consistent with the result that the supplementation of the diet with Met and Cys significantly suppressed the HSL activity in AH109A-bearing rats (Kawasaki et al. 2010) in which the serum TNF-α concentration was significantly elevated. On the other hand, these findings were inconsistent with the result that Met and Cys significantly elevated the LPL activity in the present study. These results suggest that mechanisms of action of sulfur amino acids against HSL activity may be different from those against LPL activity. It is considered that this difference may be due to the different metabolisms of these two lipases in that LPL hydrolyzes triglyceride in the blood and the hydrolysate is resynthesized to triglyceride and is accumulated in adipose tissue, whereas HSL hydrolyzes this accumulated triglyceride in adipose tissue and releases it to the blood. Sulfur amino acids may have a triglyceride accumulative effect, especially in the presence of TNF-α in 3T3-L1 adipocytes.
In conclusion, sulfur amino acids including Met, Cys and CysH affected the LPL activity and mRNA level and the HSL activity in 3T3-L1 adipocytes. Some of these effects of sulfur amino acids were different between LPL and HSL, between the absence and the presence of TNF-α, and between 3T3-L1 adipocytes and the adipose tissue from rats. The mechanisms responsible for the observed actions of these lipases resulting from sulfur amino acids and TNF-α treatments were not determined in the present study. The question of how these mechanisms govern these changes in LPL and HSL due to sulfur amino acids and TNF-α treatments remains to be answered in future studies.
Acknowledgments
We are indebted to Dr. Teruo Kawada, Kyoto University, for the gift of mouse 3T3-L1 cells, to Dr. Michael C. Schotz, University of California, Los Angeles, for presenting the LPL cDNA probe and to Dr. Toshio Watanabe, University of Tokyo, for providing the GAPDH cDNA probe.
References
- Barclay M, Skipski VP, Terebus-Kekish O, Greene EM, Kaufman RJ, Stock CC. Effects of cancer upon high-density and other lipoproteins. Cancer Res. 1970;30:2420–2430. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein using principles of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol–chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Fort P, Marty L, Piechaczyk M, El Sabrouty S, Dani C, Jeanteur P, Blanchard JM. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985;13:1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld C, Gulli R, Moser AH, Gavin LA, Feingold KR. Effect of tumor necrosis factor administration in vivo on lipoprotein lipase activity in various tissues of the rat. J Lipid Res. 1989;30:579–585. [PubMed] [Google Scholar]
- Irikura T, Takagi K, Okada K, Yagasaki K. Effect of KCD-232, a new hypolipidemic agent, on serum lipoprotein changes in hepatoma-bearing rats. Lipids. 1985;20:420–424. doi: 10.1007/BF02534232. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Pekala PH, Lane MD, Cerami A. Lipoprotein lipase suppression in 3T3–L1 cells by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci USA. 1982;79:912–916. doi: 10.1073/pnas.79.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, Jensen DF, Wancewicz EV, Joy LL, Khoo JC, Steinberg D. Hormone-sensitive lipase in differentiated 3T3–L1 cells and its activation by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci USA. 1981;78:732–736. doi: 10.1073/pnas.78.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M, Yagasaki K, Miura Y, Funabiki R. Reduction of hyperlipidemia in hepatoma-bearing rats by dietary fish oil. Lipids. 1995;30:431–436. doi: 10.1007/BF02536301. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Yagasaki K, Miura Y, Funabiki R. Comparison of the changes in lipid metabolism between hepatoma-bearing and lipopolysaccharide-treated rats. Biosci Biotechnol Biochem. 2004;68:72–78. doi: 10.1271/bbb.68.72. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Miura Y, Funabiki R, Yagasaki K. Comparison of the effects on lipid metabolism of dietary methionine and cystine between hepatoma-bearing and normal rats. Biosci Biotechnol Biochem. 2010;74:153–167. doi: 10.1271/bbb.90673. [DOI] [PubMed] [Google Scholar]
- Muntoni S, Atzori L, Mereu R, Satta G, Macis MD, Congia M, Tedde A, Desogus A, Muntoni S. Serum lipoproteins and cancer. Nutr Metab Cardiovasc Dis. 2009;19:218–225. doi: 10.1016/j.numecd.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Nakai T, Yamada S, Tamai T, Kobayashi T, Hayashi T, Takeda R. The effects of streptozotocin diabetes on hepatic triglyceride lipase activity in the rat. Metabolism. 1979;28:30–40. doi: 10.1016/0026-0495(79)90165-3. [DOI] [PubMed] [Google Scholar]
- Nilsson-Ehle P, Schotz MC. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res. 1976;17:536–541. [PubMed] [Google Scholar]
- Nydegger UE, Butler RE. Serum lipoprotein levels in patients with cancer. Cancer Res. 1972;32:1756–1760. [PubMed] [Google Scholar]
- Price SR, Olivecrona T, Pekala PH. Regulation of lipoprotein lipase synthesis by recombinant tumor necrosis factor-the primary regulatory role of the hormone in 3T3–L1 adipocytes. Arch Biochem Biophys. 1986;251:738–746. doi: 10.1016/0003-9861(86)90384-X. [DOI] [PubMed] [Google Scholar]
- Qadir MI, Malik SA. Plasma lipid profile in gynecologic cancers. Eur J Gynaecol Oncol. 2008;29:158–161. [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning, a laboratory manual. 2. New York: Cold Spring Harbor Laboratory Press; 1989. pp. 7.53–7.55. [Google Scholar]
- Sugiyama K, Kushima Y, Muramatsu K. Effects of methionine, cystine and taurine on plasma cholesterol level in rats fed a high cholesterol diet. Agric Biol Chem. 1984;48:2897–2899. [Google Scholar]
- Wion KL, Kirchgessner TG, Lusis AJ, Schotz MC, Lawn RM. Human lipoprotein lipase complementary DNA sequence. Science. 1987;235:1638–1641. doi: 10.1126/science.3823907. [DOI] [PubMed] [Google Scholar]
- Yagasaki K, Okada K, Takagi K, Irikura T. Effect of 4-(4′-chlorobenzyloxy) benzyl nicotinate (KCD-232) on cholesterol metabolism in rats fed an amino acid imbalance diet. Agric Biol Chem. 1984;48:1417–1423. [Google Scholar]
- Yagasaki K, Machida M, Funabiki R. Effects of dietary methionine, cystine, and glycine on endogenous hypercholesterolemia in hepatoma-bearing rats. J Nutr Sci Vitaminol. 1986;32:643–651. doi: 10.3177/jnsv.32.643. [DOI] [PubMed] [Google Scholar]