Abstract
AIM: To determine the general risk factors affecting the failure rate of first-line eradication therapy in Japanese patients with Helicobacter pylori (H. pylori) infection.
METHODS: The present study enrolled 253 patients who had an H. pylori infection, underwent gastro-endoscopy, and were treated with H. pylori eradication therapy. Eradication therapy consisted of 30 mg lansoprazole plus 750 mg amoxicillin and 400 mg clarithromycin twice daily for 7 d. All of the patients underwent a 13C urea breath test at least 1 mo after the completion of eradication therapy. The current study investigated the independent factors associated with successful H. pylori eradication using a multiple logistic regression analysis.
RESULTS: The overall success rate in the patients was 85.8%. Among the general factors examined in the multivariate analyses, only having an age less than 50 years was found to be significantly associated with a poor response to H. pylori eradication. Moreover, side effects were the only clinical factors in the patients who were under 50 years of age that significantly influenced the poor response to H. pylori eradication.
CONCLUSION: H. pylori-positive elderly patients should undergo eradication therapy. In addition, it is necessary to improve H. pylori eradication therapy in younger patients.
Keywords: Helicobacter pylori, Eradication, Treatment, Age, Side effect
INTRODUCTION
Helicobacter pylori (H. pylori) infections cause chronic gastritis and are associated with an increased risk of major upper gastrointestinal diseases, such as peptic ulcer disease, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma[1]. Proton-pump inhibitor (PPI)-based triple therapy, with a PPI, clarithromycin (CAM), and either amoxicillin (AMPC) or metronidazole, is a widely recommended eradication therapy[2]. In addition, this triple therapy has been approved by the medical insurance system in Japan, and the eradication therapy is administered by physicians in general practice. Consensus conferences have recommended therapeutic regimens that achieve H. pylori cure rates of over 80% on an intent-to-treat basis[3-5]. However, several large-scale clinical trials and meta-analyses have demonstrated that the most common first-line therapies fail in up to 20% of patients[6,7], and in the clinical setting, the actual treatment failure rate may be even higher[8]. Few studies have examined the causes of patient failure in these therapies among the general clinical factors in Japan. The aims of the current study were to determine the general risk factors that affect the failure rate of first-line eradication therapy in Japanese patients with H. pylori infection using a multivariate analysis.
MATERIALS AND METHODS
The current series included 253 patients who underwent a gastro-endoscopy between January 2006 and September 2007 in the Shinko hospital in Kobe, Japan, and who were diagnosed with an H. pylori infection by the presence of the bacterium at endoscopy, detection of the bacteria or an antibody in patient urine, or a positive 13C urea breath test (UBT; with a cut-off value of 2.5‰; Ubit, Otsuka Pharmaceuticals, Tokyo, Japan). All patients were treated with H. pylori eradication therapy, consisting of 30 mg lansoprazole, 750 mg amoxicillin, and 400 mg clarithromycin twice daily for 7 d. Patient compliance and treatment-related side effects were assessed at the end of the treatment period. The patients underwent a UBT at least 1 mo after the completion of eradication therapy. Successful H. pylori eradication was defined as a negative result on the UBT. Independent factors that may have been associated with successful H. pylori eradication were studied using multiple logistic regression analysis. The following variables were evaluated as independent factors: sex, age (generation), diagnosis, and side effects. The StatView system software package (version 5.0) was used for the statistical analysis. A P-value of less than 0.05 was considered to be statistically significant.
RESULTS
Demographic and clinical features of the patients who underwent first-line eradication therapy
Table 1 lists the demographic and clinical features of the patients who underwent H. pylori eradication therapy. Adverse events were observed in 41 patients within the first week after the initiation of the eradication therapy. The most common adverse events included diarrhea and gustatory dysfunction. None of the patients withdrew from the treatment course due to eradication therapy-related side effects.
Table 1.
Demographic and clinical characteristics of patients undergoing eradication therapy for an Helicobacter pylori infection
| n = 253 | |
| Sex | |
| Male | 174 |
| Female | 79 |
| Generation (s) | |
| 20 | 10 |
| 30 | 15 |
| 40 | 50 |
| 50 | 92 |
| 60 | 54 |
| 70 | 27 |
| > 80 | 5 |
| Diagnosis | |
| Ulcer | 165 |
| Non-ulcer | 88 |
| Side effect | |
| No | 212 |
| Yes | 41 |
Ulcer: A peptic ulcer was detected by gastroscopy; Non-ulcer: No peptic ulcer was detected by gastroscopy (instead, for example, atrophic gastritis, among others); Side effects: Diarrhea (n = 23), gustatory dysfunction (n = 10), among others.
Success rate of first-line eradication therapy
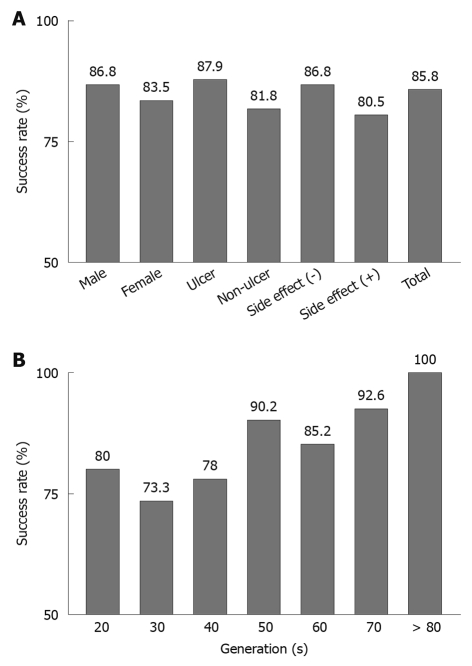
The overall success rate in the patients was 85.8%. Figure 1A shows the success rate of the first-line eradication therapy in patients with H. pylori infection when correlated with the patients’ sex, diagnoses, and side effects. The success rate tended to be higher in patients with peptic ulcers than in those without peptic ulcers (87.9% vs 81.8%). Figure 1B shows the success rates according to the patient’s age. A good success rate was observed, even among elderly patients.
Figure 1.
Success rate of first-line eradication therapy. A: Success rates according to sex (male, female), diagnosis (ulcer or non-ulcer), side effects [no (-) or yes (+)], and the total patient success rate of eradication therapy for the treatment of Helicobacter pylori (H. pylori); B: Success rates according to the patient generation of eradication therapy for H. pylori.
Multivariate analysis of independent factors for the patient response to first-line eradication therapy
The independent factors associated with the response to first-line H. pylori eradication therapy were evaluated by a multivariate analysis. The only factor (among sex, age, diagnosis, and side effects) examined in the multivariate analysis that was found to be significantly associated with a poor response to first eradication therapy was patient age of less than 50 years of age (P = 0.015, Table 2). Moreover, side effects were the only independent factor significantly associated with a poor response in patients under 50 years of age (P = 0.033, Table 3).
Table 2.
A multivariate analysis of all patients (n = 253)
| Variable | Multivariate OR | 95% CI | P-value |
| Sex (male vs female) | 1.29 | 0.62-2.71 | 0.50 |
| Age (under 50 yr vs over 50 yr) | 0.41 | 0.20-0.84 | 0.015a |
| Disease (ulcer vs non-ulcer) | 1.61 | 0.79-3.30 | 0.19 |
| Side effect (no vs yes) | 1.59 | 0.67-3.80 | 0.29 |
Statistically significant. OR: Odds ratio; CI: Confidence interval.
Table 3.
Multivariate analysis of under 50-yr-old patients (n = 75)
| Variable | Multivariate OR | 95% CI | P-value |
| Sex (male vs female) | 1.46 | 0.49-4.33 | 0.50 |
| Disease (ulcer vs non-ulcer) | 0.79 | 0.24-2.57 | 0.70 |
| Side effect (no vs yes) | 3.97 | 1.12-14.16 | 0.033a |
Statistically significant. OR: Odds ratio; CI: Confidence interval.
DISCUSSION
The current study showed that first-line eradication treatment failures occurred significantly more frequently in patients aged less than 50 years of age. Elderly patients are frequently infected with H. pylori for a longer period of time, present with enlarged atrophic gastritis, and progress to intestinal metaplasia. These patients may experience easier H. pylori eradication with first-line eradication treatment. In addition, the gastric mucosa becomes more atrophic in elderly patients in comparison to younger patients, and elderly patients also display gastric acid hyposecretion. This can compromise their ability to inactivate the AMPC and CAM. These conditions may contribute to the current results.
Pretreatment antibiotic resistance is the primary reason some patients do not respond to initial treatment[9-13]. In particular, antibiotic CAM resistance has been identified as a major factor affecting the ability to cure H. pylori infection, and the rate of resistance to this antibiotic is increasing in many geographical areas[14,15]. A similar tendency is believed to be occurring in Japan. The efficacy of PPI-based regimens is decreasing, and several studies have reported intention-to-treat eradication rates lower than 75%[16-24]. The overall success rate in patients was greater than 85% in the current series. This high success rate may be due to patients having been diagnosed with an H. pylori infection during the medical examination, and the fact that few patients were receiving any medication. Future studies will examine whether patients with H. pylori infections display antibiotic resistance.
The only factors that significantly influenced the response to eradication therapy in patients were being under 50 years of age and diarrhea, which was the most common side effect for half of the patients. It is necessary to determine how to increase the treatment success rate among younger patients. A previous study from Japan reported that the use of the probiotic bacterium Clostridium butyricum MIYAIRI 588 strain reduced fluctuations in the intestinal flora and decreased the incidence of gastrointestinal side effects[25]. Supplementation with probiotics may have several beneficial effects on H. pylori eradication, especially in younger patients.
Elderly patients also exhibited a good success rate in the current series. A previous report showed that approximately 53%-73% of elderly peptic ulcer patients are H. pylori-positive; however, the percentage of H. pylori-positive elderly patients who are treated for an H. pylori infection remains low. One-week PPI-based triple therapy regimens are highly effective and well tolerated in elderly patients[26]. Therefore, it is recommended that H. pylori-positive elderly patients be treated with eradication therapy.
In conclusion, the current study was conducted to determine the general risk factors that affect the failure rate of first-line eradication therapy in Japanese patients with an H. pylori infection. The overall success rate in patients was 85.8%. Among the general factors examined in the multivariate analyses, a patient age of less than 50 years significantly influenced the response to H. pylori eradication. Moreover, among the other clinical factors in patients under 50 years of age, only the presence of side effects was found to be significantly associated with the response to H. pylori eradication. It is necessary to determine how to increase the eradication success rate among younger patients.
COMMENTS
Background
Proton-pump inhibitor (PPI)-based triple therapy with PPI, clarithromycin, and amoxicillin is a widely recommended eradication therapy for Helicobacter pylori (H. pylori) in Japan.
Research frontiers
Few studies have described the causes of patient failure with these therapies with regard to general clinical factors in Japan.
Innovations and breakthroughs
Among the general factors examined in the multivariate analyses, only a patient age of less than 50 years was significantly related to the response to H. pylori eradication treatments. Moreover, among the other clinical factors in patients under 50 years of age, only the presence of treatment side effects was found to be significantly associated with the patient’s response to H. pylori eradication.
Applications
H. pylori-positive elderly patients should undergo eradication therapy, and it is necessary to determine how to increase the success rate among younger patients in Japan.
Peer review
The subject of this article to examine the results of H. pylori eradication is important. There are geographic differences in antibiotic resistance affecting the eradication results and the antimicrobial resistance may vary in time. Also, reports of other factors affecting the clinical treatment results are welcome.
Footnotes
Peer reviewer: Dr. Lea Veijola, Consultant gastroenterologist, Herttoniemi Hospital, Health Care of City of Helsinki, Kettutie 8, Helsinki, 00800, Finland
S- Editor Tian L L- Editor Logan S E- Editor Lin YP
References
- 1.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 2.Fuccio L, Minardi ME, Zagari RM, Grilli D, Magrini N, Bazzoli F. Meta-analysis: duration of first-line proton-pump inhibitor based triple therapy for Helicobacter pylori eradication. Ann Intern Med. 2007;147:553–562. doi: 10.7326/0003-4819-147-8-200710160-00008. [DOI] [PubMed] [Google Scholar]
- 3.Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2330–2338. doi: 10.1111/j.1572-0241.1998.00684.x. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P, Mégraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection--the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167–180. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 5.Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gisbert JP, González L, Calvet X, García N, López T, Roqué M, Gabriel R, Pajares JM. Proton pump inhibitor, clarithromycin and either amoxycillin or nitroimidazole: a meta-analysis of eradication of Helicobacter pylori. Aliment Pharmacol Ther. 2000;14:1319–1328. doi: 10.1046/j.1365-2036.2000.00844.x. [DOI] [PubMed] [Google Scholar]
- 7.Gisbert JP, Pajares R, Pajares JM. Evolution of Helicobacter pylori therapy from a meta-analytical perspective. Helicobacter. 2007;12 Suppl 2:50–58. doi: 10.1111/j.1523-5378.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- 8.Gisbert JP. "Rescue" regimens after Helicobacter pylori treatment failure. World J Gastroenterol. 2008;14:5385–5402. doi: 10.3748/wjg.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houben MH, van de Beek D, Hensen EF, Craen AJ, Rauws EA, Tytgat GN. A systematic review of Helicobacter pylori eradication therapy--the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999;13:1047–1055. doi: 10.1046/j.1365-2036.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- 10.Peitz U, Hackelsberger A, Malfertheiner P. A practical approach to patients with refractory Helicobacter pylori infection, or who are re-infected after standard therapy. Drugs. 1999;57:905–920. doi: 10.2165/00003495-199957060-00006. [DOI] [PubMed] [Google Scholar]
- 11.Dore MP, Leandro G, Realdi G, Sepulveda AR, Graham DY. Effect of pretreatment antibiotic resistance to metronidazole and clarithromycin on outcome of Helicobacter pylori therapy: a meta-analytical approach. Dig Dis Sci. 2000;45:68–76. doi: 10.1023/a:1005457226341. [DOI] [PubMed] [Google Scholar]
- 12.van der Wouden EJ, Thijs JC, van Zwet AA, Sluiter WJ, Kleibeuker JH. The influence of in vitro nitroimidazole resistance on the efficacy of nitroimidazole-containing anti-Helicobacter pylori regimens: a meta-analysis. Am J Gastroenterol. 1999;94:1751–1759. doi: 10.1111/j.1572-0241.1999.01202.x. [DOI] [PubMed] [Google Scholar]
- 13.Tompkins DS, Perkin J, Smith C. Failed treatment of Helicobacter pylori infection associated with resistance to clarithromycin. Helicobacter. 1997;2:185–187. doi: 10.1111/j.1523-5378.1997.tb00085.x. [DOI] [PubMed] [Google Scholar]
- 14.Vakil N, Hahn B, McSorley D. Clarithromycin-resistant Helicobacter pylori in patients with duodenal ulcer in the United States. Am J Gastroenterol. 1998;93:1432–1435. doi: 10.1111/j.1572-0241.1998.455_t.x. [DOI] [PubMed] [Google Scholar]
- 15.Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53:1374–1384. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkey CJ, Atherton JC, Treichel HC, Thjodleifsson B, Ravic M. Safety and efficacy of 7-day rabeprazole- and omeprazole-based triple therapy regimens for the eradication of Helicobacter pylori in patients with documented peptic ulcer disease. Aliment Pharmacol Ther. 2003;17:1065–1074. doi: 10.1046/j.1365-2036.2003.01492.x. [DOI] [PubMed] [Google Scholar]
- 17.Paoluzi P, Iacopini F, Crispino P, Nardi F, Bella A, Rivera M, Rossi P, Gurnari M, Caracciolo F, Zippi M, et al. 2-week triple therapy for Helicobacter pylori infection is better than 1-week in clinical practice: a large prospective single-center randomized study. Helicobacter. 2006;11:562–568. doi: 10.1111/j.1523-5378.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- 18.Veldhuyzen Van Zanten S, Machado S, Lee J. One-week triple therapy with esomeprazole, clarithromycin and metronidazole provides effective eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;17:1381–1387. doi: 10.1046/j.1365-2036.2003.01554.x. [DOI] [PubMed] [Google Scholar]
- 19.Vakil N, Lanza F, Schwartz H, Barth J. Seven-day therapy for Helicobacter pylori in the United States. Aliment Pharmacol Ther. 2004;20:99–107. doi: 10.1111/j.1365-2036.2004.02029.x. [DOI] [PubMed] [Google Scholar]
- 20.Laine L, Fennerty MB, Osato M, Sugg J, Suchower L, Probst P, Levine JG. Esomeprazole-based Helicobacter pylori eradication therapy and the effect of antibiotic resistance: results of three US multicenter, double-blind trials. Am J Gastroenterol. 2000;95:3393–3398. doi: 10.1111/j.1572-0241.2000.03349.x. [DOI] [PubMed] [Google Scholar]
- 21.Laine L, Suchower L, Frantz J, Connors A, Neil G. Twice-daily, 10-day triple therapy with omeprazole, amoxicillin, and clarithromycin for Helicobacter pylori eradication in duodenal ulcer disease: results of three multicenter, double-blind, United States trials. Am J Gastroenterol. 1998;93:2106–2112. doi: 10.1111/j.1572-0241.1998.00602.x. [DOI] [PubMed] [Google Scholar]
- 22.Calvet X, Ducons J, Bujanda L, Bory F, Montserrat A, Gisbert JP. Seven versus ten days of rabeprazole triple therapy for Helicobacter pylori eradication: a multicenter randomized trial. Am J Gastroenterol. 2005;100:1696–1701. doi: 10.1111/j.1572-0241.2005.50019.x. [DOI] [PubMed] [Google Scholar]
- 23.Gisbert JP, Domínguez-Muñoz A, Domínguez-Martín A, Gisbert JL, Marcos S. Esomeprazole-based therapy in Helicobacter pylori eradication: any effect by increasing the dose of esomeprazole or prolonging the treatment? Am J Gastroenterol. 2005;100:1935–1940. doi: 10.1111/j.1572-0241.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- 24.Della Monica P, Lavagna A, Masoero G, Lombardo L, Crocellá L, Pera A. Effectiveness of Helicobacter pylori eradication treatments in a primary care setting in Italy. Aliment Pharmacol Ther. 2002;16:1269–1275. doi: 10.1046/j.1365-2036.2002.01244.x. [DOI] [PubMed] [Google Scholar]
- 25.Shimbo I, Yamaguchi T, Odaka T, Nakajima K, Koide A, Koyama H, Saisho H. Effect of Clostridium butyricum on fecal flora in Helicobacter pylori eradication therapy. World J Gastroenterol. 2005;11:7520–7524. doi: 10.3748/wjg.v11.i47.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilotto A. Aging and upper gastrointestinal disorders. Best Pract Res Clin Gastroenterol. 2004;18 Suppl:73–81. doi: 10.1016/j.bpg.2004.06.015. [DOI] [PubMed] [Google Scholar]