Abstract
The mechanical behavior of a living cell is highly dynamic and constantly adapts to its local environment. Changes in temperature and chemical stimuli, such as pH, may alter the structure of the cell and its mechanical response. Thus, the mechanical properties may serve as an indicator for the cellular state. We applied dielectrophoretic forces to suspension cells by means of two microelectrodes. The resultant stretching was analyzed on consecutive cultivation days with respect to the influence of medium consumption. Systematic experiments clearly showed that the medium consumption affected the viscoelastic properties of the investigated human leukemia cells HL-60. The shift in pH value and the culture medium depletion were identified as potentially responsible for the differing temporal development of the cell deformation. Both factors were investigated separately and a detailed analysis indicated that the changes observed in the cellular stiffness were primarily attributable to nutrient depletion.
Keywords: Nutrient depletion, Dielectrophoresis, Stiffness, Cell cultivation
Introduction
The viscoelasticity of cells plays a crucial role in their behavior and is a key factor in the regulation of the shape of resting and moving cells. It is determined in a complex way by the membrane, organelles, the nucleus and the filamentous cytoskeleton that interconnects all these elements and extends through the viscous cytosol.
It was common knowledge at the turn of the last century that mechanical forces are important factors in biology, since they serve as regulators at the cell and molecular levels (Ingber 2003; Suresh et al. 2005). For example, cell-generated tensional forces are thought to regulate functions as diverse as chromosome movements, cell proliferation, tissue morphogenesis, cell contractility and motility (Ingber 2003). The application of external mechanical stimuli can induce biochemical reactions, including the synthesis of new biomolecules. Thus, physical forces and change in cell mechanics play a fundamental role in the normal or pathological development of cells.
Various techniques have been developed to analyze the mechanical properties of cells and their influence on the cellular functions, such as atomic force microscopy (Rotsch et al. 1997), micropipette aspiration (Hochmuth 2000), substrate deformation (Brown 2000), magnetic twisting cytometry (Puig-De-Morales et al. 2001; Wang et al. 1993), optical stretcher (Guck et al. 2005) etc.
In contrast to most inanimate systems, the mechanical behavior of a living cell cannot be characterized simply in terms of fixed mechanical properties, as the cell structure is a dynamic system that adapts to its local mechanochemical environment (Van Vliet et al. 2003). Changes in chemical stimuli, including pH, temperature and biomolecular activity, can alter the structure of the cell and its mechanical response.
In this paper, we present an approach to characterize the influence of external factors on the mechanical properties of cells and in particular the effect of the culture conditions on the deformability of cells.
Here, deformation was elicited by means of dielectrophoresis, i.e. the electric force exerted between two microelectrodes (Pethig and Markx 1997). In the inhomogeneous electric field around the microelectrodes, polarizable objects, like living cells, may be attracted to the electrode edges (positive dielectrophoresis, pDEP). In the gap between the two electrodes, the cells then undergo a stretching through the interaction of charges at the interface between cell and buffer with the electric field (Gimsa and Wachner 1999; Thom and Gollek 2006). By means of this novel dielectrophoretic stretcher, we investigated the influence of the pH value and nutrient depletion on the stiffness of cells in culture.
In this study we used the human leukemia cell line HL-60 as a model system and measured the evolution of cellular stiffness over several cultivation days.
Theory
The motion of an uncharged particle in an inhomogeneous electric field is called dielectrophoresis (Pohl 1978). The origin of the force causing this motion lies in the interaction of the electric field with charges induced in the particle. The magnitude and direction of this force depend on the electric properties of the particle—permittivity, electric conductivity and size—and those of its surrounding medium. If the polarizability of the particle exceeds that of the suspension medium it is attracted towards regions of stronger electric field (pDEP). The magnitude and frequency of the applied electric field also influence the dielectrophoretic force.
The cell membrane can be modeled as an electric circuit with a resistance and a capacitance in parallel. Thus, a frequency range exists in which the capacitive reactance of the membrane begins to short-out the membrane resistance and the field penetrates into the electrically conducting cytoplasmic electrolyte. So, in this range, the particle is more polarizable than the surrounding medium and pDEP occurs. Therefore, the cytosolic electric conductivity is the main determinant of the deformation response (Sukhorukov et al. 1998).
We also used this range of frequencies to avoid potentially adverse effects on the living cells caused by membrane polarization.
Materials and methods
Cell culture
Cells of the human leukemia cell line HL-60 (DSMZ, Braunschweig, Germany) were cultivated in RPMI 1640 medium supplemented with 10% fetal bovine serum (Biochrom, Berlin, Germany) and were kept in 25 cm2 plastic flasks under a 5% CO2, 95% air atmosphere at 37 °C in a humidified incubator. HL-60 cells were seeded at a density of 5 × 104/mL. The cells remained in culture for up to 5 days.
Cell preparation
For the experiments, the cells were cultivated according to different protocols, described in the following:
Protocol A: After 1 day in culture, the cells were supplied with the used-up medium removed from a 4-days-old cell culture.
Protocol B: After 4 days in culture, the cells were supplied with fresh medium.
Protocol C: The pH value of 5-days-old medium was measured. By adding hydrochloric acid (HCl), the pH value of fresh medium was adjusted to the value of the old medium. One-day-old cells as well as 4-days-old cells were cultivated for 24 h with the adjusted medium.
Protocol D: The cells were cultivated using 6-well microtiter plates with well inserts with 1 μm pore size. The medium was removed daily on five consecutive cultivation days and the cells were supplied with fresh medium.
Experimental procedure
The required aliquot of suspension cells (105 cells) was transferred by centrifugation into an isotonic solution consisting of 0.3 M inositol and PBS. The amount of the latter was titrated to obtain an electric conductivity of 5.5 mS/m. Finally, 0.5 weight percent of a commercially available, soluble polymer (Cytocon, Perkin Elmer, Hamburg, Germany) was added that effectively reduces non-specific sticking of the cells to the microchip surfaces.
Microchip
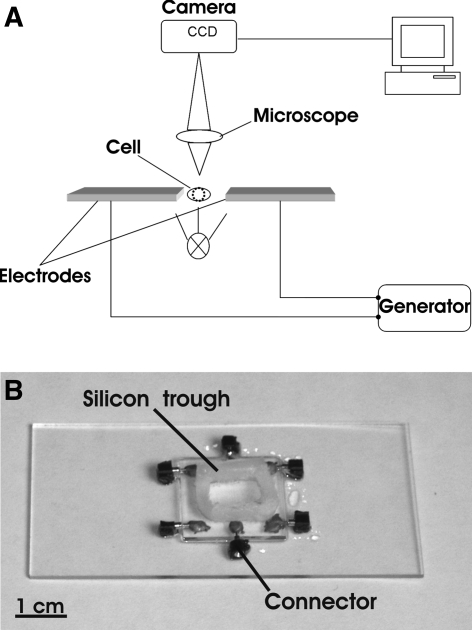
The dielectrophoretic stretchers were purpose-made, planar microstructures of two microelectrodes with a gap of 20 μm between them (Fig. 1). They were made of 20 × 20 mm2 glass slides plated with indium tin oxide, a transparent conductive material (Schott AG, Gruenenplan, Germany). The structures were processed by UV ablation with a pulsed KrF laser at 248 nm (Lextra 100, Lambda-Physik Goettingen, Germany). Silicone troughs with a volume of approximately 200 μL were mounted on the slides after processing to reduce buffer evaporation effects during the experiments.
Fig. 1.
a Experimental set-up. b Purpose-made microchip employed in the experiments
Image processing
Optical microscopy was performed using a microscope equipped with a 20×/0.35 objective (BX40 Olympus, Hamburg, Germany). The images were recorded by a computer-controlled CCD camera (Orca ER, Hamamatsu Photonics) controlled by a program provided by the camera manufacturer (Simple-PCI). The chip electrodes were connected to a function generator set to 15 MHz and a square waveform (33120A, Agilent). The voltage applied was 4 Vrms. The data are presented as the mean value ±s.e.m.
Results and discussion
Through the action of convection, the cells in the silicone trough come close to the gap between the microelectrodes and there experience the dielectrophoretic force which attracts them towards the nearest electrode edge. When the cells reach the gap, the field between the electrodes causes a cell elongation, as described earlier (Sukhorukov et al. 1998). This elongation ε is measured as the change Δl in the long axis of the cell normalized by its initial length l0. We analyzed this dielectrophoretic stretching on a single-cell level as a measure of the mechanical properties of HL-60 cells.
The standard procedure is to split these cells in culture every 3 days. We compared cultivation durations without splitting of up to 5 days. To investigate the influence of the cultivation day on the viscoelasticity of the cells, we electrically stretched samples of cells for 90 s on the cultivation days 2–5.
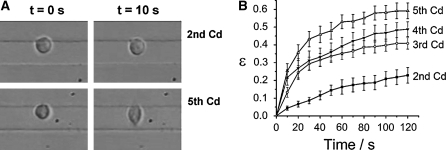
The experiments showed that the cellular stiffness effectively depends on the cultivation day: the longer the cells were cultivated without splitting, the softer they appeared in the electric field (Fig. 2).
Fig. 2.
Deformation ε of HL-60 cells on different cultivation days. a Deformation on the second and fifth cultivation day (Cd). The difference in stiffness of the cells is clearly visible after 10 s. The gap between the upper and lower electrode is 20 μm. b Deformation as a function of time. There was a considerable loss in cellular rigidity when the cells were cultivated without splitting (n ≥ 13)
The next step was to investigate to which changes in the medium that occur over time the differences in the stretching response were due. For this approach, cells cultivated for 1 day as well as cells cultivated for 4 days were prepared according to the protocols described in the “Materials and methods” section and summarized in Table 1.
Table 1.
Cell cultivation protocols for preparation of the experiments
| 1-Day-old cells | 2-Days-old cells | 3-Days-old cells | 4-Days-old cells | |
|---|---|---|---|---|
| Protocol A | 4-Days-old medium | |||
| Protocol B | Fresh medium | |||
| Protocol C | Medium with modified pH | Medium with modified pH | ||
| Protocol D | Fresh medium | Fresh medium | Fresh medium | Fresh medium |
In each case, the stretching of the cells was measured 24 h after the application of the respective protocol.
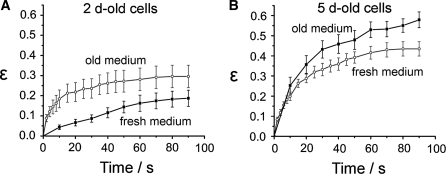
We found that the cells cultivated according to the protocol B were stiffer than control cells that had been without fresh medium for 5 days. Conversely, cells prepared according to the protocol A responded softer to the electric deformation than control cells in 2-days-old medium (Fig. 3).
Fig. 3.
a Experimental data for cells stretched on the second cultivation day. These 2-days-old cells were either grown in fresh medium (2d-old, n = 13) or in 5-days-old medium (i.e. nutrient depleted, n = 10). The latter exhibit a clearly reduced cellular rigidity, in particular in the first 40 s of the stretching. b Cell stretching on the fifth cultivation day. These cells had either been exposed to the same medium for 5 days without exchange (n = 16) or they had been supplied with fresh medium 24 h before the measurements (n = 13). A tendency towards an increased stiffness is visible in the experiments using the cells supplied with fresh medium. It can be interpreted as a recovery from the effect of old medium
The purpose of medium change in cell culture is to maintain the correct pH value and to replenish nutrients. Therefore, these two factors were separately investigated next, to determine which of them causes the change in the cellular stiffness of the HL-60 cells.
Influence of the pH value
The pH value of the cell culture medium was measured daily for five cultivation days. The values decreased from 7.6 to 7.1.
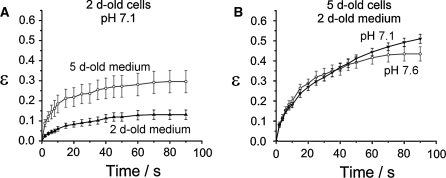
By means of adding HCl, fresh medium was obtained with the same pH value as that of 5-days-old medium (7.1) as described in protocol C. The cells were cultured for 24 h according to the protocol C. Subsequently, they were stretched (Fig. 4) and the results were compared to the corresponding data shown above (Fig. 3).
Fig. 4.
a HL-60 cells on the second cultivation day in either 5-days-old medium (open circles, n = 10) or in pH-reduced fresh medium (full triangles, n = 22). The two media had the same pH value but different effects on the cellular stiffness. The fresh medium was the decisive factor in the HL-60 cell rigidity, as shown in (b). Here, the two 5-days-old HL-60 populations exhibited the same electric deformation, although the pH values of the media were different. Both populations were cultivated in fresh medium for 24 h (n ≥ 13)
From these experiments, the two following comparisons can be made: as depicted in Fig. 4a, 2-days-old cells cultivated according to the protocol C were stiffer than 2-days-old cells cultivated according to the protocol A (with the same pH value of 7.1). Conversely (Fig. 4b), 5-days-old cells cultivated according to the protocol B (pH 7.6) and according to the protocol C (pH 7.1) showed comparable results, i.e. an increased stiffness, although in both cases the cells were in fresh medium. These results show that in the range that we investigated the pH value was not the factor responsible for the cellular rigidity. Instead, the rigidity was apparently influenced primarily by whether the medium was fresh or not, irrespective of its pH value.
Influence of nutrient depletion
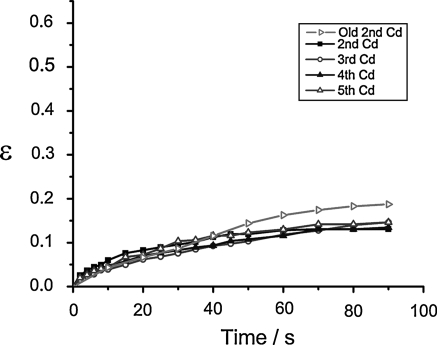
The next step was to investigate whether the differences in the stretching behavior of HL-60 cells were caused by nutrient depletion of the medium. For this purpose, we cultivated the cells according to protocol D. Every 24 h, a sample of the cells was stretched. No difference was observed in the resulting deformation curves (Fig. 5). This in turn strongly suggests that the viscoelasticity of the HL-60 cells is primarily determined by nutrient depletion.
Fig. 5.
The deformation response of HL-60 cells is independent of the cultivation day if the medium is exchanged daily. The fresh medium supply leads to constant cellular mechanical properties (n ≥ 10). For comparison, the loss in the rigidity of the HL-60 cells after cultivation for 2 days without medium exchange is also shown (Old 2nd Cd, n = 13). Error bars omitted for clarity. Length of ordinate (ε) chosen to accord with previous figures
Evidently, the purely mechanical characterization by stretching provides no information on intracellular reasons for the change in cellular stiffness. However, general conclusions can be drawn from studies showing that not only metabolic enzymes but also structural proteins are both spatially and functionally organized into dynamic complexes that assemble and disassemble dependent upon the supply status of the cell (Narayanaswamy et al. 2009). During times of starvation, cells increasingly degrade otherwise stable proteins (Gottesman and Maurizi 2001). The actin cytoskeleton is highly responsive to a vast number of signaling pathways that coordinate the rapid assembly and disassembly of filaments and regulate their incorporation into various structures such as stress fibers. This network is also subject to degradation processes during starvation. When glucose is depleted, F-actin disappears (Sagot et al. 2006). In the light of these results, we suppose that in our experiments HL-60 cells cultivated in aging medium undergo a cytoskeletal protein degradation that in turn causes a progressive structural softening. This, then, becomes visible when the cells are stretched on different cultivation days.
Conclusion
Cells were stretched between microelectrodes through dielectrophoresis and the responses to stretching after cultivation without splitting the cells of up to 5 days were compared.
By using HL-60 cells as model system, we obtained reliable data that showed that cellular stiffness effectively depends on the cultivation duration. This allowed assessing that the chemical environment of the cells influenced their viscoelastic properties over time.
The pH value and the culture medium depletion were identified as potential factors responsible for the different deformation over time. Both aspects were investigated separately. The results showed that the rigidity was primarily influenced by whether the medium was fresh or not, irrespective of its pH value which only had a minor influence on the cellular rigidity. The analysis of the response to stretching of cells which were continuously supplied with fresh culture medium for the entire cultivation duration strongly suggests that the changes in the stiffness of the HL-60 cells in our experiments were primarily attributable to nutrient depletion.
Acknowledgments
We thank Beate Morgenstern for cell culture support. The German Research Foundation (DFG) and the Foundation of German Business (SDW) are gratefully acknowledged for funding through the Priority Programme JA 1717/1-3 and a scholarship to IG, respectively.
References
- Brown TD. Techniques for mechanical stimulation of cells in vitro: a review. J Biomech. 2000;33:3–14. doi: 10.1016/S0021-9290(99)00177-3. [DOI] [PubMed] [Google Scholar]
- Gimsa J, Wachner D. A polarization model overcoming the geometric restrictions of the laplace solution for spheroidal cells: obtaining new equations for field-induced forces and transmembrane potential. Biophys J. 1999;77:1316–1326. doi: 10.1016/S0006-3495(99)76981-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S, Maurizi MR. Surviving starvation. Science. 2001;293:614–615. doi: 10.1126/science.1063371. [DOI] [PubMed] [Google Scholar]
- Guck J, Schinkinger S, Lincoln B, Wottawah F, Ebert S, Romeyke M, Lenz D, Erickson HM, Ananthakrishnan R, Mitchell D, Kaes J, Ulvick S, Bilby C. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J. 2005;88:3689–3698. doi: 10.1529/biophysj.104.045476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth RM. Micropipette aspiration of living cells. J Biomech. 2000;33:15–22. doi: 10.1016/S0021-9290(99)00175-X. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35:564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- Narayanaswamy R, Levy M, Tsechansky M, Stovall GM, O’Connell JD, Mirrielees J, Ellington AD, Marcotte EM. Widespread reorganization of metabolic enzymes into reversible assemblies upon nutrient starvation. Proc Natl Acad Sci USA. 2009;106:10147–10152. doi: 10.1073/pnas.0812771106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethig R, Markx GH. Applications of dielectrophoresis in biotechnology. Trends Biotechnol. 1997;15:426–432. doi: 10.1016/S0167-7799(97)01096-2. [DOI] [PubMed] [Google Scholar]
- Pohl HA. Dielectrophoresis. Cambridge: Cambridge University Press; 1978. [Google Scholar]
- Puig-De-Morales M, Grabulosa M, Alcaraz J, Mullol J, Maksym GN, Fredberg JJ, Navajas D. Measurement of cell microrheology by magnetic twisting cytometry with frequency domain demodulation. J Appl Physiol. 2001;91:1152–1159. doi: 10.1152/jappl.2001.91.3.1152. [DOI] [PubMed] [Google Scholar]
- Rotsch C, Braet F, Wisse E, Radmacher M. AFM imaging and elasticity measurements on living rat liver macrophages. Cell Biol Int. 1997;21:685–696. doi: 10.1006/cbir.1997.0213. [DOI] [PubMed] [Google Scholar]
- Sagot I, Pinson B, Salin B, Daignan-Fornier B. Actin bodies in yeast quiescent cells: an immediately available actin reserve? Mol Biol Cell. 2006;17:4645–4655. doi: 10.1091/mbc.E06-04-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhorukov VL, Mussauer H, Zimmermann U. The effect of electrical deformation forces on the electropermeabilization of erythrocyte membranes in low- and high-conductivity media. J Membr Biol. 1998;163:235–245. doi: 10.1007/s002329900387. [DOI] [PubMed] [Google Scholar]
- Suresh S, Spatz J, Mills JP, Micoulet A, Dao M, Lim CT, Beil M, Seufferlein T. Connections between single-cell biomechanics and human disease states: gastrointestinal cancer and malaria. Acta Biomater. 2005;1:15–30. doi: 10.1016/j.actbio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Thom F, Gollek H. Calculation of mechanical properties of human red cells based on electrically induced deformation experiments. J Electrostat. 2006;64:53–61. doi: 10.1016/j.elstat.2005.04.006. [DOI] [Google Scholar]
- Vliet KJ, Bao G, Suresh S. The biomechanics toolbox: experimental approaches for living cells and biomolecules. Acta Mater. 2003;51:5881–5905. doi: 10.1016/j.actamat.2003.09.001. [DOI] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]