Abstract
AIM: To investigate the ability of curcumin to counteract the impact of bile acids on gene expression of esophageal epithelial cells.
METHODS: An esophageal epithelial cell line (HET-1A) was treated with curcumin in the presence of deoxycholic acid. Cell proliferation and viability assays were used to establish an appropriate dose range for curcumin. The combined and individual effects of curcumin and bile acid on cyclooxygenase-2 (COX-2) and superoxide dismutase (SOD-1 and SOD-2) gene expression were also assessed.
RESULTS: Curcumin in a dose range of 10-100 μmol/L displayed minimal inhibition of HET-1A cell viability. Deoxycholic acid at a concentration of 200 μmol/L caused a 2.4-fold increase in COX-2 gene expression compared to vehicle control. The increased expression of COX-2 induced by deoxycholic acid was partially reversed by the addition of curcumin, and curcumin reduced COX-2 expression 3.3- to 1.3-fold. HET-1A cells exposed to bile acid yielded reduced expression of SOD-1 and SOD-2 genes with the exception that high dose deoxycholic acid at 200 μmol/L led to a 3-fold increase in SOD-2 expression. The addition of curcumin treatment partially reversed the bile acid-induced reduction in SOD-1 expression at all concentrations of curcumin tested.
CONCLUSION: Curcumin reverses bile acid suppression of gene expression of SOD-1. Curcumin is also able to inhibit bile acid induction of COX-2 gene expression.
Keywords: Esophageal cancer, Curcumin, Cyclooxygenase-2, Superoxide dismutase, Chemoprevention
INTRODUCTION
Over the past three decades the incidence of esophageal adenocarcinoma has increased over four-fold, and the current five year survival remains only 10%-20%[1]. Esophageal adenocarcinoma is known to develop in the distal esophagus in the setting of exposure to both bile acids and low pH[2-4]. The exact cellular mechanisms underlying this process are largely unknown. Oxidative stress has been theorized to play a significant role in the staged progression from reflux esophagitis to Barrett’s esophagus and eventually to esophageal adenocarcinoma. Increased levels of reactive oxygen species (ROS) and oxidative injury have been detected in esophageal cells exposed to low pH and bile acids[5-7], and the level of free radicals in esophageal tissue has also been associated with the degree of esophagitis[8]. In addition, we have previously shown an association between the development of esophageal adenocarcinoma and increased levels of 8-hydroxy-deoxyguanosine, an indicator of oxidative damage, using an esophageal duodenal anastomosis rat model of reflux esophagitis[9].
Based on these findings, compounds with antioxidant properties may hold promise as chemopreventative agents in the Barrett’s metaplasia-dyplasia-adenocarcinoma sequence. Curcumin is a phenolic compound derived from the plant Curcuma longa. Curcumin is known to have both anti-inflammatory and antioxidant properties. A number of animal and in vitro models have also shown curcumin to be a potent chemopreventative agent[10,11]. Previous work by Li et al[12] demonstrated in a rat model of esophageal reflux that intraperitoneal injections of curcuma aromatic oil, a volatile oil extract of Curcuma aromatica, helped prevent the development of esophageal adenocarcinoma. However, the molecular mechanisms by which curcumin may inhibit the development of esophageal adenocarcinoma have not been fully defined.
In the current study, HET-1A cells were used as an in vitro model to study the potential impact of curcumin on the initial changes induced by bile acids in the esophagus. HET-1A cells are a well characterized, non-cancerous, SV-40 T-antigen immortalized human esophageal epithelial cell line[13]. HET-1A cells have been shown to produce ROS after brief exposures to low pH and to bile acids[6,14]. HET-1A cells develop gene expression changes consistent with the development of Barrett’s esophagus upon exposure to deoxycholic acid[15], and carcinogens have been found to induce tumorigenic characteristics within these cells[16,17]. Furthermore, previous work by Rafiee et al[18] has shown that curcumin has an anti-inflammatory effect on HET-1A cells by inhibition of acidic pH-induced secretion of cytokines interleukin (IL)-8 and IL-9.
We tested the hypothesis that curcumin prevents the bile acid-mediated impairment of HET-1A cellular mechanisms for managing oxidative stress. Specifically, we investigated the impact of bile acid and curcumin on expression of the human gene forms of superoxide dismutase (SOD-1 and SOD-2). The ideal curcumin dose in combination with bile acid in vitro was determined through cell proliferation and viability testing. Curcumin- and bile acid-induced alterations of cyclooxygenase-2 (COX-2) gene expression were also studied as an additional mechanism of esophageal adenocarcinoma prevention. The aim of this study was to determine whether curcumin has effects on esophageal cell gene expression consistent with a chemopreventative in the setting of bile acid exposure.
MATERIALS AND METHODS
Chemicals
Curcumin was obtained from LKT Laboratories, Inc. (St. Paul, MN). 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium (MTT) and cDNA Trizol reagent were obtained from Sigma Chemical Co. (St. Louis, MO). 5-bromo-2’-deoxyuridine (BrdU) ELISA assay kits were purchased from Roche Applied Science (Indianapolis, IN). All other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise stated.
Cell culture
HET-1A cells, a SV-40 immortalized human esophageal epithelial cell line, were acquired from the American Type Culture Collection and grown in bronchial epithelial growth media (BEGM) and recommended supplements (BEGM Bullet Kit) obtained from Lonza Bio Science (Walkersville, MD). The growth media was also supplemented with 10% fetal bovine serum. Cells were grown in a monolayer and incubated at 37°C in 5% CO2 and 90% relative humidity.
For cellular proliferation and viability studies, HET-1A cells were plated in 96-well microtiter plates at a density of 1 × 104 cells per well and allowed to attach and grow for 48 h prior to treatment. The cells were treated with varying doses (100 nmol/L-1 mmol/L) of curcumin dissolved in ethanol and added to fresh media for a total ethanol concentration that did not exceed 2.5%. Treatment with deoxycholic acid (in ethanol) at a concentration of 100 μmol/L combined with curcumin was also performed. A vehicle control of media and 2.5% ethanol was used to study the effect of ethanol, and all treated groups were compared to this control. Treatments were conducted for 24 h.
For mRNA expression studies, HET-1A cells were plated in 6-well plates at a density of 1.5 × 105 cells per well and grown for 48 h. Curcumin and deoxycholic acid dissolved in ethanol were added with fresh media at varying concentrations. The final ethanol concentration was maintained at 0.2%, and 0.2% ethanol solution was used as a vehicle control. All studies were performed in triplicate to confirm reproducibility.
Cell viability and cell proliferation assays
MTT assays were conducted as described previously[19]. Briefly, treated media was aspirated without disrupting the cells at the bottom of the plate. MTT at a concentration of 5 mg/mL was dissolved in phosphate buffered saline (PBS) and added to each well. After incubation at 37°C for 2 h, the MTT solution was aspirated and the wells were air-dried. DMSO was added to the wells and the absorbance was read at 570 nm in a spectrophotometer.
Cell proliferation assay based on incorporation of BrdU was used to investigate the effect of the various treatments. After the 24 h treatments, the BrdU ELISA was performed according to the manufacturer’s directions.
RNA extraction and real-time polymerase chain reaction
RNA was isolated using the Trizol® method (Invitrogen, Carlsbad, CA). All procedures were carried out in an RNase free environment. The quality of the RNA was ascertained by gel electrophoresis and quantitated using NanoDrop® (NanoDrop Technologies, Wilmington, DE). The RNA was then diluted to 5 ng/μL concentration and stored at -80°C until use. Equal amounts of RNA (0.1 μg) were reverse transcribed using a high-capacity cDNA archive kit (Applied Biosystems, Forster City, CA) according to the manufacturer’s instructions.
Primers for quantitative real-time polymerase chain reaction (RT-PCR) were designed across exon boundaries to avoid amplification of genomic DNA, using Primer express® 3.0 software (Applied Biosystems, Foster City, CA) and synthesized by Integrated DNA Technologies, Inc., (Coralville, IA). The sequences of the forward and reverse primers for each gene tested are listed in Table 1.
Table 1.
Primer sequences for quantitative real-time polymerase chain reaction
| Gene | Forward | Reverse |
| COX-2 | 5'-CAGGGTTGCTGGTGGTAGGA-3' | 5'-CGTTTGCGGTACTCATTAAAAGACT-3' |
| SOD-1 | 5'-GTGGCCGATGTGTCTATTGAAG-3' | 5'-CGTTTCCTGTCTTTGTACTTTCTT-3' |
| SOD-2 | 5'-TGGCCAAGGGAGATGTTACAG-3' | 5'-CTTCCAGCAACTCCCCTTTG-3' |
| β-actin | 5'-TTCAACTTCATCATGAAGTGTGACGTG-3' | 5'-CTAAGTCATAGTCCGCCTAGAAGCATT-3' |
COX: Cyclooxygenase; SOD: Superoxide dismutase.
The PCR amplification was carried out in a final reaction volume of 20 μL containing 1 × Power SYBR® Green PCR master mix (Applied Biosystems, CA); 10 nmol/L each of forward and reverse primers specific for each gene and 40 ng of cDNA. Quantitative PCR was performed using a 7300 Prizm (Applied Biosystems, Foster City, CA) using the relative quantification protocol. The PCR conditions were: 50°C for 2 min; DNA polymerase activation at 95°C for 10 min; followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The 2-ΔΔCt method was used to determine gene quantification with human β-actin used as an endogenous reference gene. Results were expressed as fold change in gene expression compared to the vehicle control.
Statistical analysis
Relative fold changes in each group were compared using one-way analysis of variance (ANOVA), followed by a Tukey’s multiple comparison post test. A P-value < 0.05 was considered significant. All statistical analyses were performed using SPSS Version 17 (SPSS Inc., Chicago, IL).
RESULTS
Dose response effects of curcumin on cell viability and proliferation
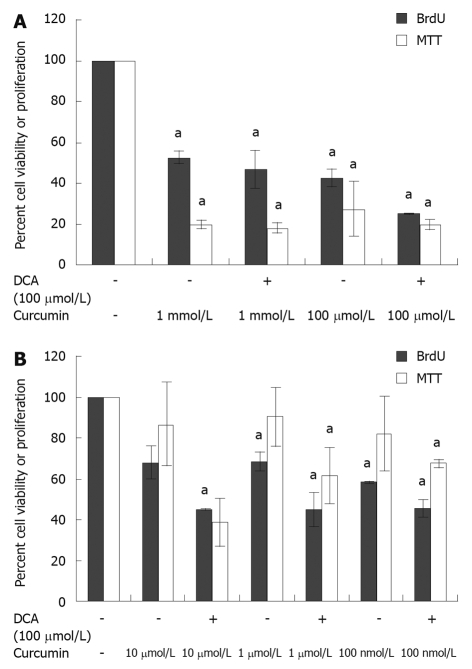
In order to establish a sub-toxic dose of curcumin, the influence of curcumin on HET-1A cell viability and proliferation was assessed using both MTT and BrdU assays, respectively. Similar trends were seen for both BrdU and MTT assays (Figure 1A and B). Compared to the vehicle control, all concentrations of curcumin tested showed significant reduction in cell viability as demonstrated by the MTT assay (33% to 58% reduction, P < 0.01). The cytotoxicity and reduction in proliferation were higher at concentrations greater than 100 μmol/L when compared to the vehicle control (MTT: 1 mmol/L - 48%, 100 μmol/L - 58%, P < 0.001; BrdU: 1 mmol/L - 80%, 100 μmol/L - 73%, P ≤ 0.01) (Figure 1A). However, less cytotoxicity was seen at curcumin concentrations less than 10 μmol/L (MTT: 30%-40%, P < 0.01), and curcumin did not adversely effect cell proliferation at these concentrations, as demonstrated by the BrdU assay (10%-14% reduction, P > 0.05) (Figure 1B). When cells were treated with deoxycholic acid (100 μmol/L) combined with varying curcumin concentrations, increased cytotoxicity was seen at each concentration of curcumin tested (Figure 1A and B). Again, the greatest cytotoxicity was demonstrated at doses of curcumin greater than 100 μmol/L (P < 0.001). All doses of curcumin tested caused synergistic cytotoxicity in the presence of deoxycholic acid (100 μmol/L).
Figure 1.
Effect of curcumin concentration on cell viability and proliferation at concentration ≥ 100 μmol/L (A) and ≤ 10 μmol/L (B). HET-1A cells incubated in 96-well plates at a density of 10 000 cells/well for 24 h demonstrated decreased cell viability and proliferation in the presence of curcumin at a concentration ≥ 100 μmol/L or ≤ 10 μmol/L. The viability and proliferation were also reduced in the presence of curcumin combined with deoxycholic acid (DCA) when compared to vehicle control (2.5% ethanol). aP < 0.05 vs vehicle control. MTT: 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium; BrdU: 5-bromo-2’-deoxyuridine; DCA: Deoxycholic acid.
Combined effects of deoxycholic acid and curcumin on COX-2 gene expression
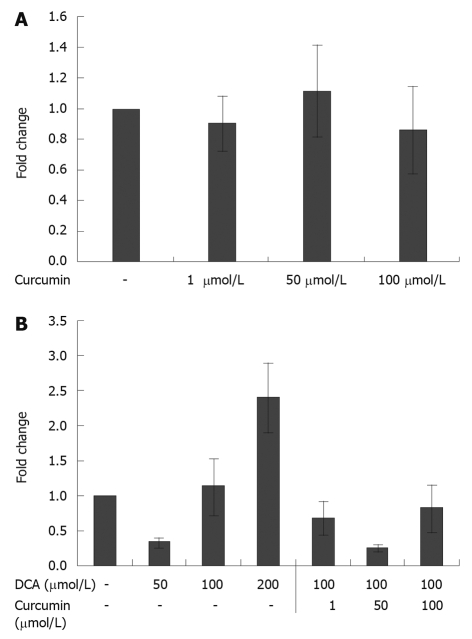
We investigated the effects of curcumin on COX-2 gene expression in HET-1A cells (Figure 2A and B). It was observed that 24 h curcumin treatment alone had minimal impact on COX-2 mRNA production. The degree of COX-2 mRNA production was unaltered even at doses of 100 μmol/L. Increased levels of COX-2 mRNA were observed in HET-1A cells treated with bile acid alone. Deoxycholic acid at 200 μmol/L caused a 2.4-fold increase in COX-2 mRNA levels compared to vehicle control. The increased expression of COX-2 genes induced by deoxycholic acid was partially reversed by the addition of curcumin. It was observed that treatment of HET-1A cells with deoxycholic acid and curcumin reduced COX-2 mRNA levels 3.3- to 1.3-fold. Curcumin inhibition of bile acid-induced COX-2 expression was observed at doses as low as 1 μmol/L curcumin. This effect was not dose-dependent, with 50 μmol/L curcumin showing the greatest effect.
Figure 2.
Effect of curcumin and deoxycholic acid on cyclooxygenase-2 gene expression. A: Effect of curcumin on cyclooxygenase-2 (COX-2) gene expression. HET-1A cells in 6-well plates at a density of 1.5 × 105 cells per well incubated with curcumin for 24 h showed unaltered gene expression of COX-2 when compared to vehicle control (0.2% ethanol). Error bars: mean ± SD (n = 4); B: Effect of both deoxycholic acid and curcumin on COX-2 gene expression. HET-1A cells in 6-well plates at a density of 1.5 × 105 cells per well incubated with deoxycholic acid (DCA) for 24 h showed increased gene expression of COX-2 with increasing doses of DCA when compared to vehicle control (0.2% ethanol). The addition of curcumin diminished the increase in gene expression. Error bars: mean ± SD (n = 4).
Combined effects of deoxycholic acid and curcumin on SOD-1 and SOD-2 gene expression
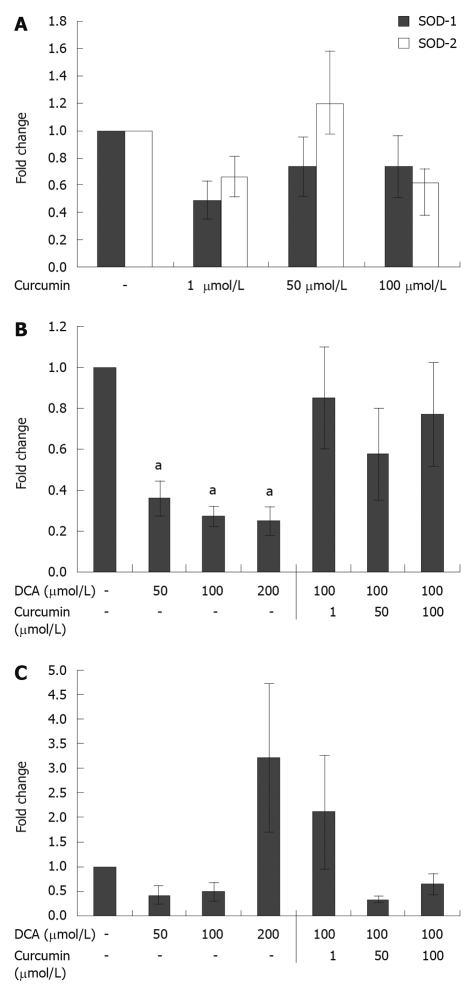
Measurements were also made of SOD gene expression following curcumin treatment of HET-1A cells for 24 h. Two human gene forms of superoxide dismutase were tested: Cu/ZnSOD (SOD-1) and MnSOD (SOD-2). Results revealed that curcumin treatment led to a non-significant 36% to 52% reduction in SOD-1 expression at all concentrations of curcumin tested. Curcumin alone also had a non-significant impact on SOD-2 expression (Figure 3A). Compared to controls, bile acid treatment of HET-1A cells yielded consistent reduction of SOD-1 and SOD-2 mRNA production, with the exception that high dose deoxycholic acid at 200 μmol/L led to a 3-fold increase in SOD-2 expression. Deoxycholic acid inhibited the expression of SOD-1 (2.8- to 4.0-fold) at concentrations ≥ 50 μmol/L (Figure 3B). The addition of curcumin treatment reversed this reduction in SOD-1 expression induced by bile acid at all concentrations of curcumin tested (Figure 3B). The combined treatment of deoxycholic acid and curcumin resulted in reduced expression of SOD-2 at concentrations ≥ 50 μmol/L (Figure 3C).
Figure 3.
Effect of curcumin and bile acid on superoxide dismutase gene expression. A: Effect of curcumin on superoxide dismutase (SOD)-1 and SOD-2 gene expression. HET-1A cells in 6-well plates at a density of 1.5 × 105 cells per well incubated with curcumin for 24 h did not show a significant impact on SOD-1 or SOD-2 gene expression. Error bars: mean ± SD (n = 4); B: Effect of curcumin and bile acid on SOD-1 gene expression. HET-1A cells in 6-well plates at a density of 1.5 × 105 cells per well incubated with deoxycholic acid (DCA) for 24 h showed a significant decrease in SOD-1 expression. When DCA treatment at 100 μmol/L was combined with curcumin the suppression in SOD-1 expression was alleviated. Error bars: mean ± SD (n = 4). aP < 0.05 vs vehicle control; C: Effect of curcumin and bile acid on SOD-2 gene expression. HET-1A cells in 6-well plates at a density of 1.5 × 105 cells per well incubated with deoxycholic acid (DCA) for 24 h showed a significant increase in SOD-2 expression at a DCA concentration of 200 μmol/L. The combined treatment of DCA and curcumin showed reduced expression of SOD-2 at concentrations ≥ 50 μmol/L. Error bars: mean ± SD (n = 4).
DISCUSSION
The purpose of this study was to investigate the potential of curcumin as a chemopreventative in the development of reflux-induced esophageal adenocarcinoma using an in vitro model of HET-1A human esophageal epithelial cells. This study demonstrates that curcumin can attenuate certain effects of bile acid on HET-1A cell gene expression. Specifically, this analysis reveals that curcumin is able to partially reverse bile acid suppression of gene expression of the free radical scavenger superoxide dismutase in the form of SOD-1; however, the impact toward SOD-2 was variable. In addition, curcumin was noted to inhibit bile acid induction of COX-2 gene expression in esophageal epithelial cells.
Several factors were taken into consideration to establish the in vitro model. Of the potentially available bile acids, the cells were exposed to deoxycholic acid because it has been commonly detected in aspirates of patients with Barrett’s esophagus[20], and deoxycholic acid has been shown to induce DNA damage in esophageal cells through oxidative injury[21]. Also important to the model was establishing an in vitro therapeutic dosage range for curcumin, as curcumin is known to induce apoptosis in both malignant and non-malignant cell types. Curcumin concentrations less than 10 μmol/L had the least impact on esophageal cell viability and proliferation in the presence of bile acid, and concentrations in the range of 50 μmol/L had the most dramatic impact on esophageal cell gene expression. This dosage range was valid because only curcumin concentrations greater than 100 μmol/L showed significant toxicity. The greatest toxicity was seen in curcumin doses higher than 100 μmol/L in combination with bile acid, which led to a 2- to 5-fold decrease in the proliferation and viability of HET 1-A cells.
The results revealed that curcumin did not inhibit the baseline expression of COX-2 genes by HET-1A cells, but curcumin did show suppression of bile acid-induced expression of COX-2 genes. Curcumin has been previously noted to inhibit both the activity and expression of COX-2 in colon cancer cells[22,23], and it has been shown that curcumin inhibits bile acid-induced COX-2 expression and activity in esophageal adenocarcinoma and squamous cell carcinoma cell lines[24]. Animal models have shown that COX-2 inhibition can potentially prevent the development of esophageal adenocarcinoma and Barrett’s esophagus[25,26]. Epidemiologic studies have also suggested that the use of COX-2 inhibitors leads to lower rates of esophageal adenocarcinoma[27]. The use of selective COX-2 inhibitors has recently fallen out of favor due to their cardiotoxic side effects[28], and non-selective cyclooxygenase inhibitors such as aspirin have potential gastrointestinal effects that make them unappealing therapeutic agents in patients already suffering from reflux. Therefore, curcumin may represent a safe alternative means of COX-2 inhibition in patients with GERD.
Curcumin and bile acid treatments of HET-1A cells revealed variable alterations of SOD gene expression. Superoxide dismutase is the primary scavenger of superoxide anion which has been implicated as the primary reactive oxygen species involved in reflux-induced oxidative damage[29-32]. Impairment of cellular antioxidant mechanisms that manage superoxide anions may contribute to the processes underlying esophageal mucosal injury by bile acids. Supplementation with SOD has been found to be protective against reflux-induced damage of the esophagus in rats[33]. Also, decreased activity of MnSOD has been measured in esophageal tissue of patients with esophagitis and Barrett’s esophagus[29,34]. Using the esophageal duodenal anastomosis-based rat model, we have previously demonstrated a decreased incidence of Barrett’s esophagus and esophageal adenocarcinoma accompanied by decreased oxidative injury in rats treated with Mn(III)tetrakis(4-benzoic acid) porphyrin (MnTBAP), an SOD mimetic[34].
Curcumin alone had minimal impact on either SOD-1 or SOD-2 expression. Bile acid induced SOD-2 gene expression, but inhibited SOD-1 gene expression. Curcumin ameliorated the effect of bile acid on SOD-1, causing a decrease in the degree of suppression. These findings are in contrast to previous work using a rat model of bile reflux demonstrating that esophagitis was associated with decreased SOD-2 enzyme production and activity and had no association with the activity and expression of SOD-1[35]. Other work by Jiménez et al[29] found increased SOD-1 and SOD-2 expression in esophageal biopsies of patients with Barrett’s and reflux esophagitis as compared to normal controls. However, the overall SOD activity was decreased in patients with esophagitis and Barrett’s esophagus compared to normal epithelium. In another investigation using human esophageal biopsy specimens, Sihvo et al[36] found that only specimens of Barrett’s associated with dysplasia showed increased SOD activity. These conflicting data illustrate that complex mechanisms related to both the timing and extent of bile acid exposure likely play a role in altering SOD expression and its impact on the development of esophageal adenocarcinoma. Curcumin as a preservative of SOD-1 expression in the early phase of bile acid exposure may provide a mechanism for the chemoprevention of esophageal adenocarcinoma.
A major difficulty of in vitro studies of reflux and Barrett’s esophagus is the inability to replicate the timing and nature of acid exposure in the distal esophagus. We were limited as to the concentration of bile acid that could be used due to the degree of cellular toxicity in the presence of curcumin. As a result, the concentrations of bile acid tested were lower than those that can occur in the distal esophagus of patients with gastroesophageal reflux, which have been measured as high as 1 to 2 mmol/L[3]. Therefore, the bile acid levels tested may have been too low to induce the degree of gene expression changes that are experienced in vivo. Adjustments were also not made for varying pH levels of the experiments, and our study looked at 24 h exposure times to bile acids and curcumin. Longer exposure times and different pH levels may yield different results with respect to gene expression.
Curcumin has previously been investigated as a chemopreventive agent in a small number of phase I and phase II trials and found to be safe and well tolerated[37-39]. However, a primary impediment to the use of curcumin has been its poor bioavailability as a result of extensive metabolization in the human gut[40]. This may be less of a drawback for curcumin use in the esophagus, as initial exposure to curcumin would occur prior to intestinal metabolism. Also, current work on developing lipolized or heat-solubilized forms of curcumin may help overcome this issue[38,41]. The current study provides a basis for possible use of curcumin for chemoprevention of esophageal adenocarcinoma through its effects on bile acid-induced alterations of COX-2 and SOD gene expression. Curcumin should be further investigated as a chemopreventative agent in the setting of reflux esophagitis and Barrett’s esophagus.
COMMENTS
Background
The incidence of esophageal adenocarcinoma has increased dramatically over the past few decades. Oxidative injury resulting from the reflux of bile acids is thought to play a role in the development of esophageal adenocarcinoma. Curcumin is a naturally occurring antioxidant derived from the herb turmeric, that might potentially counteract the effects of bile acids on esophageal cells.
Research frontiers
Curcumin has demonstrated anticancer properties in a variety of tumor types. Curcumin is also known to impact the gene expression of a variety of enzymes. This study analyzes the possible effects of curcumin on bile acid-induced alterations of gene expression by esophageal cells.
Innovations and breakthroughs
This study is one of the first to report that curcumin may have chemopreventative effects on esophageal cells in the presence of bile acids. The results of this research also suggest a dosage range of curcumin that is effective in altering esophageal cell gene expression. Understanding this therapeutic dosage range has important implications for future in vivo studies of curcumin, as it is known to have limited bioavailability.
Applications
By demonstrating that curcumin effects the production of cyclooxygenase-2 (COX-2) and antioxidant enzymes, this study suggests a possible use for curcumin as a chemopreventative against esophageal adenocarcinoma.
Terminology
HET-1A cells are an epithelial cell line derived from human esophagus. Superoxide dismutase (SOD) is an important enzyme responsible for managing the reactive oxygen species, superoxide. COX-2 is an enzyme involved in the formation of certain inflammatory mediators.
Peer review
In this manuscript, the authors determined a basis for possible use of curcumin for chemoprevention of esophageal adenocarcinoma through its effects on bile acid-induced alterations of COX-2 and SOD gene expression. This is a very interesting paper for the scientific community involved in Barrett’s esophagus and Barrett’s cancer research. Good design, good technical performance and critical discussion.
Footnotes
Supported by National Institutes of Health Grant, No. 1R03CA137801
Peer reviewers: Paul M Schneider, MD, Professor, Department of Surgery, University Hospital Zurich, Raemistrasse 100, Zurich, 8091, Switzerland; Tomohiko Shimatani, Assistant Professor, Department of General Medicine, Hiroshima University Hospital, 1-2-3 Kasumi, Minami-ku, Hiroshima 7348551, Japan; Robert J Korst, MD, Department of Cardiothoracic Surgery, Weill Medical College of Cornell University, Room M404, 525 East 68th Street, New York, NY 10032, United States
S- Editor Wang JL L- Editor Logan S E- Editor Ma WH
References
- 1.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Iftikhar SY, Ledingham S, Steele RJ, Evans DF, Lendrum K, Atkinson M, Hardcastle JD. Bile reflux in columnar-lined Barrett’s oesophagus. Ann R Coll Surg Engl. 1993;75:411–416. [PMC free article] [PubMed] [Google Scholar]
- 3.Kauer WK, Peters JH, DeMeester TR, Feussner H, Ireland AP, Stein HJ, Siewert RJ. Composition and concentration of bile acid reflux into the esophagus of patients with gastroesophageal reflux disease. Surgery. 1997;122:874–881. doi: 10.1016/s0039-6060(97)90327-5. [DOI] [PubMed] [Google Scholar]
- 4.Kauer WK, Peters JH, DeMeester TR, Ireland AP, Bremner CG, Hagen JA. Mixed reflux of gastric and duodenal juices is more harmful to the esophagus than gastric juice alone. The need for surgical therapy re-emphasized. Ann Surg. 1995;222:525–531; discussion 531-533. doi: 10.1097/00000658-199522240-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dvorak K, Payne CM, Chavarria M, Ramsey L, Dvorakova B, Bernstein H, Holubec H, Sampliner RE, Guy N, Condon A, et al. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett’s oesophagus. Gut. 2007;56:763–771. doi: 10.1136/gut.2006.103697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res. 2005;589:47–65. doi: 10.1016/j.mrrev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Sokol RJ, Winklhofer-Roob BM, Devereaux MW, McKim JM Jr. Generation of hydroperoxides in isolated rat hepatocytes and hepatic mitochondria exposed to hydrophobic bile acids. Gastroenterology. 1995;109:1249–1256. doi: 10.1016/0016-5085(95)90585-5. [DOI] [PubMed] [Google Scholar]
- 8.Wetscher GJ, Hinder RA, Bagchi D, Hinder PR, Bagchi M, Perdikis G, McGinn T. Reflux esophagitis in humans is mediated by oxygen-derived free radicals. Am J Surg. 1995;170:552–556; discussion 556-557. doi: 10.1016/s0002-9610(99)80014-2. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Wo JM, Su RR, Ray MB, Martin RC. Alterations in manganese superoxide dismutase expression in the progression from reflux esophagitis to esophageal adenocarcinoma. Ann Surg Oncol. 2007;14:2045–2055. doi: 10.1245/s10434-007-9387-7. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 11.Huang MT, Lou YR, Ma W, Newmark HL, Reuhl KR, Conney AH. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994;54:5841–5847. [PubMed] [Google Scholar]
- 12.Li Y, Wo JM, Liu Q, Li X, Martin RC. Chemoprotective effects of Curcuma aromatica on esophageal carcinogenesis. Ann Surg Oncol. 2009;16:515–523. doi: 10.1245/s10434-008-0228-0. [DOI] [PubMed] [Google Scholar]
- 13.Stoner GD, Kaighn ME, Reddel RR, Resau JH, Bowman D, Naito Z, Matsukura N, You M, Galati AJ, Harris CC. Establishment and characterization of SV40 T-antigen immortalized human esophageal epithelial cells. Cancer Res. 1991;51:365–371. [PubMed] [Google Scholar]
- 14.Dvorak K, Fass R, Dekel R, Payne CM, Chavarria M, Dvorakova B, Bernstein H, Bernstein C, Garewal H. Esophageal acid exposure at pH < or = 2 is more common in Barrett’s esophagus patients and is associated with oxidative stress. Dis Esophagus. 2006;19:366–372. doi: 10.1111/j.1442-2050.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Jones C, Gellersen O, Williams VA, Watson TJ, Peters JH. Pathogenesis of Barrett esophagus: deoxycholic acid up-regulates goblet-specific gene MUC2 in concert with CDX2 in human esophageal cells. Arch Surg. 2007;142:540–544; discussion 544-545. doi: 10.1001/archsurg.142.6.540. [DOI] [PubMed] [Google Scholar]
- 16.Arredondo J, Chernyavsky AI, Grando SA. Nicotinic receptors mediate tumorigenic action of tobacco-derived nitrosamines on immortalized oral epithelial cells. Cancer Biol Ther. 2006;5:511–517. doi: 10.4161/cbt.5.5.2601. [DOI] [PubMed] [Google Scholar]
- 17.Milo GE, Shuler CF, Stoner G, Chen JC. Conversion of premalignant human cells to tumorigenic cells by methylmethane sulfonate and methylnitronitrosoguanidine. Cell Biol Toxicol. 1992;8:193–205. doi: 10.1007/BF00156730. [DOI] [PubMed] [Google Scholar]
- 18.Rafiee P, Nelson VM, Manley S, Wellner M, Floer M, Binion DG, Shaker R. Effect of curcumin on acidic pH-induced expression of IL-6 and IL-8 in human esophageal epithelial cells (HET-1A): role of PKC, MAPKs, and NF-kappaB. Am J Physiol Gastrointest Liver Physiol. 2009;296:G388–G398. doi: 10.1152/ajpgi.90428.2008. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Nehra D, Howell P, Williams CP, Pye JK, Beynon J. Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut. 1999;44:598–602. doi: 10.1136/gut.44.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins GJ, D'Souza FR, Suzen SH, Eltahir ZS, James SA, Parry JM, Griffiths PA, Baxter JN. Deoxycholic acid at neutral and acid pH, is genotoxic to oesophageal cells through the induction of ROS: The potential role of anti-oxidants in Barrett’s oesophagus. Carcinogenesis. 2007;28:136–142. doi: 10.1093/carcin/bgl147. [DOI] [PubMed] [Google Scholar]
- 22.Lev-Ari S, Strier L, Kazanov D, Madar-Shapiro L, Dvory-Sobol H, Pinchuk I, Marian B, Lichtenberg D, Arber N. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin Cancer Res. 2005;11:6738–6744. doi: 10.1158/1078-0432.CCR-05-0171. [DOI] [PubMed] [Google Scholar]
- 23.Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, Howells L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 24.Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, Dannenberg AJ. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis. 1999;20:445–451. doi: 10.1093/carcin/20.3.445. [DOI] [PubMed] [Google Scholar]
- 25.Buttar NS, Wang KK, Leontovich O, Westcott JY, Pacifico RJ, Anderson MA, Krishnadath KK, Lutzke LS, Burgart LJ. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett’s esophagus. Gastroenterology. 2002;122:1101–1112. doi: 10.1053/gast.2002.32371. [DOI] [PubMed] [Google Scholar]
- 26.Kaur BS, Triadafilopoulos G. Acid- and bile-induced PGE(2) release and hyperproliferation in Barrett's esophagus are COX-2 and PKC-epsilon dependent. Am J Physiol Gastrointest Liver Physiol. 2002;283:G327–G334. doi: 10.1152/ajpgi.00543.2001. [DOI] [PubMed] [Google Scholar]
- 27.Bardou M, Barkun AN, Ghosn J, Hudson M, Rahme E. Effect of chronic intake of NSAIDs and cyclooxygenase 2-selective inhibitors on esophageal cancer incidence. Clin Gastroenterol Hepatol. 2004;2:880–887. doi: 10.1016/s1542-3565(04)00389-1. [DOI] [PubMed] [Google Scholar]
- 28.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 29.Jiménez P, Piazuelo E, Sánchez MT, Ortego J, Soteras F, Lanas A. Free radicals and antioxidant systems in reflux esophagitis and Barrett's esophagus. World J Gastroenterol. 2005;11:2697–2703. doi: 10.3748/wjg.v11.i18.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naya MJ, Pereboom D, Ortego J, Alda JO, Lanas A. Superoxide anions produced by inflammatory cells play an important part in the pathogenesis of acid and pepsin induced oesophagitis in rabbits. Gut. 1997;40:175–181. doi: 10.1136/gut.40.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piazuelo E, Cebrián C, Escartín A, Jiménez P, Soteras F, Ortego J, Lanas A. Superoxide dismutase prevents development of adenocarcinoma in a rat model of Barrett's esophagus. World J Gastroenterol. 2005;11:7436–7443. doi: 10.3748/wjg.v11.i47.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wetscher GJ, Hinder PR, Bagchi D, Perdikis G, Redmond EJ, Glaser K, Adrian TE, Hinder RA. Free radical scavengers prevent reflux esophagitis in rats. Dig Dis Sci. 1995;40:1292–1296. doi: 10.1007/BF02065541. [DOI] [PubMed] [Google Scholar]
- 33.Wetscher GJ, Perdikis G, Kretchmar DH, Stinson RG, Bagchi D, Redmond EJ, Adrian TE, Hinder RA. Esophagitis in Sprague-Dawley rats is mediated by free radicals. Dig Dis Sci. 1995;40:1297–1305. doi: 10.1007/BF02065542. [DOI] [PubMed] [Google Scholar]
- 34.Martin RC, Liu Q, Wo JM, Ray MB, Li Y. Chemoprevention of carcinogenic progression to esophageal adenocarcinoma by the manganese superoxide dismutase supplementation. Clin Cancer Res. 2007;13:5176–5182. doi: 10.1158/1078-0432.CCR-07-1152. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Wo JM, Su RR, Ray MB, Martin RC. Loss of manganese superoxide dismutase expression and activity in rat esophagus with external esophageal perfusion. Surgery. 2007;141:359–367. doi: 10.1016/j.surg.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 36.Sihvo EI, Salminen JT, Rantanen TK, Rämö OJ, Ahotupa M, Färkkilä M, Auvinen MI, Salo JA. Oxidative stress has a role in malignant transformation in Barrett's oesophagus. Int J Cancer. 2002;102:551–555. doi: 10.1002/ijc.10755. [DOI] [PubMed] [Google Scholar]
- 37.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 38.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 39.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 40.Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11:105–111. [PubMed] [Google Scholar]
- 41.Kurien BT, Singh A, Matsumoto H, Scofield RH. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev Technol. 2007;5:567–576. doi: 10.1089/adt.2007.064. [DOI] [PubMed] [Google Scholar]