Abstract
Cell death is a fundamentally important problem in cell lines used by the biopharmaceutical industry. Environmental stress, which can result from nutrient depletion, by-product accumulation and chemical agents, activates through signalling cascades regulators that promote death. The best known key regulators of death process are the Bcl-2 family proteins which constitute a critical intracellular checkpoint of apoptosis cell death within a common death pathway. Engineering of several members of the anti-apoptosis Bcl-2 family genes in several cell types has extended the knowledge of their molecular function and interaction with other proteins, and their regulation of cell death. In this review, we describe the various modes of cell death and their death pathways at molecular and organelle level and discuss the relevance of the growing knowledge of anti-apoptotic engineering strategies to inhibit cell death and increase productivity in mammalian cell culture.
Keywords: Bcl-2, Apoptosis, Autophagy, Cell engineering, Signalling pathways, Cell death
Introduction
It has been over two decades since the US food and drug administration (FDA) approved the first biopharmaceutical product. By 2006 some 165 biopharmaceutical products (recombinant proteins, monoclonal antibodies and nucleic acid–based drugs) have gained marketing approval (Walsh 2006). Most of these products are produced in CHO cell line which is considered as the best workhorse for commercial therapeutic protein production, generating the desired glycosylation structure (Jayapal et al. 2006), although NS0 mouse myeloma, BHK, HEK-293 and few human-derived cell lines are also used in production.
A major challenge in mammalian cell line development is that the volumetric yield of protein is typically ~10–100 folds lower than those achieved when using microbial host systems due to slower growth, lower cell based productivity and high death rate of mammalian cells. Hence, to meet the market demand cells have to be grown at high densities in large bioreactors and fed for a prolonged period of time (Al-Rubeai and Singh 1998). The problem with high density culture production is the enhanced environmental perturbation, thus stressing cells due to nutrient and oxygen transport limitation, accumulation of metabolic by-products and elevated osmolarity (Mercille et al. 2000; Al-Rubeai et al. 1992; Zhu et al. 2008). Consequently, often the first sign to cell stress is a prolongation of the cell cycle, up-regulation of the transcription factor NF-kB and Bcl-2 family of protein and triggering of the death receptors which play important role in the transduction of the apoptotic signal. During sever and sustainable stress cells are led to their death by one of the two mechanisms; passive cell death (necrosis) and programmed cell death (apoptosis and autophagy). Hence, developing methods to prevent cell death in bioreactors has been pursued with great interest.
Supplementing nutrients to culture media has been an efficient approach to overcome cell death. Alternatively, cell line engineering with anti-apoptotic genes has also successfully shown to delay the onset of cell death. The main focus of research in this area has been on the bcl-2 and bcl-xL genes. In cells over-expressing bcl-2 the results show a mixed bag of significant increase in viable cell number and modest or no increase in productivity (Tey et al. 2000a, b; Tey and Al-Rubeai 2005a, b; Meents et al. 2002). Other notable findings are the increase in macromolecular synthesis and ATP production (Imahashi et al. 2003; Janumyam et al. 2003) as well as cell cycle prolongation (Simpson et al. 1999; Zinkel et al. 2006).
The development and deployment of the ‘omic’ technologies (genomic, proteomic, metabolomic) to study cell death will certainly help to discover novel targets for cell death engineering (Kuystermans et al. 2007; Wong et al. 2006). Additionally, with the concept of cell death by autophagy in mind, it seems worthwhile to revisit the issue of cell death in production processes (Mohan et al. 2009; Zustiak et al. 2008).
This paper begins with a brief overview of the morphology and molecular mechanism of cell death, mainly apoptosis and autophagy. This is followed by a review of the work on cell line performance as influenced by the over-expression of anti-apoptotic genes and by other cell engineering strategies which delay cell death.
Morphological features of cell death
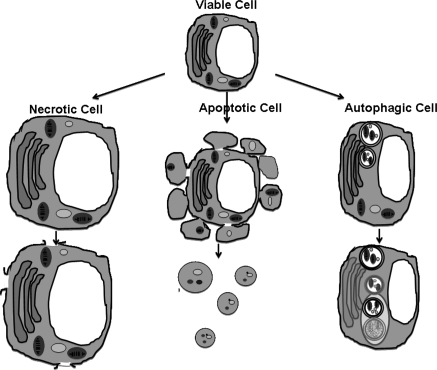
The two forms of cell death, passive death (necrosis) and programmed death (apoptosis and autophagy), are illustrated in Fig. 1. Necrosis occurs when cells are subjected to sudden and sever external stress (Singh et al. 1994). Morphologically cells feature swelling, chromatin digestion, disruption of the plasma membrane, ATP depletion, extensive DNA hydrolysis, vacuolation of the endoplasmic reticulum, organelle breakdown and cell lyses (Cotter and Al-Rubeai 1995; Leist et al. 1997).
Fig. 1.
Sketch showing the morphological features of necrotic, apoptotic and autophagic cell death. Necrosis involves clear swelling of mitochondria and golgi organelles, followed by breakdown of the cellular membrane leading to leakage of intracellular proteins. Apoptotic cells undergo organized destruction of the cellular cytoskeleton and formation of apoptotic bodies. It also involves chromatin condensation and fragmentation into spherical particles. Autophagy sequesters cytosolic proteins and organelles by a double membrane vesicle which docks and fuses with the lysosome
Cell death by apoptosis was identified and named in 1972 by Kerr et al. (1972). This form of cell death has a remarkable implication in the field of cancer research, infectious diseases and other medical areas as well as in cell culture technology. Early morphological characteristics of apoptosis include cell shrinkage, and loss of surface microvilli, followed by chromatin condensation, mitochondrial depolarization and membrane blebbing. Among the biochemical changes are an increased cytosolic Ca2+ ion concentration, cellular acidification and DNA fragmentation (into 180–200 bp) (Cummings et al. 1997). The energetically demanding process of apoptosis can be triggered by extra- or intra-cellular stimuli which activate a variety of cellular signaling cascades. One early apoptotic sign is the increase of the expression of pro-apoptotic compared to anti-apoptotic Bcl-2 family members.
Research on autophagy or so called “new apopotosis” has been on-going for over 40 years, but the process gained more attention recently when further knowledge has been obtained about the molecular machinery due to the application of the ‘omic’ technologies. Autophagy promotes survival but only under prolonged stress stimuli through nutrient limitation it induces cell death (Klionsky 2007). The catabolic cellular function is characterized by the degradation of the cell’s own components through the lysosomal machinery. Thus, a characteristic feature of autophagy is the appearance of a double-or multi-membrane cytosolic vesicle surrounding mitochondria, endoplasmic reticulum (ER) and ribosome and their delivery to and subsequent degradation by the cell’s own lysosomal system. The decomposed cell compartments are recycled for macromolecular synthesis and ATP generation. The detailed morphological difference between apoptosis and autophagy is illustrated in Mohan et al. (2009).
Molecular mechanism of cell death
Apoptosis
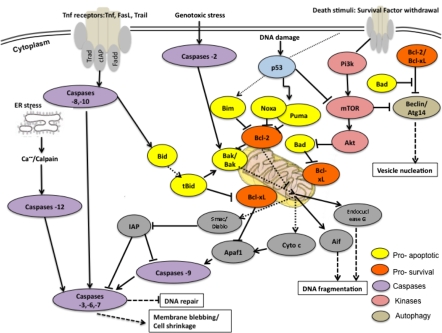
At the molecular level the process of apoptosis is controlled by a diverse range of cell signals which may originate either extra-cellulary (extrinsic inducers) or intra-cellulary (intrinsic inducers). Three main apoptotic signalling pathways can be activated by different sources of stimuli: (1) the extrinsic death receptor pathways, (2) the intrinsic or mitochondria-mediated pathway and (3) the intrinsic endoplasmic reticulum (ER) stress pathway (Jin and El Deiry 2005). All three death-inducing pathways recruit downstream caspase effectors as illustrated in Fig. 2.
Fig. 2.
Simplified apoptotic (and partly autophagic) signalling pathways (adapted pathways from “Cell Signalling Technology”)
In summary, the death receptor pathway is triggered by ligation of the so-called ‘death receptors’ (TNF receptor family; FasL, TRAIL or TNF) which can recruit and activate caspases-8 through the adaptor protein FADD at the cell surface. This recruitment causes subsequent activation of downstream (effectors) caspases without involvement of Bcl-2 family proteins or in some cases through activation of Bcl-2 family member Bid (Ashkenazi and Dixit 1998).
The mitochondrial death pathway, on the other hand, is regulated by the Bcl-2 family of proteins which represent a critical checkpoint upstream of the mitochondria for the cell decision of life or death (reviewed by Chipuk and Green 2008; Youle and Strasser 2008; Cory and Adams 2002). The pro-apoptotic Bcl-2 family members (e.g. Bax and Bak) are able to trigger mitochondrial membrane permeabilization in response to apoptotic stimuli such as DNA damage. This causes the release of pro-apoptotic factors including cytochrome c, AIF, and SMAC/DIABLO through mitochondrial permeability transition pores (PT) into the cytosol where they participate in cellular destruction. Mitochondrial efflux of cytochrome c drives the generation of the apoptosome (apoptotic body) by binding to the adapter protein APAF1 which leads to caspase activation.
Caspases can be divided into two groups, whereby the initiator caspases (caspases -8, -9, -10 and -12) activate the executor caspases (caspases -3, -6 and -7) which are responsible for the cleavage of key cellular substrates that lead to the morphological changes unique to apoptosis (Degterev et al. 2003).
The endoplasmic reticulum (ER) induces the stress pathway in response to oxidative stress, perturbation of Ca2+ or energy stores and unfolded/misfolded protein accumulation. This can lead once to proteolytic cleavage of caspase-12 and/or unfolded protein response (UPR), which can also trigger apoptosis induction under prolonged ER stress (reviewed by Schroder and Kaufman 2005 and Wu and Kaufman 2006). Subsequently, the ER derived Ca2+ signaling may provide a sensitizing signal for mitochondrial cytochome c release (Heath-Engel et al. 2008).
Autophagy
The molecular process of autophagy has mainly been studied in yeast (Tsukada and Ohsumi 1993) and until today, there have been over 30 autophagy-related proteins (Atgs) discovered, regulating multiple signaling pathways which prolong survival during a short period of cell starvation before switching the direction to cell death (Levine and Klionsky 2004). Overall, the term “autophagy” covers three processes: micro-, macro- and chaperone-mediated autophagy whereas macro-autophagy is the most active form (Reggiori and Klionsky 2002). Hence, in mammalian cells the central regulator and gatekeeper of macro-autophagy induction is the mammalian target of rapamycin (mTOR) controlled by the PI3K and PKB/Akt pathway. Under starving conditions, mTOR is inactivated, leading to transcriptional activation of Atg genes (Fig. 2). Additionally, macro-autophagy is initiated in response to endoplasmic reticulum (ER) stress caused by misfolded proteins, via the ER-activated autophagy pathway, which activates a partial unfolded protein response (Ding and Yin 2008). An in depth overview of key components involved in autophagic progression is illustrated by Zustiak et al. (2008). Notably, autophagy and apoptosis are seen to interact in various ways such as (a) autophagy may be indispensible for apoptotic occurrence, (b) autophagy may antagonize apoptosis, and (c) apoptosis and autophagy may occur independent of each other (Gonzuacik and Kimchi 2004). There are also examples where the two processes may be mutually exclusive in that inhibition of apoptosis may convert cell death morphology to autophagic and vice versa (Gonzuacik and Kimchi 2004).
Bcl-2 family members
The Bcl-2 (B-cell lymphoma) family proteins represent a critical intracellular checkpoint for inducing or preventing cell death at both the ER and mitochondria (Heath-Engel et al. 2008). In the mitochondrial-mediated pathway (illustrated in Fig. 2) they control the mitochondrial outer membrane permeabilization (MOMP) which plays a key role in the apoptotic process (Cory and Adams 2002).
Next to the well documented anti-apoptotic Bcl-2 protein, there are over 25 Bcl-2 family proteins possessing up to 4 conserved Bcl-2 homology (BH) domains. The anti-apoptotic members Bcl-2, Bcl-xL, MCL-1 and A1 contain all 4 BH domains. Some of the pro-apoptotic members (Bax, Bak, and Bok) contain 3 BH domains (BH1-3) whereas other members (Bid, Bad, Bim, Bik, Noxa and Puma) contain only the BH3 domain, also called ‘BH3-only’ members (Youle and Strasser 2008). The ‘BH3-only’ proteins are connected to proximal death and survival signals. Under sustainable cellular stress, Bim and Bid induce Bax and Bak which oligomerize at the outer mitochondrial membrane and alone or in combination with other mitochondrial proteins, such as voltage dependent anion channel (VDAC) protein form large membrane transition pores which permeabilize the membrane and release the pro-apoptotic effectors (e.g. cytochrome c) (Chipuk and Green 2008; Willis et al. 2007). Nonetheless, Bax and Bak death activity can be sequestered by the anti-apoptotic Bcl-2 and Bcl-xL proteins at the mitochondria (Cheng et al. 2001). However, when growth/survival factors are withdrawn the pro-apoptotic sensitizer/de-repressor BH3 protein Bad is thought to bind Bcl-2 to block its anti-apoptotic function and thereby can prevent Bcl-2’s inhibition of Bax and Bak (Danial et al. 2003).
The Bcl-2 family members have been recently discovered to play a dual role in the control of apoptosis and autophagy at the ER. The binding of Bcl-2/Bcl-xL to the newly identified BH3 protein Beclin-1 inhibits autophagy until the interaction is disrupted (Ku et al. 2008; Pattingre et al. 2005). On the other hand, it has been confirmed that autophagy can be induced by BH3-mimetic compounds and BH3 only proteins (e.g. Bad) disrupting the interaction between Beclin-1 and Bcl-2 and Bcl-xL. Hence, the anti-autophagy function of Bcl-2 may help maintain autophagy at levels that are compatible with cell survival, rather than cell death when expressed at higher level (Levine et al. 2008).
On the whole, how the interaction of anti- and pro-apoptotic proteins with each other is regulated at the molecular level and how they dictate the balance between survival and death remains controversial. The recent hypotheses regarding apoptosis regulation are illustrated and discussed by Chipuk and Green (2008) who provide analysis of the two models (the anti-apoptotic protein neutralization model and the direct activation of Bax and Bak model) to describe the process of mitochondrial outer membrane permeabilization that is controlled by the Bcl-2 family, which consequently dictates cellular fate and conclude that the BH3-only proteins serve as both inhibitors to the anti-apoptotic proteins and direct activators of effector molecules.
Molecular interaction of the anti-apoptotic Bcl-2
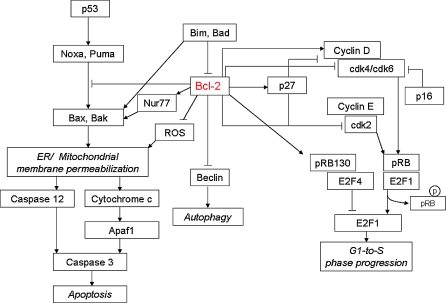
The anti-apoptotic Bcl-2 protein which protects against cell death (Vaux et al. 1988), is located in the endoplasmic reticulum (ER) membrane, the nuclear envelope and the mitochondrial outer membrane (MOM) (Krajewski et al. 1993). At the molecular level certain functions of anti-apoptotic Bcl-2 protein are well described (Fig. 3), whereas some others remain unclear or unknown. Bcl-2 is a key member of the Bcl-2 family, which modulates induction of the caspase-9-dependent apoptosis pathway at the outer mitochondrial membrane and has been the prime choice of anti-apoptosis engineering in the biotechnology community.
Fig. 3.
Overview of anti-apoptotic Bcl-2 interaction with molecules involved in apoptosis and cell cycle regulation
Bcl-2 suppression of apoptosis is primarily by protection against disruption of mitochondrial membrane potential and by involvement of cellular bioenergetics. Thus, Bcl-2 has been suggested to inhibit apoptosis by binding or sequestering pro-apoptotic proteins Bax and Bak or by regulating Bax/Bak binding partners such as voltage dependent anion channel (VDAC) or adenine nucleotide translocator (ANT) at the mitochondria. To the latter, conflicting data exist regarding the regulation of opening or closing of VDAC to regulate the proton efflux (Vander Heiden et al. 2000; Shimizu et al. 2000; Shimizu et al. 1999). Moreover, Bcl-2 was shown to restore the high ATP-to-ADP ratio in the cytosol by facilitating in the mitochondrial ATP/ADP exchange (Vander Heiden et al. 1999) and to directly enhance the antioxidant defense mechanisms thus reducing the generation of reactive oxygen species (ROS) (Deng et al. 2003; Hockenbery et al. 1993; Imahashi et al. 2003). Bcl-2 also regulates Ca2+ content in the mitochondria and endoplasmic reticulum (Lam et al. 1994). However, the exact regulatory function of the bioenergetics has not been fully elucidated (Murphy et al. 2005). In addition to these well-characterized effects, the anti-apoptotic Bcl-2 protein has also been suggested to prevent apoptosis by, at least in part, stricter control of K+ channel and mitochondrial potassium efflux (reviewed by Remillard and Yuan 2004). On the other hand, as mentioned before recent studies demonstrated that Bcl-2 can sequester Beclin-1, a protein necessary for assembly of the autophagosome for autophagic cell death (Pattingre et al. 2005).
In contrast to promoting cell survival, Bcl-2 has also been shown to interact with nuclear receptor NUR77 and can, in turn, activate Bax and Bak to induce apoptosis (Lin et al. 2004). A crucial factor for its pro- or anti-apoptotic effect has been discovered to be the Bcl-2 cellular concentration and its sub-cellular localization (Hanson et al. 2008).
Other cell functions
In contrast to most proto-oncogenes, the anti-apoptotic Bcl-2 inhibits cell cycle progression (Hockenbery et al. 1993; Mazel et al. 1996). The inhibitory effect of Bcl-2 on the cell cycle occurs at the critical control point of G1-to-S phase transition. In this regard, it has been found that Bcl-2 over-expression can elevate the expression level of both cell cycle inhibitor genes p27 and the retinoblastoma pRB relative p130 and maintain the later repressive complexes with the transcription factor E2F4, perhaps to delay the transcription factor E2F1 activity which controls expression of S phase cell cycle entry genes (Vairo et al. 2000). In addition, cyclin D/cdk4 and cyclin E/cdk2 activity has been found to be markedly diminished in Bcl-2 and Bcl-xL expressing cells (Greider et al. 2002). Using a continuous culture, Simpson et al. (1999) have been able to demonstrate a significant increase in the duration of G1 phase for the Bcl-2 over-expressing culture. They have noted that G1 extension was only evident when cells were cycling at reduced rates thus confirming that Bcl-2 can only potentiate cell cycle progression in response to environmental cues. These authors argued that the difference in G1 duration between the Bcl-2 and control cells is simply a reflection of the Bcl-2 mediated survival of cells arrested in the G1 phase due to nutrient limitation. Additionally, Bcl-2 cells revealed a consistently smaller cell size and RNA content and did not initiated macromolecular synthesis despite the induction of c-MYC and cyclin D compared to the control cells (Janumyam et al. 2003; Cory and Adams 2002). These findings suggest that Bcl-2 prevents progression of events in G1 phase which delays entry to S phase. In an unpublished study of NS0-Bcl-2 cell line we have found that the reduced energy utilization rate of these cells was directly related to Bcl-2 over-expression. The diminished energy availability was assumed to have led to a reduction in energy consuming processes such as protein biosynthesis which may have accounted for the lower cell volume, protein content and antibody synthesis that were found in this study (Krampe and Al-Rubeai 2009).
Recently, the first transcriptome profiling of cells over-expressing Bcl-2 has been established which proposed that Bcl-2 impacts several master-regulatory transcription factors, such as NF-B/Rel, AP1 and CREB, to control endothelial cell maturation and survival (Enis et al. 2008). In this context, it has been reported that Bcl-2, when localized at the nuclear fraction, may affect nuclear trafficking of multiple factors by regulating nuclear pores, and thus, the activity of transcription factors and transcription itself (Massaad et al. 2004).
In conclusion, a better understanding of signaling pathway(s) for cell cycle control, survival, and other potential cell function regulations impacted by Bcl-2 would greatly contribute to the fundamental understanding of Bcl-2 anti-apoptotic mechanism. Nevertheless, the suppression of apoptosis by Bcl-2 and other family members, the drawback of the use of these genes in cell technology is their negative impact on ‘apoptosis-unrelated’ characteristics, including specific productivity (Simpson et al. 1999; Tey et al. 2000a, b; Meents et al. 2002; Tey and Al-Rubeai 2005a, b).
Induction of cell death in mammalian cell cultures
The study of cell death, particularly apoptosis, has been an important area in biomedical research since its discovery in 1972. However, first evidence of the occurring process of apoptosis in cell culture was provided by Al-Rubeai et al. (1990). Since this report, all industrially important cell lines have been found to be dying by apoptosis. At the end of a batch culture, cells readily undergo apoptosis due to the deprivation of nutrients such as glucose, glutamine, growth factors and oxygen or due to presence of toxic metabolites such as ammonia and lactate (Simpson et al. 1998; Singh et al. 1994; Mercille and Massie 1994). In fed-batch, perfusion and chemostat cultures the situation is more complex since nutrients are constantly replenished and in some cases metabolites are removed. Cells can undergo apoptosis when nutrients are in critically low concentration or in response to various environmental stress factors such as high osmolality, pH fluctuations, oxygen gradients and deprivation due to insufficient mixing at high cell densities and potential generation of reactive oxygen species (Perani et al. 1998; Singh et al. 1994; Al-Rubeai et al. 1995a; Simpson et al. 1998; Singh and Al-Rubeai 1998; Ryu and Lee 1999). Also, exposure to increasing hydrodynamic forces by bubble bursting at the liquid surface, gas sparging, liquid flow or gas entrainment and energy dissipation of the impeller stream can induce apoptosis (Al-Rubeai et al. 1995a; Chisti 2000). Consequently, increasing level of apoptosis can lead to a decrease in productivity, product quality, growth and viable cell number (Al-Rubeai and Singh 1998; Goswami et al. 1999; Majid et al. 2007).
More recently, evidence implies that the programmed cell death by autophagy also plays a role in cell culture (Zustiak et al. 2008). Recent publication by Hwang and Lee (2008) showed that CHO cells in batch culture exhibited hallmarks of both apoptosis and autophagy due to nutrient depletion; hence, feeding would inhibit or reduce autophagy. To bear in mind, any attempt to disturb autophagy may render cells vulnerable to ER stress, suggesting that autophagy plays important roles in cell survival (Ogata et al. 2006).
Prevention of death by cell engineering
Apoptosis engineering
The importance of modification of the intracellular cell death pathways to enhance the robustness, survival and productivity of production cell lines has been suggested (Cohen and Al-Rubeai 1995) and demonstrated by several research groups in the last 15 years (reviewed by Kuystermans et al. 2007) (Table 1).
Table 1.
Summary of anti- apoptotic and autophagic cell engineering strategies carried out in cell lines used for the production of recombinant proteins
| Gene symbol | Gene name | Cell line | Author |
|---|---|---|---|
| Apoptosis: over-expression | |||
| Aven | Apoptosis, caspase activation inhibitor | CHO | Figueroa et al. (2007) |
| Bcl-2 | B-cell/lymphoma 2 | CHO, hybridoma, NS0, BHK, BL | Tey et al. (2000a), Itoh et al. (1995), Simpson et al. (1997) and Singh et al (1996) etc. |
| Bcl-xl | Bcl-2-like 1 | CHO, hybridoma, BHK | Meents et al. (2002), Mastrangelo et al. (2000) and Figueroa et al. (2004) |
| Bag-1 | Bcl-2-associated athanogene 1 | Hybridoma | Terada et al. (1997) |
| Bhrf1 | BCL2-interacting killer (apoptosis-inducing) | Hybridom | Juanola et al. (2009) |
| CrmA | Caspase 1, apoptosis-related cysteine protease inhibitor | CHO, HEK293 | Sauerwald et al. (2003) |
| E1b-19k | Bcl-2/adenovirus E1B 19kDa protein-interacting protein 3 | CHO, NS0, BHK | Figueroa et al. (2007) |
| Fadd | Fas (TNFRSF6)-associated via death domain | CHO | Wong et al. (2006) |
| Faim | Fas apoptotic inhibitory molecule | CHO | Wong et al. (2006) |
| Hsp70 | Heat shock 70kDa protein | NS0 | Lasunskaia et al. (2003) |
| Htert | Telomerase reverse transcriptase | CHO | Crea et al. (2006) |
| Mcl-1 | Myeloid cell leukemia sequence 1 (Bcl2-related) | CHO | Major and Betenbaugh (2009) |
| Mdm2 | Mdm2 p53 binding protein homolog | CHO | Arden et al. (2006) |
| Xiap | X-linked inhibitor of apoptosis | CHO, HEK293 | Sauerwald et al. (2003) |
| Apoptosis: knock-out (small interference RNA) | |||
| Alg2 | Asparagine-linked glycosylation protein 2 | CHO | Wong et al. (2006) |
| Bak | Bcl-2-antagonist/killer 1 | CHO | Lim et al. (2006) and Cost et al. (2009) |
| Bax | Bcl-2-associated X protein | CHO | Lim et al. (2006) and Cost et al. (2009) |
| Caspase 3, 7 | Caspases | CHO | Sung et al. (2005) |
| Caspase 8, 9 | Caspases | CHO | Yun et al. (2007) |
| Requiem | Apoptosis response zinc finger | CHO | Wong et al. (2006) |
| Autophagy: over-expression | |||
| ca-Akt | Protein kinase B | CHO | Hwang and Lee (2009) |
| Bcl-xl | Bcl-2-like 1 | CHO | Kim et al. (2009) |
The most common genetic modification reported in literature involves over-expression of either Bcl-2 or Bcl-xl, although other genes such as E1B19K, XIAP and Bhrf-1 (Mercille and Massie 1999; Sauerwald et al. 2003; Juanola et al. 2009) has also been used to confer apoptosis resistance. The bcl-2 gene was the first to be recognized as a survival gene involved in the apoptotic pathway and demonstrated increase in cell density and viability correlated with delay of apoptosis in hybridoma (Itoh et al. 1995; Al-Rubeai et al. 1995a). In other studies bcl-2 and bcl-xL over-expression was reported to enhance target protein in engineered CHO cells (Meents et al. 2002; Tey et al. 2000b; Chiang and Sisk 2005). Major and Betenbaugh (2009) found that transient over-expression of bcl-2 in CHO culture resulted in an increased yield of 70–270% with adequate product quality and maintenance of high viable cell numbers. Although, in one early report Bcl-2 failed to protect murine plasmacytoma NS0 cells against apoptosis induction in a batch culture (Murray et al. 1996), in a variety of environmental stress Bcl-2 has been recognized to mediate suppression of apoptosis following ammonium toxicity, oxygen limitation, hyperosmotic pressure and pH variation, occurring at late stage batch/fed-batch culture or high density culture of cell lines used for the production of recombinant proteins (Simpson et al. 1997; Perani et al. 1998; Mercille and Massie 1994; Kim and Lee 2002). Perani et al. (1998) also showed that Bcl-2 cells under shear stress condition increased in their viable cell number by nearly fivefold compared to the control. Apparently in all the above studies bcl-2 homologs demonstrated varying degree of protection depending on the nature and strength of insult which may be partially explained by the anti-apoptotic gene expression level at the time of insult which was shown to vary with time during batch culture (results not published), cell to cell or clonal variability resulted from genetic instability or functionally redundancy of the gene presumably due to their low expression. Indeed, a threshold level of expression was reported to be required E1B-19K (Mercille and Massie 1999) activity to protect cells from apoptosis.
A key factor in long-term cultivation is the role of various nutrients in culture medium. Thus, quite a few studies have been disclosed that death due to deprivation of glucose (Tey and Al-Rubeai 2005a), serum (Al-Rubeai et al. 1992), one or several B-group media vitamins (Ishaque and Al-Rubeai 1999) and all individual amino acids, with exception of theronine (Al-Rubeai et al. 1995b; Simpson et al. 1998), as well as three different ions (Ca2+, Mg2+ and K+) (Ishaque and Al-Rubeai 2002) can be inhibited by the over-expression of Bcl-2 protein. Similarly, cells over-expressing the Bcl-2 family member Bcl-xL showed improved survival properties upon exposure to culture insults initiated by the deprivation of glucose, ammonium chloride, or serum (Figueroa et al. 2004; Mastrangelo et al. 2000). Subsequently, suggestion was made that the reduced amino acid and nutrient utilization in Bcl-2/Bcl-xL transfected cells presumably lead to down-regulation of bioenergetic status of the cell and thus to reduce the nonessential or energy consuming cellular functions (Murphy et al. 2005). Such suggestion would provide explanation to the observation of lower or unchanged specific antibody in bcl-2 or bcl-xL over-expressed cell lines (Tey et al. 2000a, b; Tey and Al-Rubeai 2005a, b; Meents et al. 2002; Simpson et al. 1999; Bierau et al. 1998; Fassnacht et al. 1998; Kim and Lee 2009).
Moreover, analysis of the biochemical characteristic and biological property of the molecular produced in cells over-expressing bcl-2 homologs showed consistent or even higher galactosylation index (GI) values in both batch and continuous culture. This was explained as partly related to the enhanced cell viability (Chiang and Sisk 2005; Majid et al. 2007).
Other strategies for improving cell survival include approaches that interfere with caspase activation such as anti-sense RNA against caspase or over-expression of caspase inhibitors such as XIAP and Crma demonstrating apoptosis protection in cell culture (Sauerwald et al. 2003). The same effect was given by over-expressing E3 ubiquitin ligase (MDM2) of p53 in CHO (Arden et al. 2006). Transfection of human telomerase reverse transcriptase (hTERT) catalytic subunit in CHO-K1 cells resulted in significantly lowering apoptosis thus allowing cells to survive and proliferate in serum deprived culture (Crea et al. 2006). Other key regulatory points in the control of apoptosis are heat shock proteins (Hsp). Over-expression of Hsp27 and Hsp70, either individually or in combination in CHO cells extended considerably the fed-batch culture time, thus, improving the IFN-γ titer by 2.5-fold (Lee et al. 2009). In addition to death protection, over-expression of HSP70 in BHK-21 cells was reported to lead to a higher specific activity of the recombinant protein (Ishaque et al. 2007).
Further successful engineering approaches used to acquire higher cell protection and cell densities were given by co-transfecting cells with, for example, Aven and Bcl-xL (Figueroa et al. 2004) or E1B-19K and Aven (Figueroa et al. 2007). Further, co-expression of c-MYC, which generally promotes cell proliferation together with the anti-apoptotic Bcl-2 in a CHO cell line resulted in enhanced proliferation and decreased apoptosis rates leading to higher maximum cell numbers (Ifandi and Al-Rubeai 2005). Other examples of co-transfection strategies to improve bioprocessing of mammalian cells include the transfection of NS0 cells with bcl-2 and p21 (Astley and Al-Rubeai 2008).
The application of siRNA technology has contributed to the development of apoptotic resistant cell lines by the manipulation of pro-apoptotic gene. Lim et al. (2006) knocked down Bak and Bax to 10% of their normal level using siRNA technology to result in a 30–50% higher viability of CHO batch and fed-batch cultures and 35% increase in interferon product titer. This study showed also that CHO containing the genetic inactivated targets clearly resisted the deleterious effect of cytotoxic lectins, UV irradiation, nutrient depletion and hyperosmotic condition. The same pro-apoptotic genes were targeted by Cost et al. (2009) who used the zinc-finger nuclease technology to cleave Bax and Bak in CHO cell line. The double knock-out cells were more resistant to apoptosis induction by starvation, staurosporin and sodium butyrate and produced two to fivefold more IgG than the wild-type when grown in small scale culture systems. Further, Wong et al. (2006) used siRNA technology to knock down the pro-apoptotic genes alg-2 and requiem which has been profiled as up-regulated in fed-batch culture by microarrays. The engineered cells revealed higher maximum cell numbers and 2.5-fold higher recombinant interferon gamma yield with a greater sialylation pattern.
A completely new dimension of the regulation of cellular apoptotis was given by the recent discovery of miRNA. To highlight its cell culture applications, Gammel (2007) clearly presented the complexity of the regulation of both apoptosis and proliferation which is more multifarious than previously assumed, as well as miRNAs importance for improving industrially relevant cell lines including CHO.
Autophagy engineering
It was reported that autophagic cell death can occur parallel to apoptosis prevention by caspase inhibitors or by blocking the apoptosis machinery (Shimuzu et al. 2004). The reported potential deleterious effect of autophagy has led to the efforts to manipulate its pathway. Hwang and Lee (2009) as well as Kim et al. (2009) published the successful approach of delaying the onset of both autophagic and apoptotic cell death during batch culture in CHO cells by the over-expression of active protein kinase B (Akt) and Bcl-xl, respectively. The outcome was enhanced cell viability for an extended period of culture time with no change in specific productivity. An overview of proposed bottleneck targets for autophagic related engineering in mammalian cells is given by Mohan et al. (2009). To bear in mind, in most cases any successful attempt of cell survival engineering should not only suppress cell death but in the same should not negatively influencing specific production rate.
Conclusion
The underlying molecular regulation of cell death and interactions of particular pro-and anti-death proteins have been well studied over the last decade. Besides, we have clear understanding of both type I programmed cell death (apoptosis) and type II programmed cell death (autophagy) which has allowed us to use genetic and environmental approaches to control their pathways. Currently, the most efficient approach to control cell death is by nutrient feeding and metabolic manipulation. This approach based on fed batch culture process has led to much increased productivity through intensive process development. The integration of cell engineering of programmed cell death with metabolic manipulation and feeding processes will likely provide strong impetus to further development to increase bioreactor productivity and enhance cell robustness if specific productivity is kept unaffected by the metabolic burden that might be created by the over-expression of the survival gene. The ultimate result is successful and profitable biopharmaceutical products that are accessible and affordable to patients.
Abbreviations
- A1
Apolipoprotein
- AIF
Apoptosis-inducing factors
- ANT
Adenine nucleotide translocator
- Akt
Protein kinase B
- Atgs
Autophagy-related proteins
- Aven
Apoptosis, caspase activation inhibitor
- Apaf1
Apoptotic protease activation factor 1
- Ap1
Jun oncogene
- Bad
Bcl-2-associated agonist of cell death
- Bak
Bcl-2-antagonist/killer 1
- Bax
Bcl-2-associated X protein
- Bcl-2
B-cell lymphoma
- Bcl-xL
Bcl-2 related gene, long isoform
- Bid
BH3 interacting domain death agonist
- Bim
Bcl-2-like 11
- Bok
Bcl-2-related ovarian killer
- c-MYC
v-myc myelocytomatosis viral oncogene homolog
- Creb
AMP responsive element binding protein 1
- ER
Endoplasmic reticulum
- E2F4
Eukaryotic transcription factor 4
- E2F1
Eukaryotic transcription factor 1
- FADD
Fas-associated death domain
- FasL
Fas ligand
- Mcl-1
Myeloid cell leukemia sequence 1 (Bcl2-related)
- mTOR
Target of rapamycin
- MOMP
Mitochondrial outer membrane permeabilization
- Noxa
PMA-induced protein
- NUR77
Nuclear receptor subfamily 4
- PI3K
Phosphoinositide 3-kinase
- PKB
Protein kinase B
- PT
Permeability transition pores
- PUMA
p53-upregulated modulator of apoptosis
- p27
Cyclin dependent kinase inhibitor protein 1B
- SMAC/DIABLO
Second mitochondria-derived activator of caspases/direct IAP-bind protein with low pI
- TNF
Tumour necrosis factor
- TRAIL
TNF-related apoptosis-inducing ligand
- VDAC
Voltage dependent anion channel
- UPR
Unfolded protein response
References
- Al-Rubeai M, Singh RP. Apoptosis in cell culture. Curr Opin Biotechnol. 1998;9:152–156. doi: 10.1016/s0958-1669(98)80108-0. [DOI] [PubMed] [Google Scholar]
- Al-Rubeai M, Mills D, Emery AN. Electron microscopy of hybridoma cells with special regard to monoclonal antibody production. Cytotechnology. 1990;4:13–28. doi: 10.1007/BF00148807. [DOI] [PubMed] [Google Scholar]
- Al-Rubeai M, Emery AN, Chalder S, Jan DC. Specific monoclonal antibody productivity and the cell cycle-comparisons of batch, continuous and perfusion cultures. Cytotech. 1992;9:85–97. doi: 10.1007/BF02521735. [DOI] [PubMed] [Google Scholar]
- Al-Rubeai M, Singh RP, Goldman MH, Emery AN. The death mechanism of animal cells in conditions of intensive agitation. Biotechnol Bioeng. 1995;45:463–472. doi: 10.1002/bit.260450602. [DOI] [PubMed] [Google Scholar]
- Al-Rubeai, Singh R, Emery AN (1995b) The transfection of mammalian cells with the anti-apoptotic bcl-2 gene can enhance survivability and reduce the need for essential amino acids and nutrients. AIChE meeting, Miami, Nov, 12–17
- Arden N, Majors BS, Ahn SH, Oyler G, Betenbaugh M. Inhibiting the apoptosis pathway using MDM2 in mammalian cell cultures. Biotechnol Bioeng. 2006;97:601–614. doi: 10.1002/bit.21254. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Astley K, Al-Rubeai M. The role of Bcl-2 and its combined effect with p21CIP1 in adaptation of CHO cells to suspension and protein-free culture. Appl Microbiol Biotechnol. 2008;78:391–399. doi: 10.1007/s00253-007-1320-2. [DOI] [PubMed] [Google Scholar]
- Bierau H, Perani A, Al-Rubeai M, Emery AN. A comparison of intensive cell culture bioreactors operating with hybridomas modified for inhibited apoptotic response. J Biotech. 1998;62:195–207. doi: 10.1016/s0168-1656(98)00064-9. [DOI] [PubMed] [Google Scholar]
- Cheng EHYA, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. Bcl-2, Bcl-xL sequester BH3 domain-only molecules preventing Bax- and Bak-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Chiang GG, Sisk WP. Bcl-xL mediates increased production of humanized monoclonal antibodies in Chinese haster ovary cells. Biotechnol Bioeng. 2005;91:779–792. doi: 10.1002/bit.20551. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Green DR. How do Bcl-2 proteins induce mitochondrial outer membrane permeabilization? Trend Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisti Y. Animal-cell damage in sparged bioreactors. Trends Biotechnol. 2000;18:420–432. doi: 10.1016/s0167-7799(00)01474-8. [DOI] [PubMed] [Google Scholar]
- Cohen JJ, Al-Rubeai M. Apoptosis-targeted therapies: the ‘next big thing’ in biotechnology? Trends Biotechnol. 1995;13:281–283. doi: 10.1016/s0167-7799(00)88964-7. [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM. The Bcl-2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Cost GJ, Freyvert Y, Vafiadis A, Santiago Y, Miller JC, Rebar E, Collingwood TN, Snowden A, Gregory PD. Bak and Bax deletion using zinc-finger nucleases yields apoptosis-resistant CHO cells. Biotechnol Bioeng. 2009;105:330–340. doi: 10.1002/bit.22541. [DOI] [PubMed] [Google Scholar]
- Cotter TG, Al-Rubeai M. Cell death (apoptosis) in cell culture systems. Trends Biotechnol. 1995;13:150–155. doi: 10.1016/S0167-7799(00)88926-X. [DOI] [PubMed] [Google Scholar]
- Crea F, Sarti D, Falciani F, Al-Rubeai M. Over-expression of hTERT in CHO K1 results in decreased apoptosis and reduced serum dependency. J Biotech. 2006;121:109–123. doi: 10.1016/j.jbiotec.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Cummings MC, Winterford CM, Walker NI. Apoptosis. Am J Surg Pathol. 1997;21:88–101. doi: 10.1097/00000478-199701000-00010. [DOI] [PubMed] [Google Scholar]
- Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, Gygi SP, Korsmeyer SJ. Bad and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- Degterev A, Boyce M, Yuan JY. A decade of caspases. Oncogene. 2003;22:8543–8567. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- Deng XM, Gao FQ, May WS. Bcl-2 retards G1/S cell cycle transition by regulating intracellular ROS. Blood. 2003;102:3179–3185. doi: 10.1182/blood-2003-04-1027. [DOI] [PubMed] [Google Scholar]
- Ding WX, Yin XM. Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy. 2008;16:141–150. doi: 10.4161/auto.5190. [DOI] [PubMed] [Google Scholar]
- Enis DR, Dunmore B, Johnson N, Pober JS, Print CG. Anti-apoptotic activities of Bcl-2 correlate with vascular maturation and transcriptional modulation of human endothelial cells. Endothelium J Endothelial Cell Res. 2008;15:59–71. doi: 10.1080/10623320802092393. [DOI] [PubMed] [Google Scholar]
- Fassnacht D, Rossing S, Franek F, Al-Rubeai M, Portner R. Effect of Bcl-2 expression on hybridoma cell growth in serum-supplemented, protein free and diluted media. Cytotech. 1998;26:219–225. doi: 10.1023/A:1007914619219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa B, Jr, Chen S, Oyler G, Hardwick JM, Betenbaugh MJ. Aven and Bcl-xL enhance protection against apoptosis for mammalian cells exposed to various culture conditions. Biotechnol Bioeng. 2004;6:589–600. doi: 10.1002/bit.10913. [DOI] [PubMed] [Google Scholar]
- Figueroa B, Jr, Ailor E, Osborne D, Hardwick JM, Reff M, Betenbaugh MJ. Enhanced cell culture performance using inducible anti-apoptotic genes E1B–19K and Aven in the production of a monoclonal antibody with Chinese hamster ovary cells. Biotechnol Bioeng. 2007;97:877–892. doi: 10.1002/bit.21222. [DOI] [PubMed] [Google Scholar]
- Gammel P. MicroRNA: recently discovered key regulators of proliferation and apoptosis in animal cells. Cytotechnology. 2007;53:55–63. doi: 10.1007/s10616-007-9049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- Goswami J, Sinskey AJ, Stelle H, Stephanopoulos GN, Wang DIC. Apoptosis in batch cultures of Chinese hamster ovary cells. Biotechnol Bioeng. 1999;62:632–640. doi: 10.1002/(sici)1097-0290(19990320)62:6<632::aid-bit2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Greider C, Chattopadhyay A, Parkhurst C, Yang E. Bcl-xL and Bcl-2 delay Myc-induced cell cycle entry through elevation of p27 and inhibition of G1 cyclin-dependent kinases. Oncogene. 2002;21:7765–7775. doi: 10.1038/sj.onc.1205928. [DOI] [PubMed] [Google Scholar]
- Hanson CJ, Bootman MD, Distelhorst CW, Maraldi T, Roderick HL. The cellular concentration of Bcl-2 determines its pro- or anti-apoptotic effect. Cell Calcium. 2008;44:243–258. doi: 10.1016/j.ceca.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Heath-Engel HM, Chang NC, Shore GC. The endoplasmic reticulum in apoptosis and autophagy: role of the Bcl-2 protein family. Oncogene. 2008;27:6419–6433. doi: 10.1038/onc.2008.309. [DOI] [PubMed] [Google Scholar]
- Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Hwang SO, Lee GM. Nutrient deprivation induces autophagy as well as apoptosis in Chinese hamster ovary cell culture. Biotechnol Bioeng. 2008;99:678–685. doi: 10.1002/bit.21589. [DOI] [PubMed] [Google Scholar]
- Hwang SO, Lee GM. Effect of Akt overexpression on programmed cell death in antibody-producing Chinese hamster ovary cells. J Biotech. 2009;139:89–94. doi: 10.1016/j.jbiotec.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Ifandi V, Al-Rubeai M. Regulation of cell proliferation and apoptosis in CHO-K1 cells by the coexpression of c-Myc and Bcl-2. Biotech Prog. 2005;21:671–677. doi: 10.1021/bp049594q. [DOI] [PubMed] [Google Scholar]
- Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism and prevents cytosolic acidification during ischemia and reduces ischemia-reperfusion injury. Circulation. 2003;95:734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- Ishaque A, Al-Rubeai M. Role of Ca, Mg and K ions in determining apoptosis and extent of suppression afforded by bcl-2 during hybridoma cell culture. Apoptosis. 1999;5:335–355. doi: 10.1023/a:1009643204200. [DOI] [PubMed] [Google Scholar]
- Ishaque A, Al-Rubeai M. Role of vitamins in determining apoptosis and extent of suppression by bcl-2 during hybridoma cell culture. Apoptosis. 2002;7:231–239. doi: 10.1023/a:1015343616059. [DOI] [PubMed] [Google Scholar]
- Ishaque A, Thrift J, Murphy J, Konstantinov K. Over-expression of Hsp70 in BHK-21 cells engineered to produce recombinant Factor VIII promotes resistance to apoptosis and enhances secretion. Biotechnol Bioeng. 2007;81:496–504. doi: 10.1002/bit.21201. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Ueda H, Suzuki E. Overexpression of Bcl-2, apoptosis suppressing gene: prolonged viable culture period of hybridoma and enhanced antibody production. Biotechnol Bioeng. 1995;48:118–122. doi: 10.1002/bit.260480205. [DOI] [PubMed] [Google Scholar]
- Janumyam YM, Sansam CG, Chattopadhyay A, Cheng NL, Soucie EL, Penn LZ, Andrews D, Knudson CM, Yang E. Bcl-xL/Bcl-2 co-ordinately regulates apoptosis, cell cycle arrest and cell cycle entry. EMBO J. 2003;22:5459–5470. doi: 10.1093/emboj/cdg533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayapal KP, Wlaschin KF, Hu WS, Yap M (2006) Recombinant protein therapeutics from CHO cells—20 years and counting. CHO Consortium. SBE Special Section. Ref Type: report
- Jin ZY, El Deiry WS. Overview of cell death signalling pathways. Cancer Biol Ther. 2005;4:139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- Juanola S, Vives J, Milian E, Prats E, Cairo JJ, Godia F. Expression of Bhrf1 improves survival of murine hybridoma cultures in batch and continuous. Appl Microbiol Biotechnol. 2009;83:43–57. doi: 10.1007/s00253-008-1820-8. [DOI] [PubMed] [Google Scholar]
- Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissure kinetics. Br J Cancer. 1972;26:239. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NS, Lee GM. Response of recombinant Chinese hamster ovary cells to hyperosmotic pressure:effect of Bcl-2 overexpression. J Biotech. 2002;95:237–248. doi: 10.1016/s0168-1656(02)00011-1. [DOI] [PubMed] [Google Scholar]
- Kim YG, Lee GM. Bcl-xL overexpression does not enhance specific erythropoietin productivity of recombinant CHO cells grown at 33C and 37C. Biotech Prog. 2009;25:252–256. doi: 10.1002/btpr.91. [DOI] [PubMed] [Google Scholar]
- Kim YG, Kim JY, Mohan C, Lee GM. Effect of Bcl-xL over-expression on apoptosis and autophagy in recombinant chinese hamster ovary cells under nutrient deprived condition. Biotechnol Bioeng. 2009;4:757–766. doi: 10.1002/bit.22298. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- Krajewski S, Tanaka S, Takayama S, Schibler MJ, Fenton W, Reed JC. Investigation of the subcellular-distribution of the Bcl-2 oncoprotein—residence in the nuclear-envelope, endoplasmic-reticulum, and outer mitochondrial-membranes. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- Krampe B, Al-Rubeai M (2009) Cellular and molecular analysis of NS0 cell line in batch, chemostat and perfusion cultures. PhD Thesis, University College Dublin, Ireland
- Ku B, Woo JS, Lian C, Lee KH, Hong HS, Xiaoefei E, Kim KS, Jung JU, Oh BH. Structural and biochemical bases for the inhibition of autophagy and apoptosis by viral bcl-2 of murine y-herpesvirus 68. PLoS Pathog. 2008;4:e25. doi: 10.1371/journal.ppat.0040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuystermans D, Krampe B, Swiderek H, Al-Rubeai M. Using cell engineering and omic tools for the improvement of cell culture processes. Cytotech. 2007;53:3–22. doi: 10.1007/s10616-007-9055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M, Dubyak G, Chen L, Nunez G, Miesfeld RL, Distelhorst CW. Evidence that Bcl-2 represses apoptosis by regulating endoplasmic Reticulum-Associated Ca2+ Fluxes. Proc Natl Acad Sci USA. 1994;91:6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasunskaia EB, Fridlianskaia II, Darieva ZA, Da Silva MS, Kanashiro MM, Margulis BA. Transfection of NS0 myeloma fusion partner cells with HSP70 gene results in higher hybridoma yield by improving cellular resisstance to apoptosis. Biotech Bioeng. 2003;8:496–504. doi: 10.1002/bit.10493. [DOI] [PubMed] [Google Scholar]
- Lee YY, Wong KTK, Tan J, Toh PC, Mao Y, Brusic V, Yap MGS. Overexpression of heat shock proteins (HSPs) in CHO cells for extended culture viability and improved recombinant protein production. J Biotech. 2009;143:34–43. doi: 10.1016/j.jbiotec.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentrations: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Levine B, Sinha S, Kroemer G. Blc-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SF, Chuan KH, Liu S, Loh SOH, Chung BYF, Ong CC, Song ZW. RNAi suppression of Bax and Bak enhances viability in fed-batch cultures of CHO cells. Metab Eng. 2006;8:509–522. doi: 10.1016/j.ymben.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Lin BZ, Kolluri SK, Lin F, Liu W, Han YH, Cao XH, Dawson MI, Reed JC, Zhang XK. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- Majid FAA, Butler M, Al-Rubeai M. Glycosylation of an immunoglobulin produced from a murine hybridoma cell line: the effect of culture mode and the anti-apoptotic gene, bcl-2. Biotechnol Bioeng. 2007;97:156–169. doi: 10.1002/bit.21207. [DOI] [PubMed] [Google Scholar]
- Major BS, Betenbaugh MJ. Mcl-1 overexpression leads to higher viabilities and increased production of humanized monoclonal antibody in Chinese hamster ovary cells. Biotech Prog. 2009;25:1161–1168. doi: 10.1002/btpr.192. [DOI] [PubMed] [Google Scholar]
- Massaad CA, Portier BP, Taglialatela G. Inhibition of transcription factor activity by nuclear compartment-associated Bcl-2. J Biol Chem. 2004;279:54470–54478. doi: 10.1074/jbc.M407659200. [DOI] [PubMed] [Google Scholar]
- Mastrangelo AJ, Hardwick JM, Zou S, Betenbaugh MJ. Part II. Overexpression of bcl-2 family members enhances survival of mammalian cells in response to various cultures insults. Biotechnol Bioeng. 2000;67:555–564. doi: 10.1002/(sici)1097-0290(20000305)67:5<555::aid-bit6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Mazel S, Burtrum D, Petrie HT. Regulation of cell division cycle progression by bcl-2 expression: a potential mechanism for inhibition of programmed cell death. J Exp Med. 1996;183:2219–2226. doi: 10.1084/jem.183.5.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meents H, Enenkel B, Eppenberger HM, Werner RG, Fussenegger M. Impact of coexpression and coamplification of sICAM and antiapoptosis determinants Bcl-2/Bcl-x(L) on productivity, cell survival, and mitochondria number in CHO-DG44 grown in suspension and serum-free media. Biotechnol Bioeng. 2002;80:706–716. doi: 10.1002/bit.10449. [DOI] [PubMed] [Google Scholar]
- Mercille S, Massie B. Induction of apoptosis in nutrient-deprived cultures of hybridoma and myeloma cells. Biotechnol Bioeng. 1994;44:1140–1154. doi: 10.1002/bit.260440916. [DOI] [PubMed] [Google Scholar]
- Mercille S, Massie B. Apoptosis-resistant E1B-19K-expressing NS/0 myeloma cells exhibit increased viability and chimeric antibody productivity under perfusion culture conditions. Biotech Bioeng. 1999;63(5):529–593. doi: 10.1002/(sici)1097-0290(19990605)63:5<529::aid-bit3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Mercille S, Johnson M, Lanthier S, Kamen AA, Massie B. Understanding factors that limit the productivity of suspension-based perfusion cultures operated at high medium renewal rates. Biotechnol Bioeng. 2000;67:435–450. doi: 10.1002/(sici)1097-0290(20000220)67:4<435::aid-bit7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Mohan C, Kim Y-G, Lee GM (2009) Apoptosis and autophagy cell engineering. Book Series Cell Line Development, Cell Engineering, vol 6. pp 195–216
- Murphy E, Imahashi K, Steenbergen C. Bcl-2 regulation of mitochondrial energetics. Trends Cardiovasc Med. 2005;15:283–290. doi: 10.1016/j.tcm.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Murray K, Ang CE, Gull K, Hickman JA, Dickson AJ. NSO myeloma cell death: influence of bcl-2 overexpression. Biotechnol Bioeng. 1996;51:298–304. doi: 10.1002/(SICI)1097-0290(19960805)51:3<298::AID-BIT5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Ogata M, Hino SI, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang X, Misushima N, Packer M, Schneider M, Levine B. Apoptosis and autophagy after mitochondrial or endoplasmic reticulum photodamage. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Perani A, Singh RP, Chauhan R, Al-Rubeai M. Variable functions of Bcl-2 in mediating bioreactor stress-induced apoptosis in hybridoma cells. Cytotech. 1998;28:177–188. doi: 10.1023/A:1008002319400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F, Klionsky DJ. Autophagy in the eukariotic cell. Eukaryotic Cell. 2002;2:11–21. doi: 10.1128/EC.01.1.11-21.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remillard CV, Yuan JXJ. Activation of K+ channels: an essential pathway in programmed cell death. Am J Physiol Lung Cell Mol Physiol. 2004;286:L49–L67. doi: 10.1152/ajplung.00041.2003. [DOI] [PubMed] [Google Scholar]
- Ryu JS, Lee GM. Application of hypoosmolar medium to fed-batch culture of hybridoma cells for improvement of culture longevity. Biotechnol Bioeng. 1999;62:120–123. doi: 10.1002/(sici)1097-0290(19990105)62:1<120::aid-bit14>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Sauerwald TM, Oyler GA, Betenbaugh MJ. Study of caspase inhibitors for limiting death in mammalian cell culture. Biotechnol Bioeng. 2003;81:329–340. doi: 10.1002/bit.10473. [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Ide T, Yanagida T, Tsujimoto Y. Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J Biol Chem. 2000;275:12321–12325. doi: 10.1074/jbc.275.16.12321. [DOI] [PubMed] [Google Scholar]
- Shimuzu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- Simpson N, Milner AE, Al-Rubeai M. Prevention of hybridoma cell death by bcl-2 during sub-optimal culture conditions. Biotechnol Bioeng. 1997;54:1–16. doi: 10.1002/(SICI)1097-0290(19970405)54:1<1::AID-BIT1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Simpson NH, Singh RP, Perani A, Goldenzon C, Al-Rubeai M. In hybridoma cultures, deprivation of any single amino acid leads to apoptotic death, which is suppressed by the expression of the bcl-2 gene. Biotechnol Bioeng. 1998;59:90–98. doi: 10.1002/(sici)1097-0290(19980705)59:1<90::aid-bit12>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Simpson NH, Singh RP, Emery AN, Al-Rubeai M. Bcl-2 over-expression reduces growth rate and prolongs G1 phase in continuous chemostat cultures of hybridoma cells. Biotechnol Bioeng. 1999;64:174–186. doi: 10.1002/(sici)1097-0290(19990720)64:2<174::aid-bit6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Singh RP, Al-Rubeai M. Apoptosis and bioprocess technology. Adv Biochem Eng Biotechnol. 1998;62:167–184. doi: 10.1007/BFb0102310. [DOI] [PubMed] [Google Scholar]
- Singh RP, Alrubeai M, Gregory CD, Emery AN. Cell-death in bioreactors—a role for apoptosis. Biotechnol Bioeng. 1994;44:720–726. doi: 10.1002/bit.260440608. [DOI] [PubMed] [Google Scholar]
- Singh RP, Emery AN, Al-Rubeai M. Enhancement of survivability of mammalian cells by over-expression of the apoptosis-suppressor gene bcl-2. Biotech Bioeng. 1996;52:166–175. doi: 10.1002/(SICI)1097-0290(19961005)52:1<166::AID-BIT17>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Sung YH, Hwang SJ, Lee GM. Influence of down-regulation of caspase-3 by siRNAs on sodium butyrate-induced apoptotic cell death of Chinese hamster ovary cells producing thrombopoietin. Metab Eng. 2005;5–6:457–466. doi: 10.1016/j.ymben.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Terada S, Fukuoka K, Fujita T, Komatsu T, Takayaa S, Reed JC, Suzuki E. Anti-apoptotic genes, bag-1 and bcl-2, enabled hybridoma cells to survive under treatment for arresting cell cycle. Cytotech. 1997;25:17–23. doi: 10.1023/A:1007954103572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tey BT, Al-Rubeai M. Effect of Bcl-2 over-expression on cell cycle and antibody productivity in chemostat cultures of myeloma NS0 cells. J Biosci Bioeng. 2005;100:303–310. doi: 10.1263/jbb.100.303. [DOI] [PubMed] [Google Scholar]
- Tey BT, Al-Rubeai M. Bcl-2 over-expression reduced the serum dependency and improved the nutrient metabolism in NS0 cell culture. Biotechnol Bioprocess Eng. 2005;10:254–261. [Google Scholar]
- Tey BT, Singh RP, Piredda L, Piacentini M, Al-Rubeai M. Bcl-2 mediated suppression of apoptosis in myeloma NS0 cultures. J Biotechnol. 2000;79:147–159. doi: 10.1016/s0168-1656(00)00223-6. [DOI] [PubMed] [Google Scholar]
- Tey BT, Singh RP, Piredda L, Piacentini M, Al-Rubeai M. Influence of bcl-2 on cell death during the cultivation of a Chinese hamster ovary cell line expressing a chimeric antibody. Biotechnol Bioeng. 2000;68:31–43. doi: 10.1002/(sici)1097-0290(20000405)68:1<31::aid-bit4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;25:169–174. doi: 10.1016/0014-5793(93)80398-e. [DOI] [PubMed] [Google Scholar]
- Vairo G, Soos TJ, Upton TM, Zalvide J, DeCaprio JA, Ewen ME, Koff A, Adams JM. Bcl-2 retards cell cycle entry through p27(Kip1), pRB relative p130, and altered E2F regulation. Mol Cell Biol. 2000;20:4745–4753. doi: 10.1128/mcb.20.13.4745-4753.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Schumacker PT, Thompson CB. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol Cell. 1999;3:159–167. doi: 10.1016/s1097-2765(00)80307-x. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Li XX, Schumacker PT, Colombini M, Thompson CB. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc Natl Acad Sci USA. 2000;97:4666–4671. doi: 10.1073/pnas.090082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Walsh G. Biopharmaceutical benchmarks 2006. Nat Biotechnol. 2006;24:769–776. doi: 10.1038/nbt0706-769. [DOI] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DCS. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Wong DCF, Wong KTK, Nissom PM, Heng CK, Yap MGS. Targeting early apoptotic genes in batch and fed-batch CHO cell cultures. Biotechnol Bioeng. 2006;95:350–361. doi: 10.1002/bit.20871. [DOI] [PubMed] [Google Scholar]
- Wu J, Kaufman RJ. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The Bcl-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Yun CY, Liu S, Lim SF, Wang T, Chung BY, Jiat Teo J, Chuan KH, Soon AS, Goh KS, Song Z (2007) Specific inhibition of caspase-8 and -9 in CHO cells enhances cell viability in batch and fed-batch cultures. Metab Eng 9:406–418 [DOI] [PubMed]
- Zhu Y, Cuenca JV, Zhou WC, Varma A. NS0 cell damage by high gas velocity sparging in protein-free and cholesterol-free cultures. Biotechnol Bioeng. 2008;101:751–760. doi: 10.1002/bit.21950. [DOI] [PubMed] [Google Scholar]
- Zinkel S, Gross A, Yang E. Bcl-2 family in DNA damage and cell cycle control. Cell Death Differ. 2006;13:1351–1359. doi: 10.1038/sj.cdd.4401987. [DOI] [PubMed] [Google Scholar]
- Zustiak M, Pollack JK, Marten MR, Betenbaugh MJ. Feast of famine: autophagy control and engineering in eukaryotic cell culture. Curr Optin Biotech. 2008;5:518–526. doi: 10.1016/j.copbio.2008.07.007. [DOI] [PubMed] [Google Scholar]