Abstract
AIM: To represent our clinical experience in the treatment of intestinal perforation arising from typhoid fever.
METHODS: The records of 22 surgically-treated patients with typhoid intestinal perforation were evaluated retrospectively.
RESULTS: There were 18 males and 4 females, mean age 37 years (range, 8-64 years). Presenting symptoms were fever, abdominal pain, diarrhea or constipation. Sixteen cases were subjected to segmental resection and end-to-end anastomosis, while 3 cases received 2-layered primary repair following debridement, one case with multiple perforations received 2-layered primary repair and end ileostomy, one case received segmental resection and end-to-end anastomosis followed by an end ileostomy, and one case received segmental resection and end ileostomy with mucous fistula operation. Postoperative morbidity was seen in 5 cases and mortality was found in one case.
CONCLUSION: Intestinal perforation resulting from Salmonella typhi is an important health problem in Eastern and Southeastern Turkey. In management of this illness, early and appropriate surgical intervention is vital.
Keywords: Intestinal perforation, Typhoid fever, Treatment
INTRODUCTION
Typhoid fever is a febrile disease caused by Salmonella typhi, a Gram-negative bacillus, which does not present as a significant health issue in developed countries, but continues to be an important problem in tropical regions[1,2]. It is generally transmitted by the fecal-oral route and may occasionally lead to an epidemic. Typhoid fever remains a notable public health issue in regions having no adequate and proper infrastructure[3].
Although intestinal hemorrhage is the most common complication of typhoid fever, intestinal perforation continues to be the most frequent reason behind high morbidity and mortality[2]. Generally, hemorrhage and perforation occur in the terminal ileum secondary to necrosis of Peyer’s patches at 2-3 wk after the onset of the disease[4,5]. Frequency of perforation varies between 0.8% and 18%[3,6,7]. Mortality rates of typhoid intestinal perforation (TIP) cases are reported to be between 5% and 62%. Perioperative mortality rates are noted to rise up to 80% in patients who received surgery due to late perforations[2,6-9].
Studies focusing on TIP in large series, are generally reported from endemic regions[3]. In a study including 229 cases, Asefa[10] notes TIPs as one of the most important causes underlying the acute abdomen.
While early surgical procedures are regarded as definitive treatments along with preoperative resuscitation and postoperative intensive care, the methods that should be used in surgery are still contentious.
The aim of the present study is to retrospectively review TIP cases and evaluate the outcomes of this complication among patients treated in the Department of General Surgery, Faculty of Medicine, Yuzuncu Yil University of Van, which has provided healthcare services since 1994 to a wide region of Turkey encompassing many provinces and districts.
MATERIALS AND METHODS
The study included 22 cases admitted with an acute abdomen profile who were diagnosed with TIP and treated in the Department of General Surgery between 1994 and 2010. By retrospectively reviewing the patient records, the cases were analyzed in terms of demographic, medical, and surgical personal data. The cases were evaluated with regard to age, gender, number of perforations, localization of the perforation, type of operation, and morbidity and mortality rates. With the exception of 3 patients who were admitted to the Department of Internal Diseases and Department of Infectious Diseases and diagnosed with typhoid fever before being transferred to the Department of Surgery upon development of acute abdomen, all cases initially presented to the Emergency Department because of abdominal pain. The cases who were considered to be prediagnosed with acute abdomen secondary to medical history and physical examination results, were subjected to erect abdominal plain film, posterior to anterior lung film, complete blood count, complete urinalysis, and biochemical analysis including amylase. None of our patients, even those who were reported to have intraabdominal free fluid by ultrasonography, received paracentesis.
RESULTS
There were 18 males (81.8%) and 4 females (18.2%), with an age range of 8-64 years (mean, 37 years). Common symptoms were fever, abdominal pain, and vomiting. Physical examination revealed generalized peritonitis in all cases. Each patient received urinary and nasogastric catheters prior to the operation. Fluid/electrolyte imbalance was corrected and antibiotherapy was started. Three cases transferred from the Departments of Internal Diseases and Infectious Diseases were diagnosed and recorded as intestinal perforation cases, whereas the remaining patients were operated on with the prediagnoses of peptic ulcer perforation, perforated appendicitis, and generalized peritonitis. In all cases, laparotomy was performed by midline incision. Six cases demonstrated multiple perforations, while 16 cases showed perforation on the antimesenteric side, appearing similar to a staple hole. One of the cases with multiple perforations had 7 perforation foci. The location of the perforations was the jejunum in 3 cases (located at an average distance of 63 cm from the Treitz ligament), the ileum in 18 cases (located at an average distance of 50 cm from the ileocaecal valve), and the cecum in one case. Based on the preference of operating surgeons and the extent of peritoneal contamination, 15 cases (68.2%) were subjected to segmental resection and end-to-end anastomosis, while 3 cases (13.6%) received 2-layered primary repair following debridement, one case (4.55%) with multiple perforations received 2-layered primary repair and end ileostomy, one case (4.55%) received segmental resection and end-to-end anastomosis, followed by an end ileostomy, one case (4.55%) received right hemicholectomy and end-to-end anastomosis, and one case (4.55%) received segmental resection and end ileostomy with mucous fistula operation.
Pezzer drains were inserted into both subhepatic and retrovesical spaces of 4 cases, while placing 2 Pezzer drains in the retrovesical space in one case, and one Pezzer drain in the retrovesical spaces of 17 cases. The preferred surgical methods are outlined in Table 1. In all the 19 patients who had no serological or bacteriological diagnosis, typhoid fever diagnosis was verified by isolation of Salmonella typhi serologically and/or from stool. The case who presented with a sepsis profile and was subjected to ileum resection and anastomosis treatment, died postoperatively at 10 h. Five cases, including a patient who received debridement and primary repair before formation of an ileal fistula that closed spontaneously during the postoperative period, and 4 patients who exhibited wound infection, developed morbidity.
Table 1.
Preferred surgical methods
| Preferred surgical methods | Patients (n = 22) |
| Segmental resection + anastomosis | 16 |
| Jejunum | 3 |
| Ileum | 12 |
| Cecum (right hemicholectomy) | 1 |
| Debridement + 2-layered primary repair | 3 |
| 2-layered primary repair + end ileostomy | 1 |
| Segmental resection + anastomosis followed by an end ileostomy | 1 |
| Segmental resection + end ileostomy with mucous fistula | 1 |
Histopathologic results
Macroscopic view: In patients who received resection, there were ulcers in the jejunum, ileum, and cecum, which had a perforated appearance, extending parallel to the axis of the intestines and displaying a length varying between 0.2 and 2 cm. Mesenteric lymphadenomegaly was determined.
Microscopic view: The sections acquired from the ulcerated areas showed loss of mucosal integrity, enclosure of the muscular mucosa by the ulcers which also appeared to have destroyed the inner circular muscle layer, a predominance of macrophages beneath the mucosa and in the adjacent areas, and infiltrating mononuclear inflammatory cells. The typhoid nodule (Figure 1A) had macrophages containing bacteria, red blood cells, and nuclear debris from small nodular aggregates in Peyer’s patches. In typhoid ulceration (Figure 1B) macrophages, which are also defined as typhoid cells, were observed to form clusters in the mesenteric lymph ganglia.
Figure 1.
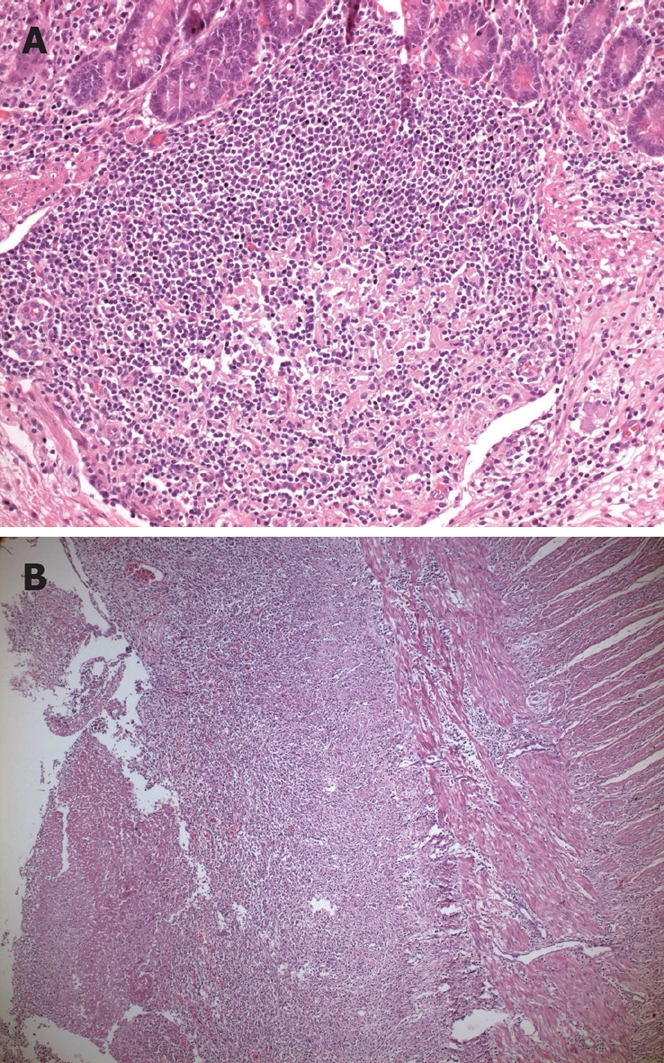
Histopathologic view of typhoid lesions. A: Typhoid nodule, there are macrophages containing bacteria, red blood cells, and nuclear debris from small nodular aggregates in Peyer’s patches (HE stain, × 20 objective); B: Typhoid ulceration (HE stain, × 5 objective).
DISCUSSION
In the current study 22 surgically treated patients with TIP were evaluated. The most common surgical intervention was segmental resection with end-to-end anastomosis.
Most fatal complications of typhoid fever are intestinal hemorrhage and enteric perforation. Those complications occur secondary to necrosis of Peyer’s patches[3,7,8]. Typhoid fever leads to hyperplasia in the reticuloendothelial system, necrosis, and ulceration limited to Peyer’s patches[1,9]. The frequency of TIP is reported to vary between 0.8% and 39%, depending on the geographic region[7-9]. Butler[11] reviewed 15 980 typhoid fever cases from the world literature and found the frequency of TIP to be 2.8%. In our country, the frequency of typhoid complications is around 20%[3]. Hosoglu et al[4] reported the frequency of TIP as 10.5%.
Those complications generally occur during the second or third week of the disease[3-5]. While typhoid fever often affects the terminal ileum, in rare cases the jejunum and cecum may also be involved[9]. TIP in appendicitis cases has been mentioned as a case report in the literature[12]. Typhoid fever is known to cause spontaneous gall bladder perforation among cases with no cholelithiasis[1]. Cecal ulcers are smaller than the ones occurring in the jejunum, and they seldom demonstrate perforation. Generally, TIP occurs as a single perforation similar to a stapler hole, and is localized on the antimesenteric side[9]. In the present study, perforations were localized in the jejunum in 3 patients, in the ileum in 18 patients, and in the antimesenteric side of the caecum in one patient. The length of the perforations localized in the jejunum and ileum, varied between 0.5 and 2 cm, while the size of the perforation in the cecum was found to be smaller, thereby being consistent with the relevant literature.
The number and size of ulcers do not have any relationship with the severity of the symptoms. Characteristically, those ulcers do not cause symptoms of peritoneal irritation before being perforated, and the peritoneal response following perforation is observed to be delayed. Unlike other perforations, in cases with TIP, the omentum does not migrate to the perforation site[9].
In the study of Ameh et al[13], fever and abdominal pain were found to be the most common symptoms, whereas guarding was observed to be the most common physical examination finding. All our 22 cases had fever, abdominal pain and peritoneal irritation signs in the physical examination. However, none of our cases demonstrated a sign of synchronous intestinal hemorrhage. Relative bradycardia is an important finding for enteric fever and it is seen more commonly among adult and adolescent patients[14]. In this series, none of the cases displayed that finding. In addition, among typhoid fever cases, hepatosplenomegaly is known to be frequent, and while it is reported to be the most common physical finding in one study[4], we did not determine even a single splenomegaly case among our patients.
TIP is encountered rarely among people under the age of 5 years and over the age of 50 years. More than 50% of the cases are seen during the second and third decades of life. Its prevalence in men is 3 times higher than in women[7,9]. Saxe et al[2] conducted a study of 112 cases with typhoid perforation and found the mean age was 20 years (range, 3-75 years) and the male/female ratio was 1.73. In another 2 similar studies, the male/female ratio was found to be 2.5 and 4[15,16]. In the stuıdy of Atamanalp et al[7], mean age was found to be 36.3 years (range, 7-68 years). In the current study, mean age was 37 years (range, 8-64 years) and the male/female ratio was 4.5. Risk factors for perforation among hospitalized patients were determined to be short symptomatic period prior to presentation, leukopenia, inadequate treatment, and being male[4]. Although the exact underlying mechanism of TIP among men is not yet known, spending longer time and consuming more food outdoors may lead to more frequent contact with the bacillus[7].
TIPs started to be treated surgically towards the end of the 1800s. As a result of understanding the pathogenesis of typhoid fever and using more effective antibiotherapies, early surgery has become the optimal treatment option for perforations[1]. However, the method to be applied in surgical treatment of TIP cases, is still contentious. From a practical point of view, the perforation site should be closed and the peritoneal cavity should be irrigated in the surgical treatment. In multiple perforations, segmental resection and anastomosis can be performed safely[2]. Rahman et al[17], found no correlation between the applied surgical procedure and the reduction in mortality. In a majority of the cases, TIP affects the ileum and primary repair is appropriate. Shah et al[18] found the rates of complications and mortality in resection-anastomosis patients were lower than in other intervention groups. Therefore, they advocated resection-anastomosis as the ideal surgical method for typhoid enteric perforations.
Similarly, Athié et al[19] performed surgery on 352 cases with typhoid ileal perforation, and found the rates of mortality and morbidity in the resection-anastomosis group were lower than in the primary closure group. They recommended a 10 cm resection from the upper and lower ends of the perforation and anastomosis (even if there is only one perforation) in cases with a perforated ileum. However, Beniwal et al[20] suggested primary closure as the first choice of treatment. Similarly, Shukla et al[21], reported a reduction in mortality rate from 35% to 10.8%, secondary to using a single-layer primary closure method. Adesunkanmi et al[22], advocated the 2-layer closure technique as the most successful surgical method regardless of the application of an omental patch. In the study of Saxe et al[2], which included 112 typhoid enteric perforation cases, 77% of the cases received primary repair for single perforation, while 19% of the cases were subjected to segmental resection because of multiple perforations. Atamanalp et al[7] performed surgery on 82 patients with typhoid ileal perforation: primary repair after debridement in 32 cases, wedge resection and primary closure in 9 cases, resection and anastomosis in 9 cases, end ileostomy after resection in 28 cases, and exteriorization in 4 cases. In multiple perforation cases where short bowel syndrome development is likely, primary repair is recommended instead of resection[3,23]. Several authors suggest ileostomy in cases with delayed multiple perforation and diffuse peritoneal contamination[22,24,25].
Recently, laparoscopic treatment methods have also been employed in TIP cases. Ramachandran et al[26] reported 6 successful laparoscopic primary closure cases. Sinha et al[27] treated 25 cases laparoscopically with a port-site infection rate of 8%.
The mortality rate in TIP cases is reported to vary between 5% and 60%[7,9,28]. Saxe[2] found a postoperative mortality rate of 16%. In the studies of Ameh[13] and Meier et al[24], the mortality rates were 20% and 39%, respectively. Atamanalp et al[7] determined a mortality rate of 11%. Although, mortality rates have shown a decrease lately, they are still at important levels. The significant differences in reported mortality rates have revealed the need to investigate the underlying reasons. Young age, inadequate medical treatment, late presentation, number of perforations and sepsis are mentioned among the factors influencing mortality[1]. Some authors claim that the number of perforations might affect prognosis[20,22]. On the other hand, Rahman et al[17] and Atamanalp et al[7] determined no significant correlation between the number of perforations, and prognosis and mortality. We found no study reporting a relationship between the localization of perforation and prognosis in the literature. In the present study, among our 22 cases, only one 62-year-old patient with sepsis died in the postoperative period. The low mortality rate in our study might be secondary to factors such as early and appropriate surgical intervention, effective perioperative resuscitation, postoperative intensive care procedures, safe anesthesia, and delivery of wide-spectrum antibiotics with low resistance.
The most common complication of TIP cases is wound infection, while the most serious is formation of a fecal fistula. Wound dehiscence, intestinal obstruction, intraabdominal abscess, empyema, bleeding diathesis, and psychosis may occur[7]. In the present study, one case developed an ileal fistula which closed spontaneously in the postoperative period and 4 cases developed wound infection (5 complications in total).
In conclusion, the treatment of TIP consists of appropriate early surgical intervention, effective resuscitation in the preoperative period, postoperative care, and use of proper antibiotics. Although primary closure is the most frequently recommended procedure, segmentary resection and end-to-end anastomosis may be reserved for patients with multiple perforations. Segmentary resection and end-to-end anastomosis has low mortality and morbidity rates. Thus resection-anastomosis should be used as a surgical treatment method for TIP. Ileostomy is associated with high mortality and morbidity. However, it may be life-saving in patients with severe abdominal contamination.
COMMENTS
Background
Typhoid fever is a febrile disease caused by Salmonella typhi and is an important problem in tropical regions. Progression of disease is associated most commonly with hemorrhage and intestinal perforation.
Research frontiers
Typhoid perforation is an important complication of typhoid fever. It is seen rarely, but shows a high mortality and morbidity. The mainstay of treatment of typhoid intestinal perforation is surgery.
Innovations and breakthroughs
Recent reports have highlighted the importance of surgical treatment. Pathogenesis of typhoid perforation indicates the need for more effective antibiotic therapies and early surgery.
Applications
Although primary closure is the most frequently recommended procedure, this research opens the way for new surgical options such as segmentary resection and end-to-end anastomosis.
Peer review
After reviewing the literature on surgical aspects, authors should recommend the surgery of choice and should be included in conclusions.
Footnotes
Peer reviewer: Radha K Dhiman, Associate Professor, Department of Hepatology, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India
S- Editor Wang YR L- Editor Cant MR E- Editor Lin YP
References
- 1.Saxena V, Basu S, Sharma CL. Perforation of the gall bladder following typhoid fever-induced ileal perforation. Hong Kong Med J. 2007;13:475–477. [PubMed] [Google Scholar]
- 2.Saxe JM, Cropsey R. Is operative management effective in treatment of perforated typhoid? Am J Surg. 2005;189:342–344. doi: 10.1016/j.amjsurg.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 3.Kotan C, Kosem M, Tuncer I, Kisli E, Sönmez R, Çıkman Ö, Söylemez Ö, Arslantürk H. Typhoid intestinal perforation:Review of 11 cases. Kolon Rektum Hast Derg. 2000;11:6–10. [Google Scholar]
- 4.Hosoglu S, Aldemir M, Akalin S, Geyik MF, Tacyildiz IH, Loeb M. Risk factors for enteric perforation in patients with typhoid Fever. Am J Epidemiol. 2004;160:46–50. doi: 10.1093/aje/kwh172. [DOI] [PubMed] [Google Scholar]
- 5.Dutta TK, Beeresha , Ghotekar LH. Atypical manifestations of typhoid fever. J Postgrad Med. 2001;47:248–251. [PubMed] [Google Scholar]
- 6.Pegues DA, Miller SI. Salmonella Species, Including Salmonella Typhi. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Elsevier Churchill Livingstone; 2009. pp. 2287–2903. [Google Scholar]
- 7.Atamanalp SS, Aydinli B, Ozturk G, Oren D, Basoglu M, Yildirgan MI. Typhoid intestinal perforations: twenty-six year experience. World J Surg. 2007;31:1883–1888. doi: 10.1007/s00268-007-9141-0. [DOI] [PubMed] [Google Scholar]
- 8.Mock CN, Amaral J, Visser LE. Improvement in survival from typhoid ileal perforation. Results of 221 operative cases. Ann Surg. 1992;215:244–249. doi: 10.1097/00000658-199203000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eggleston FC, Santoshi B, Singh CM. Typhoid perforation of the bowel. Experiences in 78 cases. Ann Surg. 1979;190:31–35. doi: 10.1097/00000658-197907000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asefa Z. Pattern of acute abdomen in Yirgalem Hospital, southern Ethiopia. Ethiop Med J. 2000;38:227–235. [PubMed] [Google Scholar]
- 11.Butler T, Knight J, Nath SK, Speelman P, Roy SK, Azad MA. Typhoid fever complicated by intestinal perforation: a persisting fatal disease requiring surgical management. Rev Infect Dis. 1985;7:244–256. doi: 10.1093/clinids/7.2.244. [DOI] [PubMed] [Google Scholar]
- 12.Golakai VK, Makunike R. Perforation of terminal ileum and appendix in typhoid enteritis: report of two cases. East Afr Med J. 1997;74:796–799. [PubMed] [Google Scholar]
- 13.Ameh EA. Typhoid ileal perforation in children: a scourge in developing countries. Ann Trop Paediatr. 1999;19:267–272. doi: 10.1080/02724939992356. [DOI] [PubMed] [Google Scholar]
- 14.Katar S, Onur H, Yaramıs A, Ozbek MN, Ecer S. Enteric fever in 19 children cases with positive hemocultures. Çocuk Sağlığı ve Hastalıkları Dergisi. 2006;49:19–23. [Google Scholar]
- 15.Onen A, Dokucu AI, Ciğdem MK, Oztürk H, Otçu S, Yücesan S. Factors effecting morbidity in typhoid intestinal perforation in children. Pediatr Surg Int. 2002;18:696–700. doi: 10.1007/s00383-002-0794-3. [DOI] [PubMed] [Google Scholar]
- 16.Agbakwuru EA, Adesunkanmi AR, Fadiora SO, Olayinka OS, Aderonmu AO, Ogundoyin OO. A review of typhoid perforation in a rural African hospital. West Afr J Med. 2003;22:22–25. doi: 10.4314/wajm.v22i1.27973. [DOI] [PubMed] [Google Scholar]
- 17.Rahman GA, Abubakar AM, Johnson AW, Adeniran JO. Typhoid ileal perforation in Nigerian children: an analysis of 106 operative cases. Pediatr Surg Int. 2001;17:628–630. doi: 10.1007/s003830100008. [DOI] [PubMed] [Google Scholar]
- 18.Shah AA, Wani KA, Wazir BS. The ideal treatment of the typhoid enteric perforation - resection anastomosis. Int Surg. 1999;84:35–38. [PubMed] [Google Scholar]
- 19.Athié CG, Guízar CB, Alcántara AV, Alcaraz GH, Montalvo EJ. Twenty-five years of experience in the surgical treatment of perforation of the ileum caused by Salmonella typhi at the General Hospital of Mexico City, Mexico. Surgery. 1998;123:632–636. doi: 10.1016/s0039-6060(98)70201-6. [DOI] [PubMed] [Google Scholar]
- 20.Beniwal US, Jindal P, Sharma J, Jain S, Shyam G. Comparative study of postoperative procedures in typhoid perforation. Indian J Surg. 2003;65:172–177. [Google Scholar]
- 21.Shukla VK, Sahoo SP, Chauhan VS, Pandey M, Gautam A. Enteric perforation--single-layer closure. Dig Dis Sci. 2004;49:161–164. doi: 10.1023/b:ddas.0000011620.56077.97. [DOI] [PubMed] [Google Scholar]
- 22.Adesunkanmi AR, Ajao OG. The prognostic factors in typhoid ileal perforation: a prospective study of 50 patients. J R Coll Surg Edinb. 1997;42:395–399. [PubMed] [Google Scholar]
- 23.Eustache JM, Kreis DJ Jr. Typhoid perforation of the intestine. Arch Surg. 1983;118:1269–1271. doi: 10.1001/archsurg.1983.01390110027007. [DOI] [PubMed] [Google Scholar]
- 24.Meier DE, Tarpley JL. Typhoid intestinal perforations in Nigerian children. World J Surg. 1998;22:319–323. doi: 10.1007/s002689900388. [DOI] [PubMed] [Google Scholar]
- 25.Gedik E, Girgin S, Taçyildiz IH, Akgün Y. Risk factors affecting morbidity in typhoid enteric perforation. Langenbecks Arch Surg. 2008;393:973–977. doi: 10.1007/s00423-007-0244-8. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran CS, Agarwal S, Dip DG, Arora V. Laparoscopic surgical management of perforative peritonitis in enteric fever: a preliminary study. Surg Laparosc Endosc Percutan Tech. 2004;14:122–124. doi: 10.1097/01.sle.0000129387.76641.29. [DOI] [PubMed] [Google Scholar]
- 27.Sinha R, Sharma N, Joshi M. Laparoscopic repair of small bowel perforation. JSLS. 2005;9:399–402. [PMC free article] [PubMed] [Google Scholar]
- 28.Ekenze SO, Ikefuna AN. Typhoid intestinal perforation under 5 years of age. Ann Trop Paediatr. 2008;28:53–58. doi: 10.1179/146532808X270680. [DOI] [PubMed] [Google Scholar]


